Abstract
The lungs are constantly exposed to inhaled debris, allergens, pollutants, commensal or pathogenic microorganisms, and respiratory viruses. As a result, innate and adaptive immune responses in the respiratory tract are tightly regulated and are in continual flux between states of enhanced pathogen clearance, immune-modulation, and tissue repair. New single-cell-sequencing techniques are expanding our knowledge of airway cellular complexity and the nuanced connections between structural and immune cell compartments. Understanding these varied interactions is critical in treatment of human pulmonary disease and infections and in next-generation vaccine design. Here, we review the innate and adaptive immune responses in the lung and airways following infection and vaccination, with particular focus on influenza virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The ongoing SARS-CoV-2 pandemic has put pulmonary research firmly into the global spotlight, challenging previously held notions of respiratory immunity and helping identify new populations at high risk for respiratory distress.
Thomas and colleagues present an overview of pulmonary immunity, covering innate and adaptive responses following infection and vaccination, with a particular focus on responses to influenza and SARS-CoV-2. They also highlight exciting recent advances and the importance of continuing research efforts into human respiratory health.
Introduction
The respiratory tract is an intricate and complex system that facilitates both gas exchange and blood oxygenation, while forming a physical and immunologic barrier between the external environment, blood, and tissue sites. The upper respiratory tract (URT), also referred to as the conducting airways, includes the nasal cavity, pharynx, and larynx and is also the site of the nasal-associated lymphoid tissue (NALT; cervical lymph nodes) (Figure 1 A). Descending into the lung, the lower respiratory tract (LRT) includes the trachea and the bronchi and bronchiole branches of the lung. The bronchial-associated lymphoid tissue (BALT; mediastinal lymph nodes) drains from these sites, training localized adaptive responses (Figure 1A). Within the lung parenchyma and extending from the ends of the bronchioles are the alveoli and lung interstitial spaces, often referred to as the “respiratory zone.” These specialized tissues are the major site of gas exchange between the lung and the blood and contain the largest vascular bed in the body (Hewitt and Lloyd, 2021). Proper airway and lung function is tightly associated with human health, and a careful balance between infection response and tissue function needs to be maintained. Multiple disease states stem from dysregulated responses in the airways, including asthma, allergy, and acute or chronic pulmonary diseases. Further, numerous infectious agents, including respiratory viral, bacterial, fungal, and protozoan pathogens, target cells that line the airways for replication and can cause direct damage to barrier sites and trigger inflammation-associated tissue damage. Indeed, respiratory pathogens remain a prominent cause of global mortality. In 2020, LRT infections constituted the fourth leading cause of death worldwide and are responsible for nearly 2.4 million annual deaths (GBD 2016 Lower Respiratory Infections Collaborators, 2018; World Health Organization, 2020). Young children, the elderly, immunocompromised individuals, and individuals with co-morbidities bear the brunt of these infections, with Haemophilus influenzae B, Streptococcus pneumoniae, respiratory syncytial virus (RSV), and influenza viruses taking a particularly high toll (GBD 2016 Lower Respiratory Infections Collaborators, 2018). Maintaining balance in the respiratory tract during insult, injury, or infection is paramount to host survival. In the lung alveoli, gas exchange is mediated by pressure balances across epithelial and endothelial cells, and changes in this homeostasis can lead to loss of blood oxygenation or accumulation of CO2. During initial infection with a respiratory pathogen, balance between pathogen clearance and immune modulation is tightly controlled by the epithelial-immune cell axis, as dysregulation can lead to severe tissue damage. At later stages of infection, tradeoffs between sustained inflammation and tissue repair must be made to re-establish proper lung function. As a result, innate and adaptive pulmonary immune responses are tightly regulated following damage and infection to maintain this balance. Here, we discuss the innate and adaptive immune responses in the respiratory tract following infection and vaccination, how these systems are broadly regulated, and new areas of ongoing research in pulmonary health and immunity. To place these responses in context, we focus on immunity to influenza and SARS-CoV-2 infection and vaccination. The continuing morbidity and mortality associated with the global SARS-CoV-2 pandemic further highlights the necessity of continued research into human respiratory biology, factors impacting innate and adaptive immunity, and identifying cellular and humoral correlates of protection and severity following infection and vaccination.
