Abstract
Escherichia coli (E. coli) was identified among the most relevant antimicrobial‐resistant (AMR) bacteria in the EU for dogs and cats, horses, swine, poultry, cattle, sheep and goats in previous scientific opinions. Thus, it has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on its eligibility to be listed, Annex IV for its categorisation according to disease prevention and control rules as in Article 9 and Article 8 for listing animal species related to the bacterium. The assessment has been performed following a methodology previously published. The outcome is the median of the probability ranges provided by the experts, which indicates whether each criterion is fulfilled (lower bound ≥ 66%) or not (upper bound ≤ 33%), or whether there is uncertainty about fulfilment. Reasoning points are reported for criteria with uncertain outcome. According to the assessment here performed, it is uncertain whether AMR E. coli can be considered eligible to be listed for Union intervention according to Article 5 of the AHL (33–66% probability). According to the criteria in Annex IV, for the purpose of categorisation related to the level of prevention and control as in Article 9 of the AHL, the AHAW Panel concluded that the bacterium does not meet the criteria in Sections 1, 2, 3 and 4 (Categories A, B, C and D; 0–5%, 5–10%, 10–33% and 10–33% probability of meeting the criteria, respectively) and the AHAW Panel was uncertain whether it meets the criteria in Section 5 (Category E, 33–66% probability of meeting the criteria). The animal species to be listed for AMR E. coli according to Article 8 criteria include mammals, birds, reptiles and fish.
Keywords: antimicrobial resistance, Escherichia coli, Animal Health Law, listing, categorisation, impact
1. Introduction
The European Food Safety Authority (EFSA) received a mandate from the European Commission to investigate the global state of play as regards antimicrobial‐resistant (AMR) animal pathogens that cause transmissible animal diseases (Term of Reference (ToR) 1), to identify the most relevant AMR bacteria in the European Union (EU) (first part of ToR 2), to summarise the existing or potential animal health impact of those identified bacteria in the EU (second part of ToR 2) and to perform the assessment of those bacteria to be listed and categorised according to the criteria in Article 5, Annex IV according to Article 9 and Article 8 within the Regulation (EU) No 2016/429 1 on transmissible animal diseases (‘Animal Health Law’) (ToR 3).
The global state of play for AMR animal pathogens that cause transmissible animal diseases (ToR 1) and the results of the assessment of the most relevant AMR bacteria in the EU (first part of ToR 2) for dogs and cats, horses, swine, poultry, cattle, sheep and goats were published in separate EFSA scientific opinions (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f).
According to the results of the assessment already conducted, Escherichia coli (E. coli) was identified among the most relevant AMR bacteria in the EU for dogs and cats, horses, swine, poultry, cattle, sheep and goats due to frequent involvement of E. coli as the causative agent of a range of diseases in all animal species and the high levels of phenotypic resistance to most of the antimicrobial classes commonly used to treat these diseases reported in strains of animal origin.
This scientific opinion presents the results of the assessment on AMR E. coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats on its eligibility to be listed and categorised within the AHL framework. Special focus is placed on the animal health impact of AMR E. coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats in the EU, which is also summarised here as part of the assessment conducted according to the profile of the infection and its impact on animal welfare (Article 7).
1.1. Background and Terms of Reference as provided by the requestor
The background and ToRs as provided by the European Commission for the present document are reported in Sections 1.1 and 1.2 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021g).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToRs is as in Sections 1.2.3 and 1.3.3 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021g).
The present document reports the results of the assessment on AMR E. coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats according to the criteria of the AHL articles as follows:
Article 7: AMR E. coli infection profile and impacts;
Article 5: eligibility of AMR E. coli infection to be listed;
Article 9: categorisation of AMR E. coli infection according to disease prevention and control rules as in Annex IV;
Article 8: list of animal species (also apart from dogs and cats, horses, swine, poultry, cattle, sheep and goats) related to AMR E. coli infection.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for listing and categorisation of animal diseases within the AHL framework (EFSA AHAW Panel, 2017).
In order to take into account the specifics related to animal diseases caused by bacteria resistant to antimicrobials, the term ‘disease’ as in the AHL was interpreted in a broader sense, referring also to colonisation by commensal and potentially opportunistic bacteria, and the general presence of the identified AMR bacteria in the EU, depending on each criterion.
The following assessment was performed by the EFSA Panel on Animal Health and Welfare (AHAW) based on the information collected and compiled in form of a fact sheet as in Section 3.1 of the present document. The outcome is the median of the probability ranges provided by the experts, which are accompanied by verbal interpretations only when they fall within the ranges as spelled out in Table 1.
Table 1.
Approximate probability scale recommended for harmonised use in EFSA (EFSA Scientific Committee, 2018)
| Probability term | Subjective probability range |
|---|---|
| Almost certain | 99–100% |
| Extremely likely | 95–99% |
| Very likely | 90–95% |
| Likely | 66–90% |
| About as likely as not | 33–66% |
| Unlikely | 10–33% |
| Very unlikely | 5–10% |
| Extremely unlikely | 1–5% |
| Almost impossible | 0–1% |
3. Assessment
3.1. Assessment of AMR Escherichia coli according to Article 7 criteria of the AHL
3.1.1. Article 7(a) Disease profile
E. coli is present in the intestinal microbiota of mammals and birds, being mostly commensal although some strains can cause severe to life‐threatening intestinal and extra‐intestinal infections in humans and animals, and can survive and even grow outside the host (Poirel et al., 2018; Loayza et al., 2020; Valat et al., 2020). Pathogenic E. coli can be classified into different pathotypes based on the presence of certain virulence factors which confer specific pathogenic characteristics. Intestinal infections result in more or less severe diarrhoea caused by different E. coli pathotypes, such as enterotoxigenic, enteropathogenic or enterohaemorrhagic E. coli (ETEC, EPEC and EHEC, respectively), potentially evolving into a haemolytic uraemic syndrome (HUS) in the case of EHEC (Bélanger et al., 2011). Extra‐intestinal pathogenic E. coli (ExPEC) are another important group of pathogenic E. coli causing a diversity of infections in animals including urinary tract infections (UTIs), meningitis, septicaemia, bovine mastitis and colibacillosis in poultry caused by avian pathogenic E. coli (APEC). Uropathogenic E. coli (UPEC) can colonise the urinary tract and cause cystitis and pyelonephritis, which can lead to urosepsis (Bélanger et al., 2011).
E. coli has been recognised by the European Centre for Disease Prevention and Control (ECDC) as an excellent indicator for antimicrobial resistance surveillance because of its ubiquity, frequent exposure to systemic antimicrobial treatment and great genomic plasticity (EFSA and ECDC, 2017). The prevalence of acquired resistance in commensal E. coli also indirectly indicates the magnitude of the selective pressure from the use of antibiotics in an animal population. Although E. coli is intrinsically susceptible to almost all clinically relevant antimicrobial agents, this species has a great capacity to acquire antibiotic resistance genes, mostly through horizontal gene transfer, including those coding for: extended‐spectrum β‐lactamases (ESBLs) (conferring resistance to penicillins, aminopenicillins, cephalosporins, third‐generation cephalosporins and the fourth‐generation cephalosporin cefquinome), AmpC β‐lactamases (conferring resistance to penicillins, third‐generation cephalosporins (ceftazidime, cefotaxime), cephamycin and (variably) aztreonam), carbapenemases (conferring resistance to carbapenems), 16S rRNA methylases (conferring pan‐resistance to aminoglycosides), plasmid‐mediated quinolone resistance (PMQR) (conferring decreased susceptibility to (fluoro)quinolones) and plasmid‐mediated polymyxin resistance (MCR) (conferring resistance to polymyxins) (Poirel et al., 2018).
Information provided in this fact sheet has been specified for E. coli resistant to antibiotics of veterinary importance, namely those categorised as B (quinolones, third‐ and fourth‐generation cephalosporins, polymyxins), C (aminoglycosides, first‐ and second‐generation cephalosporins, cephamycins, macrolides, lincosamides, pleuromutilins, rifaximin) and D (aminopenicillins, natural penicillins, isoxazolyl penicillin) by the Antimicrobial Advice ad hoc Expert Group (AMEG), and adopted by both the European Medicines Agency (EMA)’s Committee for Veterinary Medicinal Products (CVMP) and Committee for Medicinal Products for Human Use (CHMP) in line with EMA’s support of a ‘One Health’ approach that promotes close and integrated cooperation between human and veterinary medicine. AMR or multidrug‐resistant (MDR) (non‐susceptible to at least one agent in ≥ three antimicrobial categories) E. coli is hereby described in the following animal species of interest: dogs and cats, horses, swine, poultry, cattle, sheep and goats. Verotoxigenic E. coli (VTEC) is not in the scope of this fact sheet, as it is listed among the zoonotic agents covered by Directive 2003/99/EC 2 . Whenever information reported in the fact sheet is not further elaborated in terms of antimicrobial resistance, it is because the information available does not specify antimicrobial resistance.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Dogs and cats. In dogs (Canis lupus familiaris) and cats (Felis catus), E. coli is the leading cause of UTIs, accounting for 50–60% of those infections (EFSA and ECDC, 2017). Other diseases such as bacteraemia and pyometra have also been reported (Greiner et al., 2008; Hagman, 2018).
Horses. In horses (Equus caballus), E. coli has been mostly associated with urinary and reproductive infections, respiratory diseases and infections of soft tissues and wounds (Maddox et al., 2015; SVARM, 2020; Isgren et al., 2021).
Cattle, sheep and goats. In cattle (Bos taurus), sheep (Ovis aries) and goats (Capra hircus), the most frequently reported infections associated with E. coli include intestinal infections and septicaemia in calves, lambs and goat kids and mastitis in adult dairy animals. The latter is non‐contagious and occurs through environmental contamination of the udder. Other less common presentations include peritonitis, cystitis/pyelonephritis, endometritis, wound infections and meningitis derived from sepsis (Gay, 1965; Besser and Gay, 1985; Smith et al., 1985; CABI, 2019; EFSA AHAW Panel, 2021e).
Poultry. Regarding poultry species, including chickens (Gallus gallus), turkeys (Meleagris gallopavo) and others (e.g. duck, geese, quail, ostrich), APEC can cause diverse localised or systemic infections, designated as avian colibacillosis. All avian species are susceptible to APEC infections, which includes colisepticaemia, haemorrhagic septicaemia, coligranuloma (Hjarre’s disease), air sac disease (chronic respiratory disease), swollen‐head syndrome, venereal colibacillosis, coliform cellulitis (inflammatory or infectious process), peritonitis, salpingitis, orchitis, osteomyelitis/synovitis (including turkey osteomyelitis complex), panophthalmitis, omphalitis/yolk sac infection and enteritis (Mellata, 2013; Nolan et al., 2020; Kathayat et al., 2021). However, the various forms of colibacillosis are most associated with broiler chickens and turkeys. In other avian species, the infections naturally occur especially when animals are kept intensively in confined conditions (Mellata, 2013; Nolan et al., 2020; EFSA AHAW Panel, 2021d; Kathayat et al., 2021).
Swine. Enteric colibacillosis is the most common disease worldwide in pigs (Sus scrofa domesticus), caused by the colonisation of ETEC strains. Although colibacillosis occurs in all age groups, it is most frequent in piglets at early age, causing neonatal diarrhoea and after weaning, post‐weaning diarrhoea (PWD). Oedema disease E. coli (EDEC) infection (oedema in the submucosa of the stomach and the mesocolon) often occurs in the same age as PWD, usually without signs of sickness (no diarrhoea or fever), and the causative E. coli strains share certain virulence factors, while some strains can cause both diseases. In contrast, older pigs develop resistance to colibacillosis. Moreover, the presence of ETEC is not always sufficient for disease development. Other factors related to feeding, weaning age, other infectious agents and season will influence the clinical course of the infection (Dubreuil, 2017; Luppi, 2017; Fairbrother and Nadeau, 2019).
Other susceptible animal species, in addition to those mentioned above, include natural hosts such as warm‐blooded animals (e.g. mammals and birds) (Loayza et al., 2020).
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/order)
There is very little information available in the scientific literature on infections and antimicrobial susceptibility of E. coli in wild animal species, despite being potential reservoirs and harbouring pathogenic and AMR E. coli in their gut (Lagerstrom and Hadly, 2021). One case of necrotising pneumonia and pleuritis was associated with an AMR ExPEC strain in a tiger (Panthera tigris) cub, resulting in death after a few hours (Carvallo et al., 2010).
Parameter 2 – Naturally susceptible domestic species (or family/order)
E. coli is an opportunistic pathogen that resides in the gut microbiota of the animal species hereby analysed (dogs and cats, horses, swine, poultry, cattle, sheep and goats) and in other warm‐blooded animals not included in the scope of this assessment (e.g. rabbits, alpacas, mice, other birds), so it can cause infections in all animal species mentioned so far. Moreover, AMR E. coli has been described in other domestic species from different animal families and in undomesticated but captive‐bred wild animals (Cercopithecidae, Falconidae, Caviidae, Columbidae, Leporidae, Camelidae), including in pet animals (Saidani et al., 2019; Salinas et al., 2019; Suay‐García et al., 2019; Ghanbarpour et al., 2020; Lengliz et al., 2021).
Parameter 3 – Experimentally susceptible wildlife species (or family/order)
No information is available on experimentally susceptible wildlife species.
Parameter 4 – Experimentally susceptible domestic species (or family/order)
Models for UTIs with UPEC have been performed in mice and rabbits (Hannan and Hunstad, 2016; Othman et al., 2021). Infectious models of chicken colibacillosis, chick colisepticaemia and rat neonatal meningitis with EXPEC strains have also been tested (Bélanger et al., 2011).
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/order)
A very small amount of information exists on E. coli (genetic diversity, virulence and antimicrobial resistance) in wild animals despite evidence that they harbour pathogenic and AMR E. coli in their gut microbiomes (Lagerstrom and Hadly, 2021). A recent literature review revealed that of over three million publications related to E. coli, less than 100 to date addressed E. coli in wild animals, from which only 29 focused on antimicrobial resistance of E. coli in wild animal hosts (Lagerstrom and Hadly, 2021). The same review revealed that E. coli has only been studied in wild animals in 40 countries and Antarctica. Nevertheless, colonisation of AMR E. coli has been reported in different animal classes, including birds (e.g. gulls, sparrow, hawk, geese, owls, pheasants), mammals (e.g. boar, deer, rabbits, cows, badger, wolf, hedgehogs, bats, foxes), fish and reptiles (e.g. turtle). The prevalence of AMR bacteria in wildlife is high enough for wildlife to be considered environmental reservoirs by many authors on the subject and may even serve as melting pots for novel AMR genetic combinations potentially harmful to human health (Lagerstrom and Hadly, 2021). In fact, Nowakiewicz et al. (2020) reported that of 78 E. coli isolates from bats (Myotis daubentonii and Plecotus auritus) in Poland, 38 genetically distinct strains were resistant to at least to one antimicrobial. 71% of these strains met the MDR criterion and the highest resistances were observed in the case of ampicillin (66%), kanamycin (84%), sulfamethoxazole/trimethoprim (61%/55%, respectively) and streptomycin (50%). In addition, Radhouani et al. (2013) study reported high rates of AMR E. coli in red foxes (Vulpes vulpes). Among the 22 E. coli isolates from faecal samples, 72.7% were resistant to one or more tested antimicrobial agents. A high percentage of E. coli isolates exhibited resistance to streptomycin, tetracycline, sulfamethoxazole/trimethoprim and ampicillin (54.4%, 50%, 31.8% and 27.3%, respectively).
Parameter 6 – Domestic reservoir species (or family/order)
AMR E. coli has been reported, either colonising or causing infections, in all domestic animal species enclosed in this assessment (Ewers et al., 2012). A literature review focusing on the distribution of ESBL‐/AmpC‐producing E. coli with respect to geographical and host origin revealed that most of the reports concern poultry (chicken and turkey), and were from European countries (Ewers et al., 2012). In fact, a growing burden of ESBL‐producing E. coli has been observed especially in dogs, cats and horses, and data on prevalence indicate high carriage and infection rates among companion animals. The same study revealed that the most frequently detected ESBLs were CTX‐M‐1, CTX‐M‐14, CTX‐M‐15 and SHV‐12, while CMY‐2 was the predominant acquired AmpC reported. CTX‐M‐1 was broadly disseminated among animals (28% in companion animals, 28% in poultry, 72% in cattle and swine) in Europe, while CTX‐M‐14 was the most prevalent in companion animals and poultry in Asia (30–33%), and to a lesser extent in cattle and pigs (14%) and in livestock (cattle and sheep, pigs and poultry) (4–7%) in Europe. CTX‐M‐15 was present in E. coli from all groups of animals studied, and CMY‐2 was described in all areas and hosts investigated, with a frequency ranging from 2% to 31% (Ewers et al., 2012).
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/incidence
The prevalence and incidence data of AMR E. coli are extremely difficult to compare, as study design, study populations, methods (different susceptibility testing methods and different clinical breakpoints), interpretive criteria, etc., vary considerably between studies and countries, and even within countries. Such a large variation makes it difficult to identify any one region or continent with particularly high or low resistance levels. Moreover, strains that were recovered from different animal species, and even from different body sites are reported together.
Dogs and cats. UTIs are the most frequently reported disease caused by E. coli in dogs and cats. In fact, data collected in 2018 by the French national surveillance network for AMR (RESAPATH) showed that kidney and urinary tract pathologies were the second and first most common infection among all the clinical dog and cat isolates received (24%, n = 3,397/14,324 isolates; 43%, n = 2017/4,659, respectively) (RESAPATH, 2018). Moreover, E. coli was the main bacterium identified among all the isolates associated with kidney and urinary tract pathologies (45% in dogs, n = 1,539/3,397; 50% in cats, n = 1,007/2,017). E. coli isolated from dogs and cats with kidney and urinary tract pathologies were mostly resistant to doxycycline (51% and 50%, respectively), amoxicillin (30% and 29%, respectively), amoxicillin–clavulanic acid (26% and 25%, respectively) and third‐generation cephalosporins (29% and 24% resistant to cephalexin; 28% and 18% to cephalothin; 25% and 20% to cefuroxime, respectively) (RESAPATH, 2018). A European multicentre study involving 14 countries and collecting data between 2008 and 2013 also showed that, overall, E. coli was the most frequently identified bacteria in UTI cases from dogs and cats, accounting for 59.5% and 59.3% of all isolates analysed (Marques et al., 2018). Information on the proportion of antimicrobial resistance in clinical E. coli from dogs and cats is reported in Table 2 according EFSA AHAW Panel (2021a).
Table 2.
Weighted arithmetic mean, minimum and maximum proportion of resistance (%R or %R + I) and weighted standard deviation in E. coli for the target antimicrobials in Europe (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f)
| Antibiotic | Animal species | No. of papers | No. of isolates | Weighted arithmetic mean % of resistance | Minimum resistance % observed | Maximum resistance % observed | Weighted standard deviation |
|---|---|---|---|---|---|---|---|
| Third‐generation cephalosporins – cefoperazone | Cattle (dairy) | 1 | 135 | 0.8 | 0.8 | 0.8 | NA |
| Third‐generation cephalosporins (Other) | Dogs and cats | 13 | 9,350 | 6.5 | 0.2 | 71.4 | 10.4 |
| Cattle (dairy) | 14 | 2,767 | 4.3 | 0 | 43.3 | 10.6 | |
| Cattle (mixed/unknown (a) ) | 3 | 4,791 | 2.9 | 0.6 | 3.1 | 0.4 | |
| Goats | 1 | 278 | 3 | 3 | 3 | NA | |
| Sheep | 2 | 390 | 11.4 | 1 | 70.7 | 24.8 | |
| Sheep and goats | 1 | 114 | 0 | 0 | 0 | NA | |
| Swine | 12 | 8,842 | 4.2 | 0 | 15.5 | 2.8 | |
| Aminopenicillins | Dogs and cats | 12 | 8,716 | 33.1 | 12.1 | 100 | 19 |
| Cattle (dairy) | 13 | 2,575 | 31.1 | 9.7 | 77.4 | 15.7 | |
| Cattle (mixed/unknown (a) ) | 5 | 4,876 | 79.7 | 46.2 | 83 | 8.7 | |
| Goats | 1 | 280 | 53 | 53 | 53 | NA | |
| Sheep | 2 | 562 | 51.3 | 46.1 | 55 | 4.4 | |
| Sheep and goats | 1 | 114 | 39.5 | 39.5 | 39.5 | NA | |
| Chickens (broilers) | 4 | 822 | 28.1 | 7 | 82 | 21.3 | |
| Chickens (layers) | 4 | 681 | 24 | 11 | 54.7 | 16.6 | |
| Ducks | 1 | 1,179 | 38 | 38 | 38 | NA | |
| Turkeys | 2 | 275 | 45.7 | 38.8 | 52 | 6.6 | |
| Swine | 13 | 8,554 | 63.9 | 26 | 98.5 | 12.7 | |
| Amoxicillin–Clavulanic acid | Dogs and cats | 12 | 13,382 | 18.6 | 0 | 100 | 17.3 |
| Cattle (dairy) | 9 | 2,418 | 13.3 | 0 | 23 | 10.3 | |
| Cattle (mixed/unknown (a) ) | 5 | 5,078 | 49.1 | 3.4 | 56 | 14.8 | |
| Goats | 1 | 281 | 32 | 32 | 32 | NA | |
| Sheep | 2 | 563 | 26.2 | 14.8 | 34 | 9.4 | |
| Sheep and goats | 1 | 114 | 7.9 | 7.9 | 7.9 | NA | |
| Swine | 6 | 3,786 | 15.7 | 2 | 29.6 | 10.7 | |
| Apramycin | Cattle (mixed/unknown (a) ) | 1 | 2,057 | 6 | 6 | 6 | NA |
| Goats | 1 | 86 | 2 | 2 | 2 | NA | |
| Sheep | 2 | 265 | 1.6 | 1.5 | 2 | 0.2 | |
| Sheep and goats | 1 | 114 | 0 | 0 | 0 | NA | |
| Swine | 6 | 6,915 | 11.5 | 5 | 73 | 16.3 | |
| Colistin | Cattle (dairy) | 5 | 414 | 0.7 | 0 | 3.2 | 1.1 |
| Chickens (layers) | 2 | 250 | 8.4 | 1 | 13.4 | 6.1 | |
| Swine | 8 | 5,15 | 9.7 | 0 | 76.9 | 13.8 | |
| Fluoroquinolones | Dogs and cats | 14 | 8,820 | 8.3 | 2.1 | 39.3 | 9.6 |
| Cattle (dairy) | 9 | 2,020 | 3 | 0 | 38.1 | 6.9 | |
| Cattle (mixed/unknown (a) ) | 3 | 4,106 | 9.9 | 9 | 29.3 | 2.9 | |
| Goats | 1 | 258 | 9 | 9 | 9 | NA | |
| Sheep | 2 | 548 | 4.5 | 4 | 5.2 | 0.6 | |
| Sheep and goats | 1 | 114 | 0 | 0 | 0 | NA | |
| Chickens (broilers) | 5 | 4,252 | 8.4 | 2 | 40 | 6 | |
| Chickens (layers) | 4 | 2,559 | 7.6 | 1.6 | 59.7 | 14.8 | |
| Ducks | 1 | 1,179 | 2 | 2 | 2 | NA | |
| Turkeys | 2 | 1,366 | 3.3 | 3 | 9.2 | 1.3 | |
| Swine | 14 | 8,934 | 8.5 | 0.1 | 56.5 | 12.3 | |
| Gentamicin | Cattle (dairy) | 1 | 63 | 20.6 | 20.6 | 20.6 | NA |
| Cattle (mixed/unknown (a) ) | 4 | 4,785 | 17 | 2.5 | 25.9 | 5.5 | |
| Goats | 1 | 270 | 9 | 9 | 9 | NA | |
| Sheep | 1 | 332 | 5 | 5 | 5 | NA | |
| Sheep and goats | 1 | 114 | 0.9 | 0.9 | 0.9 | NA | |
| Chickens (broilers) | 2 | 3,727 | 2.9 | 0 | 3 | 0.5 | |
| Chickens (layers) | 3 | 2,402 | 1.8 | 0.5 | 2 | 0.5 | |
| Ducks | 1 | 1,153 | 1 | 1 | 1 | NA | |
| Turkeys | 3 | 1,524 | 3.7 | 2 | 18.4 | 5 | |
| Poultry (mixed/unknown) | 1 | 141 | 14.8 | 14.8 | 14.8 | NA | |
| Swine | 12 | 8,216 | 11.7 | 0 | 70 | 16.2 | |
| Neomycin | Cattle (dairy) | 4 | 1,168 | 9 | 0 | 12 | 4.3 |
| Cattle (mixed/unknown (a) ) | 1 | 99 | 14.9 | 14.9 | 14.9 | NA | |
| Goats | 1 | 190 | 18 | 18 | 18 | NA | |
| Sheep | 2 | 363 | 23.7 | 9 | 34.5 | 12.6 | |
| Sheep and goats | 1 | 114 | 20.2 | 20.2 | 20.2 | NA | |
| Chickens (broilers) | 1 | 1,787 | 2 | 2 | 2 | NA | |
| Chickens (layers) | 3 | 162 | 2.9 | 0 | 12.7 | 3.2 | |
| Ducks | 1 | 672 | 3 | 3 | 3 | NA | |
| Turkeys | 1 | 527 | 3 | 3 | 3 | NA | |
| Swine | 6 | 6,654 | 15.7 | 3.8 | 20 | 3.8 | |
| Nitrofurantoin | Dogs and cats | 2 | 2,056 | 1.1 | 1 | 1.6 | 0.2 |
| Spectinomycin | Chickens (broilers) | 1 | 1,267 | 14 | 14 | 14 | NA |
| Chickens (layers) | 1 | 436 | 13 | 13 | 13 | NA | |
| Ducks | 1 | 564 | 5 | 5 | 5 | NA | |
| Turkeys | 1 | 524 | 10 | 10 | 10 | NA | |
| Swine | 5 | 6,262 | 35.7 | 30.3 | 51 | 7.7 | |
| Streptomycin | Chickens (layers) | 1 | 262 | 68.7 | 50 | 93.2 | NA |
| Poultry (mixed/unknown) | 1 | 141 | 58.2 | 58.2 | 58.2 | NA | |
| Sulfonamide–Trimethoprim | Dogs and cats | 12 | 14,481 | 11.5 | 4.3 | 61.2 | 9 |
| Cattle (dairy) | 7 | 2,050 | 12.6 | 3 | 40 | 7 | |
| Cattle (mixed/unknown (a) ) | 4 | 4,983 | 38.4 | 14.2 | 50 | 6 | |
| Goats | 1 | 280 | 36 | 36 | 36 | NA | |
| Sheep | 2 | 564 | 44.3 | 20 | 61 | 20.2 | |
| Sheep and goats | 1 | 114 | 22.8 | 22.8 | 22.8 | NA | |
| Chickens (broilers) | 3 | 3,912 | 24.9 | 17.3 | 29.5 | 1.4 | |
| Chickens (layers) | 3 | 2,248 | 11.8 | 3 | 42 | 8.2 | |
| Ducks | 1 | 1,179 | 37 | 37 | 37 | NA | |
| Turkeys | 3 | 1,525 | 25.2 | 7.7 | 67.1 | 14.5 | |
| Poultry (mixed/unknown) | 1 | 141 | 56.7 | 56.7 | 56.7 | NA | |
| Swine | 9 | 4,309 | 51.1 | 26.1 | 79.1 | 10.7 | |
| Sulfonamides | Swine | 4 | 1,495 | 65.4 | 35.2 | 75 | 9.7 |
| Tetracyclines | Cattle (dairy) | 2 | 343 | 22.4 | 14.3 | 58.5 | 17.1 |
| Cattle (mixed/unknown (a) ) | 5 | 4,867 | 71.8 | 28.8 | 76 | 12.3 | |
| Goats | 1 | 268 | 57 | 57 | 57 | NA | |
| Sheep | 2 | 541 | 58.3 | 47.9 | 66 | 9 | |
| Sheep and goats | 1 | 114 | 58.8 | 58.8 | 58.8 | NA | |
| Chickens (broilers) | 3 | 3,273 | 41.2 | 9.3 | 44 | 8.9 | |
| Chickens (layers) | 4 | 2,305 | 28.9 | 13 | 69.4 | 12.2 | |
| Ducks | 1 | 1,591 | 52.9 | 52 | 55 | 1.4 | |
| Turkeys | 2 | 1,571 | 41.3 | 16.9 | 43 | 5.1 | |
| Swine | 13 | 8,503 | 71.5 | 25 | 96.7 | 11.4 |
R: resistant; I: intermediate; NA: standard deviation cannot be calculated because only one study was included.
Cattle data is presented according to type of production: dairy or mixed/unknown.