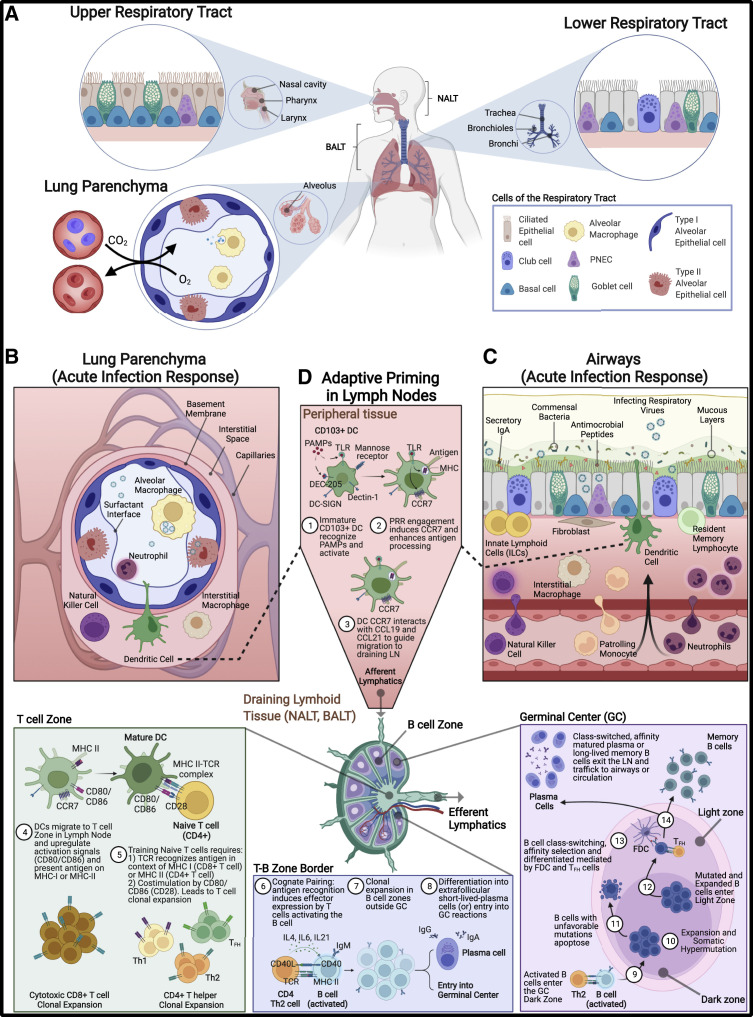
Figure 1.
Respiratory tract compartments and immune environment
(A) The upper (nasal cavity, pharynx, larynx), lower (trachea, bronchi, bronchioles), and the lung parenchyma (alveoli and lung interstitial spaces) compartments of the respiratory tract. Specific structural cells in the URT include undifferentiated basal cells, ciliated epithelial, and secretory goblet cells. Ciliated epithelium in the URT along with mucus that is synthesized by goblet cells provide a mechanical means of removing debris, allergens, and potential pathogens from the URT. These cells are also present in LRT, with additional inclusion of club cells, non-ciliated epithelia that moderate tolerance responses. Like the URT, mechanical removal of materials from the LRT is accomplished by mucociliary activities of the ciliated epithelia and goblet cells along with coughing to dislodge materials impacted on bronchial branch points.
(B and C) Cells and immune factors present during mucosal immune response and acute inflammation in the (B) lung parenchyma and in the (C) airway tissues. Embedded within the mucous layer are secreted innate immune factors including antimicrobial peptides, proteases, lactoferrin, and complement that act to further reduce passage of foreign material into the lungs. Secretory immunoglobulins (sIgA and sIgM), produced by sub-epithelial plasma cells, can also be found coating the surface of the URT and LRT in the mucous layer and provide antigen-specific targeting of foreign antigens in parallel to their innate immune counterparts. Among innate cellular responders during acute infection, neutrophils predominate and produce additional factors (IL8 and elastase) to further amplify immune cell recruitment. In addition to neutrophils, natural killer (NK) cells, monocytes, and eosinophils are also recruited from circulation. Mesenchymal (fibroblasts), epithelial, and endothelial cells produce proteases to reshape the extracellular matrix landscape, the products of which signal for further recruitment of immune cells and open physical space for immune cells to occupy. Epithelial cells also release localized cytokine signals to activate resident innate lymphoid cells (ILCs), interstitial macrophages (IM), and dendritic cells (DCs). Each cell type, tissue-resident or recruited, produces a carefully orchestrated balance of either inflammatory and chemotactic cytokines (TNFɑ, IL1β, IL6, IL8, IFN-I, -II, -III, GM-CSF) or immune-regulatory (IL10, TGFβ, IL1Ra) factors in an effort to resist and eliminate pathogens without causing excessive inflammation.
(D) Professional antigen-presenting cells, mainly CD103+ dendritic cells (cDCs), capture antigen in the airways, become activated, and traffic to draining lymph nodes (LNs) via afferent lymphatics to prime adaptive responses. In LN T cell zones, externally derived antigens are presented on class II MHC, prompting CD4+ T cell training, while internally derived antigens are processed and presented on class I MHC to CD8+ T cells. CD103+ respiratory DCs can also cross-present peptides from external sources on class I MHC, possibly via efferocytosis, thus allowing CD8+ T cell priming (Ho et al., 2011). APCs promote maturation and expansion of naive CD4+ and CD8+ T cells through pMHC:TCR and co-stimulation (CD80/CD86). CD8+ cytotoxic T cells and subsets of CD4+ T helper cells traffic back to the site of infection. CD4+ Th2 cells migrate to T-B border and form cognate pairs with activated B cells (IgM+) via TCR:pMHC engagement, cytokine release (IL4, IL6, IL21), and co-stimulation (CD40L), leading to B cell clonal expansion and differentiation into low-affinity IgG or IgA-producing plasma cells (traffic to sites of infection) or entry into germinal centers (GCs). In GC dark zone, activated B cells undergo expansion and somatic hypermutation of the B cell receptor (BCR), resulting in either an unfavorable BCR specificity (leading to apoptosis) or desirable BCR specificity that binds strongly to peptides maintained on the surface of follicular dendritic cells (FDCs) in the light zone. B cells cycle through iterative rounds of expansion/somatic hypermutation (dark zone) and affinity selection (light zone), resulting in selection of high-affinity BCRs. Interactions with T follicular helper (Tfh) cells lead to B cell differentiation and class-switching to long-lived memory B cells and high-affinity plasma cells, which traffic to sites of infection or are maintained as long-lived memory populations. PNEC, pulmonary neuroendocrine cell; NALT, nasal-associated lymphoid tissue; BALT, bronchial-associated lymphoid tissue. Figure created with BioRender.com.
The respiratory immune environment
Due to the importance of proper lung function to host survival, immune responses in the respiratory tract constitute a thoroughly choreographed dance between pathogen clearance and maintenance of tissue function. This interplay is often discussed in terms of resistance and tolerance, with resistance referring to the innate and adaptive immune responses mounted to clear (“resist”) an infection and tolerance referring to the mechanisms by which the immune system minimizes (“tolerates”) both pathogen- and immune-mediated damage to respiratory tissue (Medzhitov et al., 2012). In the airways, states of resistance are specifically calibrated, tightly controlled, and, importantly, balanced by anti-inflammatory and repair-focused tolerance responses. The consequences of dysregulated airway resistance include hyper-inflammatory syndromes, barrier site destruction, and long-term sequelae such as lung fibrosis. As the consequences of dysregulated inflammation carry a heavy cost of morbidity and mortality spanning all age groups and demographics, they are important areas of ongoing research.