Horses. E. coli has been the causative agent of different horse diseases. In the UK, data from six large equine diagnostic laboratories from 2018 reported that E. coli represented the most common Gram‐negative bacterium recovered from diseased horses (38.3%; n = 958/2,499), being the most common AMR urogenital pathogen (31.9%; n = 391/1,227) and the second most common AMR isolate from surgical site/catheter‐related/orthopaedic infections (SSIs/CRIs/OIs) (18.8%; n = 99/526) (Isgren et al., 2021). Among clinical E. coli analysed from urogenital and SSIs/CRIs/OIs, 21.5% and 50.5% were MDR, respectively. Data collected in 2018 by RESAPATH revealed that reproductive pathology was the most common disease reported among all clinical horse isolates received (45%, n = 1,844/4,107), with E. coli being the second main bacteria isolated (12%, n = 480/1,844), and mostly resistant to amoxicillin (35%), trimethoprim–sulfonamides (34%) and streptomycin (33%) (RESAPATH, 2018). The last data of the Swedish Veterinary Antibiotic Resistance Monitoring (SVARM) showed that clinical E. coli isolated from the genital tract of mares were commonly resistant to trimethoprim–sulfamethoxazole, which gradually increased from 10% to 17% between 2013 and 2018, and 15% in 2019 and 2020 (SVARM, 2020). In 2020, 79% (201/253) of the isolates were susceptible to all tested antibiotics, and the proportion of MDR isolates was 5% (13/253; 69% (9/13)) resistant to three antibiotics, and 31% (4/13) to four antibiotics. The most common phenotype in E. coli isolated from the genital tract of horses was resistance to ampicillin, tetracycline and trimethoprim–sulfamethoxazole, occurring in ten of the MDR isolates (77%).
Cattle. The estimated prevalence of E. coli among diarrhoeic calves from studies in the Netherlands and Switzerland varied between 4.9% and 5.5% (Uhde et al., 2008; Bartels et al., 2010). In addition, E. coli is one of the most common environmental bovine mastitis pathogens, with studies from France and the UK reporting a prevalence of 16.0–19.8% among all the pathogens isolated (Bradley et al., 2007; Botrel et al., 2010). It is important to note that approximately 60–70% of all antimicrobials administered on dairy farms are for preventing and treating mastitis, which affects herds in all countries and is the most economically burdensome disease encountered by dairy farmers (Cobirka et al., 2020). However, treatment of mastitis due to Gram‐negative bacteria is discouraged by certain guidelines and only recommended as parenteral therapy if there is systemic involvement (NZVA, 2018). On the other hand, antimicrobial therapy is often needed to treat gastrointestinal colibacillosis (EFSA AHAW Panel, 2021e). In a recent assessment conducted by the EFSA AHAW Panel (2021e), collected data suggested higher levels of resistance among E. coli isolates from gastrointestinal cases compared to mastitis cases. According to the same assessment, there was a marked difference in the proportion of resistance to third‐generation cephalosporins considering the production type, with a weighted mean proportion of 10.9% resistance in dairy isolates (obtained from milk or udder) and 36.5% in isolates of mixed/unknown origin. However, the authors note that, considering only European studies, less than 8% of E. coli isolates were resistant to third‐generation cephalosporins, with exception of a Ukrainian study that reported 43.3% of mastitis isolates to be resistant to ceftiofur. For other β‐lactams, resistance levels were generally high for aminopenicillins although with much variation between countries. Differences were also observed according to the site of infection, with French and German monitoring reports presenting resistance rates to aminopenicillins of 81–83% among isolates from calf diarrhoea, while the same reports refer resistance proportions of 12–34% to aminopenicillins among E. coli isolates from mastitis (RESAPATH, 2018; GERM‐Vet, 2020). Resistance proportions to fluoroquinolones in Europe were low, but differing according to type of production, accounting for 3% and 10% among E. coli isolates of dairy and unknown/mixed origin, respectively. It is of note, however, that GERM‐Vet (2020) reported 29.3% of German isolates from calf diarrhoea to be resistant to ciprofloxacin based on (human) clinical breakpoints (EFSA AHAW Panel, 2021e). More recently, a survey published in Europe in 2018, concerning a total of 207 E. coli isolates obtained between 2009 and 2012 from mastitis in nine EU countries, revealed high resistance to cephapirin (23.2%), moderate to tetracycline (14.5%), low to amoxicillin/clavulanic acid (3.9%), cephalexin (4.8%), cephalonium (5.3%) and very low to ceftiofur (1%) (de Jong et al., 2018). In another survey from the UK involving E. coli isolates from diseased (62.7% with diarrhoea, 11.7% dead, 6.7% with malaise) cattle (n = 534), sheep (n = 101) and goats (n = 13), a high prevalence of resistance to tetracycline (70.7%), sulfonamides (73.6%), ampicillin (69.5%), streptomycin (48.5%), trimethoprim/sulfametoxazole (36.4%), chloramphenicol (43.4%) and neomycin (33.1%) was observed. These data seem consistent with the fact that tetracyclines, β‐lactams and trimethoprim/sulfonamides account for most therapeutic antimicrobials sold for veterinary use. Regarding other antimicrobials tested, the resistance proportion for amoxicillin–clavulanic acid was 25.4%, nalidixic acid 17.4%, ciprofloxacin 14.3%, cefotaxime 3.2%, cefuroxime 1.6%, gentamicin 2.5%, apramycin 4.5% and urazolidone 2.5% (Cheney et al., 2015).
Sheep and goats. High proportion of resistance to third‐generation cephalosporins (71%) was reported among 58 isolates from sheep, including isolates from neonatal lambs and adult sheep, in the UK (EFSA AHAW Panel, 2021f), although another British study reported no third‐generation cephalosporin resistance among the 114 E. coli isolates from goats and sheep (Cheney et al., 2015). Nevertheless, it should be highlighted that the two studies tested different animal populations (sheep vs. sheep/goat), included isolates from different time periods (2019 vs. 2005–2007), although data regarding infection type is not detailed (EFSA AHAW Panel, 2021f). Data collected in 2018 by RESAPATH revealed that digestive pathology was the second most common disease reported among all clinical sheep isolates received (32.7%, n = 383/1,172), with E. coli being the main bacterium isolated (87.2%, n = 334/383) and mostly resistant to streptomycin (59%), amoxicillin (55%), tetracycline (56%), sulfonamides (56%) and amoxicillin–clavulanic acid (34%) (RESAPATH, 2018).
Poultry. Multiple E. coli serogroups (O1, O2, O5, O8, O18 and O78) have been associated with APEC isolates (56.5%), being O1, O2 and O78 the most frequently identified in Europe (Guabiraba and Schouler, 2015; Nolan et al., 2020; Kathayat et al., 2021). Besides, in Europe, high rates of resistance to several classes of antibiotics have been observed among APEC strains from several poultry species, as observed in Table 2 (EFSA AHAW Panel, 2021d). Overall, APEC strains from chickens presented higher rates of resistance to antibiotics compared with other poultry species (despite the number of studies available being much higher for chickens). Interestingly, higher rates of resistance to some antibiotics (commonly used in poultry production) was observed in APEC strains (Table 2) recovered from chicken broilers (ampicillin–50%, ciprofloxacin–61.6%, gentamicin–5.3%, sulfamethoxazole–38.5% and trimethoprim–29.4%) and turkeys (ampicillin–62.2%, gentamicin–4.2%, ciprofloxacin–44.6%, sulfamethoxazole–37.3%, trimethoprim–27.6% and tetracycline–60.8%) compared with E. coli isolates from healthy animals (EFSA AHAW Panel, 2021d).
Swine. ETEC causing colibacillosis present specific virulence factors, which can be transferred horizontally between strains. Moreover, ETEC with specific fimbriae adhesins (Fs) tend to be associated with specific serogroups (neonatal diarrhoea: F4 with O8, O138, O141, O145, O147, O149, O157 serogroups; and PWD: F4‐O149 and F18‐O138, ‐O147 and ‐O149 serogroups). Of note, O149 is the most prevalent serogroup of ETEC in Europe, America and Australia (Dubreuil et al., 2016; Luppi, 2017; Fairbrother and Nadeau, 2019). Outbreaks of F4‐positive ETEC tend to involve only one strain at any one time (Fairbrother and Nadeau, 2019). Antibiotics have been used extensively for disease control, with resultant high levels of antimicrobial resistance detected in ETEC strains worldwide (e.g. apramycin, neomycin, sulfonamide‐trimethoprim and colistin) (Luppi, 2017; EFSA AHAW Panel, 2021c). The EFSA AHAW Panel (2021c) revealed that, in Europe, clinical swine E. coli isolates presented a high proportion of resistance to several antibiotics, particularly to aminopenicillins, sulfonamides and tetracycline with average levels of resistance from 63% to 70% (Table 2) (EFSA AHAW Panel, 2021c). Lower rates of resistance to clinically important antibiotics were observed, such as to fluoroquinolones (7.9%) and third‐generation cephalosporins (4.2%). Of note, the average of resistance to colistin was relatively low, namely 9.7% (EFSA AHAW Panel, 2021c), but a Spanish study reported 77% of colistin resistance associated with the presence of mcr genes in MDR ST10 and ST131 ETEC isolates (García et al., 2018; García‐Meniño et al., 2018). Also, in Europe, the proportion of resistance among E. coli isolates from healthy animals were lower (third‐generation cephalosporins–0.7%, ampicillin–35.7%, gentamicin–2.4%, sulfamethoxazole–35.1% and tetracycline–46.5%) (EFSA and ECDC, 2021) compared to clinical isolates (third‐generation cephalosporins–4.2%, ampicillin–63.9%, gentamicin–11.7%, sulfonamides–65.4% and tetracycline–71.5%) (Table 2) (EFSA AHAW Panel, 2021c).
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
Available data on case‐morbidity rate of E. coli infection in animals are scanty. A retrospective study performed in Germany over five years revealed that among the 192 bacterial isolates recovered from 150 cats, 103 were isolated from animals showing clinical signs of UTI (54.7%; n = 82/150), 73 were from cats with subclinical bacteriuria (38%; n = 57/150) and the remaining were from cats with clinical signs not evaluable/not documented (Teichmann‐Knorrn et al., 2018). In the same study, E. coli was identified in 52.4% (n = 54/103) of isolates recovered from cats showing clinical signs of UTI (Teichmann‐Knorrn et al., 2018). In calves with diarrhoea, the prevalence of E. coli ranged from 2.6 to 5.5% in faecal samples of diarrhoeic neonatal calves (Uhde et al., 2008; Bartels et al., 2010). One study reported that among broiler chickens with clinical manifestation of colibacillosis, the prevalence rate of APEC was 53.4% (Ibrahim et al., 2019). Information on case‐morbidity rate is not available for the remaining animal species of interest.
Mortality
Parameter 3 – Case‐fatality rate
The mortality rates of life‐threatening infections are not well documented in dogs, cats and horses. Additionally, case‐fatality rates depend on infection type. While the most common infection associated with E. coli, namely UTI in dogs and cats and reproductive disease in horses, are usually not a cause of death, others such as septicaemia, meningoencephalitis, pneumonia or septic synovitis can result in individual case‐fatality (Brooks et al., 2013; Li et al., 2019). An outbreak associated with the MDR E. coli ST58 has been reported in bulldog puppies, with a fatality rate of 100% (n = 8) (Mattioni Marchetti et al., 2020).
In cattle, acute diarrhoea is the main reason behind 75% of neonatal calf mortality during the pre‐weaning period in dairy herds (Muktar et al., 2015). The prevalence of E. coli ranged from 4.9% to 5.5% in neonatal calf diarrhoea (Uhde et al., 2008; Bartels et al., 2010).
In a study conducted in Norway evaluating the causes of early neonatal lamb mortality, it was estimated that E. coli accounted for 14% of neonatal lamb mortality, mainly associated with septicaemic cases (Holmøy et al., 2017), but there is no global data on the mortality associated with E. coli infection in small ruminants.
In poultry, all ages are susceptible to APEC diseases, and most, if not all, commercial avian species experience some degree of morbidity and mortality, which are highly variable. Colibacillosis is the leading cause of mortality (up to 20%) and morbidity in poultry, being often manifested in older birds as an acute septicaemia. Besides, salpingitis (oviduct inflammation) results in the decreased egg production and sporadic mortality, being one of the most common causes of mortality in commercial layers and breeders (Nolan et al., 2020). In contrast, young birds, including developing embryos, are more frequently affected, presenting much higher mortality rates (up to 50%, 983 dead chickens within the first week of life) (Olsen et al., 2012), due to the severity of the infection and/or poor chick quality and sanitation in the hatchery. Outbreaks have been associated with caged layers and specific serotypes (e.g. O111 causing mortality, septicaemia and polyserositis in egg‐laying chickens) (Guabiraba and Schouler, 2015; Nolan et al., 2020). Since the ability to cause embryos or chick mortality differentiates APEC from commensal E. coli, it was possible to determine the APEC strains virulence in vitro by testing the embryo lethality (Nolan et al., 2020). Assays showed that mortality within two days was greater than 29% for virulent strains (Wooley et al., 2000).
Enteric colibacillosis in swine is associated with high morbidity and mortality (Dubreuil, 2017; Fairbrother and Nadeau, 2019). In general, mortality can reach up to 70% in neonatal piglets with severe watery diarrhoea, 1.5–2% in post‐weaned and/or grow‐finish pigs with moderate diarrhoea, and up to 25% in untreated pigs with severe to moderate diarrhoea. In the case of oedema disease, it is associated with rates of mortality ranging from 50% to over 90%, also in post‐weaned and/or grow‐finish pigs (Fairbrother and Nadeau, 2019).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Parameter 1 – Report of zoonotic human cases (anywhere)
Companion animals, such as dogs and cats, might be sources of sporadic zoonotic cases related to AMR E. coli, in part due to close and prolonged contact with humans, although conflicting data have been reported. Some studies have described human and companion animal isolates sharing the same genes and indistinguishable E. coli strains, suggesting transmission of the bacteria between dogs or cats and humans (Harada et al., 2012; Carvalho et al., 2016), while others observed a high clonal diversity of MDR E. coli recovered from these animals and their owners (Abbas et al., 2019). This inconsistency in genetic linkage may be because different E. coli strains acquire the AMR phenotypes and genotypes from the same source or because the same strain carrying MDR phenotypes and genotypes was transmitted either to animals or owners. Nonetheless, a single case report of UTI in a companion animal caused by an E. coli strain concurrently present in a human household contact suggests that UTI may sometimes be a zoonosis in either direction (human to dog or dog to human) (Johnson et al., 2008). In a study from the Czech Republic, similar ESBL‐carrying isolates were found in a horse and a human, indicating a zoonotic potential and/or occupational hazard (Dolejska et al., 2011).
E. coli transmission from ruminants to humans occurs mainly through food (meat, seeds and vegetables) contaminated by ruminant manure, but also from direct contact and contact with contaminated fomites and/or the environment involving the Shiga toxin‐producing E. coli (STEC), VTEC and EHEC serotypes. Following zoonotic transmission, the human incubation period ranges between 1 and 16 days with most signs in 3–4 days, involving a greater risk to children under 5 years of age. The carriage may be asymptomatic but may also involve gastrointestinal symptoms such as watery diarrhoea, haemorrhagic colitis, nausea, vomiting, abdominal pain and cramping, but systemic involvement may also occur with fever and, ultimately, the HUS (The Center for Food Security and Public Health, 2021). In a previous study from the USA, an ST69 strain was detected, isolated from a cow (with no data as to whether the animal was healthy or diseased), that showed 94% similarity by pulsed‐field gel electrophoresis (PFGE) to a human UTI isolate (Ramchandani et al., 2005).
Several studies have suggested a zoonotic transmission of APEC from poultry, as well as a source or reservoir of extra‐intestinal infections in humans. In fact, genetic similarities were found between APEC strains, namely the presence of ColV plasmids, essential for poultry adaptation, in human ExPEC (Ge et al., 2014; Jørgensen et al., 2019). Furthermore, common virulence genes were also found between APEC and ExPEC, namely UPEC and neonatal meningitis E. coli (NMEC), evidencing the ability to cause UTIs and meningitis in humans (Ewers et al., 2007; Cunha et al., 2017; Stromberg et al., 2017; Najafi et al., 2019). Notably, phylogenetic studies have also demonstrated similarities (by multilocus sequence typing (MLST) and PFGE, or whole genome sequencing (WGS) analysis) of several APEC strains with human ExPEC, belonging to the clinically relevant MDR clonal lineages ST73, ST95 and ST131 (Johnson et al., 2007; Mora et al., 2009; Ge et al., 2014; Cunha et al., 2017; Liu et al., 2018; Jørgensen et al., 2019). Moreover, this potential transmission was investigated particularly among the ST95 lineage, which comprises not only strains that have been prevalent causes of human disease but is also the predominant ST causing avian colibacillosis, confirming that multiple lineages of ExPEC belonging to ST95 exist, of which the majority may cause infection in humans, while only part of the ST95 cluster seems to be avian pathogenic (Jørgensen et al., 2019). Furthermore, other STs (e.g. ST10, ST23, ST117, ST359, ST617, ST746) detected in APEC isolates presented similarities with ExPEC isolates (Kathayat et al., 2021). In fact, it was already suggested that some human ExPEC strains, as UPEC, might have evolved from APEC clonal lineages (Manges and Johnson, 2012; Jørgensen et al., 2019).
The E. coli transmission from swine to humans may occur through ingestion of contaminated food and/or water and by direct contact with faeces and contaminated surfaces/environment (Monger et al., 2021). In fact, particular strains known as human ExPEC were found on pig farms, in pigs and retail pork meat (Wasiński, 2019). Also, similarities between ST131 strains from swine ETEC and human isolates have been demonstrated, showing a potential zoonotic source of this clonal lineage (García et al., 2018).
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment, even at laboratory level
Dogs and cats. Resistance to antibiotics varied tremendously between studies and countries and even within countries. The proportion of resistance reported in individual studies with at least 50 E. coli isolates, sorted by continent, is presented in Figure 1 (EFSA AHAW Panel, 2021a).
Figure 1.
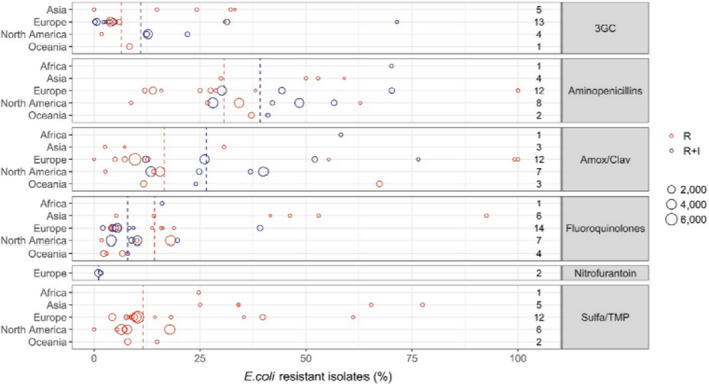
E. coli resistance data for each included study sorted by continent (obtained from EFSA AHAW Panel (2021a)). The total number of studies by continent and antimicrobial is reported on the right side. Each circle represents one study and the size of each circle reflects how many isolates were included in the study. The colour of a circle illustrates whether the proportion represents resistance only (red circle) or resistance merged with intermediate (blue circle). The dashed lines indicate, for each antibiotic, the weighted arithmetic mean of %R (red dashed line) or %R + I (blue dashed line)
Resistance levels were relatively low in many European studies for sulfonamide–TMP combinations, although one Italian study reported 61.2% of E. coli resistant to sulfonamide–TMP in dogs (Rampacci et al., 2018). Resistance to aminopenicillins was somewhat higher than for sulfonamide–TMP combinations. However, data for ampicillin should be interpreted with caution, as the Clinical and Laboratory Standards Institute (CLSI) has very different veterinary breakpoints for UTIs (> 8 mg/L), and skin and SSTIs (> 0.5 mg/L). One study reported 100% resistance to ampicillin for E. coli isolated from dogs’ skin (de Jong et al., 2020). The breakpoints for amoxicillin–clavulanic acid are the same as for aminopenicillins without β‐lactamase inhibitors; hence, there are the same challenges of interpretation and comparison across studies. As expected, the addition of the β‐lactamase inhibitor leads to higher susceptibility. In fact, resistance levels to amoxicillin–clavulanic acid in Europe were around half of those observed for aminopenicillins alone. Resistance to third‐generation cephalosporins varied but was generally lower than for other antimicrobials considered here, and never exceeded 35%. Resistance to fluoroquinolones was assessed using data for ciprofloxacin and enrofloxacin, and resistance never exceeded 25% in Europe. Resistance levels to nitrofurantoin were reported only by two studies from Sweden and were less than 2% (Windahl et al., 2014; SVARM, 2019).
Horses. While there are several studies reporting on carriage of AMR E. coli isolates, there is currently a lack of data on antimicrobial susceptibility patterns in bacterial isolates from equine clinical submissions globally. Recent publications from France have reported susceptibility patterns from a variety of bacteria from clinical submissions from 2012 to 2016 and identified increasing resistance to trimethoprim–sulfamethoxazole in E. coli (Isgren et al., 2021). Another report from France identified a decrease in MDR E. coli clinical isolates from 2006 to 2016; however, prevalence of MDR still remained above 22.5% for E. coli (Isgren et al., 2021). The proportion of resistance reported in a study performed in the UK, involving six large equine diagnostic laboratories, and including 958 clinical E. coli isolates, is presented in Table 3 (Isgren et al., 2021).
Table 3.
Proportion of resistance (in %) of E. coli isolated from clinical infections in horses, classified by sample site, in the UK in 2018 (obtained from Isgren et al. (2021))
| Antibiotic | Total no. of isolated tested | Proportion of resistance (%) | Proportion of resistant isolates by sample site (% total tested) | ||||
|---|---|---|---|---|---|---|---|
| Respiratory (2,187) | Urogenital (1,227) | Skin/Wound (1,163) | SSI/CRI/OI (526) | Unknown and other (595) | |||
| Total | 958 | – | 8.4 (183) | 31.9 (391) | 13.1 (152) | 18.8 (99) | 22.4 (133) |
| Aminopenicillins | 627 | 35.4 | 39.0 (141) | 27.3 (300) | 44.9 (91) | 64.6 (48) | 29.8 (47) |
| Β‐lactamase inhibitor combinations | 402 | 8.7 | 7.0 (158) | 12.2 (41) | 9.6 (104) | 12.2 (49) | 6.0 (50) |
| Third/fourth‐generation cephalosporins | 955 | 14.0 | 11.5 (183) | 9.0 (390) | 14.6 (151) | 23.5 (98) | 24.8 (133) |
| Aminoglycosides | 955 | 23.4 | 18.0 (183) | 18.0 (389) | 25.0 (152) | 43.9 (98) | 29.3 (133) |
| Tetracyclines | 954 | 48.0 | 42.1 (183) | 37.1 (388) | 55.3 (152) | 60.2 (98) | 70.7 (133) |
| Folate pathway inhibitors | 945 | 44.3 | 37.0 (181) | 38.1 (381) | 53.3 (152) | 60.2 (98) | 50.4 (133) |
| Fluoroquinolones | 955 | 10.7 | 9.3 (183) | 5.9 (389) | 17.1 (152) | 21.4 (98) | 11.3 (133) |
| Phenicols | 204 | 26.5 | 28.0 (25) | 11.8 (34) | 24.4 (41) | 28.0 (25) | 32.9 (79) |
| MDR | 958 | 31.7 | 30.6 (183) | 21.5 (391) | 37.5 (152) | 50.5 (99) | 42.9 (133) |
–: not indicated; MDR: multidrug‐resistant.
Cattle. Most European studies reported less than 8% of E. coli isolates resistant to third‐generation cephalosporins (EFSA AHAW Panel, 2021e). For other β‐lactams, resistance levels were generally high for aminopenicillins (EFSA AHAW Panel, 2021e). In France, 83% and 34% of E. coli from calf diarrhoea and mastitis, respectively, were resistant to amoxicillin. In Germany, resistance to ampicillin was 81% and 12% among E. coli isolated from calf diarrhoea and mastitis, respectively. It therefore appears that E. coli causing gastrointestinal disorders are much more likely to be resistant to aminopenicillins than mastitis isolates. Although not described in further detail, French and German reports showed the same trend for other antibiotics (amoxicillin–clavulanic acid, sulfonamide–trimethoprim and fluoroquinolones). Mean resistance levels were lower for amoxicillin–clavulanic acid compared with ampicillin. The resistance to tetracycline was high, ranging between 22.4% and 76%, while for sulfonamide–trimethoprim, it ranged between 12.6% and 50% (RESAPATH, 2018; GERM‐Vet, 2020).
Sheep and goats. European data concern antimicrobial resistance results from the UK and surveillance reports from France (EFSA AHAW Panel, 2021f). In France, E. coli from sheep (digestive pathologies) and goat (all pathologies) showed high proportions of resistance (> 50%) to tetracycline and amoxicillin, followed by sulfonamide–trimethoprim and amoxicillin−clavulanic acid (ranging mostly between 20% and 40%), and low levels for ceftiofur and apramycin (≤ 4%) (RESAPATH, 2018). In the UK, clinical E. coli retrieved from sheep (including isolates from neonatal lambs and adult sheep) showed high resistance levels to tetracyclines and ampicillin (35–65%), followed by sulfonamide–trimethoprim, amoxicillin−clavulanic acid and neomycin (ranging between 6% and 35%), unlike that observed for neomycin in Northern Ireland with all isolates consistently reported as resistant over the years (2015–2019) (UK‐VARSS, 2019).
Poultry. In poultry industry, several veterinary critically important antimicrobial agents have been used worldwide for the control of APEC infections (OIE, 2021), either in flocks to prevent illness (prophylaxis) or in flocks where some birds are already ill with the intention to prevent further illness or mortality (metaphylaxis) (Singer and Hofacre, 2006). However, including in Europe, APEC strains have been reported presenting MDR profiles and resistance to several antibiotics commonly used for the treatment of APEC infections, as ampicillin, sulfamethoxazole, tetracycline and trimethoprim (Table 2) (EFSA AHAW Panel, 2021d; Kathayat et al., 2021).
Swine. In swine production, antibiotics should be administered to sick piglets/pigs showing clinical signs of colibacillosis (Luppi, 2017). However, in practice, when mortality occurs, a metaphylactic approach is applied in all animals (Luppi, 2017). In Europe, clinical ETEC isolates from swine presented high levels of resistance to antibiotics commonly used for enteric colibacillosis treatment, as aminopenicillins, sulfonamides and tetracycline (Table 2), indicating that in many countries, these antibiotic classes may have limited efficacy against ETEC infections (EFSA AHAW Panel, 2021c). Moreover, particularly in Spain, high rates of resistance to colistin (77%) were detected. They were associated with MDR ST10 and ST131 ETEC strains presenting the emerging plasmid‐mediated colistin resistance mcr genes, including associated ESBL genes (García et al., 2018; García‐Meniño et al., 2018). The emergence and potential dissemination of these resistance mechanisms in both ETEC and commensal E. coli, including through the food chain, together with the lack of current data on AMR prevalence of ETEC strains, highlights the need for surveillance/monitoring studies in ETEC from swine (Madec and Haenni, 2018; Laird et al., 2021).
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
Duration of the infectious period for sick animals depends on the infection type, site and severity. In cats and dogs, uncomplicated UTIs usually resolve in 5−7 days, although these animals can experience persistent or recurrent UTIs caused by E. coli (Drazenovich et al., 2004; Freitag et al., 2006; Johnson et al., 2008).
E. coli carriage in cattle may be asymptomatic but can lead to diarrhoea and septicaemia in calves. Calves may be affected with diarrhoea for prolonged periods of time, or they may die suddenly from acute septicaemia or dehydration and acidosis that may result in anorexia and ataxia (Berchtold, 2009). For peracute and acute disease, the clinical course is short (3−8 h), and signs are related to the development of septic shock (Bashahun and Amina, 2017). Diarrhoeic calves can shed ETEC within 12 h and recovered calves can continue to shed for several months. Moreover, colostrum‐deprived calves that were infected experimentally with as few as 70 ETEC of serogroup 09:K35:K99 remained clinically normal but shed up to 106 ETEC/g of faeces for several days. Adult animals also can serve as a reservoir for infection, and a study analysing a dairy herd showed that 15 of 152 (10%) cows were shedding 102 to 104 ETEC/g of faeces when sampled within 1 week of parturition (Acres, 1985).
In sheep and goats, the clinical signs are mainly intestinal in lambs.
Regarding poultry, colibacillosis clinical signs vary from inapparent to total unresponsiveness just prior to death depending on the specific type of infection produced by APEC. Localised infections generally result in fewer and milder clinical signs than systemic diseases. Besides, colibacillosis often occurs concurrently with other diseases, making it difficult to determine the contribution of each agent to the overall clinical disease (Nolan et al., 2020).
In swine, ETEC infections are associated with acute watery diarrhoea with or without vomiting by disrupting intestinal cell homeostasis due to enterotoxins production. These symptoms rapidly lead to dehydration. Diarrhoea of newborn piglets is observed in an endemic condition; litters from first‐parity sows could be more involved due to a lack of protection by passive immunity. Moreover, when infection occurs post‐weaning, diarrhoea in piglets lasts from 1 to 5 days. Also, affected pigs are usually depressed with a reduced appetite and a rough sticky wet hair coat. Sudden deaths can occur, particularly at the start of the outbreak. Oedema disease is associated with sudden death (sporadic mortality up to 65%), possibly paralysis and eyelid oedema. The disease varies from 4 to 14 days and typically disappears abruptly as it appears (Dubreuil et al., 2016; Luppi, 2017; Fairbrother and Nadeau, 2019).