The respiratory tract comprises a rich cellular environment (Figure 1). Structural cell populations, including epithelial, endothelial, and mesenchymal cells, form a continuous physical barrier spanning the entirety of the airway and lung tissues (Holt et al., 2008; Ardain et al., 2020; Flerlage et al., 2021). Ciliated epithelial cells produce growth factors, are important sensors of infection or tissue damage, and secrete antimicrobial factors including surfactant, complement, mucins, and antimicrobial peptides (AMPs). Within the lung parenchyma, epithelial cells form the structure of alveoli and, along with endothelial cells, mediate gas exchange to and from the blood (Figure 1B). Endothelial cells line the large and small blood vessels of the lung and, during inflammation, mediate extravasation of immune cells into respiratory tissue. Mesenchymal cells populate the space below the epithelial layer and have numerous functions during homeostasis, including synthesis of extracellular matrix (ECM) components and production of growth factors. During acute inflammation, mesenchymal cells produce pro-inflammatory cytokines and chemokines as well as matrix proteases that restructure the ECM. In addition to structural cells, numerous non-recirculating, tissue-resident immune cells including alveolar macrophages (AMs), innate lymphoid cells (ILCs), unconventional T cells, and adaptive tissue-resident lymphocytes comprise the dedicated immune compartment of the respiratory tract. Following the introduction of an immunogen, sentinel fibroblasts and dynamic recirculating cells from the lung interstitium (interstitial macrophages [IMs], dendritic cells [DCs]) or periphery (neutrophils, natural killer [NK] cells, monocytes) respond to the site of insult. Adaptive immune cells including plasma B cells and CD4 and CD8 T cells provide antigen-specific responses across the lung tissue.
A major characteristic of pulmonary mucosal sites is the ubiquitous thin layer of fluid covering the luminal surface of the airway, called mucus. Mucus, produced by secretory cells (goblet cells), is formed by a complex of mucin proteins, cytokines, complement factors, antimicrobial peptides, secretory IgA, and commensal bacteria that line the airways and mucosal barrier sites (Figure 1C). Mucins are particularly important players in viral transmission, as these diverse glycoprotein complexes may trap and prevent viral entry in an infected host. Evidence for interference of influenza virus entry via binding and membrane fusion was recently reported for a group of mucin-like polypeptides in the airways (Delaveris et al., 2020). Conversely, during other respiratory virus infections, viral attachment to mucin sugars can enhance adhesion and cell entry and, further, may facilitate formation of virus-laden particles during expulsion, enhancing both aerosol and fomite transmission. Interestingly, early laboratory studies of SARS-CoV-2 predicted high rates of fomite and environmental transmission due to the stability of the virus within mucous droplets (Kampf et al., 2020; Kratzel et al., 2020; van Doremalen et al., 2020; Liu et al., 2021); however, these high rates were never observed during community transmission (Meyerowitz et al., 2020; NCIRD, 2020). A recent study found that bovine mucins could effectively neutralize human coronavirus OC43 rather than supporting infectivity, suggesting a possible mechanism for reduced fomite transmission of SARS-CoV-2 (Wardzala et al., 2022).
The lipoprotein surfactant is a vital component of the alveolar space composed of lipids, phosphatidylcholine, and surfactant proteins (SF-A, -B, -C, and -D) produced in part by type II airway epithelial cells (AEC2 cells) and regulated by AMs (Nobs, 2020). During homeostasis, surfactant lines the luminal face of alveoli, forming the air-liquid interface, reducing surface tension, preventing alveolar collapse, and facilitating gas exchange (Knudsen and Ochs, 2018). Surfactant acts as an opsonin (Sorensen, 2018), but also has numerous immunomodulatory roles including suppression of activated DCs and engaging AM Toll-like receptors (TLRs) to reduce pro-inflammatory cytokine secretion (Nathan et al., 2016; Puttur et al., 2019). Conversely, as an immune enhancer, surfactant can directly interact with airway immune cells, including DCs and macrophages, to induce activation and production of inflammatory cytokines and to increase cell chemotaxis (Sorensen, 2018).
Innate respiratory response: Acute pulmonary inflammation
Acute pulmonary inflammation is a complex and highly regulated immune response to infection, damage, or other significant pyrogenic insults to airway tissues and is broadly characterized by vasodilation, increased vascular permeability, and enhanced inflammatory cell infiltration into tissue spaces in the upper, lower, and lung parenchyma spaces. This process progresses sequentially from initiation to pathogen-response calibration, signal amplification, effector function, and immune relay, all leading to the final resolution and tissue-repair phases (Figure 2 ). Numerous cells are involved in mediating acute inflammation in the respiratory tract, including structural cells, non-circulating tissue-resident immune cells, and recruited innate and adaptive responses acting in coordination to clear an infection and limit tissue damage.
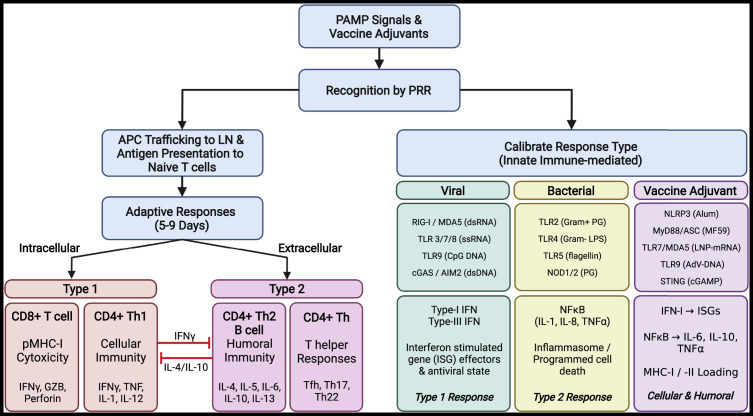
Figure 2.
Acute pulmonary inflammation and downstream responses
Initiation: Lung insults often involving infectious etiologic agents or vaccine components are recognized by structural cells, including epithelial and endothelial cells, along with tissue-resident antigen-presenting cells (airway macrophages and dendritic cells). Together, these cells recognize pathogen-associated molecular patterns (PAMPs), tissue damage-associated molecular patterns (DAMPs), or vaccine adjuvants via a complex network or surface, endosomal, and cytoplasmic sensors, collectively termed pattern recognition receptors (PRRs). Calibration: Detection of viral and bacterial PAMPs or vaccine-derived adjuvants initiates differential response pathways depending on the source (viral, bacterial, or adjuvant). Detection of viral PAMPS leads to production of IFN-I and/or IFN-III, TNFα, IL1β, IL6, IL8, IL12, MCP1, and inflammasome activation. Distinctive bacterial PAMPs (endotoxins, lipopolysaccharide [LPS], and peptidoglycan [PG]) trigger NF-κB signaling axis, leading to production of IL6, IL8, IL18, TNFɑ/β, and inflammasome activation. Detection and response to vaccine adjuvants can be tailored to promote type 1 or type 2 immune responses and specific development of cellular and humoral immunity. Trafficking: Specialized APCs capture and process protein components from pathogen or vaccine sources, traffic to airway-associated draining lymph nodes, and present antigen to naive T cells. Adaptive responses: Priming of adaptive responses begins around day 4 post-exposure and occurs in respiratory-associated lymphoid tissues. Following antigen-specific training, CD8+ T cell and CD4+ Th1 responses (IFNγ, TNF, IL1, IL12) are typically mounted against intracellular pathogens (antigens presented on MHC-I), while CD4+ Th2 (IL4, 5, 6, 10, 13), humoral (Tfh, B cells; antibody), and CD4 T helper (Th17, Th22) responses are increased in response to extracellular insults. Cross-presentation of external-derived antigens on MHC-I to CD8+ T cells allows development of cellular immunity to external insults. Figure created with BioRender.com.