Parameter 2 – Presence and duration of latent infection period
There is no data to estimate the duration of latent infection period of AMR E. coli causing infections in dogs, cats or horses. In calves, it was observed that diarrhoea was established between 12 and 15 h after inoculation with ETEC under experimental conditions (Tzipori et al., 1981a), while in lambs, the incubation period was 12 h (Tzipori et al., 1981b). In goats, however, this period has not been established yet. In poultry colibacillosis, the time between infection and the onset of clinical symptoms varies according to the type of infection. The incubation period is short, generally between 1 and 3 days, in experimental studies in which birds are exposed to high numbers of virulent organisms (Nolan et al., 2020). Regarding swine production, neonatal diarrhoea is observed during the first 3–5 days of piglets’ life, while in PWD, diarrhoea is observed after 3–10 days. Oedema disease mostly occurs during the first few weeks after weaning and is characterised by sudden death without sickness signs (usually no diarrhoea or fever) (Dubreuil et al., 2016; Luppi, 2017; Fairbrother and Nadeau, 2019).
Parameter 3 – Presence and duration of the pathogen in healthy carriers
Carriage of AMR E. coli in healthy animals has been reported by many authors (Ewers et al., 2012), yet little is known about its persistence. Longitudinal studies focused on specific antimicrobial resistance mechanisms (e.g. ESBL, ESBL/AmpC, carbapenemases) during specific time periods (e.g. up to 6 months) and used different methods to determine susceptibility to antimicrobial drugs, making it difficult to compare and estimate the presence and duration of AMR E. coli in healthy animals.
In dogs and cats, prevalence of AMR E. coli carriage varied between 22–63% and 1.4–10.2%, with persistence being reported during 101 days to 3 years and 36–108 days, respectively (Johnson et al., 2008; Wedley et al., 2011; Baede et al., 2015; Schmidt et al., 2015; Aslantaş and Yilmaz et al., 2017; van den Bunt et al., 2020). Moreover, it seems that some dogs are non‐carriers of ESBL‐producing E. coli, whereas others are intermittent or persistent carriers (Baede et al., 2015; van den Bunt et al., 2020).
In horses, the prevalence of faecal carriage with E. coli strains resistant to at least one antimicrobial ranged from 13.4% to 24.5%, although a prevalence of 69.5% was identified by one large study on 650 samples from 692 horses (Maddox et al., 2015). The estimated prevalence of carriage of MDR isolates varied between 2.6% and 37.6%, and the prevalence of faecal ESBL‐producing E. coli between 4 and 6.7% (Maddox et al., 2015; Kaspar et al., 2019). No longitudinal studies have been performed to estimate its persistence in healthy horses.
A US study found colonisation with cefotaxime‐resistant bacteria (predominantly E. coli) in more than 92% of young beef calves sampled in their study. Notably, the investigated calves had never been treated with antibiotics, suggesting acquisition from another source (Mir et al., 2018).
In Europe, previous studies carried out in Sweden and Germany, revealed prevalence of ESBL/AmpC E. coli‐positive calves of 18% and 93%, respectively (Weber et al., 2021). In Spain, ESBL‐/AmpC‐producing E. coli was isolated in 32.9% of dairy cattle herds, 9.6% of beef cattle herds and 7.0% of sheep flocks (Tello et al., 2020).
In poultry, APEC strains can colonise healthy birds in the mucosal sites of gastrointestinal, respiratory and reproductive tracts without causing disease. Only in the presence of stressors (production‐related stress, immunosuppression and concurrent infections), APEC can invade the mucosal layers and reach extra‐intestinal organs, as an opportunistic pathogen, resulting in multisystemic infections, colibacillosis (Guabiraba and Schouler, 2015; Nolan et al., 2020; Kathayat et al., 2021). In fact, a study revealed that E. coli recovered from healthy birds and their environment were phylogenetically similar to APEC strains isolated from colisepticaemic birds (Ewers et al., 2009).
Enteric colibacillosis in swine requires the presence, by ingestion, of ETEC and specific predisposing environmental conditions and host factors, so that isolates proliferate in the intestine and cause disease due to specific virulence factors. The degree of colonisation and proliferation of ETEC determine the occurrence of the disease (Luppi, 2017). In fact, it was already demonstrated that ETEC strains were present in 16.6% of non‐diarrhoeic pigs during the piglets’ suckling period, 66% in the nursery phase and 17.3% in the finisher population. Moreover, ETEC strains can be shed in faeces from healthy pigs (Luppi, 2017).
Environment
Parameter 4 – Length of survival of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment
The ability of E. coli strains to survive and grow in the environment is likely to vary by strain and genotype (Jang et al., 2017). E. coli strains, including AMR ones, have been reported to survive and, in some cases even grow, in a variety of natural environments, including subtropical and temperate soils, surface water and sediments, estuary water environments and even treated drinking water (Yu et al., 2021). Moreover, survival is reported for up to 260 days in autoclaved river water (dark, 4 and 15°C) (Flint, 1987) and over 6 months in sun‐dried algal mats stored in airtight plastic bags at 4°C (Jang et al., 2017). In laboratory studies, E. coli can grow and replicate to high cell densities, up to 4.2 × 105 CFU/g soil in non‐sterile soils when incubated at 30 or 37°C, and survived longer than 1 month when soil temperatures were < or = 25°C (Jang et al., 2017). Three EPEC isolates resistant to tetracycline or ampicillin and mecillinam or ampicillin, mecillinam, cefixime, ceftriaxone and cefotaxime were able to persist in autoclaved standard soil (Montealegre et al., 2018). Specifically, substantial growth was observed from days 0 (seeded at a concentration of ~ 103 CFU/g dry soil) to 3 (all isolates were detected at concentrations of 108 CFU/g dry soil). Beyond day 3, the concentration decreased but remained higher than the concentrations observed immediately after spiking, and all the isolates persisted up to 84 days of experimental study.
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
Although AMR E. coli can be transmitted both vertically and horizontally between diseased and susceptible animals, transmission of antimicrobial resistance genes seems to occur more frequently by horizontal gene transfer (Loayza et al., 2020).
UTI caused by AMR E. coli in dogs and cats are commonly considered as individual or isolated opportunistic infections. Nonetheless, some studies reported that similar AMR E. coli strains can effectively colonise the same or different animal species, sharing the same household or admitted to the same veterinarian clinic, which suggests a potential clonal cross‐species transmission (Bélanger et al., 2011; Damborg et al., 2012; Nigg et al., 2019; Mattioni Marchetti et al., 2020; van den Bunt et al., 2020). A recent study reported a single AMR E. coli clone causing infection in puppies living in a breeding kennel, implying a clonal outbreak (Mattioni Marchetti et al., 2020). The source of the infectious disease of the entire litter remains unknown, yet the breeding kennel had a previous history of E. coli infections. Other studies identified blaCTX‐M‐1 on IncHI1 and IncI1 plasmids present in E. coli isolates belonging to diverse sequence types, recovered from diseased horses of different countries (Lupo et al., 2018), highlighting their contribution to the horizontal dissemination of antimicrobial resistance genes.
In horses, it is possible that UTI results from individual faecal contamination of the urogenital tract, while reproductive pathologies (e.g. uterine infections) in mares occur by natural mating, artificial insemination, reproductive examination or parturition (Satué and Gardon, 2016).
E. coli transmission among ruminants may occur through ingestion of contaminated soil, food, water and by direct contact with faeces and fomites (The Center for Food Security and Public Health, 2021). It is also possible for calves to become infected via the nasopharyngeal mucosa (i.e. inhalation) (Bashahun and Amina, 2017). It is of note, however, that antimicrobials are more frequently used in cows in dairy systems for the treatment of some diseases such as mastitis, metritis and lameness, as well as a prophylactic treatment for mastitis before the dry period starts, which results in milk contaminated with antimicrobial residues that is frequently used to feed calves and may contribute to further selection of resistant bacteria (Astorga et al., 2019).
Poultry species can be infected by APEC strains through contaminated feed and water, being further spread to other birds through the faecal‐oral or aerosol routes. Besides, APEC can be vertically transmitted from infected breeders via contaminated eggs (Nolan et al., 2020; Kathayat et al., 2021). In fact, several colibacillosis outbreaks were reported (among broilers and layer chickens), including associated with MDR strains (Dhillon and Jack, 1996; Zanella et al., 2000; Vandekerchove et al., 2005; Solà‐Ginés et al., 2015). Besides, the spread of ST117 O78:H4, previously associated with human UPEC, was recently recovered from diseased broilers and breeders in distantly located chicken farms across Denmark, Finland and Norway by vertical transmission through the broiler breeding pyramid (Ronco et al., 2017).
In swine, ETEC is transmitted to healthy animals from symptomatic or asymptomatic carrier piglets, sows or possibly other animal species, faecal‐contaminated feed, water, soil and the environment of the pig barn, but also via aerosols (Dubreuil et al., 2016; Fairbrother and Nadeau, 2019).
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
Although UTI caused by AMR E. coli is a common human disease, evidence of transmission between diseased dogs, cats, horses and humans is lacking. Nevertheless, healthy dogs and cats might be potential reservoirs of AMR determinants that can be transmitted to humans through direct or indirect contact through environmental contamination of households, veterinary clinics and public spaces (VKM, 2015).
E. coli transmission from cattle, sheep and goats to humans may occur through ingestion of contaminated food and/or water and by direct contact with faeces and contaminated surfaces (Pelzer and Currin, 2005). In fact, raw milk and dairy products (e.g. cheese) have been associated with diarrhoeagenic E. coli contamination. Furthermore, high prevalence of potentially pathogenic E. coli strains in raw milk and raw milk cheeses is reported in several countries worldwide (Ribeiro et al., 2019).
APEC transmission from poultry to humans may occur through ingestion of contaminated poultry products (meat and eggs) and/or water, and by direct contact with faeces and contaminated surfaces/environment (Mellata, 2013; Nolan et al., 2020; Kathayat et al., 2021). Transmission of ETEC from swine to humans may occur through ingestion of contaminated pork meat and/or water, and by direct contact with faeces and contaminated surfaces/environment (Wasiński, 2019; Monger et al., 2021).
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
It is not established if there is any significant transmission between cats, dogs, horses or between these animals and humans during the clinical urogenital disease phase caused by AMR E. coli. Furthermore, no data is available about the incidence and/or transmission speed of pathogenic/AMR E. coli transmission between ruminants, poultry, pigs or between these animals and humans during the infectious period. However, in a Dutch study, it was observed that calves presented a higher prevalence of E. coli among diarrhoeagenic animals in the first week of age (4.6%), decreasing in the second week (0.7%) and increasing again by the third week of age (2%) (Bartels et al., 2010).
Parameter 4 – Transmission rate (β) (from R0 and infectious period) between animals and, when relevant, between animals and humans
There is no data published on transmission rate of pathogenic E. coli/AMR E. coli between dogs, cats, horses, cattle, sheep, goats, poultry and swine, and between these animals and humans during the infectious period.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
The distribution of AMR E. coli is clearly endemic, since this is a worldwide ubiquitous bacterial species. Infections caused by AMR E. coli in animals have been reported in all EU MSs.
Risk of introduction
This section is not relevant due to the ubiquitous occurrence of AMR E. coli.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
Routine diagnostics is based on sample culture from animals presenting clinical signs of bacterial infection and bacterial isolation. E. coli can be identified by using commercial biochemical tests including analytical profile index kits and matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry (MALDI‐TOF MS) for E. coli identification. Additionally, several methods to differentiate and/or subtype E. coli pathotypes are available, including multilocus enzyme electrophoresis (MLEE), pathotyping/virulence factor typing, MLST, PFGE, multiplex polymerase chain reaction (PCR), MALDI‐TOF MS based on flagellar antigen and WGS (Chui et al., 2015; Riley, 2020).
Antimicrobial susceptibility testing (AST) can be performed using the disk diffusion or minimum inhibitory concentration (MIC) determination methods, for which some commercial devices are available (broth microdilution methods, gradient tests, semi‐automated devices). Currently, there is no standard method to perform AST and interpret clinical breakpoints in veterinary laboratories. Some followed the guidelines from CLSI (https://clsi.org/) and others from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (https://eucast.org/).
Parameter 2 – Existence of control tools
Different animal infections caused by E. coli can be controlled by vaccination (colibacillosis in poultry and swine, mastitis in dairy cattle, intestinal infection in calves (Section 3.1.4.2)), antimicrobials (all animals) (Section 3.1.4.3) and biosecurity measures (all animals) (Section 3.1.4.4).
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
AMR E. coli causing infections in cats and dogs was reported in 14 MSs (Austria, Belgium, Denmark, Finland, France, Germany, Greece, Italy, the Netherlands, Norway, Poland, Portugal, Spain and Sweden), in horses in three MSs (France, Italy and Sweden), in sheep and goats in France, in cattle in nine MSs (Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Poland and Sweden), in swine in eight MSs (Austria, Denmark, Finland, France, Germany, Italy, Spain and Sweden), and in poultry in 10 MSs (Belgium, Czech Republic, Finland, France, Germany, Greece, Italy, Romania, Slovakia and Sweden) (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f).
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
Losses depend on infection type, animal sector and vary among countries. In terms of milk yield losses, E. coli is considered the most harmful pathogen causing mastitis. Economic losses due to mastitis include direct costs due to diagnostic testing, veterinary service, medication, discarded milk and labour, as well as indirect costs associated with future milk production loss, reduced reproduction and premature culling and replacement of mastitic cows. The costs of preventive measures also need to be considered (Heikkilä et al., 2018). Monthly economic losses (treatment period and cost per cow, withdrawal period, daily milk production and milk value data) associated with cattle clinical mastitis ranged between US$12,000 and 76,000/farm/month (He et al., 2020).
Calf diarrhoea is a major cause of economic loss to cattle producers, with estimates of 53.4–57% of calf mortality being due to diarrhoea (Cho and Yoon, 2014). Calf diarrhoea has a cost of £11 million to the UK cattle industry per year, with an estimated cost of £58 per animal (Bennett and IJpelaar, 2005). Economic losses of meat (2% decline in live weight, 2.7% deterioration in feed conversion ratio) and egg production (up to 20%) due to E. coli causing colibacillosis in poultry have been reported (Guabiraba and Schouler, 2015; Kathayat et al., 2021). In a study from the Netherlands concerning four layer and two broiler breeder flocks and referring to prices of 2014, it was estimated that the mean numbers of eggs lost were 10 and 11 per hen housed (PHH), while mean slaughter weight loss was 0.2 and 0.5 kg PHH. Losses per flock ranged from €3,635 to €21,766 (Landman and van Eck, 2015).
Concerning swine production, in severe cases of disease, a decline of 30–40% of pigs’ body weight can occur (Fairbrother and Nadeau, 2019).
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Transmissibility between animals and humans
Parameter 1 – Types of routes of transmission between animals and humans
Most people are infected with E. coli from contaminated food (e.g. undercooked meat), unpasteurised milk or contact with animal faeces from the environment. Discussion on the sources of human exposure to E. coli is given in Section 3.1.1.6.
Parameter 2 – Incidence of zoonotic cases
Data reporting zoonotic incidence is lacking, however evidence demonstrating possible zoonotic potential of particular AMR E. coli clonal lineages have been described. APEC is presumed to be zoonotic and to represent an external reservoir for extra‐intestinal infections in humans, including UTIs and meningitis in humans. Particular high‐risk clonal lineages recovered from poultry, ST73, ST95 and ST131, were reported to present high similarity with human ExPEC strains, using MLST, PFGE and/or WGS (Johnson et al., 2007; Mora et al., 2009; Ge et al., 2014; Cunha et al., 2017; Liu et al., 2018; Jørgensen et al., 2019). In addition, other STs (e.g. ST10, ST23, ST117, ST359, ST617, ST746) associated with APEC presented similarities with ExPEC isolates (Kathayat et al., 2021). Moreover, high similarity was also observed between ST131 from clinical ETEC swine strains and human isolates (García et al., 2018).
Transmissibility between humans
Parameter 3 – Human‐to‐human transmission is sufficient to sustain sporadic cases or community‐level outbreak
Transmission of AMR E. coli between humans has been reported in hospital, community and household settings. However, most of the studies showed that human transmission was more frequent in community and household settings. Hilty et al. (2012) found that the overall transmission rate, in the nosocomial setting, for ESBL‐producing E. coli was 4.5% (4 of 88 exposed contacts), corresponding to an incidence of transmission of 5.6 cases per 1,000 exposure days; while for the household setting, the ESBL‐producing E. coli carriage was found in 31 (35.2%) of 88 household contacts, but based on the molecular analysis, transmission was plausible for only 20 (22.7%) (Hilty et al., 2012). These differences may be justified by the longer exposure times in the outpatient household compared with the hospital setting, and the control measures usually implemented in the hospitals for infection control (Valverde et al., 2008; Hilty et al., 2012; Madigan et al., 2015; Whitmer et al., 2019).
Parameter 4 – Sporadic, epidemic or pandemic potential
Particular AMR E. coli clonal lineages have the potential for pandemic spread. As mentioned in Section 3.1.1.3, several pandemic ExPEC lineages were demonstrated to have zoonotic potential. Nowadays, E. coli ST131 is recognised as the major ExPEC clonal lineage responsible for the spread of MDR and clinically relevant resistance genes (e.g. mcr and ESBL genes). Notably, similarities between ST131 from APEC or ETEC swine strains and humans were demonstrated (Riley, 2014; García‐Meniño et al., 2018; Kondratyeva et al., 2020). Furthermore, other pandemic MDR lineages, such as E. coli ST 95 and ST73, were associated with APEC strains (Riley, 2014; Kondratyeva et al., 2020). Moreover, the pandemic ExPEC ST73 (O6‐B2‐ST73 clonal group) was first described in poultry, as the cause of avian colibacillosis in Brazil (Cunha et al., 2017). Other clonal lineages, such as ST10, ST23, ST117, ST359, ST617 and ST746 from poultry and/or swine, also appear to have zoonotic potential (Wasiński, 2019; Kondratyeva et al., 2020). ST69 E. coli strains recovered from cows revealed similarities to a human UTI isolate (Ramchandani et al., 2005). In addition, closely related PFGE types of ST73 strains isolated from humans, dogs and cats allowed to suggest a possible cross‐species transmission (Riley, 2014).
Furthermore, other relevant high‐risk human ExPEC clonal lineages were reported in animals, but without evidence of potential transmission, as MDR ST131 recovered from cattle, dogs, cats and horses, and ST95, ST393 and ST410 in dogs and/or cats (Riley, 2014; Brilhante et al., 2020; Nittayasut et al., 2021).
The severity of human forms of the disease
Parameter 5 – Disability‐adjusted life year (DALY)
From EARS‐Net data collected in 2015, 148,727 (95%, 131,757–166,361) cases of infections with third‐generation cephalosporin‐resistant E. coli occurred in the EU and European Economic Area (EEA) (Cassini et al., 2019). These infections accounted for 7,049 (308–7,863) attributable deaths and 32,600 (29,800–35,600) DALYs. The number of deaths attributable to third‐generation cephalosporin‐resistant E. coli infections increased by 4.12 times during 2007–2015. According to 2016 data, diarrhoea caused by ETEC was responsible for a mean of 51,186 deaths among people of all ages, with a fatal attributable fraction of 0.76% (GBD, 2016 Diarrhoeal Disease Collaborators, 2018).
The availability of effective prevention or medical treatment in humans
Parameter 6 – Availability of medical treatment and their effectiveness (therapeutic effect and any resistance)
E. coli remains one of the most frequent causes of nosocomial and community‐acquired bacterial infections including UTIs, enteric infections and systemic infections in humans. The recommended first‐line empiric antibiotic therapy for acute uncomplicated UTI in otherwise healthy adult non‐pregnant females is a 5‐day course of nitrofurantoin, a 3‐g single dose of fosfomycin tromethamine or a 5‐day course of pivmecillinam. Second‐line options include oral cephalosporins such as cephalexin or cefixime, fluoroquinolones and β‐lactams, such as amoxicillin–clavulanate. Current treatment options for UTIs due to AmpC‐β‐lactamase‐producing E. coli include nitrofurantoin, fosfomycin, pivmecillinam, fluoroquinolones, cefepime, piperacillin–tazobactam and carbapenems. Oral treatment options for UTIs due to ESBLs‐producing E. coli include nitrofurantoin, fosfomycin, pivmecillinam, amoxicillin–clavulanate, finafloxacin and sitafloxacin. Parenteral treatment options for UTIs due to ESBLs‐producing E. coli include piperacillin–tazobactam, carbapenems including meropenem/vaborbactam, imipenem/cilastatin–relebactam and sulopenem, ceftazidime–avibactam, ceftolozane–tazobactam, aminoglycosides including plazomicin, cefiderocol, fosfomycin, sitafloxacin and finafloxacin. In the case of carbapenem‐resistant E. coli, ceftazidime‐avibactam, meropenem/vaborbactam, imipenem/cilastatin–relebactam, colistin, fosfomycin, aztreonam and ceftazidime–avibactam, aztreonam and amoxicillin–clavulanate, aminoglycosides including plazomicin, cefiderocol and tigecycline are the last‐resource treatment options.
E. coli has been increasingly associated with the resistance to antibiotics, especially to the third‐/fourth‐/fifth‐generation cephalosporins and fluoroquinolones. Considering antimicrobial resistance data reported by EU/EEA countries to EARS‐Net for 2020, more than half of the E. coli isolates were resistant to at least one antimicrobial group under surveillance, and combined resistance to several antimicrobial groups was a frequent occurrence (WHO Regional Office for Europe and ECDC, 2021). According to ECDC surveillance data from 2020 in Europe, the percentage of E. coli resistant isolates to third‐generation cephalosporins varied between 5.8% in Norway and 41.4% in Bulgaria, while resistance to fluoroquinolones varied between 10% in Norway and 48.2% in Cyprus, reflecting the North‐to‐South and West‐to‐East gradient of resistance, with higher rates observed in the southern and eastern parts of the region. Although carbapenem resistance in E. coli is still rare, there are already some reports, with higher expression in Eastern European countries, such as Bulgaria (0.8% resistant isolates) and Romania (0.7% resistant isolates) (ECDC, 2021).
Regarding treatment clinical failure due to antimicrobial resistance, it was reported that patients with third‐generation cephalosporin‐resistant E. coli infections had significantly increased odds of dying within 30 days of the onset of their infection compared to patients with third‐generation cephalosporin‐susceptible E. coli infections (summary odds ratio (sOR) 2.02, 95% CI 1.66–2.46, p < 0.001). In addition, patients with quinolone‐resistant E. coli infections had significantly increased odds of dying within 30 days of the onset of their infection in comparison with patients with quinolone‐susceptible E. coli infections (sOR 1.49, 95% CI 1.23–1.82, p = 0.002) (MacKinnon et al., 2020).
Parameter 7 – Availability of vaccines and their effectiveness (reduced morbidity)
Currently, there are no licensed human vaccines against E. coli.
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level, and duration of impairment
Information concerning persistence and duration of diseases in animals is described in detail in Section 3.1.1.5. Information concerning the severity of clinical signs is referred to below.
Dogs and cats. In dogs and cats, E. coli is the leading cause of UTIs, accounting for 50–60% of those infections (EFSA and ECDC, 2017). Clinical signs of lower UTI include pollakiuria, gross haematuria, periuria, dysuria and stranguria. These are non‐specific and can be seen in any disease of the lower urinary tract, of which idiopathic cystitis is the most common in cats. Acute pyelonephritis (upper UTI) may be associated with distinct clinical signs such as fever and painful kidneys, as well as anorexia, lethargy, polyuria and polydipsia, vomiting and diarrhoea (Dorsch et al., 2019). According to one study of 17 histopathologically confirmed cases of pyelonephritis, the most common clinical signs were non‐specific, such as anorexia, lethargy and vomiting; renal pain and pyrexia were only observed in 3/17 and 2/17 cats, respectively (Dorsch et al., 2019). In dogs, chronic pyelonephritis is considered to produce only mild or absent clinical signs. In cats, there is a lack of knowledge regarding this disease entity (Dorsch et al., 2019). Other diseases such as bacteraemia and pyometra have also been reported (Greiner et al., 2008; Hagman, 2018).
Horses. In horses, E. coli has been mostly associated with urinary and reproductive infections, respiratory diseases and infections of soft tissues and wounds (Maddox et al., 2015; SVARM, 2020; Isgren et al., 2021). Clinical signs of endometritis in mares may be hidden, but vaginal discharge, short inter‐oestrus intervals and/or a shortened luteal phase and reduced fertility can be detected (Pasolini et al., 2016).
Cattle, sheep and goats. In cattle, sheep and goats, the most frequently reported infections associated with E. coli include intestinal infections and septicaemia in calves, lambs and goat kids, and mastitis in adult dairy animals. The latter is non‐contagious and occurs through environmental contamination of the udder. Clinical mastitis is characterised by sudden onset with redness and swelling of the udder with the milk of an affected quarter being altered, presenting flakes or clots and/or has a watery consistency. Cows may become lethargic, with reduced appetite, and usually have fever. Subclinical mastitis, by contrast, is characterised by a lack of visible signs in the milk or in the udder but results in decreased milk production; it is more difficult to detect but occurs 15–40 times more often than the clinical form, and its duration is longer (Cobirka et al., 2020). Severe dehydration caused by faecal loss of fluids and electrolytes is a frequent complication of diarrhoea that may result with the development of strong ion (metabolic) acidosis that is accompanied by central nervous system depression, ability to stand and suckling force (Berchtold, 2009). Other less common presentations include peritonitis, cystitis/pyelonephritis, endometritis, wound infections and meningitis derived from sepsis (Gay, 1965; Besser and Gay, 1985; Smith et al., 1985; CABI, 2019; EFSA AHAW Panel, 2021e).
Poultry. Regarding poultry species, including chickens, turkeys and others (e.g. duck, goose, quail, ostrich), APEC can cause diverse localised or systemic infections, designated as avian colibacillosis. All avian species are susceptible to APEC infections, which includes colisepticaemia, haemorrhagic septicaemia, coligranuloma (Hjarre’s disease), air sac disease (chronic respiratory disease), swollen head syndrome, venereal colibacillosis, coliform cellulitis (inflammatory or infectious process), peritonitis, salpingitis, orchitis, osteomyelitis/synovitis (including turkey osteomyelitis complex), panophthalmitis, omphalitis/yolk sac infection and enteritis (Mellata, 2013; Nolan et al., 2020; Kathayat et al., 2021). However, the various forms of colibacillosis are most associated with broiler chickens and turkeys. In other avian species, the infections naturally occur especially when animals are kept intensively in confined conditions (Mellata, 2013; Nolan et al., 2020; EFSA AHAW Panel, 2021d; Kathayat et al., 2021).
Swine. Enteric colibacillosis is the most common disease worldwide in pigs, caused by the colonisation of ETEC strains. Although colibacillosis occurs in all age groups, it is most frequent in piglets at early age, causing neonatal diarrhoea and after weaning, PWD. EDEC infection (oedema in the submucosa of the stomach and the mesocolon) often occurs in the same age as PWD, usually without signs of sickness (no diarrhoea or fever), and the causative E. coli strains share certain virulence factors, while some strains can cause both diseases. In contrast, older pigs develop resistance to colibacillosis. Moreover, the presence of ETEC is not always sufficient for disease development. Other factors related to feeding, weaning age, other infectious agents and season will influence the clinical course of the infection (Dubreuil, 2017; Luppi, 2017; Fairbrother and Nadeau, 2019).
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
There are no reports regarding E. coli infection affecting endangered wild species listed in CITES and/or IUCN; however, warm‐blood animals are gut E. coli carriers. Since E. coli is an opportunistic pathogen, there is the potential of infection occurrence with variable severity.
Parameter 2 – Mortality in wild species
Studies on mortality caused by AMR E. coli in wild animals are scanty and are individual case reports, namely the death of a tiger with necrotising pneumonia and pleuritis caused by an AMR ExPEC strain (Carvallo et al., 2010).
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
As previously stated, E. coli strains, including antimicrobial resistant ones, have been reported to survive and, in some cases even grow, in a variety of natural environments (Yu et al., 2021). Whilst wildlife may potentially carry the pathogen, potentially acquired from contaminated environments, there is no evidence of a capacity to cause substantial mortality in wildlife.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
AMR E. coli are not listed by the OIE. E. coli is listed as a zoonotic disease (colibacillosis) of cattle, sheep, goats, swine and poultry by CFSPH, which should be reported to human/public health authorities (MS reporting may vary).
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
AMR E. coli are not listed.
Parameter 3 – Included in any other list of potential bio‐agro‐terrorism agents
AMR E. coli are not included in any other list of potential bio‐agro‐terrorism agents.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tools, OIE‐certified
There are no officially/internationally recognised diagnostic tests. Diagnosis of E. coli infection is based on clinical signs and standard bacterial culture and identification. Detection of antibiotic resistance is based on phenotypic (antimicrobial susceptibility testing), proteomic (MALDI‐TOF MS) and genotypic methods (e.g. WGS, PCR).