During the initiation phase, unique molecular signatures present on viruses, bacteria, fungi, protozoans, or vaccine immunogens, collectively termed pathogen-associated molecular patterns (PAMPs), are detected by surface, vesicular, and cytosolic pattern recognition receptors (PRRs) expressed by epithelial and resident immune cells. To establish an appropriate resistant or tolerant environment, responses to different agents (bacteria, fungus, virus) are carefully calibrated through engagement of distinct PRR combinations, resulting in tailored recruitment of immune cell types and cytokines produced, which has been reviewed extensively (Moldoveanu et al., 2009; Zhang et al., 2021; Diamond and Kanneganti, 2022) (Figures 2, 3A, and 3B ). Following detection of airway and lung insults, epithelial, endothelial, and tissue-resident immune cells secrete factors that form a chemotactic gradient, cajoling circulating innate immune responders to the site of injury. Endothelial cells and pericytes surrounding pulmonary blood vessels and capillary sites vasodilate, becoming more permeable, and increase expression of surface adhesion molecules to allow extravasation of peripheral immune cells from the blood. A new area of investigation, culminating in an initial 2022 study, aimed to capture the numerous and rapid cellular transitional states (e.g., motility and morphology) of individual cells during acute inflammatory responses—a task that had been, until recently, technologically limited (Crainiciuc et al., 2022). Using 4D live imaging of individual leukocytes at sites of active inflammation paired with constructed behavioral landscapes, investigators were able to characterize the continuous morphokinetic and behavioral changes in single leukocytes as they adapted to local cues, tissue barriers, and acute states of inflammation. Importantly, the study identifies a continuum of neutrophil states within blood vessels that was associated with pathogenic inflammation and, further, found that the kinase Fgr was a driver of this pathogenic state. This study and others underscore the importance and heterogeneity of the recruited cellular dynamics and intrinsic cell changes that occur between circulation and inflamed tissue sites. Together, the activities of these resident and recruited immune cells coordinate pathogen clearance through phagocytosis of opsonized pathogens and apoptotic cellular debris, killing of infected cells via antigen-agnostic (NK) or antigen-specific (resident CD8+ T cells) cytolysis, and neutralization through localized antibody responses. During viral infections, interferon (IFN)-stimulated gene (ISG) effectors also act to restrict virus-cell membrane fusion, increase PRR expression, direct viral restriction, and trigger host cell apoptosis (Sen, 2002). Specialized professional antigen-presenting cells (APCs), predominantly airway DCs, sample materials from the site of infection and traffic to the draining lymphoid tissues to train antigen-specific T and B cells (discussed in detail below) (Figure 1D). After pathogen neutralization and clearance, the pro-inflammatory resistance signals are quickly replaced by immune-modulators and anti-inflammatory signals, predominantly IL10 and TGFβ, produced by airway macrophages, T regulatory (Treg) cells, and others. Tissue-repair mechanisms begin the process of repairing damaged airway cells and restoring lung function (Figures 3C–3E).
Table 1.
Innate and adaptive immune response dynamics
| Figure 3 | Innate response | Description | Cell types or factors involved | References |
|---|---|---|---|---|
| A | Low immunogenic clearance | Respiration of microbes, viruses, or environmental debris may be cleared by low-immunogenic or physical means in the airway lumen, maintaining tolerance including expulsion, direct killing, and inactivation (antimicrobial peptides; complement, lysozyme), or immediate opsonin (complement, surfactant, IgA)-mediated phagocytosis. |
|
Watford et al., 2000 |
| B | Initiation Calibration Amplification |