Effectiveness
Parameter 2 – Sensitivity and specificity of diagnostic tests
One study reported the comparative sensitivity, specificity, positive predictive value and negative predictive value between MALDI‐TOF MS and biochemical methods (PHOENIX® identification cardsor API® identification strips) for E. coli identification (Benagli et al., 2011). MALDI‐TOF MS sensitivity was higher than API®/Phoenix® (95.58% vs. 90.82%). Despite the importance of antimicrobial susceptibility testing for clinical management of infection and antimicrobial resistance surveillance, the breakpoints of CLSI and EUCAST are far from harmonised and it is unclear how the discrepancies between the two systems will be addressed. A study comparing susceptibility interpretation between CLSI and EUCAST revealed discrepancies concerning rates of ciprofloxacin and amoxicillin–clavulanic acid resistant E. coli (Cusack et al., 2019). The proportions of E. coli resistant to ciprofloxacin would have been markedly higher using clinical EUCAST breakpoints (59.1% vs. 46.5%), and resistance to amoxicillin–clavulanic acid would have also increased (52.3% vs. 19.9%) (Table 4). Other studies also highlighted the low agreement between EUCAST and CLSI methodologies when performing MIC testing of amoxicillin/clavulanic acid, with a higher degree of resistant‐categorised E. coli strains by applying EUCAST guidelines (Vanstokstraeten et al., 2021).
Table 4.
Comparison of susceptibilities of E. coli (n = 428) to antibiotics using CLSI and EUCAST clinical breakpoints (Cusack et al., 2019)
| Antibiotic | CLSI 2018 (%) | EUCAST 2018 (%) | Category agreement (%) | ||||
|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ||
| Amoxicillin–clavulanic acid | 55.6 | 24.5 | 19.9 | 47.7 | – | 52.3 | 64.7 |
| Ampicillin | 5.8 | 2.3 | 91.8 | 8.2 | – | 91.8 | 97.7 |
| Ciprofloxacin | 50.5 | 3 | 46.5 | 31.3 | 9.6 | 59.1 | 77.8 |
| Gentamicin | 58.4 | 0 | 41.6 | 55.1 | 2.8 | 42.1 | 96.7 |
| Ceftriaxone | 41.8 | 0.2 | 57.9 | 40.4 | 1.4 | 58.2 | 98.4 |
| Trimethoprim–sulfamethoxazole | 25.9 | 0.5 | 73.6 | 25.9 | 0.2 | 73.8 | 99.8 |
I: intermediate; S: susceptible; R: resistant; –: no intermediate category.
Proposals to update the harmonised monitoring and reporting of antimicrobial resistance from a public health perspective in E. coli from food‐producing animals in the EU were presented in 2019 in an EFSA scientific report (EFSA, 2019). Phenotypic monitoring of antimicrobial resistance in bacterial isolates, using microdilution methods for testing susceptibility and interpreting resistance using epidemiological cut‐off values was encouraged, including further characterisation of E. coli isolates showing resistance to extended‐spectrum cephalosporins and carbapenems, as well as the specific monitoring of ESBL/AmpC/carbapenemase‐producing E. coli. As regards the laboratory methodologies, it was stated that broth microdilution is the preferred method and EUCAST epidemiological cut‐off values should be used as interpretative criteria to define microbiological resistance.
This scientific report also considered the advantages inherent in the WGS technology but also its current limitations, as well as the expected evolution of the present situation, and proposed to follow a gradual, phased approach to integration of WGS within the harmonised antimicrobial resistance monitoring. In fact, effectiveness of WGS in identifying resistance genotypes of MDR E. coli isolated from farm cattle, and whether these correlate with observed phenotypes have also been measured (Tyson et al., 2015). The study showed that resistance genotypes correlated with 97.8% specificity and 99.6% sensitivity to the identified phenotypes. Most of the discordant results were attributable to the aminoglycoside streptomycin, whereas there was a perfect genotype–phenotype correlation for most antibiotic classes such as tetracycline, quinolones and phenicols.
MALDI‐TOF MS has also been evaluated for rapid detection of amoxicillin‐ and cefotaxime‐resistant E. coli isolates from positive blood cultures (Florio et al., 2020). Categorical agreement between the MALDI‐TOF MS and the reference method was 97 and 83% for amoxicillin and cefotaxime, and correctly classified 95% and 84% of the amoxicillin‐ and cefotaxime‐susceptible E. coli isolates, respectively.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
The type of sample matrix used for bacterial culture depends on the infection type (e.g. urine in the case of suspected UTI, faeces in the case of diarrhoea, blood for confirming septicaemia).
3.1.4.2. Article 7(d)(ii) Vaccination
Availability
Parameter 1 – Types of vaccines available on the market (live, inactivated, DIVA, etc.)
Only few vaccines are available on the market to prevent animal infections caused by E. coli, with some of them being represented in Table 5. However, the major drawback of these vaccines is the lack of protection against animal infections caused by different E. coli strains. There are no vaccines available on the market to treat UTI caused by E. coli in dogs and cats, reproductive pathologies in horses, or infections in goats and sheep.
Table 5.
Examples of commercially available vaccines to prevent different infections caused by E. coli in animals
| Animals | Commercial vaccines (composition) | Authorised for use in the EU | Route of administration | Used for | Onset/Duration if immunity | Field protection | Reference |
|---|---|---|---|---|---|---|---|
| Poultry | Poulvac E. coli (live; E. coli aroA gene deleted, type O78, strain EC34195) | Yes | Spray, oral | Chickens and turkeys for active immunisation against colibacillosis caused by E. coli serotype O78 |
Onset: 2 weeks after vaccination in chickens, 3 weeks after second vaccination in turkeys Duration: 8 weeks for the reduction of lesions and 12 weeks for the reduction of mortality in chickens by spray vaccination, 12 weeks for the reduction of lesions and mortality in chickens by oral administration, not established in turkeys |
Significant reduction in colibacillosis lesions and deaths in vaccinated animals Positive effect on average daily weight gain, number of antibiotic treatment days and percentage of animals marketed compared to controls |
EMA (2022) |
| Gall N tect CBL (live attenuated; APEC, Δcrp, type O78, strain AESN1331) | No | Spray | Chickens for active immunisation against colibacillosis caused by E. coli serotype O78 | Not indicated |
Prevents avian colibacillosis infection Improves productivity |
Uotani et al. (2017) | |
| Swine | Coliprotec F4/F18 (live; E. coli O8:K87 and O141:K94) | Yes | Oral | Pigs from 18 days of age against PWD caused by E. coli |
Onset: 7 days after vaccination Duration: 21 days after vaccination |
Significantly reduced colonisation of pig intestines after challenge with a virulent F4+ ETEC strain Significantly reduced the duration and severity of diarrhoea and accumulation of fluids in the intestines after infection |
EMA (2021a) |
| Enteroporc Coli (recombinant, inactivated; contains parts of the E. coli bacterium called fimbrial adhesins F4ab, F4ac, F5 and F6) | Yes | Injection | Sows (female pigs that have already given birth to piglets) or gilts (female pigs that have not yet given birth to piglets) to protect their offspring from intestinal disease caused by E. coli |
Onset (after uptake of colostrum): within 12 h after birth Duration (after uptake of colostrum): first days of life |
Reduce death and/or the clinical signs of E. coli infection such as neonatal piglet diarrhoea | EMA (2021b) | |
| Porcilis ColiClos (recombinant, inactivated; contains parts of the E. coli bacterium called fimbrial adhesins F4ab, F4ac, F5 and F6 and LT toxoid, and toxoid of the Clostridium perfringens type C) | Yes | Injection | Sows (female pigs that have already given birth to piglets) or gilts (female pigs that have not yet given birth to piglets) to protect their offspring from intestinal disease caused by E. coli strains that express the components F4ab, F4ac, F5 or F6, and by Clostridium perfringens type C | For the passive immunisation of progeny by active immunisation of sows and gilts |
Reduce death and the clinical signs of neonatal piglet diarrhoea and necrotic enterotoxaemia May reduce the use of antimicrobials in pig production units |
EMA (2020) | |
| Neocolipor (inactivated; E. coli expressing the adhesins F4ab, F4ac, F4ad, F5, F6, F41) | Yes | Injection | Female pigs (sows and gilts) | For the passive immunisation of progeny by active immunisation of sows and gilts | Induces the specific seroconversion of vaccinated animals, piglets are passively immunised against neonatal enterotoxicosis by intake of colostrum and milk containing adhesin‐specific antibodies | EMA (2021c) | |
| Cattle | Locatim (inactivated; bovine concentrated lactoserum containing Anti‐E. coli F5‐specific IgGs) | Yes | Oral | Newborn calves | Not indicated |
Reduce mortality in newborn calves caused by enterotoxicosis due to the bacterium E. coli during the first days of life Less severe clinical signs of diarrhoea and better survival time in calves that received the vaccine than those that did not receive it |
EMA (2021d) |
| Enviracor™ J‐5 (inactivated; E. coli J‐5 strain) | No | Injection | Healthy dairy cattle | Not indicated |
Reduced duration of E. coli mastitis (64 h shorter) Higher antibody titres in milk and serum |
Drugs.com (2021a) | |
| Startvac (inactivated; E. coli and Staphylococcus aureus) | Yes | Injection | Healthy cows in a herd, during and after pregnancy |
Onset: ≈ 13 days after the first injection Duration: ≈ 130 days post‐parturition |
Reduced the number of cows with mastitis due to Staphylococcus aureus and related bacteria Reduced the severity of the symptoms in the cows that had mastitis Increased number of cows being cured of the infection Reduction in the number of cows that needed treatment for mastitis Increase in the quantity and quality of milk production |
EMA (2018) | |
| ScourGuard 4KC (inactivated; bovine rotavirus serotypes G6 and G10, bovine coronavirus, enterotoxigenic strains of E. coli having the K99 pili adherence factor, Clostridium perfringens type C) | No | Oral | Healthy, pregnant cows and heifers as an aid in preventing diarrhoea in their calves | Not indicated |
Prevention of diarrhoea in calves Significant reduction in mortality |
Drugs.com (2021b) | |
| Bovilis Rotavec Corona (inactivated; Bovine rotavirus inactivated, strain UK‐Compton, serotype G6 P5, Bovine coronavirus inactivated, strain Mebus, E. coli F5 (K99) adhesin) | No | Injection | Healthy cows, during pregnancy |
Onset: from the start of colostrum feeding Duration: in calves artificially fed with pooled colostrum, protection will continue until colostrum feeding ceases; in naturally suckled calves, protection against rotavirus will persist for at least 7 days and against coronavirus for at least 14 days |
Reduce the severity of diarrhoea caused by E. coli F5 (K99) Reduce the incidence of scours caused by rotavirus Reduce the shedding of virus by calves infected with rotavirus or coronavirus |
– |
Parameter 2 – Availability/production capacity (per year)
The availability of vaccines in Europe is variable, according to national regulatory policies. Vaccines presented in Table 5 have been widely produced by companies for many years, yet their production capacity is unknown.
Effectiveness
Parameter 3 – Field protection as reduced morbidity (as reduced susceptibility to infection and/or to disease)
According to producer information, vaccines can prevent infections, reduce clinical signs of infections and decrease morbidity and mortality of animals. Field protection of specific vaccines is presented in Table 5.
Parameter 4 – Duration of protection
Duration of protection has been indicated for most of the vaccines and is variable (Table 5).
Feasibility
Parameter 5 – Way of administration
This is variable according to the vaccine (e.g. oral, intramuscular injections, spray) (Table 5).
3.1.4.3. Article 7(d)(iii) Medical treatments
Availability
Parameter 1 – Types of drugs available on the market
Dogs and cats. Antibiotics are the only medical treatments used to treat E. coli infections. Ampicillin, amoxicillin, amoxicillin with clavulanic acid and sulfonamides with trimethoprim are commonly recommended as first‐choice drugs for UTIs, and fluoroquinolones and cephalexin for other infections. Bacteriophage therapy to combat canine and feline E. coli UTIs seems to be a promising strategy as therapeutic agents, yet the lack of regulation for this type of pharmaceutical hinders its potential commercialisation (Ferriol‐González and Domingo‐Calap, 2021). Other therapeutic strategies, such as the use of probiotics and antimicrobial peptides, are considered against UTIs, yet limited success has been achieved.
Horses. Different antibiotics are available to treat urogenital infections (e.g. amoxicillin with clavulanic acid, trimethoprim/sulfonamide combinations, enrofloxacin, ciprofloxacin) and bacteraemia (e.g. gentamicin, third‐generation cephalosporins – cefpodoxime, cefotaxime, ceftazidime, ceftriaxone or ceftiofur). Cefquinome (fourth‐generation cephalosporin, a protected antibiotic) is only recommended to treat respiratory diseases, septicaemia and severe sepsis of neonate horses (less than 3 weeks). Other medical treatments used for preventing uterine infections in mares after mating or insemination includes uterine lavage with sterile saline or lactated Ringer’s solution.
Cattle, sheep and goats. The specific antibiotic to be used depends on the infection type (De Briyne et al., 2014). For example, antibiotics recommended for treatment of mastitis may include aminopenicillins, first‐ to fourth‐generation cephalosporins, aminoglycosides and macrolides, while polymyxins are commonly used in diarrhoea (De Briyne et al., 2014). Other authorised medical treatments include probiotics (reduction of diarrhoeal infection in calves) and teat sealants (subnitrate for protection against intramammary infections of mastitis) (EMA CVMP and EFSA BIOHAZ Panel, 2017).
Poultry. Antibiotics belonging to different classes, including tetracyclines, sulfonamides, aminoglycosides, penicillins, cephalosporins (ceftiofur), quinolones, polymyxins (colistin), chloramphenicols (florfenicol), macrolides (erythromycin) and lincosamides (lincomycin) are used for the treatment of APEC infections. Other medical treatments include prebiotics, probiotics and essential oils for treatment of colibacillosis (EMA CVMP and EFSA BIOHAZ Panel, 2017).
Swine. Antibiotics such as ampicillin, apramycin, third‐generation cephalosporins, gentamicin, neomycin, spectinomycin, amongst others, have been used to treat neonatal diarrhoea; while enrofloxacin, apramycin, ceftiofur, neomycin, gentamicin, amoxicillin/clavulanic acid, trimethoprim/sulfonamide and colistin are commonly used to treat PWD. Other medical treatments include electrolyte treatment for the treatment of acidosis and dehydration, organic acids and probiotics for reducing diarrhoea (EMA CVMP and EFSA BIOHAZ Panel, 2017).
Parameter 2 – Availability/production capacity (per year)
The AMEG of EMA considers third‐generation cephalosporins, quinolones and polymyxins as classes of antibiotics for which there should be special restrictions regarding their use in animals (category B, restrict) (EMA, 2019). These restricted antibiotics should only be used for the treatment of clinical conditions when there are no alternative antibiotics in a lower category that could be clinically effective. All the licensed drugs/classes are produced in volume.
Effectiveness
Parameter 3 – Therapeutic effects in the field (effectiveness)
Antibiotic therapy is generally effective. Nonetheless, resistance of pathogenic E. coli to the antibiotic used will lead to treatment failure, and in some cases will lead to the use of second‐ or third‐tier antimicrobial options. Moreover, there is no data to assess the frequency and impact of treatment failure. Data on efficacy of probiotics as preventing diseases are strictly strain dependent, and of prebiotics and botanicals (e.g. essential oils) are strictly product/formulation dependent.
Feasibility
Parameter 4 – Way of administration
The way of administration is variable given that antibiotics in each (sub)class are available in several different formulations and for administration by different routes (from intramammary treatment of individual cows to treatment of many hundreds of broiler chickens by medication of drinking water). A suggested listing of routes of administration and formulations, ranked in order from those with in general lower effect on the selection of antimicrobial resistance to those that would be expected to have higher impact on resistance, was proposed by EMA (EMA, 2019) as follows: local individual treatment (e.g. udder injector, eye or ear drops); parenteral individual treatment (intravenously, intramuscularly, subcutaneously); oral individual treatment (e.g. tablets, oral bolus); injectable group medication (metaphylaxis), only if appropriately justified; oral group medication via drinking water/milk replacer (metaphylaxis), only if appropriately justified; oral group medication via feed/premixes (metaphylaxis), only if appropriately justified.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
Various disinfectant products are available for prevention and control of E. coli infections in veterinary clinics and hospitals. Regular cleaning and disinfection of environmental surfaces and medical equipment (e.g. stethoscopes, thermometers) are important to prevent hospital‐acquired infections.
Biosecurity measures are recommended and often implemented for reducing the risk of introduction and spread of infectious diseases in farm animals (horses, swine, poultry, cattle, sheep and goats). They include farm sanitation (e.g. protective clothing used by employees and visitors, cleaned and disinfected equipment and vehicles); facility biosecurity (e.g. disinfectant footbaths; quarantine facilities); animal biosecurity (e.g. animal quarantine, vaccination, dead animal management); feed and water biosecurity (e.g. cleaning and disinfection of food storage areas and farrowing crates, filtration and chemical sterilisation of water and regular testing of water quality); manure biosecurity (manure removal and storage in an area inaccessible to animals).
Moreover, preventive measures concerning dairy animals include scrupulous attention to colostrum quality and delivery, prevention of overcrowding and frequent sanitisation of maternity areas. In particular, the following list of prevention measures has been proposed: (1) to milk cows with (sub)clinical mastitis last; (2) to use separate cloths during preparation of udder; (3) to wash dirty udders during preparation of udder; (4) pre‐stripping; (5) use of milkers’ gloves during milking; (6) to disinfect teats post‐milking; (7) to back‐flush clusters after milking a cow with (sub)clinical mastitis; (8) to replace teat‐cup liners in time; (9) application of blanket/selective dry cow therapy; (10) to keep cows standing after milking; (11) to feed additional dry cow minerals; (12) to prevent over‐crowding; (13) to clean cubicles; (14) to clean yards; (15) to optimise feed ration (Baraitareanu and Vidu, 2020).
In addition, biosecurity measures in poultry farms include cleaning and disinfection of entire poultry houses and equipment inside after the removal of each flock and before the introduction of a new flock (Zhou et al., 2020). After removal of each flock, surface litter and caged droppings need to be removed and disposed of at a place away from the poultry production area (Zhou et al., 2020). Once sick and dead birds are identified, they should be isolated and kept in a separate place for further diagnosis. Subsequent measures will depend on the specific cause of the disease (Zhou et al., 2020). All carcasses and ill birds should be removed as soon as possible, since they act as reservoirs and source of infectious agents for other birds in the same shed.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
Biosecurity measures based on cleaning and disinfection are effective in reducing and/or eliminating E. coli (Becker et al., 2021). There is little or no quantitative data on the effectiveness of other biosecurity measures with respect to prevention of animal infections by E. coli.
Feasibility
Parameter 3 – Feasibility of biosecurity measures
Measures such as limited co‐mingling, adequate ventilation and temperature controls, appropriate nutrition and housing and quality assurance programmes are commonly used in modern animal production to reduce the risk of introduction and spread of infections. However, these risk management measures usually require training and incentivising staff. Biosecurity measures based on disinfection are feasible and inexpensive.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
There is no EU legislation affecting animal movements and dealing with AMR E. coli infection, with the exception of VTEC, which is not covered in this fact sheet.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between‐farm spread
There are no measures concerning restricting movement of animals infected with E. coli, thereby effectiveness cannot be measured. In theory, restricting the movement of diseased animals (horses, cattle, sheep, goats, swine, poultry) to another farm will prevent the spread of AMR E. coli. However, animal movement restriction within farm premises may not be effective if the transmission source occurs through ingestion of contaminated food, water or fomites.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
There are no measures concerning restricting movement of animals infected by E. coli, thereby feasibility cannot be measured. However, restricting movement of animals infected by MDR pandemic E. coli clones should be feasible as Council Directives dealing with restrictions of animal movement have been successfully and feasibly implemented by MSs for other bacterial infections.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
There are several methods available for killing of animal species, as reported in the EU according to Council Regulation (EC) No 1099/2009 3 , including mechanical, electrical and gas methods, and lethal injection. However, killing sick animals infected by AMR E. coli is not a measure implemented for disease eradication, with the exception of VTEC not covered in this fact sheet.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
There are no measures concerning killing of animals infected with AMR E. coli, thereby effectiveness cannot be measured. However, if an infected animal is killed, the disease will not spread further from this animal.
Feasibility
Parameter 3 – Feasibility of killing animals
There are no measures concerning killing animals infected with AMR E. coli, thereby feasibility cannot be measured. The economic loss by killing diseased farm animals can be very high. If farm animals can be effectively treated with antibiotics, killing the infected animal will not be necessary. If the antibiotic treatment is not effective, killing will be an option. Concerning dogs and cats, the feasibility depends on acceptance by animal owners.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Availability
Parameter 1 – Available disposal option
Incineration or rendering is allowed, while burial and composting are not permitted in the EU, as it may adversely affect the quality of the soil and water near the burial sites. Disposal of carcasses from animals infected with AMR E. coli are the same as those used for animals that died from diseases caused by other pathogens (incineration, rendering).
Effectiveness
Parameter 2 – Effectiveness of disposal option
All disposal options are well established and may be used successfully.
Feasibility
Parameter 3 – Feasibility of disposal option
The use of incineration depends on the existence of an accessible incinerator, properly licensed and of adequate capacity. It is unlikely to be universally available.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
Cost of treatment in dogs, cats and horses can increase when infections are caused by AMR or MDR E. coli, as they may result in treatment failure with the consequence of increasing veterinary expenditures due to prolonged hospitalisation or additional visits, diagnostic tests and therapies. However, specific information on actual costs is not available.
Regarding the economic costs of mastitis in dairy animals (e.g. cows, goats and sheep), it consists of losses in milk production per animal per year, expenditure with treatment (e.g. antibiotics) and preventive measures. In fact, it was estimated economic losses of clinical mastitis ranging from €61 to €97 per cow worldwide, although with differences between farms, e.g. in the Netherlands, losses due to clinical and subclinical mastitis varied between €17 and €198 per cow per year (Hogeveen et al., 2011). Concerning the costs of an outbreak of diarrhoea in lambs, a recent Italian study estimated that the in‐farm production losses fluctuated from €50 to €1,200 (accounting for losses of meat production due to mortality and the reduced weight gain); if we consider the maximum mortality (80%) observed in one of the analysed farms; however, then economic losses due to an outbreak of diarrhoea in lambs may reach up to €2500 (Mariano et al., 2018). Despite the significant economic impact of colibacillosis in poultry industry, which comprises treatment and prophylaxis expenses, data estimating the real cost are hard to find (Guabiraba and Schouler, 2015). In swine production, enteric colibacillosis may result in economic losses due to mortality, decreased weight gain and cost for treatments, vaccinations and feed supplements. The overall cost of PWD depends on the disease severity and was estimated to range from €40 to €314 per sow (Luppi, 2017). Two longitudinal studies which also accounted for the indirect economic impact due to changes in technical parameters found that farrow‐to‐finish pig farms exhibiting a higher biosecurity and health status were correlated with improved technical parameters and a higher economic margin (net farm profit) of approximately €180/sow/year and €200/sow/year than the farms with the lowest biosecurity status (Rojo‐Gimeno et al., 2016).
Parameter 2 – Cost of eradication (culling, compensation)
There is no data available about the cost of animal eradication caused by AMR E. coli infections.
Parameter 3 – Cost of surveillance and monitoring
There is no data available estimating surveillance and monitoring costs of pathogenic/AMR E. coli. The surveillance and monitoring costs of pathogenic/AMR bacteria are the responsibility of national surveillance programmes, which exists in few European countries (Norway (NORM‐VET), Sweden (Swedres‐SVARM), Finland (FINRES‐Vet), France (RESAPATH), Germany (GERM‐Vet)).
Parameter 4 – Trade loss (bans, embargoes, sanctions) by animal product
There is no data to estimate trade loss caused by these AMR E. coli infections, due to bans, embargoes or sanctions.
Parameter 5 – Importance of the disease for the affected sector (% loss or € lost compared to business amount of the sector)
The cost of an average case of mastitis affecting a dairy cow with a production of 7,000 kg of milk per lactation has been estimated as approximately £131 (€198). This value includes labour, treatment, drugs, veterinary charges, discarded milk, milk production loss, feed intake needed for production of discarded milk and occasional fatality of the disease. These numbers mostly depend on the actual price of milk and medication as well as the severity and duration of the disease. According to statistics from the National Mastitis Council in the USA, losses due to reduced production as a result of mastitis plus prevention and control costs exceeded US$2 billion annually and approximately one‐third of all cows were affected (Cobirka et al., 2020). The economic losses of colibacillosis in poultry industry can be due to mortality (up to 20%) and decreased meat (2% decline in live weight, 2.7% deterioration in feed conversion ratio) and egg production (up to 20%), decreased hatching rates, and increased condemnation of carcasses (up to 43%). In fact, APEC costs hundreds of millions of dollars in economic losses worldwide. Particularly, in the USA, losses of US$40 million annually were estimated only due to carcass condemnation (Guabiraba and Schouler, 2015; Kathayat et al., 2021). In swine, the leading cause of economic losses is the high mortality associated with enteric colibacillosis, namely up to 70% in neonatal piglets and up to 25% in untreated animals. Moreover, in severe cases of disease, 30–40% of total body weight may be lost (Fairbrother and Nadeau, 2019).
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
Disease prevention measures are likely to be acceptable by general society, except for pet owners, which may not be able to sustain the veterinary expenditures, and animal farmers who believe that implementing or altering disease prevention and control measures are expensive or impractical.
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
Despite vaccination being a common and effective strategy to prevent animal diseases, it is reported as a stressful and painful event for animals (e.g. pain, necrosis and self‐mutilation in response to intramuscular injection in animals) (Temple et al., 2020). For other measures to control pathogenic AMR E. coli infections, there is no data available regarding their welfare impact on domestic animals.
Parameter 2 – Wildlife depopulation as control measure
This is not applied.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
There is no data available in Europe estimating the real environmental impact of the use of antimicrobial compounds or residues; however, the amount of several veterinary antibiotic residues was detected in manure from several animal farms in China (fleroxacin: chicken – 99.43 mg/kg; norfloxacin: chicken – 225.45 mg/kg; ciprofloxacin: swine/chicken/cattle – 33.98/45.59/29.59 mg/kg; enrofloxacin: swine/chicken/cattle – 33.26/1,420.76/46.70 mg/kg; oxytetracycline: swine/cattle – 59.06/59.59 mg/kg; chlortetracycline: swine/cattle – 21.06/27.59 mg/kg; tetracyclines: swine – 34.58mg/kg; sulfonamides – 0.17 mg/kg; macrolides: swine – 80 mg/kg; nitrofurans: swine, chicken, cattle – 0.085 mg/kg) (Ma et al., 2021). Furthermore, it is well known that antibiotics used in farms for the control of animals infections can be released in the environment in many ways (e.g. farm manure, used as fertiliser, wastewater), contributing to the selection and spread of AMR bacteria, including E. coli (Larsson and Flach, 2021; Monger et al., 2021). Moreover, biocides (e.g. microbicidal component, as formaldehyde, peroxygen, peracetic acid, chlorocresol) are routinely used in farms, veterinary clinics/hospitals for cleaning and disinfection proposes and not specifically for E. coli infections treatment and/or prevention (Kampf, 2018). Therefore, the use and amount of such antimicrobial agents cannot be ascribed specifically to E. coli control.
Biodiversity
Parameter 1 – Mortality in wild species
There is no data available about wild species mortality by measures used to control AMR E. coli infections.
3.2. Assessment of AMR Escherichia coli according to Article 5 criteria of the AHL on its eligibility to be listed
3.2.1. Detailed outcome on Article 5 criteria
In Table 6 and Figure 2, the results of the expert judgement on the Article 5 criteria of the AHL for AMR E. coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats are presented.
Table 6.
Outcome of the expert judgement on Article 5 criteria
|
Criteria to be met by the disease: According to the AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| A(i) | The disease is transmissible | 90–99 | Fulfilled | 0 | 11 |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | 99–100 | Fulfilled | 0 | 11 |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | 95–100 | Fulfilled | 0 | 11 |
| A(iv) | Diagnostic tools are available for the disease | 90–100 | Fulfilled | 0 | 11 |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | 33–66 | Uncertain | 0 | 11 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point A(i)–A(v), the disease needs to fulfil at least one of the following criteria | |||||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | 66–95 | Fulfilled | 0 | 11 |
| B(ii) | The disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union | 90–95 | Fulfilled | 0 | 11 |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | 66–90 | Fulfilled | 0 | 11 |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | 5–25 | Not fulfilled | 0 | 11 |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | 10–33 | Not fulfilled | 0 | 11 |
na: not applicable.
Figure 2.
Outcome of the expert judgement on Article 5 criteria and overall probability of AMR E. coli on its eligibility to be listed
- Listing: the probability of the disease to be listed according to Article 5 criteria of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion is reported in Sections A.1 and A.2 of Appendix A.
In Figure 2, the outcome of the expert judgement is graphically shown together with the estimated overall probability of the AMR bacterium meeting the criteria of Article 5 on its eligibility to be listed.
3.2.1.1. Reasoning for uncertain outcome on Article 5 criteria
Criterion A(v) (risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union):
As the bacterium is ubiquitous and farming environment is a predisposing factor, several different risk mitigation measures can be applied: vaccination, biosecurity, changes in animal husbandry for reducing risk factors, application of cleaning and disinfection procedures, etc.
Biosecurity and management measures are available and in general effective and proportionate to the risk posed by AMR E. coli.
Antimicrobial therapy is generally effective. Nonetheless, resistance of pathogenic E. coli to the antimicrobials used will lead to treatment failure. Data to assess the current frequency and impact of treatment failure are lacking.
A low efficacy is described for the few available vaccines.
There is surveillance in place for VTEC and the commensal bacterium, but not for pathogenic E. coli strains, so uncertainty remains.
Altogether, (effective) risk mitigation measures are available, but uncertainty remains as to whether they are being well implemented for all species, and there may be an excessive use of antimicrobials of second and third tiers to deal with AMR E. coli, which would not be proportionate to the risk posed.
3.2.2. Overall outcome on Article 5 criteria
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
According to the results shown in Table 6, AMR E. coli complies with four criteria of the first set (A(i)–A(iv)), but there is uncertainty (33–66% probability) on the assessment on compliance with Criterion A(v). Therefore, it is uncertain whether AMR E. coli can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL. The estimated overall probability range for the AMR bacterium being eligible to be listed is 33–66% (Figure 2).
3.3. Assessment of AMR Escherichia coli according to criteria in Annex IV for the purpose of categorisation as in Article 9 of the AHL
In Tables 7, 8, 9, 10, 11 and related graphs (Figures 3, 4–5), the results of the expert judgement on AMR E. coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats according to the criteria in Annex IV of the AHL, for the purpose of categorisation as in Article 9, are presented.
Table 7.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (Category A of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present in only in a very limited part of the territory of the Union | 0–5 | Not fulfilled | 0 | 11 |
| 2.1 | The disease is highly transmissible | 10–33 | Not fulfilled | 0 | 11 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 66–90 | Fulfilled | 0 | 11 |
| 2.3 | The disease affects multiple species of kept and wild animals or single species of kept animals of economic importance | 99–100 | Fulfilled | 0 | 11 |
| 2.4 | The disease may result in high morbidity and significant mortality rates | 66–90 | Fulfilled | 0 | 11 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential, or possible significant threats to food safety | 66–90 | Fulfilled | 0 | 11 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 66–90 | Fulfilled | 0 | 11 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 10–66 | Uncertain | 0 | 11 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–95 | Fulfilled | 0 | 11 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 33–75 | Uncertain | 0 | 11 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 10–50 | Uncertain | 0 | 11 |
na: not applicable.
Table 8.
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (Category B of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease | 5–10 | Not fulfilled | 0 | 11 |
| 2.1 | The disease is moderately to highly transmissible | 33–66 | Uncertain | 0 | 11 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 66–90 | Fulfilled | 0 | 11 |
| 2.3 | The disease affects single or multiple species | ‐ | Fulfilled | 0 | 11 |
| 2.4 | The disease may result in high morbidity with in general low mortality | 33–66 | Uncertain | 0 | 11 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic potential, or possible significant threats to food safety | 66–90 | Fulfilled | 0 | 11 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 66–90 | Fulfilled | 0 | 11 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 10–66 | Uncertain | 0 | 11 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–95 | Fulfilled | 0 | 11 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 33–75 | Uncertain | 0 | 11 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 10–50 | Uncertain | 0 | 11 |
na: not applicable.
Table 9.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (Category C of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character | 90–99 | Fulfilled | 0 | 11 |
| 2.1 | The disease is moderately to highly transmissible | 33–66 | Uncertain | 0 | 11 |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | – | Fulfilled | 0 | 11 |
| 2.3 | The disease affects single or multiple species | – | Fulfilled | 0 | 11 |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss | 10–33 | Not fulfilled | 0 | 11 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety | 66–90 | Fulfilled | 0 | 11 |
| 4 | The disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems | 50–90 | Uncertain | 0 | 11 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 10–66 | Uncertain | 0 | 11 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–95 | Fulfilled | 0 | 11 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 33–75 | Uncertain | 0 | 11 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 10–50 | Uncertain | 0 | 11 |
na: not applicable.
Figure 3.
Outcome of the expert judgement on criteria of Section 1 of Annex IV and overall probability of the AMR bacterium to be fitting in Category A of Article 9
- Category A: The probability of the disease to be categorised according to Section 1 of Annex IV of the AHL (overall outcome).
Figure 4.
Outcome of the expert judgement on criteria of Section 2 of Annex IV and overall probability of the AMR bacterium to be fitting in Category B of Article 9
- Category B: the probability of the disease to be categorised according to Section 2 of Annex IV of the AHL (overall outcome).
Figure 5.
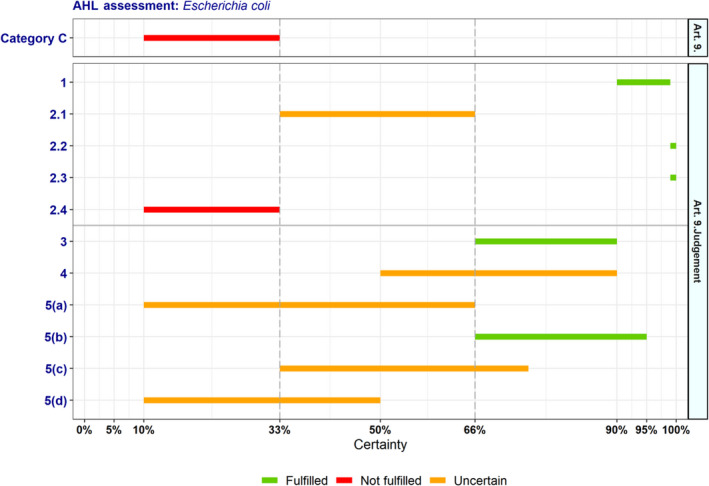
Outcome of the expert judgement on criteria of Section 3 of Annex IV and overall probability of the AMR bacterium to be fitting in Category C of Article 9
- Category C: the probability of the disease to be categorised according to Section 3 of Annex IV of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion is reported in Sections B.1 and B.2 of Appendix B.
3.3.1. Detailed outcome on Category A criteria
3.3.1.1. Reasoning for uncertain outcome on Category A criteria
Criterion 5(a) (the disease has a significant impact on society, with in particular an impact on labour markets):
The effects of the diseases caused by AMR E. coli are already present and do not seem to have a major effect.
AMR E. coli causes significant impact on animal production, but an impact on labour markets or society is unlikely.
For workers in the animal sector, there may be effects on workdays lost for zoonotic cases, reduced productivity and income.
Criterion 5(c) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it):
AMR E. coli can be found in the environment, as well as residues of the antimicrobials that are used for its control. There is no data available in Europe or elsewhere estimating the real environmental impact.
E. coli is an opportunistic pathogen, wildlife may potentially carry the pathogen acquired from contaminated environments, and thus there is the potential of occurring infections with variable severity. However, there is no obvious link between antimicrobial resistance and the impact on the environment and biodiversity.
There is no data available about mortality in wild animal species by measures used to control AMR E. coli infections, but it may increase in wildlife if AMR strains spread.
Direct impact on the environment does not seem to occur. However, contamination of the environment with AMR E. coli may lead to transmission to other animals or humans.
Criterion 5(d) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds):
Transmission from domestic to wild animals is potentially possible, and the presence of AMR E. coli has been reported in wild animal species.
It can be supposed to cause disease and mortality in some species or under certain circumstances, although not in a relevant manner, but it may become significant for endangered breeds if AMR strains spread.
There is no obvious link between antimicrobial resistance and the impact on the environment and biodiversity.
E. coli are ubiquitous commensals that are heavily monitored. If this has not already been described as a problem, then the risk seems to be unlikely.
A lot of uncertainty remains due to the lack of data about impact on wildlife.
3.3.2. Detailed outcome on Category B criteria
3.3.2.1. Reasoning for uncertain outcome on Category B criteria
Criterion 2.1 (the disease is moderately to highly transmissible):
Although in general considered an environmental bacterium which may result in opportunistic infections, available evidence clearly show that E. coli may be transmitted from infected to non‐infected animals, and that although transmission of AMR genes seems to occur more frequently by horizontal gene transfer, both vertical and horizontal transmission may occur also for AMR E. coli.
Direct transmission is possible, but usually transmission is via the faecal routes, where transmission depends on, e.g. stocking density and other management factors.
The speed of transmission does not seem to be high in general, but given the high mortality rates that can be observed for certain species, it can be highly transmissible in the context of certain production systems with high density of animals (e.g. poultry farms, calves/piglets stables).
Criterion 2.4 (the disease may result in high morbidity with in general low mortality):
Clinical presentation as well as morbidity and mortality vary between infection type, animal species, age and production system. Therefore, it is difficult to make an overall statement.
It can cause high morbidity and mortality in poultry, calves, piglets and lambs, less in dogs, cats, horses and adult cattle.
Diseases caused by AMR E. coli may also lead to more moderate pictures, particularly when good management is in place. In immunologically mature animals, the disease may only lead to production loss.
Criterion 5(a) (the disease has a significant impact on society, with in particular an impact on labour markets): See above in Section 3.3.1.1.
Criterion 5(c) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it): See above in Section 3.3.1.1.
Criterion 5(d) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds): See above in Section 3.3.1.1.
3.3.3. Detailed outcome on Category C criteria
3.3.3.1. Reasoning for uncertain outcome on Category C criteria
Criterion 2.1 (the disease is moderately to highly transmissible): See above in Section 3.3.1.1.
Criterion 4 (the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems):
E. coli is a common cause of disease in many animal species with a significant negative impact on animal health. Therefore, the overall economic impact due to costs of prevention and treatment as well as production loss may increase with the spread of AMR strains.
AMR E. coli are frequent in multiple species and may as such not affect certain types of production systems more than others. Therefore, a specific impact of AMR E. coli on the economy is uncertain.
The significance of its impact is hard to quantify, but E. coli is among the main pathogens routinely affecting all relevant livestock species.
The highest economic impact is related to high levels of mortality that occur in specific production systems (piglets, poultry, calves, etc.).
Criterion 5(a) (The disease has a significant impact on society, with in particular an impact on labour markets): See above in Section 3.3.1.1.
Criterion 5(c) (The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it): See above in Section 3.3.1.1.
Criterion 5(d) (The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds): See above in Section 3.3.1.1.
3.3.4. Detailed outcome on Category D criteria
Table 10.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (Category D of Article 9)
| Diseases in Category D need to fulfil criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL and the following: | Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| D | The risk posed by the disease can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | 10–33 | Not fulfilled | 0 | 11 |
na: not applicable.
3.3.5. Detailed outcome on Category E criteria
Table 11.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (Category E of Article 9)
| Diseases in Category E need to fulfil criteria of Section 1, 2 or 3 of Annex IV of the AHL and/or the following: | Outcome | ||
|---|---|---|---|
|
Median range (%) |
Fulfilment | ||
| E |
Surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently Category E would apply.) |
33–66 | Uncertain |
3.3.6. Overall outcome on criteria in Annex IV for the purpose of categorisation as in Article 9
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D, or E – corresponding to points (a) to (e) of Article 9(1) of the AHL) if it fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d), as shown in Tables 7–11. According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
The overall outcome of the assessment on criteria in Annex IV of the AHL, for the purpose of categorisation of AMR E. coli as in Article 9, is presented in Table 12 and Figure 6.
Table 12.
Outcome of the assessment on criteria in Annex IV of the AHL for the purpose of categorisation as in Article 9
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5(a) | 5(b) | 5(c) | 5(d) | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | 0–5 | 10–33 | 66–90 | 99–100 | 66–90 | 66–90 | 66–90 | 10–66 | 66–95 | 33–75 | 10–50 |
| B | 5–10 | 33–66 | 66–90 | – | 33–66 | 66–90 | 66–90 | 10–66 | 66–95 | 33–75 | 10–50 |
| C | 90–99 | 33–66 | – | – | 10–33 | 66–90 | 50–90 | 10–66 | 66–95 | 33–75 | 10–50 |
| D | 10–33 | ||||||||||
| E | 33–66 | ||||||||||
Probability ranges (% certainty; –: criterion fulfilled by default) and fulfilment of criteria (green: fulfilled; red: not fulfilled; orange: uncertain) (EFSA AHAW Panel, 2017).
Figure 6.
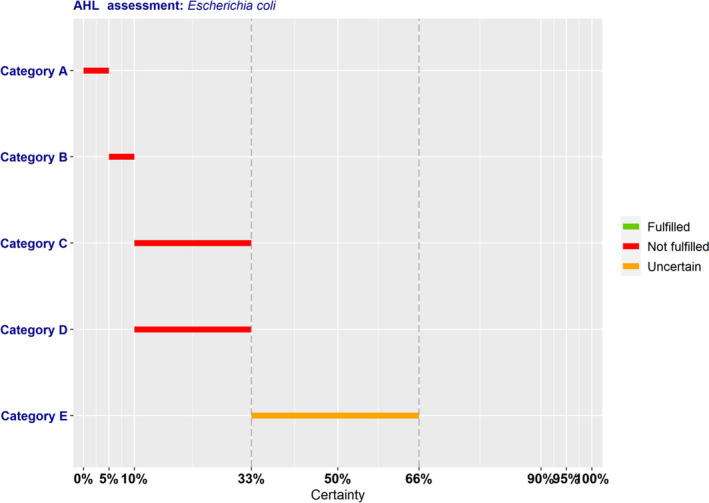
Outcome of the expert judgement on criteria in Annex IV and overall probabilities for categorisation of the AMR bacterium in accordance with Article 9
According to the assessment here performed, AMR E. coli complies with the following criteria of Section 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a) to (e) of Article 9(1):
To be assigned to Category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR E. coli complies only with Criteria 2.2 (66–90% probability), 2.3 (99–100% probability) and 2.4 (66–90% probability). To be eligible for Category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)), and AMR E. coli complies with Criteria 3 (66–90% probability), 4 (66–90% probability) and 5(b) (66–95% probability). The assessment was inconclusive on compliance with Criteria 5(a) (10–66% probability), 5(c) (33–75% probability) and 5(d) (10–50% probability). Overall, it was assessed with 0–5% probability that AMR E. coli may be assigned to Category A according to criteria in Section 1 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR E. coli complies only with Criteria 2.2 (66–90% probability) and 2.3 (fulfilled by default). The assessment was inconclusive on compliance with Criteria 2.1 (33–66% probability) and 2.4 (33–66% probability). To be eligible for Category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)), and AMR E. coli complies with Criteria 3 (66–90% probability), 4 (66–90% probability) and 5(b) (66–95% probability). The assessment was inconclusive on compliance with Criteria 5(a) (10–66% probability), 5(c) (33–75% probability) and 5(d) (10–50% probability). Overall, it was assessed with 5–10% probability that AMR E. coli may be assigned to Category B according to criteria in Section 2 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR E. coli complies only with Criteria 1 (90–99% probability), 2.2 and 2.3 (both fulfilled by default). The assessment was inconclusive on compliance with Criterion 2.1 (33–66% probability). To be eligible for Category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)), and AMR E. coli complies with Criteria 3 (66–90% probability) and 5(b) (66–95% probability). The assessment was inconclusive on compliance with Criteria 4 (50–90% probability), 5(a) (10–66% probability), 5(c) (33–75% probability) and 5(d) (10–50% probability). Overall, it was assessed with 10–33% probability that AMR E. coli may be assigned to Category C according to criteria in Section 3 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category D, a disease needs to comply with criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL and with the specific Criterion D of Section 4, with which AMR E. coli does not comply (10–33% probability).
To be assigned to Category E, a disease needs to comply with criteria of Section 1, 2 or 3 of Annex IV of the AHL, and/or the surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, for which the assessment is inconclusive (33–66% probability of fulfilling the criteria).
3.4. Assessment of AMR Escherichia coli according to Article 8 criteria of the AHL
In this section, the results of the assessment on the criteria of Article 8(3) of the AHL for AMR E. coli are presented. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to the list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible to a specific listed disease, or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely’.
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also the possible role of biological or mechanical vectors. 4
According to the mapping, as presented in Table 5, Section 3.2, of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the animal species to be listed for AMR E. coli according to the criteria of Article 8(3) of the AHL are mammals, birds, reptiles and fish.
4. Conclusions
The AHAW Panel emphasises that the assessment of impacts, as well as prevention and control measures, related to AMR bacteria using the criteria as laid down in Articles 5 and 9 of the AHL is particularly challenging for opportunistic pathogens that can also be found as commensal bacteria in healthy animals.
TOR 1: For each of those identified AMR bacteria considered most relevant in the EU, following the criteria laid down in Article 7 of the AHL, an assessment on its eligibility to be listed for Union intervention as laid down in Article 5(3) of the AHL;
It is uncertain (30–66% probability, ‘as likely as not’) whether AMR E. coli can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL.
TOR 2: For each of the AMR bacteria which was found eligible to be listed for Union intervention, an assessment on its compliance with the criteria in Annex IV for the purpose of categorisation in accordance with Article 9 of the AHL;
The AHAW Panel considered with 0–5% probability (from ‘almost impossible’ to ‘extremely unlikely’) that AMR E. coli meets the criteria as in Section 1 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (a) of Article 9(1) of the AHL.
The AHAW Panel considered with 5–10% probability (‘very unlikely’) that AMR E. coli meets the criteria as in Section 2 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (b) of Article 9(1) of the AHL.
The AHAW Panel considered with 10–33% probability (‘unlikely’) that AMR E. coli meets the criteria as in Section 3 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (c) of Article 9(1) of the AHL.
The AHAW Panel considered with 10–33% probability (‘unlikely’) that AMR E. coli meets the criteria as in Section 4 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (d) of Article 9(1) of the AHL.
The AHAW Panel was uncertain (33–66% probability, ‘as likely as not’) whether AMR E. coli meets the criteria as in Section 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (e) of Article 9(1) of the AHL.
TOR 3: For each of the AMR bacteria which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL;
The animal species that can be considered to be listed for AMR E. coli according to Article 8(3) of the AHL include mammals, birds, reptiles and fish.
The AHAW Panel highlights that monitoring of antimicrobial resistance in opportunistic bacteria could help to assess their impacts. Therefore, even though the assessment on AMR E. coli is inconclusive on its eligibility to be listed for Union intervention, specific initiatives (e.g. monitoring or applied research) into various aspects of AMR E. coli can be useful to better understand its distribution and to assess its impact on animal health and welfare in the EU.
Abbreviations
- AHAW
Animal Health and Welfare
- AHL
Animal Health Law
- AMEG
Antimicrobial Advice ad hoc Expert Group
- AMR
Antimicrobial‐resistant
- APEC
Avian pathogenic E. coli
- AST
Antimicrobial susceptibility testing
- CFSPH
Center for Food Security and Public Health
- CHMP
Committee for Medicinal Products for Human Use
- CI
Current impact
- CITES
Convention on International Trade in Endangered Species
- CLSI
Clinical and Laboratory Standards Institute
- CRI
Catheter‐related infection
- CVMP
Committee for Veterinary Medicinal Products
- DALY
Disability‐adjusted life year
- DIVA
Differentiation of infected from vaccinated animals
- EDEC
Oedema disease E. coli
- EEA
European Economic Area
- EHEC
Enterohaemorrhagic E. coli
- EPEC
Enteropathogenic E. coli
- ESBL
Extended‐spectrum β‐lactamase
- ETEC
Enterotoxigenic E. coli
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- ExPEC
Extra‐intestinal pathogenic E. coli
- F
Fimbriae adhesin
- HUS
Haemolytic uremic syndrome
- I
Intermediate
- IUCN
International Union for Conservation of Nature
- MALDI‐TOF MS
Matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry
- MCR
Plasmid‐mediated polymyxin resistance
- MDR
Multidrug‐resistant
- MIC
Minimum inhibitory concentration
- MLEE
Multi‐locus enzyme electrophoresis
- MLST
Multi‐locus sequence typing
- MS
Member State
- NMEC
Neonatal meningitis E. coli
- OI
Orthopaedic infection
- OIE
Office International des Épizooties (World Organisation for Animal Health)
- PCR
Polymerase chain reaction
- PFGE
Pulsed‐field gel electrophoresis
- PHH
Per hen housed
- PI
Potential impact
- PMQR
Plasmid‐mediated quinolone resistance
- PWD
Post‐weaning diarrhoea
- R
Resistant
- sOR
Summary odds ratio
- SSI
Surgical site infection
- ToR
Term of Reference
- UPEC
Uropathogenic E. coli
- UTI
Urinary tract infection
- STEC
Shiga toxin‐producing E. coli
- VTEC
Verotoxigenic E. coli
- WGS
Whole genome sequencing
Appendix A – Criteria with certain outcome
A.1. Article 5 criteria
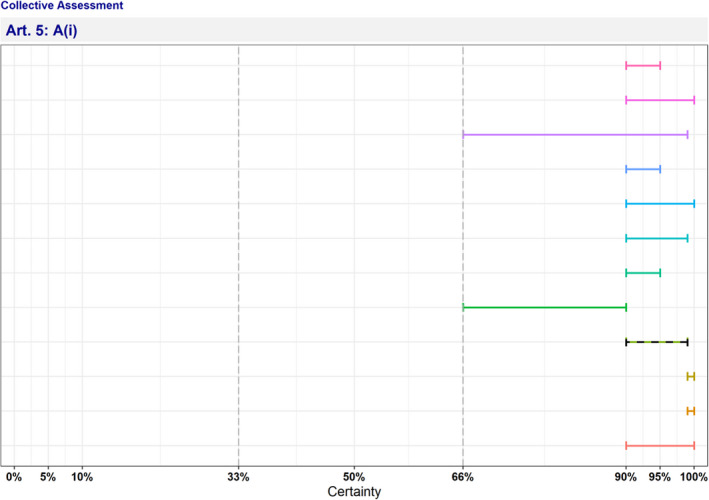
Figure A.1 Individual probability ranges reflecting fulfilment of Criterion A(i) (the disease is transmissible) after the collective judgement
- The median range is displayed as a dashed line.
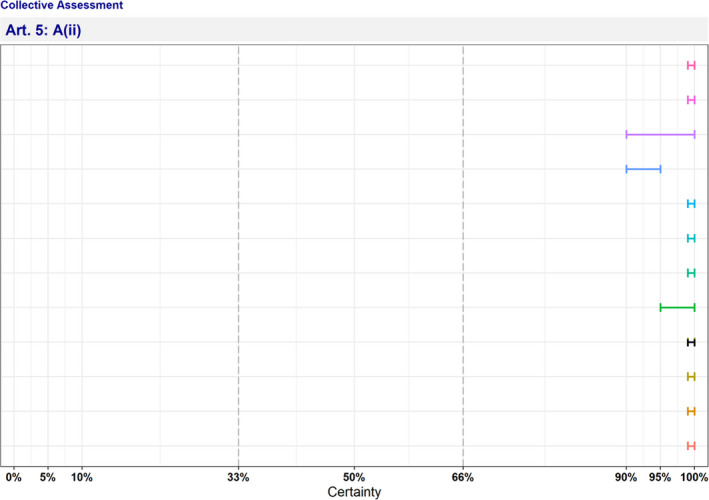
Figure A.2 Individual probability ranges reflecting fulfilment of Criterion A(ii) (animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union) after the collective judgement
- The median range is displayed as a dashed line.
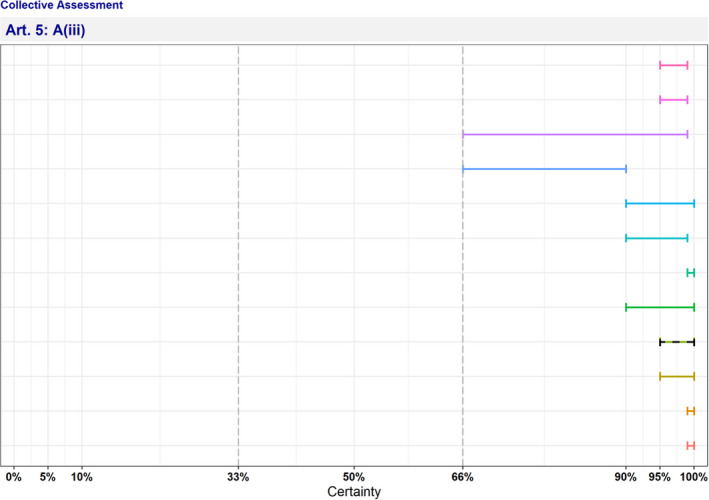
Figure A.3 Individual probability ranges reflecting fulfilment of Criterion A(iii) (the disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character) after the collective judgement
- The median range is displayed as a dashed line.
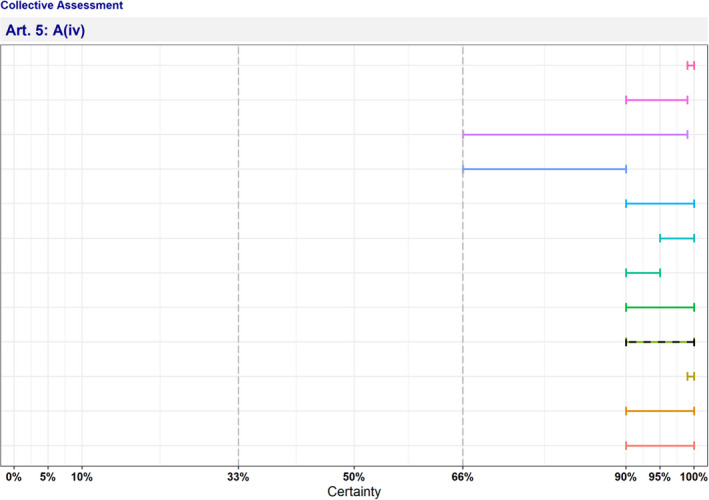
Figure A.4 Individual probability ranges reflecting fulfilment of Criterion A(iv) (diagnostic tools are available for the disease) after the collective judgement
- The median range is displayed as a dashed line.
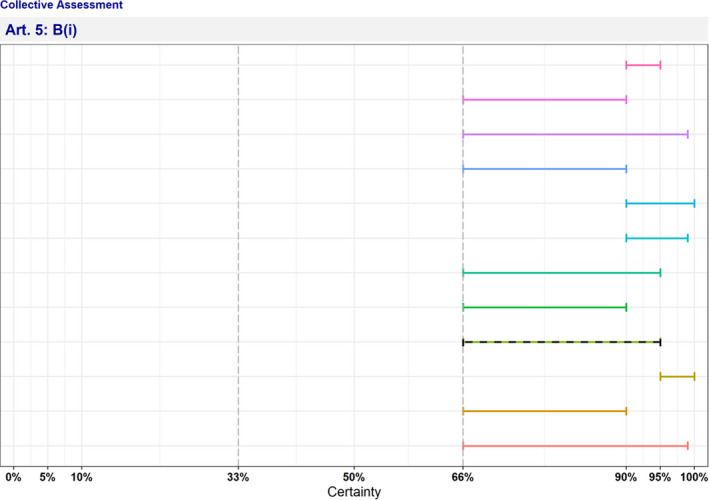
Figure A.5 Individual probability ranges reflecting fulfilment of Criterion B(i) (the disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character) after the collective judgement
- The median range is displayed as a dashed line.
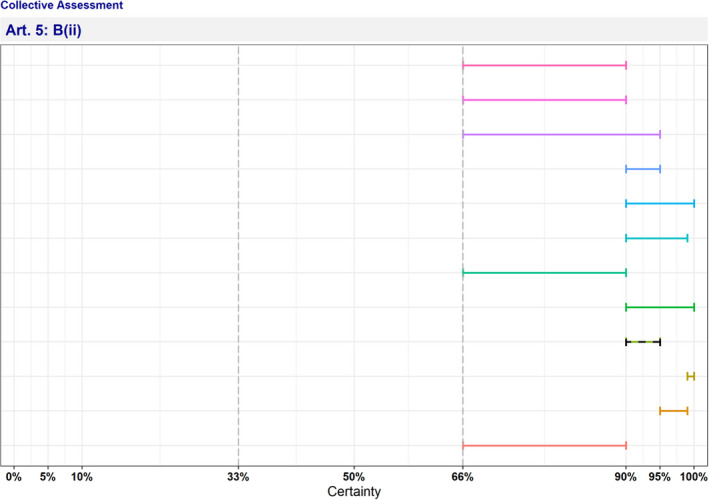
Figure A.6 Individual probability ranges reflecting fulfilment of Criterion B(ii) (the disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union) after the collective judgement
- The median range is displayed as a dashed line.
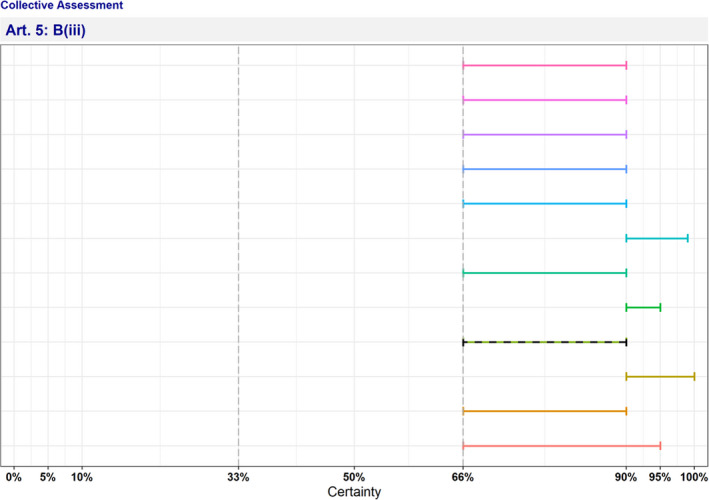
Figure A.7 Individual probability ranges reflecting fulfilment of Criterion B(iii) (the disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union) after the collective judgement
- The median range is displayed as a dashed line.