Structural cells, including epithelial and endothelial cells, along with tissue-resident antigen-presenting cells (airway macrophages and dendritic cells) recognize PAMPs or tissue DAMPs via surface, endosomal, and cytoplasmic sensors termed PRRs. Engagement of PAMPs from infection or vaccination leads to calibrated responses and “decisions” to maintain tolerance or promote resistance. Pro-inflammatory and chemokine signaling is amplified. Innate immune cells are recruited from the periphery. Following detection of airway and lung insults, epithelial, endothelial, and tissue-resident immune cells secrete factors that form a chemotactic gradient cajoling circulating innate immune responders to the site of injury. |
|
Reviewed in Alon et al. (2021); Ardain et al. (2020); Diamond and Kanneganti (2022); Hewitt et al. (2021); Holt et al. (2008); Moldoveanu et al. (2009); Sen (2002); Zhang et al. (2021) |
| C | Effector functions Clearance Trafficking to draining LNs |
Granulation tissue comprising a cellular matrix of fibroblasts, leukocytes, and epithelial cells forms in the respiratory tissue to facilitate and intensify antigen-agnostic clearance (phagocytosis, NET production, cell killing). Activated phagocytes, such as AMs, DCs, and recruited monocytes and neutrophils, engulf opsonized pathogens and apoptotic cellular debris while cytolytic NK cells mediate direct killing of infected cells. ECM is remodeled, allowing further immune cell infiltration. APCs collect and process antigen, traffic to LN, and present antigen to adaptive cells |
|
|
| D | Resolution Tissue repair |
After pathogen neutralization and clearance, the pro-inflammatory resistant signals are quickly replaced by immune-modulators and anti-inflammatory signals, predominantly IL10 and TGFβ, produced by airway macrophages, Treg cells, and others. Phagocytes clear apoptotic cell debris while numerous cell types produce anti-inflammatory signals reducing cell recruitment and release of pro-inflammatory cytokines. Tissue repair mechanisms begin the process of repairing damaged airways cells and restoring barrier, ECM, and lung function. |
|
|
| E | Tolerant response | Metabolic and epigenetic reprogramming. Tolerance to subsequent challenges |
|
Reviewed in: Netea et al. (2016); Netea et al. (2020) |
| F | Trained response | Metabolic and epigenetic reprogramming. Priming and enhancement of subsequent challenges |
|
| Figure 3 | Adaptive response | Description | Cell types or factors involved | References |
|---|---|---|---|---|
| I | Antigen priming in LN T and B cell expansion Cytotoxic T cells |
All airway DCs, but most regularly CD103+ cDCs, direct adaptive lymphocyte responses by (1) sampling antigen from the airways and alveolar spaces, (2) trafficking to the draining LN (2–4 days post-infection), and (3) processing and presenting peptide antigens on MHC-I or MHC-II to naive lymphocytes, allowing clonal expansion of CD4+ (helper) and CD8+ (cytotoxic) T cells. Effector T cells traffic back to airways. Th2 cells train short-lived plasma B cells (IgA and IgG). Low-level antibody production |
|
Guilliams et al. (2014); Kim and Braciale, (2009); Lambrecht and Hammad, (2012); Moltedo et al. (2011); Okada et al. (2005); Shulman et al. (2013); Worbs et al. (2017) |
| II | GC reactions Lung T cell responses Antibody production |
Effector T cells amplify innate responses (CD4+) or engage in antigen-specific cell killing (CD8+). Antigen-experienced B cells engage Tfh cells in LN GCs leading to class-switch recombination (CSR), affinity maturation (somatic hypermutation), and differentiation into the long-lived plasma or memory B cells secreting high-affinity immunoglobulins |
|
Coffey et al. (2009); Pereira et al. (2010); Ripperger and Bhattacharya (2021); Victora et al. (2010) |
| III | Memory cell development | Activities of CD8+, CD4+ (Th), Treg, and γδ T cells reduce inflammation and repair tissue. Short-lived effector cells apoptose. Memory T cell subsets include CD45RA− CCR7+ central memory (Tcm) cells circulating in lymphoid tissues, CD45RA− CCR7− effector memory (Tem) in the peripheral tissues, and CD45RA+ CCR7− Tem (TEMRA) develop. CD8+ TRM cells accumulate and self-renew in regions of the lung undergoing tissue repair termed repair-associated memory depots (RAMD) where they seed the airways with de novo TRMs. CD4 and CD8 TRMs accumulate with resident memory B cells (BRM) within inducible bronchus-associated lymphoid tissue (iBALT) |