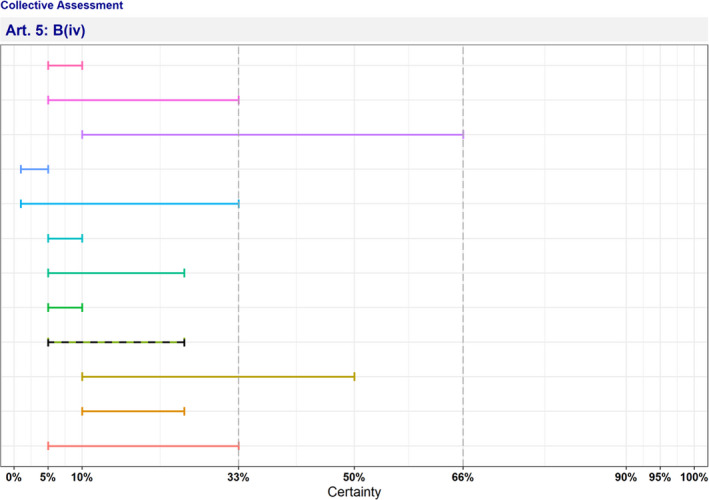
Figure A.8 Individual probability ranges reflecting non‐fulfilment of Criterion B(iv) (the disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism) after the collective judgement
- The median range is displayed as a dashed line.
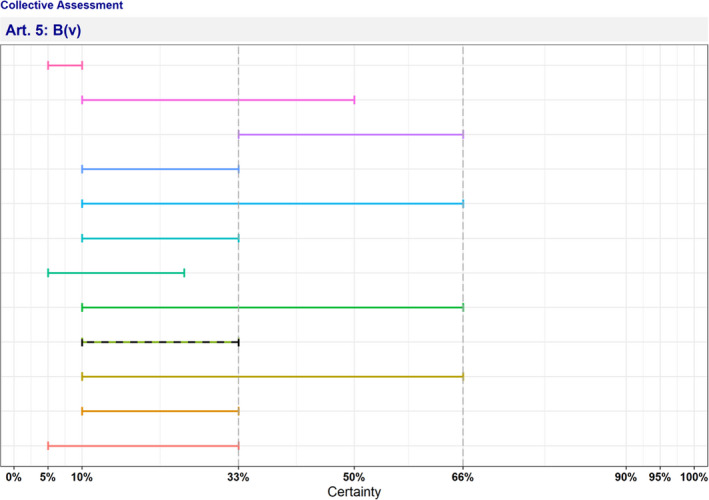
Figure A.9 Individual probability ranges reflecting non‐fulfilment of Criterion B(v) (the disease has or could have a significant negative impact on the environment, including biodiversity, of the Union) after the collective judgement
- The median range is displayed as a dashed line.
A.2. Article 9 criteria
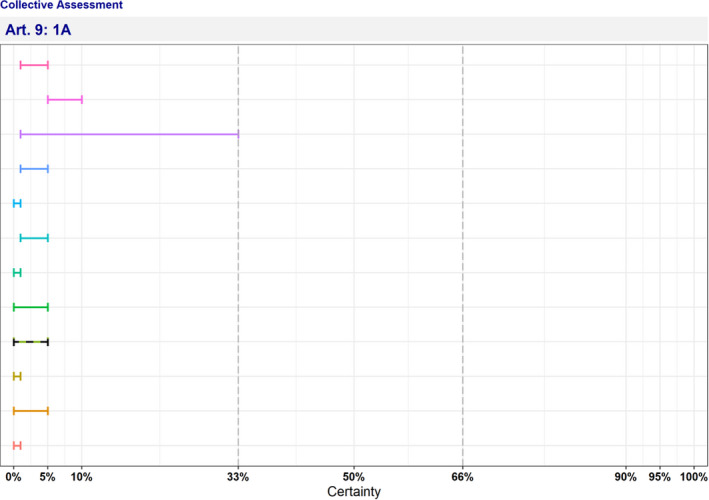
Figure A.10 Individual probability ranges reflecting non‐fulfilment of Criterion 1A (the disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present in only in a very limited part of the territory of the Union) after the collective judgement
- The median range is displayed as a dashed line.
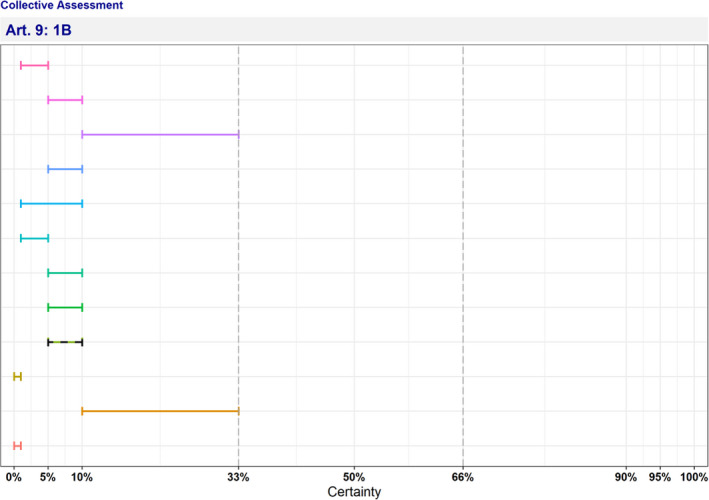
Figure A.11 Individual probability ranges reflecting non‐fulfilment of Criterion 1B (the disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease) after the collective judgement
- The median range is displayed as a dashed line.
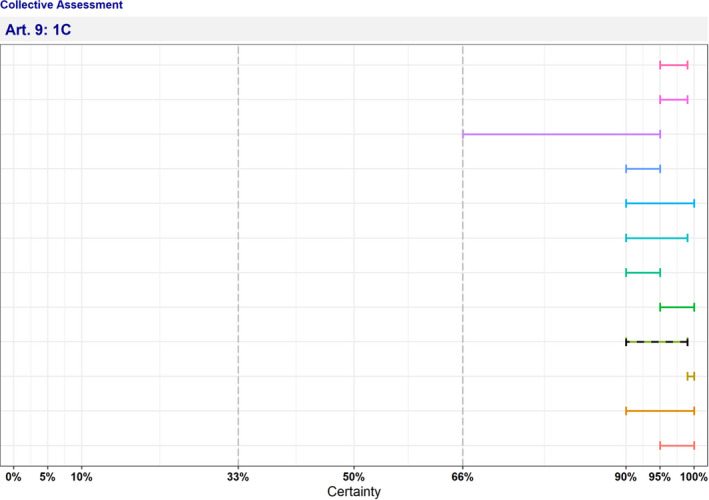
Figure A.12 Individual probability ranges reflecting fulfilment of Criterion 1C (the disease is present in the whole or part of the Union territory with an endemic character) after the collective judgement
- The median range is displayed as a dashed line.
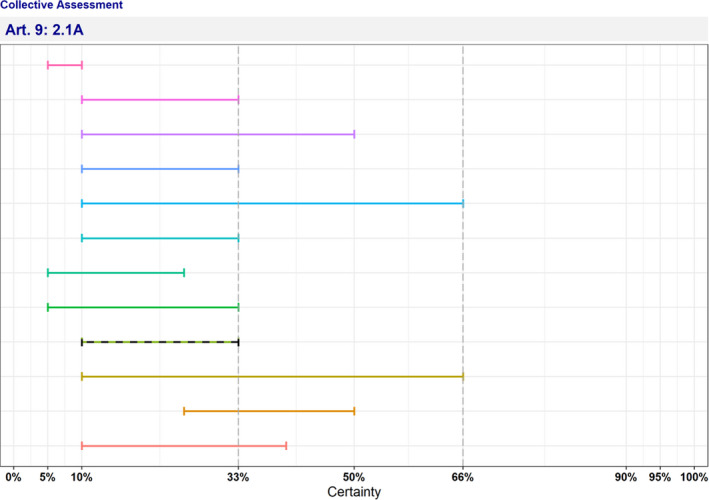
Figure A.13 Individual probability ranges reflecting non‐fulfilment of Criterion 2.1A (the disease is highly transmissible) after the collective judgement
- The median range is displayed as a dashed line.
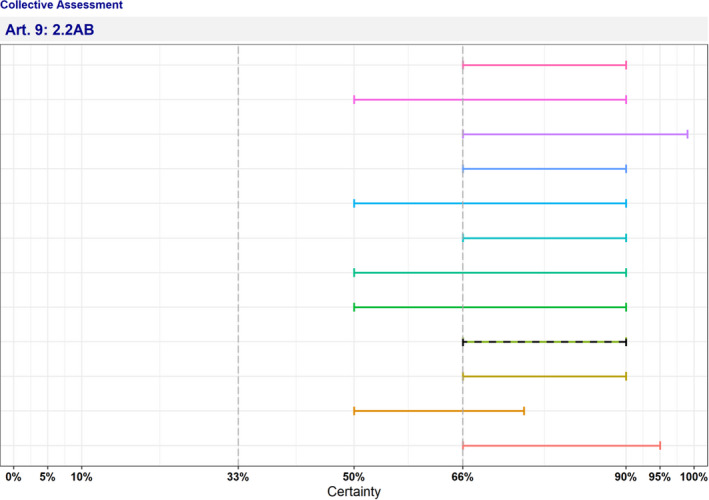
Figure A.14 Individual probability ranges reflecting fulfilment of Criterion 2.2AB (there are possibilities of airborne or waterborne or vector‐borne spread) after the collective judgement
- The median range is displayed as a dashed line.
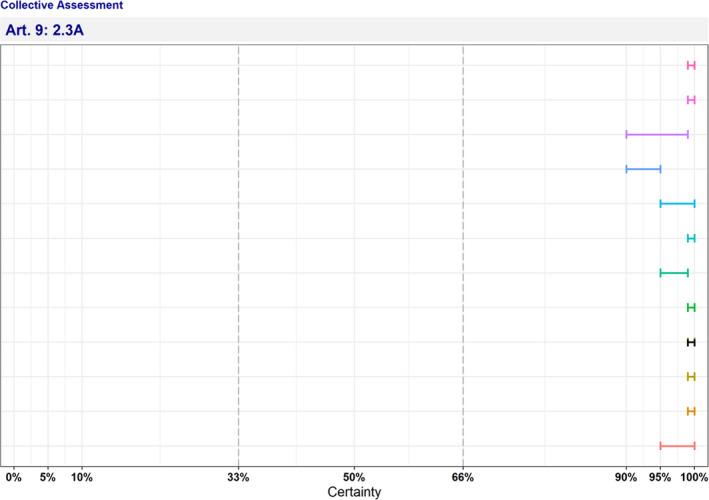
Figure A.15 Individual probability ranges reflecting fulfilment of Criterion 2.3A (the disease affects multiple species of kept and wild animals or single species of kept animals of economic importance) after the collective judgement
- The median range is displayed as a dashed line.
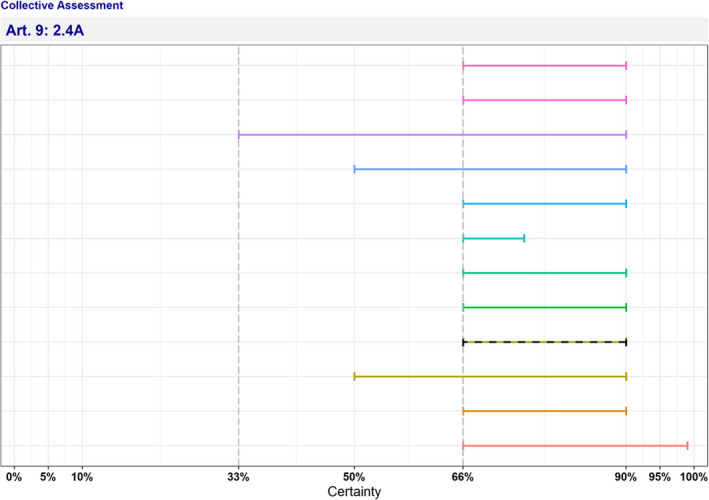
Figure A.16 Individual probability ranges reflecting fulfilment of Criterion 2.4A (the disease may result in high morbidity and significant mortality rates) after the collective judgement
- The median range is displayed as a dashed line.
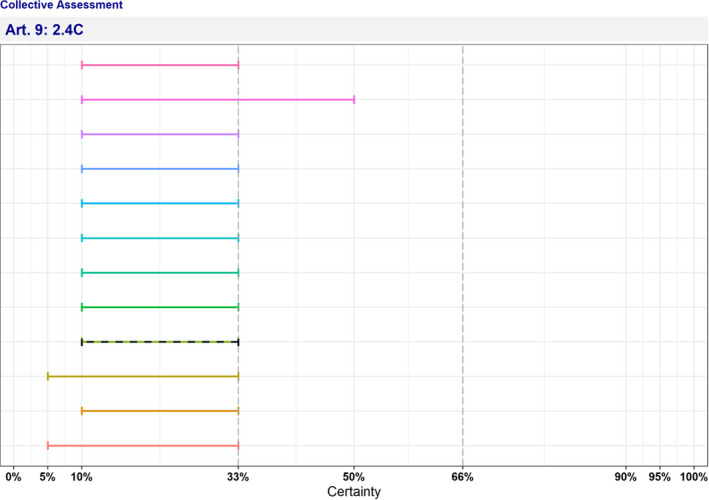
Figure A.17 Individual probability ranges reflecting non‐fulfilment of Criterion 2.4C (the disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss) after the collective judgement
- The median range is displayed as a dashed line.
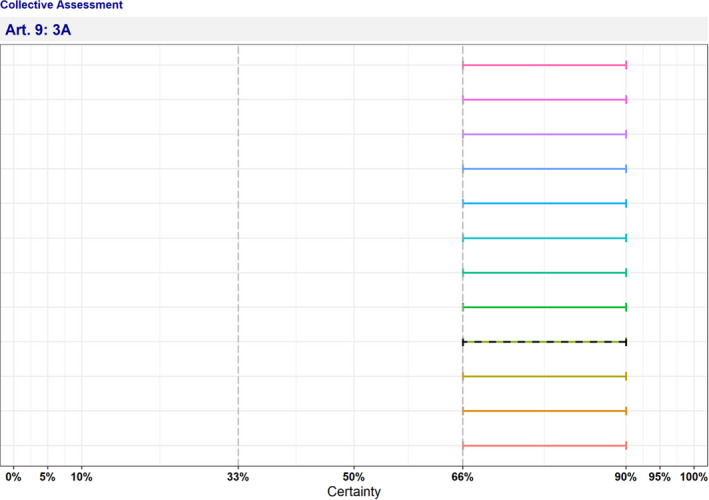
Figure A.18 Individual probability ranges reflecting fulfilment of Criterion 3A (the disease has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.
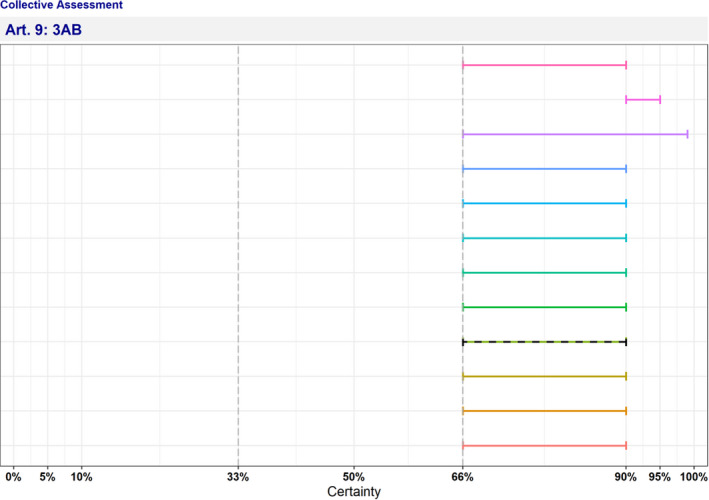
Figure A.19 Individual probability ranges reflecting fulfilment of Criterion 3AB (the disease has a zoonotic potential with significant consequences for public health, including epidemic potential or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.
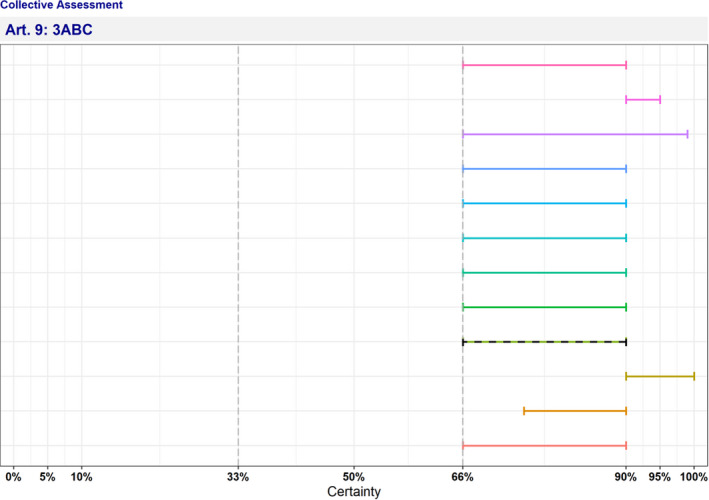
Figure A.20 Individual probability ranges reflecting fulfilment of Criterion 3ABC (the disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.
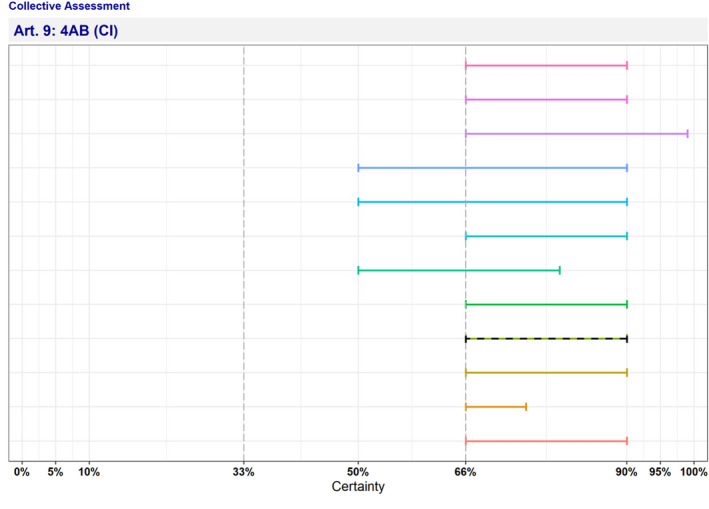
Figure A.21 Individual probability ranges reflecting fulfilment of Criterion 4AB (current impact) (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals) after the collective judgement
- CI: current impact. The median range is displayed as a dashed line.
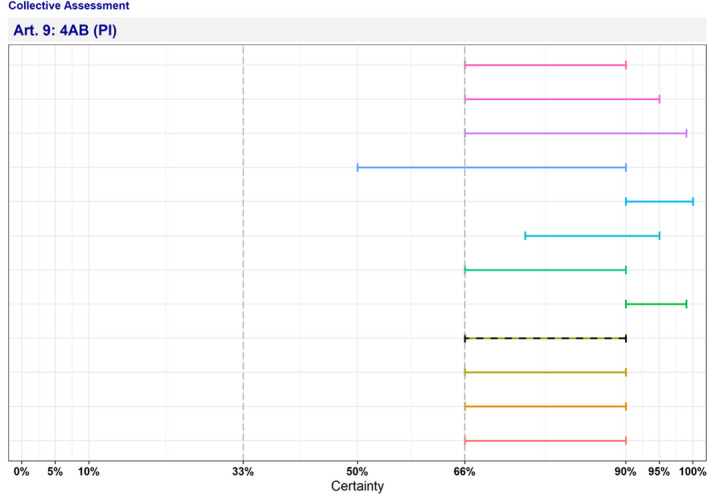
Figure A.22 Individual probability ranges reflecting fulfilment of Criterion 4AB (potential impact) (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals) after the collective judgement
- PI: potential impact. The median range is displayed as a dashed line.
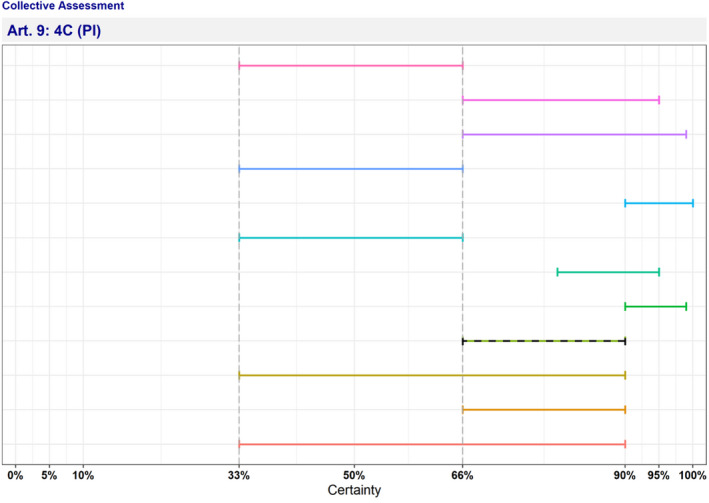
Figure A.23 Individual probability ranges reflecting fulfilment of Criterion 4C (potential impact) (the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems) after the collective judgement
- PI: potential impact. The median range is displayed as a dashed line.
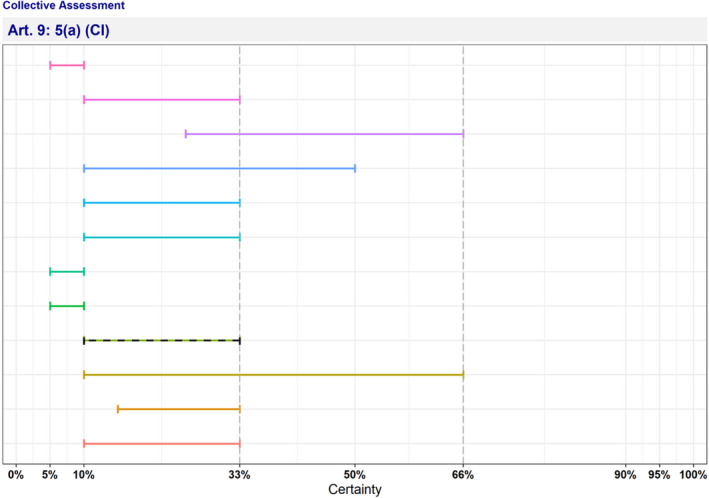
Figure A.24 Individual probability ranges reflecting non‐fulfilment of Criterion 5(a) (current impact) (the disease has a significant impact on society, with in particular an impact on labour markets) after the collective judgement
- CI: current impact. The median range is displayed as a dashed line.
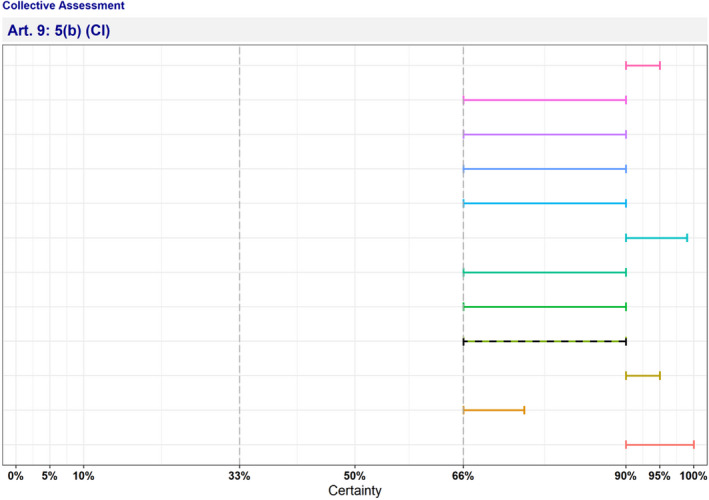
Figure A.25 Individual probability ranges reflecting fulfilment of Criterion 5(b) (current impact) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
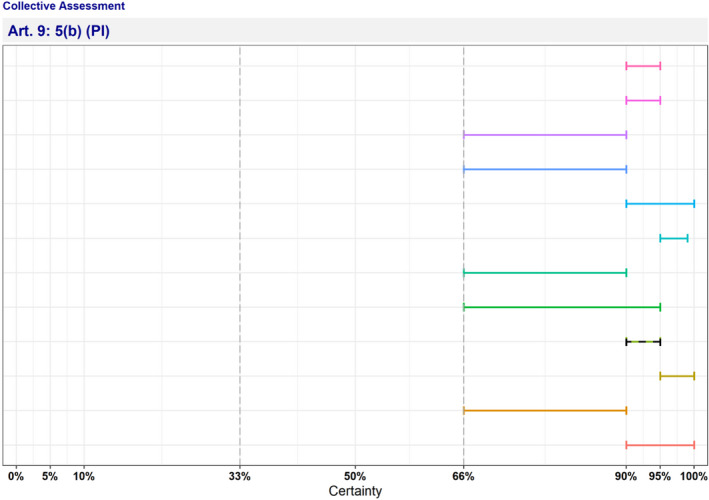
Figure A.26 Individual probability ranges reflecting fulfilment of Criterion 5(b) (potential impact) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.
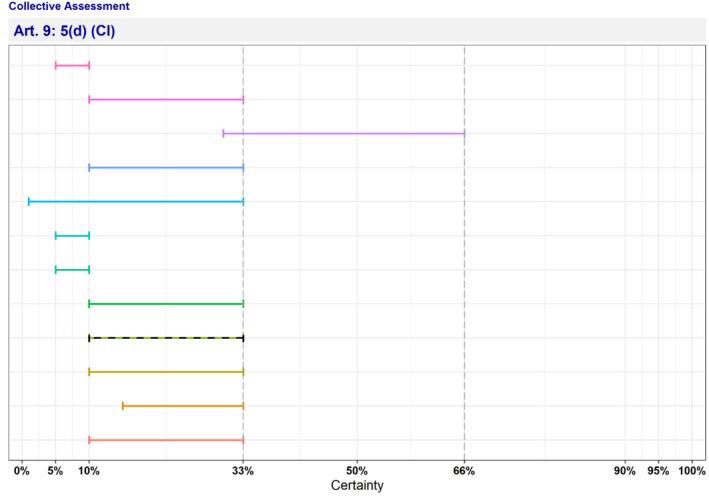
Figure A.27 Individual probability ranges reflecting non‐fulfilment of Criterion 5(d) (current impact) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
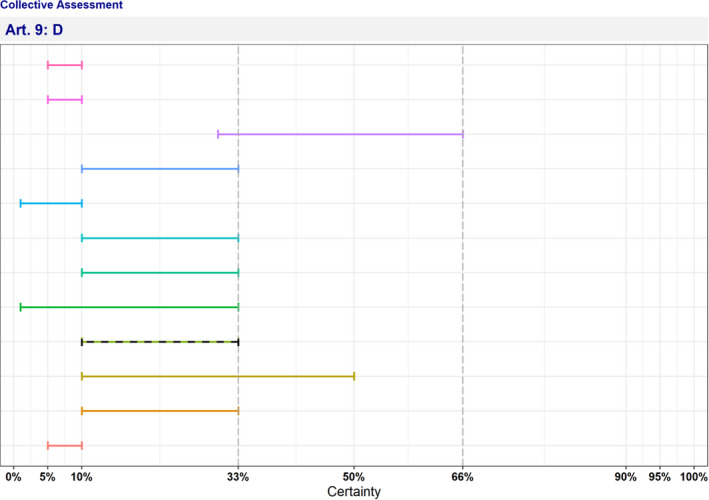
Figure A.28 Individual probability ranges reflecting non‐fulfilment of Criterion D (the risk posed by the disease can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread) after the collective judgement
- The median range is displayed as a dashed line.
Appendix B – Criteria with uncertain outcome
B.1. Article 5 criteria
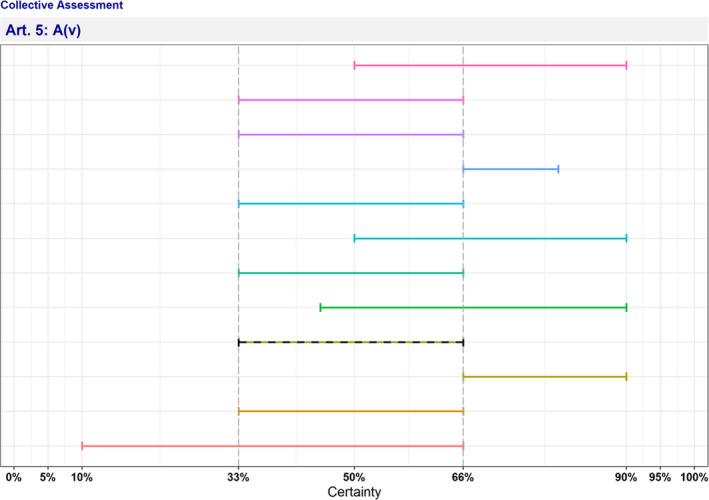
Figure B.1 Individual probability ranges reflecting uncertain outcome on Criterion A(v) (risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union) after the collective judgement
- The median range is displayed as a dashed line.
B.2. Article 9 criteria
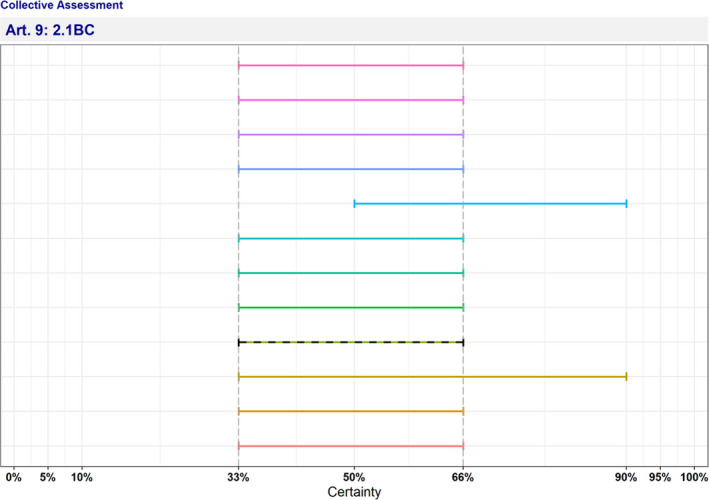
Figure B.2 Individual probability ranges reflecting uncertain outcome on Criterion 2.1BC (the disease is moderately to highly transmissible) after the collective judgement
- The median range is displayed as a dashed line.
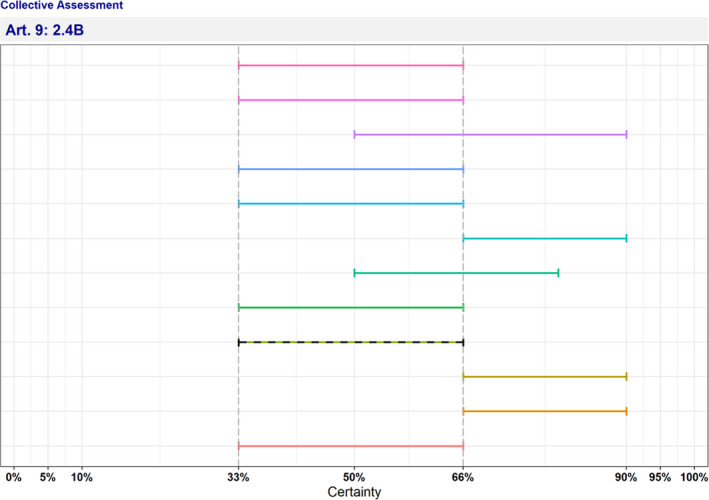
Figure B.3 Individual probability ranges reflecting uncertain outcome on Criterion 2.4B (the disease may result in high morbidity with in general low mortality) after the collective judgement
- The median range is displayed as a dashed line.
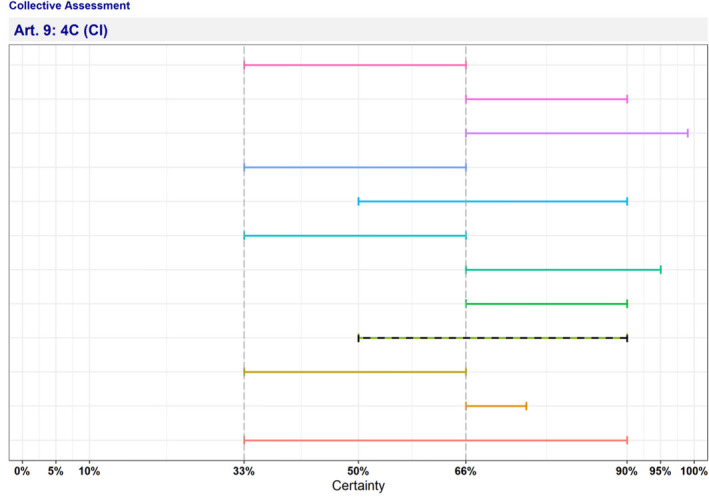
Figure B.4 Individual probability ranges reflecting uncertain outcome on Criterion 4C (current impact) (the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
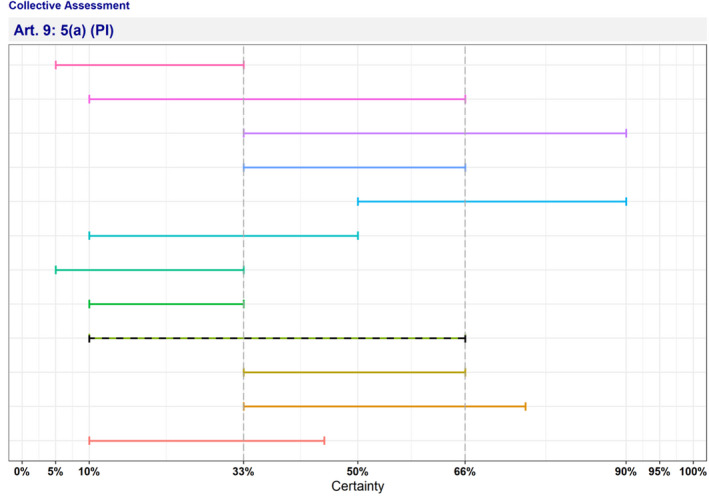
Figure B.5 Individual probability ranges reflecting uncertain outcome on Criterion 5(a) (potential impact) (the disease has a significant impact on society, with in particular an impact on labour markets) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.
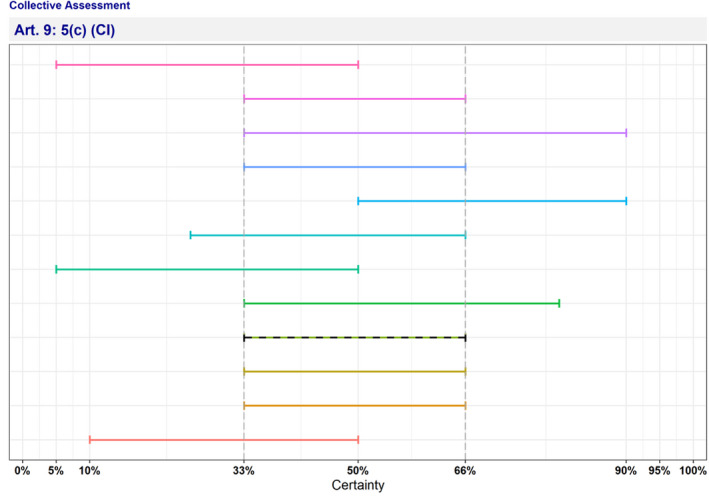
Figure B.6 Individual probability ranges reflecting uncertain outcome on Criterion 5(c) (current impact) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
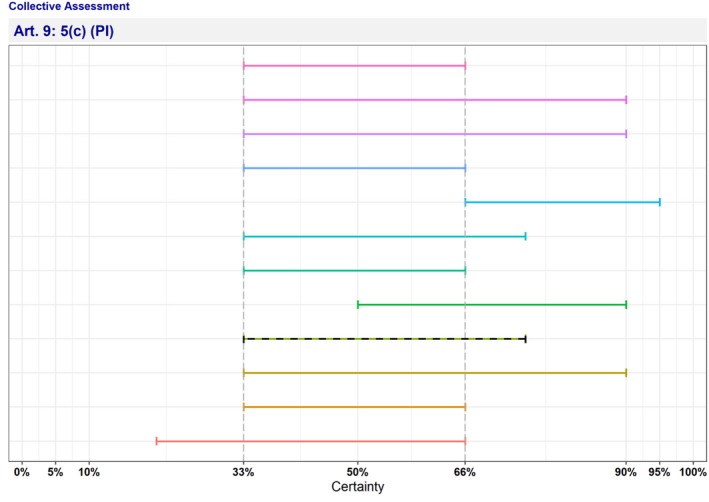
Figure B.7 Individual probability ranges reflecting uncertain outcome on Criterion 5(c) (potential impact) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.
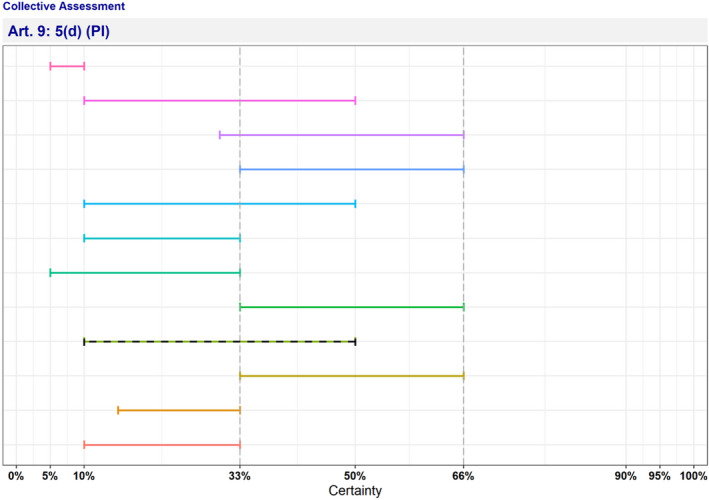
Figure B.8 Individual probability ranges reflecting uncertain outcome on Criterion 5(d) (potential impact) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Baldinelli F, Broglia A, Kohnle L and Alvarez J, 2022. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): antimicrobial‐resistant Escherichia coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats. EFSA Journal 2022;20(5):7311, 93 pp. 10.2903/j.efsa.2022.7311
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00093
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The AHAW Panel wishes to thank Teresa Gonçalves Ribeiro, Filipa Grosso Ledo and Joana Araújo Alves de Campos from the University of Porto, Portugal, for conducting the extensive literature review under the contract PO/EFSA/ALPHA/2021/01. The AHAW Panel also wishes to thank Verena Oswaldi from EFSA for the support provided for this scientific output.
Adopted: 30 March 2022
Notes
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. OJ L 303, 18.11.2009, p. 1–30.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- Abbas G, Khan I, Mohsin M, Sajjad‐Ur‐Rahman YT and Ali S, 2019. High rates of CTX‐M group‐1 extended‐spectrum β‐lactamases producing Escherichia coli from pets and their owners in Faisalabad, Pakistan. Infection and Drug Resistance, 12, 571–578. 10.2147/IDR.S189884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acres SD, 1985. Enterotoxigenic Escherichia coli infections in newborn calves: a review. Journal of Dairy Science, 68, 229–256. 10.3168/jds.S0022-0302(85)80814-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslantaş Ö and Yilmaz EŞ, 2017. Prevalence and molecular characterization of extended‐spectrum β‐lactamase (ESBL) and plasmidic AmpC β‐lactamase (pAmpC) producing Escherichia coli in dogs. The Journal of Veterinary Medicine Science, 79, 1024–1030. 10.1292/jvms.16-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astorga F, Navarrete‐Talloni MJ, Miró MP, Bravo V, Toro M, Blondel CJ and Hervé‐Claude LP, 2019. Antimicrobial resistance in E. coli isolated from dairy calves and bedding material. Heliyon, 5, e02773. 10.1016/j.heliyon.2019.e02773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baede VO, Wagenaar JA, Broens EM, Duim B, Dohmen W, Nijsse R, Timmerman AJ and Hordijk J, 2015. Longitudinal study of extended‐spectrum‐β‐lactamase‐ and AmpC‐producing Enterobacteriaceae in household dogs. Antimicrobial Agents and Chemotherapy, 59, 3117–3124. 10.1128/AAC.04576-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraitareanu S and Vidu L, 2020. Dairy farms biosecurity to protect against infectious diseases and antibiotics overuse. In: Mareș M, Lim SHE, Lai K‐S and Cristina R‐T (eds.), Antimicrobial Resistance – A One Health Perspective. Rijeka: IntechOpen. 10.5772/intechopen.93200 [DOI]
- Bartels CJM, Holzhauer M, Jorritsma R, Swart WAJM and Lam TJGM, 2010. Prevalence, prediction and risk factors of enteropathogens in normal and non‐normal faeces of young Dutch dairy calves. Preventive Veterinary Medicine, 93, 162–169. 10.1016/j.prevetmed.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashahun GM and Amina A, 2017. Colibacillosis in calves: a review of literature. Journal of Animal Science and Veterinary Medicine, 2, 62–71. 10.31248/jasvm2017.041 [DOI] [Google Scholar]
- Becker E, Projahn M, Burow E and Käsbohrer A, 2021. Are there effective intervention measures in broiler production against the ESBL/AmpC producer Escherichia coli? Pathogens, 10, 608. 10.3390/pathogens10050608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger L, Garenaux A, Harel J, Boulianne M, Nadeau E and Dozois CM, 2011. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli . FEMS Immunology and Medical Microbiology, 62, 1–10. 10.1111/j.1574-695X.2011.00797.x [DOI] [PubMed] [Google Scholar]
- Benagli C, Rossi V, Dolina M, Tonolla M and Petrini O, 2011. Matrix‐assisted laser desorption ionization‐time of flight mass spectrometry for the identification of clinically relevant bacteria. PLoS One, 6, e16424. 10.1371/journal.pone.0016424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R and IJpelaar J, 2005. Updated estimates of the costs associated with thirty four endemic livestock diseases in great britain: a note. Journal of Agricultural Economics, 56, 135–144. 10.1111/j.1477-9552.2005.tb00126.x [DOI] [Google Scholar]
- Berchtold J, 2009. Treatment of calf diarrhea: intravenous fluid therapy. The Veterinary Clinics of North America. Food Animal Practice, 25, 73–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser TE and Gay CC, 1985. Septicemic Colibacillosis and failure of passive transfer of Colostral immunoglobulin in calves. Veterinary Clinics of North America: Food Animal Practice, 1 3, 445–459. 10.1016/S0749-0720(15)31295-0 [DOI] [PubMed] [Google Scholar]
- Botrel M‐A, Haenni M, Morignat E, Sulpice P, Madec J‐Y and Calavas D, 2010. Distribution and antimicrobial resistance of clinical and subclinical mastitis pathogens in dairy cows in Rhône‐Alpes. France. Foodborne Pathogens and Disease, 7, 479–487. 10.1089/fpd.2009.0425 [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Leach KA, Breen JE, Green LE and Green MJ, 2007. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. The Veterinary Record, 160, 253–257. 10.1136/vr.160.8.253 [DOI] [PubMed] [Google Scholar]
- Brilhante M, Menezes J, Belas A, Feudi C, Schwarz S, Pomba C and Perreten V, 2020. OXA‐181‐producing extraintestinal pathogenic Escherichia coli sequence type 410 isolated from a dog in Portugal. Antimicrobial Agents and Chemotherapy, 64, e02298–e2319. 10.1128/AAC.02298-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JW, Roberts EL, Kocher K, Kariyawasam S and DebRoy C, 2013. Fatal pneumonia caused by Extraintestinal pathogenic Escherichia coli (ExPEC) in a juvenile cat recovered from an animal hoarding incident. Veterinary Microbiology, 167, 704–707. 10.1016/j.vetmic.2013.08.015 [DOI] [PubMed] [Google Scholar]
- CABI , 2019. Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/83003 [Google Scholar]
- Carvalho AC, Barbosa AV, Arais LR, Ribeiro PF, Carneiro VC and Cerqueira AMF, 2016. Resistance patterns, ESBL genes, and genetic relatedness of Escherichia coli from dogs and owners. Brazilian Journal of Microbiology, 47, 150–158. 10.1016/j.bjm.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvallo FR, Debroy C, Baeza E, Hinckley L, Gilbert K, Choi SJ, Risatti G and Smyth JA, 2010. Necrotizing pneumonia and pleuritis associated with extraintestinal pathogenic Escherichia coli in a tiger (Panthera tigris) cub. Journal of Veterinary Diagnostic Investigation, 22, 136–140. 10.1177/104063871002200130 [DOI] [PubMed] [Google Scholar]
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb‐Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C and Monnet DL, 2019. Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European Economic Area in 2015: a population‐level modelling analysis. The Lancet Infectious Diseases, 19, 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney TEA, Smith RP, Hutchinson JP, Brunton LA, Pritchard G and Teale CJ, 2015. Cross‐sectional survey of antibiotic resistance in Escherichia coli isolated from diseased farm livestock in England and Wales. Epidemiology and Infection, 143, 2653–2659. 10.1017/S0950268814003963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y‐I and Yoon K‐J, 2014. An overview of calf diarrhea – infectious etiology, diagnosis, and intervention. Journal of Veterinary Science, 15, 1–17. 10.4142/jvs.2014.15.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui H, Chan M, Hernandez D, Chong P, McCorrister S, Robinson A, Walker M, Peterson LA, Ratnam S, Haldane DJ, Bekal S, Wylie J, Chui L, Westmacott G, Xu B, Drebot M, Nadon C, Knox JD, Wang G and Cheng K, 2015. Rapid, sensitive, and specific Escherichia coli H antigen typing by matrix‐assisted laser desorption ionization‐time of flight‐based peptide mass fingerprinting. Journal of Clinical Microbiology, 53, 2480–2485. 10.1128/JCM.00593-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobirka M, Tancin V and Slama P, 2020. Epidemiology and Classification of Mastitis. Animals, 10, 2212. 10.3390/ani10122212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MPV, Saidenberg AB, Moreno AM, Ferreira AJP, Vieira MAM, Gomes TAT and Knöbl T, 2017. Pandemic extra‐intestinal pathogenic Escherichia coli (ExPEC) clonal group O6–B2‐ST73 as a cause of avian colibacillosis in Brazil. PLoS One, 12, e0178970. 10.1371/journal.pone.0178970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack TP, Ashley EA, Ling CL, Rattanavong S, Roberts T, Turner P, Wangrangsimakul T and Dance DAB, 2019. Impact of CLSI and EUCAST breakpoint discrepancies on reporting of antimicrobial susceptibility and AMR surveillance. Clinical Microbiology and Infection, 25, 910–911. 10.1016/j.cmi.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborg P, Marskar P, Baptiste KE and Guardabassi L, 2012. Faecal shedding of CTX‐M‐producing Escherichia coli in horses receiving broad‐spectrum antimicrobial prophylaxis after hospital admission. Veterinary Microbiology, 154, 298–304. 10.1016/j.vetmic.2011.07.005 [DOI] [PubMed] [Google Scholar]
- De Briyne N, Atkinson J, Pokludová L and Borriello SP, 2014. Antibiotics used most commonly to treat animals in Europe. Veterinary Record, 175, 325. 10.1136/vr.102462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS and Jack OK, 1996. Two outbreaks of colibacillosis in commercial caged layers. Avian Diseases, 40, 742–746. [PubMed] [Google Scholar]
- Dolejska M, Duskova E, Rybarikova J, Janoszowska D, Roubalova E, Dibdakova K, Maceckova G, Kohoutova L, Literak I, Smola J and Cizek A, 2011. Plasmids carrying blaCTX‐M‐1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. Journal of Antimicrobial Chemotherapy, 66, 757–764. 10.1093/jac/dkq500 [DOI] [PubMed] [Google Scholar]
- Dorsch R, Teichmann‐Knorrn S and Sjetne LH, 2019. Urinary tract infection and subclinical bacteriuria in cats: a clinical update. Journal of Feline Medicine and Surgery, 21, 1023–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazenovich N, Ling GV and Foley J, 2004. Molecular investigation of Escherichia coli strains associated with apparently persistent urinary tract infection in dogs. Journal of Veterinary Internal Medicine, 18, 301–306. [DOI] [PubMed] [Google Scholar]
- Drugs.com , 2021a. Enviracor J‐5 (Canada). Available online: https://www.drugs.com/vet/enviracor‐j‐5‐can.html
- Drugs.com , 2021b. ScourGuard 4KC (Canada). Available online: https://www.drugs.com/vet/scourguard‐4kc‐can.html
- Dubreuil JD, 2017. Enterotoxigenic Escherichia coli and probiotics in swine: what the bleep do we know? Bioscience of Microbiota, Food and Health, 36, 75–90. 10.12938/bmfh.16-030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil JD, Isaacson RE and Schifferli DM, 2016. Animal Enterotoxigenic Escherichia coli . EcoSal plus, 7. 10.1128/ecosalplus.ESP-0006-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) , 2021. Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=4
- EFSA (European Food Safety Authority) , Aerts M, Battisti A, Hendriksen R, Kempf I, Teale C, Tenhagen B‐A, Veldman K, Wasyl D, Guerra B, Liébana E, Thomas‐López D and Belœil P‐A, 2019. Scientific report on the technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food. EFSA Journal 2019;17(6):5709, 122 pp. 10.2903/j.efsa.2019.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and (European Centre for Disease Prevention and Control) , 2017. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal, 2017;15(2):4694, 212 pp. 10.2903/j.efsa.2017.4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2021. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA Journal 2021;19(4):6490, 179 pp. 10.2903/j.efsa.2021.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(7):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Guardabassi L, Hilbert F, Mader R, Aznar I, Baldinelli F and Alvarez J, 2021a. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Dogs and cats. EFSA Journal 2021;19(6):6680, 58 pp. 10.2903/j.efsa.2021.6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021b. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Horses. EFSA Journal 2021;19(12):7112, 43 pp. 10.2903/j.efsa.2021.7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021c. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Swine. EFSA Journal 2021;19(12):7113, 66 pp. 10.2903/j.efsa.2021.7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021d. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Poultry. EFSA Journal 2021;19(12):7114, 47 pp. 10.2903/j.efsa.2021.7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021e. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: cattle. EFSA Journal 2021;19(12):6955, 89 pp. 10.2903/j.efsa.2021.6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021f. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: sheep and goats. EFSA Journal 2021;19(12):6956, 37 pp. 10.2903/j.efsa.2021.6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Ahaw Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Guardabassi L, Hilbert F, Mader R, Smith P, Aznar I, Muñoz Guajardo I, Baldinelli F and Alvarez J, 2021g. Scientific Opinion on the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials. EFSA Journal 2021;19(6):6645, 29 pp. 10.2903/j.efsa.2021.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed]
- EMA (European Medicines Agency) and EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2017. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobialagents in animal husbandry in the European Union, and the resulting impacts on food safety(RONAFA). [EMA/CVMP/570771/2015]. EFSA Journal 2017;15(1):4666, 245 pp. 10.2903/j.efsa.2017.4666 [DOI] [PMC free article] [PubMed]
- EMA (European Medicines Agency) , 2018. Startvac. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/startvac
- EMA (European Medicines Agency) , 2019. Categorisation of antibiotics in the European Union. Available online: https://www.ema.europa.eu/en/documents/report/categorisation‐antibiotics‐european‐union‐answer‐request‐european‐commission‐updating‐scientific_en.pdf
- EMA (European Medicines Agency) , 2020. Porcilis ColiClos. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/porcilis‐coliclos
- EMA (European Medicines Agency) , 2021a. Coliprotec F4/F18. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/coliprotec‐f4‐f18
- EMA (European Medicines Agency) , 2021b. Enteroporc Coli. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/enteroporc‐coli
- EMA (European Medicines Agency) , 2021c. Neocolipor. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/neocolipor
- EMA (European Medicines Agency) , 2021d. Locatim (previously Serinucoli). Available online: https://www.EMA..europa.eu/en/medicines/veterinary/EPAR/locatim
- EMA (European Medicines Agency) , 2022. Poulvac E. coli. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/poulvac‐e‐coli
- Ewers C, Li G, Wilking H, Kiessling S, Alt K, Antáo E‐M, Laturnus C, Diehl I, Glodde S, Homeier T, Böhnke U, Steinrück H, Philipp H‐C and Wieler LH, 2007. Avian pathogenic, uropathogenic, and newborn meningitis‐causing Escherichia coli: how closely related are they? International Journal of Medical Microbiology, 297, 163–176. 10.1016/j.ijmm.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Ewers C, Antão E‐M, Diehl I, Philipp H‐C and Wieler LH, 2009. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Applied and Environmental Microbiology, 75, 184–192. 10.1128/AEM.01324-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers C, Bethe A, Semmler T, Guenther S and Wieler LH, 2012. Extended‐spectrum β‐lactamase‐producing and AmpC‐producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clinical Microbiology and Infection, 18, 646–655. 10.1111/j.1469-0691.2012.03850.x [DOI] [PubMed] [Google Scholar]
- Fairbrother JM and Nadeau É, 2019. Colibacillosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW and Zhang J (eds.), Diseases of Swine, John Wiley and Sons Inc, New York, USA. 807–834. [Google Scholar]
- Ferriol‐González C and Domingo‐Calap P, 2021. Phage therapy in livestock and companion animals. Antibiotics (Basel), 10, 559. 10.3390/antibiotics10050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint KP, 1987. The long‐term survival of Escherichia coli in river water. Journal of Applied Microbiology, 63, 261–270. [DOI] [PubMed] [Google Scholar]
- Florio W, Baldeschi L, Rizzato C, Tavanti A, Ghelardi E and Lupetti A, 2020. Detection of antibiotic‐resistance by MALDI‐TOF mass spectrometry: an expanding area. Frontiers in Cellular and Infection Microbiology, 10. 10.3389/fcimb.2020.572909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag T, Squires RA, Schmid J, Elliott J and Rycroft AN, 2006. Antibiotic sensitivity profiles do not reliably distinguish relapsing or persisting infections from reinfections in cats with chronic renal failure and multiple diagnoses of Escherichia coli urinary tract infection. Journal of Veterinary Internal Medicine, 20, 245–249. 10.1892/0891-6640(2006)20[245:aspdnr]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- García V, García‐Meniño I, Mora A, Flament‐Simon SC, Díaz‐Jiménez D, Blanco JE, Alonso MP and Blanco J, 2018. Co‐occurrence of mcr‐1, mcr‐4 and mcr‐5 genes in multidrug‐resistant ST10 Enterotoxigenic and Shiga toxin‐producing Escherichia coli in Spain (2006–2017). International Journal of Antimicrobial Agents, 52, 104–108. 10.1016/j.ijantimicag.2018.03.022 [DOI] [PubMed] [Google Scholar]
- García‐Meniño I, García V, Mora A, Díaz‐Jiménez D, Flament‐Simon SC, Alonso MP, Blanco JE, Blanco M and Blanco J, 2018. Swine Enteric Colibacillosis in Spain: pathogenic potential of mcr‐1 ST10 and ST131 E. coli isolates. Frontiers in Microbiology, 9, 2659. 10.3389/fmicb.2018.02659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CC, 1965. Escherichia coli and neonatal disease of calves. Bacteriology Reviews, 29, 75–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Diarrhoeal Disease Collaborators , 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet, Infectious Diseases, 18, 1211–1228. 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XZ, Jiang J, Pan Z, Hu L, Wang S, Wang H, Leung FC, Dai J and Fan H, 2014. Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS One, 9, e112048. 10.1371/journal.pone.0112048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERM‐Vet , 2020. Bericht zur Resistenzmonitoringstudie 2018 – Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien, Berlin, Germany. Available online: https://www.bvl.bund.de/SharedDocs/Berichte/07_Resistenzmonitoringstudie/Bericht_Resistenzmonitoring_2018.pdf;jsessionid=73681DF7A5486A5AF1AA037A6A7D47A0.1_cid298?__blob=publicationFile&v=6 [Google Scholar]
- Ghanbarpour R, Aflatoonian MR, Askari A, Abiri Z, Naderi Z, Bagheri M, Jajarmi M, Shobeiri S, Molaei R and Askari N, 2020. Domestic and game pigeons as reservoirs for Escherichia coli harbouring antimicrobial resistance genes. Journal of Global Antimicrobial Resistance, 22, 571–577. 10.1016/j.jgar.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Greiner M, Wolf G and Hartmann K, 2008. A retrospective study of the clinical presentation of 140 dogs and 39 cats with bacteraemia. Journal of Small Animal Practice, 49, 378–383. 10.1111/j.1748-5827.2008.00546.x [DOI] [PubMed] [Google Scholar]
- Guabiraba R and Schouler C, 2015. Avian colibacillosis: still many black holes. FEMS Microbiology Letters, 362, fnv118. 10.1093/femsle/fnv118 [DOI] [PubMed] [Google Scholar]
- Hagman R, 2018. Pyometra in small animals. The Veterinary Clinics of North America. Small Animal Practice, 48, 639–661. 10.1016/j.cvsm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Hannan TJ and Hunstad DA, 2016. A murine model for Escherichia coli urinary tract infection. Methods in Molecular Biology, 1333, 159–175. 10.1007/978-1-4939-2854-5_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Okada E, Shimizu T, Kataoka Y, Sawada T and Takahashi T, 2012. Antimicrobial resistance, virulence profiles, and phylogenetic groups of fecal Escherichia coli isolates: a comparative analysis between dogs and their owners in Japan. Comparative Immunology, Microbiology and Infectious Diseases, 35, 139–144. 10.1016/j.cimid.2011.12.005 [DOI] [PubMed] [Google Scholar]
- He W, Ma S, Lei L, He J, Li X, Tao J, Wang X, Song S, Wang Y, Wang Y, Shen J, Cai C and Wu C, 2020. Prevalence, etiology, and economic impact of clinical mastitis on large dairy farms in China. Veterinary Microbiology, 242. 10.1016/j.vetmic.2019.108570 [DOI] [PubMed] [Google Scholar]
- Heikkilä AM, Liski E, Pyörälä S and Taponen S, 2018. Pathogen‐specific production losses in bovine mastitis. Journal of Dairy Science, 101, 9493–9504. 10.3168/jds.2018-14824 [DOI] [PubMed] [Google Scholar]
- Hilty M, Betsch BY, Bögli‐Stuber K, Heiniger N, Stadler M, Küffer M, Kronenberg A, Rohrer C, Aebi S, Endimiani A, Droz S and Mühlemann K, 2012. Transmission dynamics of extended‐spectrum β‐lactamase‐producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clinical Infectious Diseases, 55, 967–975. 10.1093/cid/cis581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen H, Huijps K and Lam TJGM, 2011. Economic aspects of mastitis: new developments. New Zealand Veterinary Journal, 59, 16–23. 10.1080/00480169.2011.547165 [DOI] [PubMed] [Google Scholar]
- Holmøy IH, Waage S, Granquist EG, L’Abée‐Lund TM, Ersdal C, Hektoen L and Sørby R, 2017. Early neonatal lamb mortality: postmortem findings. Animal, 11, 295–305. 10.1017/S175173111600152X [DOI] [PubMed] [Google Scholar]
- Ibrahim RA, Cryer TL, Lafi SQ, Basha E‐A, Good L and Tarazi YH, 2019. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Veterinary Research, 15, 159. 10.1186/s12917-019-1901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgren CM, Williams NJ, Fletcher OD, Timofte D, Newton RJ, Maddox TW, Clegg PD and Pinchbeck GL, 2021. Antimicrobial resistance in clinical bacterial isolates from horses in the UK. Equine Veterinary Journal, 54, 390–414. 10.1111/evj.13437 [DOI] [PubMed] [Google Scholar]
- Jang J, Hur H‐G, Sadowsky MJ, Byappanahalli MN, Yan T and Ishii S, 2017. Environmental Escherichia coli: ecology and public health implications‐a review. Journal of Applied Microbiology, 123, 570–581. 10.1111/jam.13468 [DOI] [PubMed] [Google Scholar]
- Johnson JR, Clabots C and Kuskowski MA, 2008. Multiple‐host sharing, long‐term persistence, and virulence of Escherichia coli clones from human and animal household members. Journal of Clinical Microbiology, 46, 4078–4082. 10.1128/JCM.00980-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ, Kariyawasam S, Wannemuehler Y, Mangiamele P, Johnson SJ, Doetkott C, Skyberg JA, Lynne AM, Johnson JR and Nolan LK, 2007. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. Journal of Bacteriology, 189, 3228–3236. 10.1128/JB.01726-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A, Garch FE, Simjee S, Moyaert H, Rose M, Youala M and Siegwart E, 2018. Monitoring of antimicrobial susceptibility of udder pathogens recovered from cases of clinical mastitis in dairy cows across Europe: VetPath results. Veterinary Microbiology, 213, 73–81. 10.1016/j.vetmic.2017.11.021 [DOI] [PubMed] [Google Scholar]
- de Jong A, Youala M, El Garch F, Simjee S, Rose M, Morrissey I and Moyaert H, 2020. Antimicrobial susceptibility monitoring of canine and feline skin and ear pathogens isolated from European veterinary clinics: results of the ComPath Surveillance programme. Veterinary Dermatology, 31, 431–e114. 10.1111/vde.12886 [DOI] [PubMed] [Google Scholar]
- Jørgensen SL, Stegger M, Kudirkiene E, Lilje B, Poulsen LL, Ronco T, Dos Santos TP, Kiil K, Bisgaard M, Pedersen K, Nolan LK, Price LB, Olsen RH, Andersen PS and Christensen H, 2019. Diversity and population overlap between avian and human Escherichia coli belonging to sequence type 95. mSphere, 4, e00333–e00318. 10.1128/mSphere.00333-18 [DOI] [PMC free article] [PubMed]
- Kampf G, 2018. Antiseptic Stewardship: Biocide Resistance and Clinical Implications, Springer International Publishing, Cham, Switzerland. 694, [Google Scholar]
- Kaspar U, von Lützau K, Schlattmann A, Rösler U, Köck R and Becker K, 2019. Zoonotic multidrug‐resistant microorganisms among non‐hospitalized horses from Germany. One Health, 7. 10.1016/j.onehlt.2019.100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathayat D, Lokesh D, Ranjit S and Rajashekara G, 2021. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens, 10, 467. 10.3390/pathogens10040467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratyeva K, Salmon‐Divon M and Navon‐Venezia S, 2020. Meta‐analysis of pandemic Escherichia coli ST131 plasmidome proves restricted plasmid‐clade associations. Scientific Reports, 10, 36. 10.1038/s41598-019-56763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman WJM and van Eck JHH, 2015. The incidence and economic impact of the Escherichia coliperitonitis syndrome in Dutch poultry farming. Avian Pathology, 44, 370–378. [DOI] [PubMed] [Google Scholar]
- Lagerstrom KM and Hadly EA, 2021. The under‐investigated wild side of Escherichia coli: genetic diversity, pathogenicity and antimicrobial resistance in wild animals. Proceedings of the Royal Society B: Biological Sciences, 288. 10.1098/rspb.2021.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird TJ, Abraham S, Jordan D, Pluske JR, Hampson DJ, Trott DJ and O'Dea M, 2021. Porcine enterotoxigenic Escherichia coli: antimicrobial resistance and development of microbial‐based alternative control strategies. Veterinary Microbiology, 258. 10.1016/j.vetmic.2021.109117 [DOI] [PubMed] [Google Scholar]
- Larsson DGJ and Flach C‐F, 2021. Antibiotic resistance in the environment. Nature Reviews Microbiology, 10.1038/s41579-021-00649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengliz S, Benlabidi S, Raddaoui A, Cheriet S, Ben Chehida N, Najar T and Abbassi MS, 2021. High occurrence of carbapenem‐resistant Escherichia coli isolates from healthy rabbits (Oryctolagus cuniculus): first report of blaIMI and blaVIM type genes from livestock in Tunisia. Letters in Applied Microbiology, 73, 708–717. 10.1111/lam.13558 [DOI] [PubMed] [Google Scholar]
- Liu CM, Stegger M, Aziz M, Johnson TJ, Waits K, Nordstrom L, Gauld L, Weaver B, Rolland D and Statham S, 2018. Escherichia coli ST131‐H22 as a Foodborne Uropathogen. MBio, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Z, Chang X, Wang X, Hu J, Lin Q, Jia Y, Yang X and Wang X, 2019. Disruption of blood‐brain barrier by an Escherichia coli isolated from canine septicemia and meningoencephalitis. Comparative Immunology, Microbiology and Infectious Diseases, 63, 44–50. 10.1016/j.cimid.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Loayza F, Graham JP and Trueba G, 2020. Factors obscuring the role of E. coli from domestic animals in the global antimicrobial resistance crisis: an evidence‐based review. International Journal of Environmental Research and Public Health, 17, 3061. 10.3390/ijerph17093061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo A, Haenni M, Saras E, Gradin J, Madec J‐Y and Börjesson S, 2018. Is blaCTX‐M‐1 riding the same plasmid among horses in Sweden and France? Microbial Drug Resistance, 24, 1580–1586. 10.1089/mdr.2017.0412 [DOI] [PubMed] [Google Scholar]
- Luppi A, 2017. Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porcine Health Management, 3, 16. 10.1186/s40813-017-0063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Xu S, Tang Z, Li Z and Zhang L, 2021. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health, 3, 32–38. 10.1016/j.bsheal.2020.09.004 [DOI] [Google Scholar]
- MacKinnon MC, Sargeant JM, Pearl DL, Reid‐Smith RJ, Carson CA, Parmley EJ and McEwen SA, 2020. Evaluation of the health and healthcare system burden due to antimicrobial‐resistant Escherichia coli infections in humans: a systematic review and meta‐analysis. Antimicrobial Resistance and Infection Control, 9, 200. 10.1186/s13756-020-00863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox TW, Clegg PD, Williams NJ and Pinchbeck GL, 2015. Antimicrobial resistance in bacteria from horses: epidemiology of antimicrobial resistance. Equine Veterinary Journal, 47, 756–765. 10.1111/evj.12471 [DOI] [PubMed] [Google Scholar]
- Madec J‐Y and Haenni M, 2018. Antimicrobial resistance plasmid reservoir in food and food‐producing animals. Plasmid, 99, 72–81. 10.1016/j.plasmid.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Madigan T, Johnson JR, Clabots C, Johnston BD, Porter SB, Slater BS and Banerjee R, 2015. Extensive household outbreak of urinary tract infection and intestinal colonization due to extended‐spectrum β‐lactamase‐producing Escherichia coli sequence type 131. Clinical Infectious Diseases, 61, e5–12. 10.1093/cid/civ273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manges AR and Johnson JR, 2012. Food‐borne origins of Escherichia coli causing extraintestinal infections. Clinical Infectious Diseases, 55, 712–719. 10.1093/cid/cis502 [DOI] [PubMed] [Google Scholar]
- Mariano V, Nardi A, Moruzzo R, di Iacovo FP and Rossignoli CM, 2018. In‐farm cost of an outbreak of diarrhoea in lambs. Small Ruminant Research, 166, 17–21. 10.1016/j.smallrumres.2018.07.008 [DOI] [Google Scholar]
- Marques C, Belas A, Franco A, Aboim C, Gama LT and Pomba C, 2018. Increase in antimicrobial resistance and emergence of major international high‐risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. Journal of Antimicrobial Chemotherapy, 73, 377–384. 10.1093/jac/dkx401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioni Marchetti V, Bitar I, Mercato A, Nucleo E, Marchesini F, Mancinelli M, Prati P, Scarsi GS, Hrabak J, Pagani L, Fabbi M and Migliavacca R, 2020. Deadly puppy infection caused by an MDR Escherichia coli O39 blaCTX‐M‐15, blaCMY‐2, blaDHA‐1, and aac(6)‐Ib‐cr – positive in a breeding Kennel in Central Italy. Frontiers in Microbiology, 11, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M, 2013. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathogens and Disease, 10, 916–932. 10.1089/fpd.2013.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir RA, Weppelmann TA, Teng L, Kirpich A, Elzo MA, Driver JD and Jeong KC, 2018. Colonization dynamics of cefotaxime resistant bacteria in beef cattle raised without cephalosporin antibiotics. Frontiers in Microbiology, 9, 500. 10.3389/fmicb.2018.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monger XC, Gilbert A‐A, Saucier L and Vincent AT, 2021. Antibiotic resistance: from pig to meat. Antibiotics, 10, 1209. 10.3390/antibiotics10101209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montealegre MC, Roy S, Böni F, Hossain MI, Navab‐Daneshmand T, Caduff L, Faruque ASG, Islam MA and Julian TR, 2018. Risk factors for detection, survival, and growth of antibiotic‐resistant and pathogenic Escherichia coli in household soils in rural Bangladesh. Applied and Environmental Microbiology, 84, e01978–e2018. 10.1128/AEM.01978-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, López C, Dabhi G, Blanco M, Blanco JE, Alonso MP, Herrera A, Mamani R, Bonacorsi S, Moulin‐Schouleur M and Blanco J, 2009. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiology, 9, 132. 10.1186/1471-2180-9-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muktar Y, Gezhagne M, Biruk T and Dinaol B, 2015. A review on major bacterial causes of calf diarrhea and its diagnostic method. Journal of Veterinary Medicine and Animal Health, 7, 173–185. 10.5897/JVMAH2014.0351 [DOI] [Google Scholar]
- Najafi S, Rahimi M and Nikousefat Z, 2019. Extra‐intestinal pathogenic Escherichia coli from human and avian origin: detection of the most common virulence‐encoding genes. Veterinary Research Forum, 10, 43–49. 10.30466/vrf.2019.34307 [DOI] [PMC free article] [PubMed]
- NZVA (New Zealand Veterinary Association) , 2018. Antibiotic judicious use guidelines for the New Zealand veterinary profession. Available online: https://www.amrvetcollective.com/assets/guidelines/guide_dairy.pdf
- Nigg A, Brilhante M, Dazio V, Clément M, Collaud A, Gobeli Brawand S, Willi B, Endimiani A, Schuller S and Perreten V, 2019. Shedding of OXA‐181 carbapenemase‐producing Escherichia coli from companion animals after hospitalisation in Switzerland: an outbreak in 2018. Eurosurveillance, 24. 10.2807/1560-7917.ES.2019.24.39.1900071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittayasut N, Yindee J, Boonkham P, Yata T, Suanpairintr N and Chanchaithong P, 2021. Multiple and high‐risk clones of extended‐spectrum cephalosporin‐resistant and blaNDM‐5‐Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics, 10, 1374. 10.3390/antibiotics10111374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan LK, Vaillancourt J‐P, Barbieri NL and Logue CM, 2020. Colibacillosis. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, de Wit S, Grimes T, Johnson D, Kromm M, Prajitno TY, Rubinoff I and Zavala G (eds.), Diseases of Poultry, John Wiley & Sons Inc, New York, USA. pp. 770–860. [Google Scholar]
- Nowakiewicz A, Zięba P, Gnat S, Trościańczyk A, Osińska M, Łagowski D, Kosior‐Korzecka U and Puzio I, 2020. Bats as a reservoir of resistant Escherichia coli: a methodical view. Can we fully estimate the scale of resistance in the reservoirs of free‐living animals? Research in Veterinary Science, 128, 49–58. 10.1016/j.rvsc.2019.10.017 [DOI] [PubMed] [Google Scholar]
- Olsen RH, Frantzen C, Christensen H and Bisgaard M, 2012. An investigation on first‐week mortality in layers. Avian Diseases, 56, 51–57. 10.1637/9777-051011-Reg.1 [DOI] [PubMed] [Google Scholar]
- Othman MA, Ezzat HM, Rizk DEE, Kamal AH, Al‐Mahameed AE, Marwani AM, Bindyna KM and Salvatore S, 2021. Induction of bacterial cystitis in female rabbits by uropathogenic Escherichia coli and the differences between the bladder dome and trigone. Ultrastructural Pathology, 45, 159–166. 10.1080/01913123.2021.1920653 [DOI] [PubMed] [Google Scholar]
- Pasolini MP, Prete CD, Fabbri S and Auletta L, 2016. Endometritis and Infertility in the Mare – The Challenge in Equine Breeding Industry–A Review. In: Darwish AM (ed.). Genital Infections and Infertility [Internet], IntechOpen, London. [Google Scholar]
- Pelzer KD and Currin N 2005. Zoonotic Diseases of Cattle. Virginia Cooperative Extension, 400–460. Available online: https://vtechworks.lib.vt.edu/handle/10919/50703 [Google Scholar]
- Poirel L, Madec J‐Y, Lupo A, Schink A‐K, Kieffer N, Nordmann P and Schwarz S, 2018. Antimicrobial resistance in Escherichia coli microbiology. Spectrum, 6. 10.1128/microbiolspec.ARBA-0026-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhouani H, Igrejas G, Gonçalves A, Pacheco R, Monteiro R, Sargo R, Brito F, Torres C and Poeta P, 2013. Antimicrobial resistance and virulence genes in Escherichia coli and enterococci from red foxes (Vulpes vulpes). Anaerobe, 23, 82–86. 10.1016/j.anaerobe.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Ramchandani M, Manges AR, DebRoy C, Smith SP, Johnson JR and Riley LW, 2005. Possible animal origin of human‐associated, multidrug‐resistant. Uropathogenic Escherichia Coli. Clinical Infectious Diseases, 40, 251–257. 10.1086/426819 [DOI] [PubMed] [Google Scholar]
- Rampacci E, Bottinelli M, Stefanetti V, Hyatt DR, Sgariglia E, Coletti M and Passamonti F, 2018. Antimicrobial susceptibility survey on bacterial agents of canine and feline urinary tract infections: weight of the empirical treatment. Journal of Global Antimicrobial Resistance, 13, 192–196. 10.1016/j.jgar.2018.01.011 [DOI] [PubMed] [Google Scholar]
- RESAPATH (French surveillance network for antimicrobial resistance in bacteria from diseased animals) , Bourély C, Cazeau G, Haenni M, Jarrige N, Jouy E, Lupo A, Madec J‐Y and Mader R, 2018. Monitoring of Antimicrobial Resistance in bacteria from diseased animals in France in 2018: Summary Report of the RESAPATH network (resapath.anses.fr). Available online: https://www.anses.fr/fr/system/files/LABO‐Ra‐Resapath2018EN.pdf [Google Scholar]
- WHO Regional Office for Europe and European Centre for Disease Prevention and Control (ECDC) , 2021. Surveillance of antimicrobial resistance in Europe, 2020 data. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Surveillance‐antimicrobial‐resistance‐in‐Europe‐2020.pdf
- Ribeiro LF, Barbosa MMC, Pinto FR, Lavezzo LF, Rossi GAM, Almeida HMS and Amaral LA, 2019. Diarrheagenic Escherichia coli in raw milk, water, and cattle feces in non‐technified dairy farms. Ciência Animal Brasileira, 20, e–47449. 10.1590/1089-6891v20e-47449 [DOI] [Google Scholar]
- Riley LW, 2014. Pandemic lineages of extraintestinal pathogenic Escherichia coli . Clinical Microbiology and Infection, 20, 380–390. 10.1111/1469-0691.12646 [DOI] [PubMed] [Google Scholar]
- Riley LW, 2020. Distinguishing pathovars from nonpathovars: Escherichia coli microbiology. Spectrum, 8. 10.1128/microbiolspec.AME-0014-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo‐Gimeno C, Postma M, Dewulf J, Hogeveen H, Lauwers L and Wauters E, 2016. Farm‐economic analysis of reducing antimicrobial use whilst adopting improved management strategies on farrow‐to‐finish pig farms. Preventive Veterinary Medicine, 129, 74–87. 10.1016/j.prevetmed.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Ronco T, Stegger M, Olsen RH, Sekse C, Nordstoga AB, Pohjanvirta T, Lilje B, Lyhs U, Andersen PS and Pedersen K, 2017. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genomics, 18, 13. 10.1186/s12864-016-3415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidani M, Messadi L, Mefteh J, Chaouechi A, Soudani A, Selmi R, Dâaloul‐Jedidi M, Ben Chehida F, Mamlouk A, Jemli MH, Madec J‐Y and Haenni M, 2019a. Various Inc‐type plasmids and lineages of Escherichia coli and Klebsiella pneumoniae spreading blaCTX‐M‐15, blaCTX‐M‐1 and mcr‐1 genes in camels in Tunisia. Journal of Global Antimicrobial Resistance, 19, 280–283. 10.1016/j.jgar.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Salinas L, Cárdenas P, Johnson TJ, Vasco K, Graham J and Trueba G, 2019b. Diverse commensal Escherichia coli clones and plasmids disseminate antimicrobial resistance genes in domestic animals and children in a semirural community in ecuador. Msphere, 4, e00316–e319. 10.1128/mSphere.00316-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satué K and Gardon J, 2016. Pregnancy Loss in Mares. In: Darwish AM (ed.). Genital Infections and Infertility [Internet], IntechOpen, London. [Google Scholar]
- Schmidt VM, Pinchbeck GL, Nuttall T, McEwan N, Dawson S and Williams NJ, 2015. Antimicrobial resistance risk factors and characterisation of faecal E. coli isolated from healthy Labrador retrievers in the United Kingdom. Preventive Veterinary Medicine, 119, 31–40. 10.1016/j.prevetmed.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Singer RS and Hofacre CL, 2006. Potential impacts of antibiotic use in poultry production. Avian Diseases, 50, 161–172. 10.1637/7569-033106R.1 [DOI] [PubMed] [Google Scholar]
- Smith KL, Todhunter D and Schoenberger P, 1985. Environmental mastitis: cause, prevalence, prevention. Journal of Dairy Science, 68, 1531–1553. [DOI] [PubMed] [Google Scholar]
- Solà‐Ginés M, Cameron‐Veas K, Badiola I, Dolz R, Majó N, Dahbi G, Viso S, Mora A, Blanco J, Piedra‐Carrasco N, González‐López JJ and Migura‐Garcia L, 2015. Diversity of multi‐drug resistant avian pathogenic Escherichia coli (APEC) causing outbreaks of colibacillosis in broilers during 2012 in Spain. PLoS One, 10, e0143191. 10.1371/journal.pone.0143191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg ZR, Johnson JR, Fairbrother JM, Kilbourne J, Van Goor A, Curtiss RR and Mellata M, 2017. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS One, 12, e0180599. 10.1371/journal.pone.0180599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suay‐García B, Galán F, Rodríguez‐Iglesias MA and Pérez‐Gracia MT, 2019. Detection and characterization of extended‐spectrum beta‐lactamases‐producing Escherichia coli in animals. Vector‐Borne and Zoonotic Diseases, 19, 115–120. 10.1089/vbz.2018.2333 [DOI] [PubMed] [Google Scholar]
- Swedish Veterinary Antibiotic Resistance Monitoring (SVARM) , 2019. Sales of antibiotics and occurrence of resistance in Sweden. Available online: https://www.sva.se/media/0hihej1c/swedres‐svarm‐2019.pdf
- Swedish Veterinary Antibiotic Resistance Monitoring (SVARM) , 2020. Sales of antibiotics and occurrence of resistance in Sweden. Available online: https://www.sva.se/media/8d9678c390929e9/swedres_svarm_2020.pdf
- Teichmann‐Knorrn S, Reese S, Wolf G, Hartmann K and Dorsch R, 2018. Prevalence of feline urinary tract pathogens and antimicrobial resistance over five years. Veterinary Record, 183, 21. 10.1136/vr.104440 [DOI] [PubMed] [Google Scholar]
- Tello M, Ocejo M, Oporto B and Hurtado A, 2020. Prevalence of cefotaxime‐resistant Escherichia coli isolates from healthy cattle and sheep in northern Spain: phenotypic and genome‐based characterization of antimicrobial susceptibility. Applied and Environmental Microbiology, 86, e00742–e820. 10.1128/AEM.00742-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple D, Jiménez M, Escribano D, Martín‐Valls G, Díaz I and Manteca X, 2020. Welfare benefits of intradermal vaccination of piglets. Animals, 10, 1898. 10.3390/ani10101898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Center for Food Security & Public Health , 2021. Zoonotic Diseases of cattle. Available online: https://www.cfsph.iastate.edu/Assets/zoonotic‐diseases‐of‐cattle‐table.pdf
- Tyson GH, McDermott PF, Li C, Chen Y, Tadesse DA, Mukherjee S, Bodeis‐Jones S, Kabera C, Gaines SA, Loneragan GH, Edrington TS, Torrence M, Harhay DM and Zhao S, 2015. WGS accurately predicts antimicrobial resistance in Escherichia coli . Journal of Antimicrobial Chemotherapy, 70, 2763–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori SR, Makin TJ, Smith ML and Krautil FL, 1981a. Clinical manifestations of diarrhea in calves infected with rotavirus and enterotoxigenic Escherichia coli . Journal of Clinical Microbiology, 13, 1011–1016. 10.1128/jcm.13.6.1011-1016.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Sherwood D, Angus KW, Campbell I and Gordon M, 1981b. Diarrhea in lambs: experimental infections with enterotoxigenic Escherichia coli, rotavirus, and Cryptosporidium sp. Infection and Immunity, 33, 401–406. 10.1128/iai.33.2.401-406.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde FL, Kaufmann T, Sager H, Albini S, Zanoni R, Schelling E and Meylan M, 2008. Prevalence of four enteropathogens in the faeces of young diarrhoeic calves in Switzerland. Veterinary Record, 163, 362–366. 10.1136/vr.163.12.362 [DOI] [PubMed] [Google Scholar]
- UK‐VARSS , 2019. UK Veterinary Antibiotic Resistance and Sales Surveillance Report (UK‐VARSS 2018). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/909987/_1587146‐v23‐VARSS_2018_Report__2019_‐accessible.pdf
- Uotani Y, Kitahara R, Imai T, Tsutsumi N, Sasakawa C, Nagai S and Nagano T, 2017. Efficacy of an avian colibacillosis live vaccine for layer breeder in Japan. Journal of Veterinary Medical Science, 79, 1215–1219. 10.1292/jvms.17-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valat C, Drapeau A, Beurlet S, Bachy V, Boulouis H‐J, Pin R, Cazeau G, Madec J‐Y and Haenni M, 2020. Pathogenic Escherichia coli in dogs reveals the predominance of ST372 and the human‐associated ST73 extra‐intestinal lineages. Frontiers in Microbiology, 11, 580. 10.3389/fmicb.2020.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde A, Grill F, Coque TM, Pintado V, Baquero F, Cantón R and Cobo J, 2008. High rate of intestinal colonization with extended‐spectrum‐beta‐lactamase‐producing organisms in household contacts of infected community patients. Journal of Clinical Microbiology, 46, 2796–2799. 10.1128/JCM.01008-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bunt G, Fluit AC, Spaninks MP, Timmerman AJ, Geurts Y, Kant A, Scharringa J, Mevius D, Wagenaar JA, Bonten MJM, van Pelt W and Hordijk J, 2020. Faecal carriage, risk factors, acquisition and persistence of ESBL‐producing Enterobacteriaceae in dogs and cats and co‐carriage with humans belonging to the same household. Journal of Antimicrobial Chemotherapy, 75, 342–350. 10.1093/jac/dkz462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstokstraeten R, Belasri N, Demuyser T, Crombé F, Barbé K and Piérard D, 2021. A comparison of E. coli susceptibility for amoxicillin/clavulanic acid according to EUCAST and CLSI guidelines. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical. Microbiology, 40, 2371–2377. [DOI] [PubMed] [Google Scholar]
- Vandekerchove D, Vandemaele F, Adriaensen C, Zaleska M, Hernalsteens J‐P, De Baets L, Butaye P, Van Immerseel F, Wattiau P, Laevens H, Mast J, Goddeeris B and Pasmans F, 2005. Virulence‐associated traits in avian Escherichia coli: comparison between isolates from colibacillosis‐affected and clinically healthy layer flocks. Veterinary Microbiology, 108, 75–87. 10.1016/j.vetmic.2005.02.009 [DOI] [PubMed] [Google Scholar]
- VKM , 2015. Assessment of the transfer of antimicrobial resistance between pets and humans in Norway. Opinion of the Panel on biological hazards of the Norwegian Scientific Committee for Food Safety, ISBN: 978‐82‐8259‐183‐6, Oslo, Norway.
- Wasiński B, 2019. Extra‐intestinal pathogenic Escherichia coli – threat connected with food‐borne infections. Annals of Agricultural and Environmental Medicine, 26, 532–537. 10.26444/aaem/111724 [DOI] [PubMed] [Google Scholar]
- Weber LP, Dreyer S, Heppelmann M, Schaufler K, Homeier‐Bachmann T and Bachmann L, 2021. Prevalence and risk factors for ESBL/AmpC‐E. coli in pre‐weaned dairy calves on dairy farms in Germany. Microorganisms, 9, 2135. 10.3390/microorganisms9102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedley AL, Maddox TW, Westgarth C, Coyne KP, Pinchbeck GL, Williams NJ and Dawson S, 2011. Prevalence of antimicrobial‐resistant Escherichia coli in dogs in a cross‐sectional, community‐based study. Veterinary Record, 168, 354. 10.1136/vr.d1540 [DOI] [PubMed] [Google Scholar]
- Whitmer GR, Moorthy G and Arshad M, 2019. The pandemic Escherichia coli sequence type 131 strain is acquired even in the absence of antibiotic exposure. PLoS Path, 15, e1008162. 10.1371/journal.ppat.1008162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl U, Holst BS, Nyman A, Grönlund U and Bengtsson B, 2014. Characterisation of bacterial growth and antimicrobial susceptibility patterns in canine urinary tract infections. BMC Veterinary Research, 10, 217. 10.1186/s12917-014-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley RE, Gibbs PS, Brown TP and Maurer JJ, 2000. Chicken embryo lethality assay for determining the virulence of avian Escherichia coli isolates. Avian Diseases, 44, 318–324. [PubMed] [Google Scholar]
- World Organisation for Animal Health (OIE) , 2021. OIE list of antimicrobial agents of veterinary importance. Available online: https://www.oie.int/app/uploads/2021/06/a‐oie‐list‐antimicrobials‐june2021.pdf
- Yu D, Banting G and Neumann NF, 2021. A review of the taxonomy, genetics, and biology of the genus Escherichia and the type species Escherichia coli . Canadian Journal of Microbiology, 67, 553–571. 10.1139/cjm-2020-0508 [DOI] [PubMed] [Google Scholar]
- Zanella A, Alborali GL, Bardotti M, Candotti P, Guadagnini PF, Martino PA and Stonfer M, 2000. Severe Escherichia coli O111 septicaemia and polyserositis in hens at the start of lay. Avian Pathology, 29, 311–317. 10.1080/03079450050118430 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Shen B and Bi D, 2020. Chapter 30 ‐ Management of pathogens in poultry. In: Bazer FW, Cliff Lamb G and Wu G (eds.), Animal Agriculture, Academic Press. pp. 515–530. ISBN 9780128170526. Available online: https://eur03.safelinks.protection.outlook.com/?url=https%3A%2F%2Fdoi.org%2F10.1016%2FB978‐0‐12‐817052‐6.00030‐6&data=05%7C01%7C%7C75bcbf862d2c4143133608da2859875f%7C406a174be31548bdaa0acdaddc44250b%7C1%7C0%7C637866662995623794%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=FFh%2FjLhihzDxsJmHEHtPCt4GHJBNhAA0NCV6FUxqHeg%3D&reserved=0https://www.sciencedirect.com/science/article/pii/B9780128170526000306?via%3Dihub. 10.1016/B978-0-12-817052-6.00030-6 [DOI]