|
Adachi et al. (2015); Halle et al. (2009); Masopust and Soerens (2019); Moyron-Quiroz et al.(2004); Ray et al. (2004); Saule et al. (2006); Tan et al. (2019); Thome et al. (2014) |
| IV | Adaptive response (Rapid – pre-existing) | Recognition of homotypic or cross-reactive antigen by mucosal TRM and BRM cells and rapid differentiation and effector functions (antibody production, cell killing, T helper). Activation of long-lived T effectors and high-affinity memory B cells |
|
Beura et al. (2019); Kumar et al. (2018); Teijaro et al. (2011a); Turner 2013; Wu et al. (2014); Zens et al. (2016) |
| V | Neutralization | Adaptive effector functions of resident and circulating antigen-activated memory T and B cells lead to direct pathogen neutralization, opsonization, innate cell activation, or killing of infected cells |
|
Adachi et al. (2015); Masopust and Soerens (2019); Wang et al. (2015); Zens et al. (2016) |
| VI | Immune memory | Antibody levels wane but remain relatively elevated and TRM and BRM cells re-establish residency. High-affinity memory B cells and antibody-secreting plasma cells traffic to the bone-marrow and continually produce antibodies. |
|
Humphries et al. (2021); Kurosaki et al. (2015); Palm and Henry (2019) |
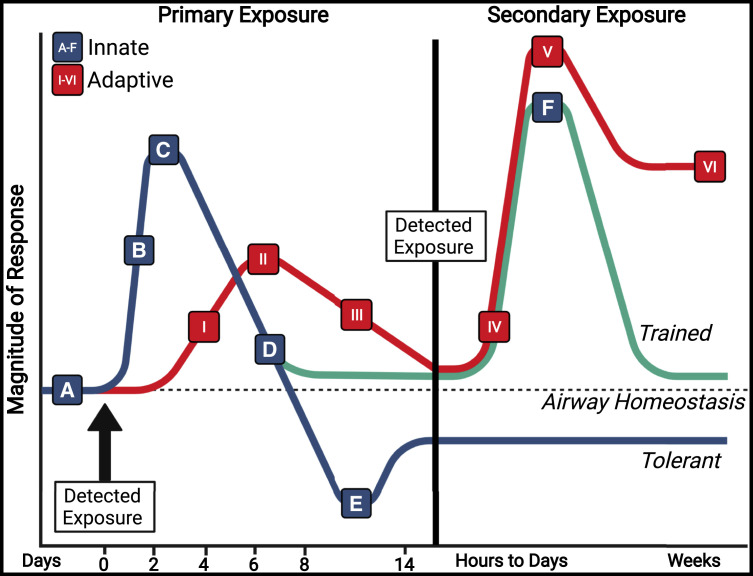
Figure 3.
Innate and adaptive immune response magnitude dynamics over time
Pulmonary infections or vaccinations that overcome low immunogenic clearance (A) trigger innate immune responses (blue/green) and initiate acute pulmonary inflammation (A and B) rapidly upon detection of PAMPs. Differential response cascades are calibrated by structural and resident immune cells to promote the appropriate inflammatory responses, coordinate effector functions of infiltrating innate cells, and activate antigen-presenting cells (with internalized antigen) to traffic to draining lymph nodes (B and C). As the infection is cleared, regulatory cells promote a return to a tolerant state, allowing tissue repair and blocking extravasation of additional immune cells (D). In certain innate immune cell types, the immune environment and PAMP type can promote metabolic and epigenetic reprogramming, driving innate cells into maintained tolerant (E, blue) or trained (F, green) phenotypes upon a subsequent challenge. Adaptive responses (red) are primed in respiratory draining lymphoid tissues (I), which promote clonal expansion of antigen-specific CD8+ cytotoxic T cells, CD4+ T helper cells, and low-affinity IgA and IgG-secreting plasma B cells that respond at tissue sites between 5 and 9 days post-challenge (II). Germinal center reactions in LN lead to class-switching, affinity selection, and generation of high-affinity plasma cells producing IgA and IgG and long-lived plasma cells (III). Tissue resident T (TRM) and B (BRM) cells are established in the lung via the iBALT and RAMD, while other lymphoid cells promote tolerance and tissue repair (III). Upon secondary exposure to homotypic or cross-reactive antigens in the lung and airways, adaptive responses are rapidly triggered, mediated first by activated TRM and BRM cells, then by recruited peripheral lymphocytes (IV and V). While innate responses activate and wane during secondary exposure, adaptive responses, mainly IgA and IgG levels, remain elevated for weeks (V). PAMPs, pathogen-associated molecular patterns; iBALT, inducible bronchus-associated lymphoid tissue; RAMD, repair-associated memory depots. Figure created with BioRender.com. See also Table 1.
Innate respiratory response: Role of IFN during viral infection
Type I (IFNα/β) and type III (IFNλ) interferons, master antiviral signaling factors, are the main drivers behind the innate immune response to viral infections. Via autocrine and paracrine signaling events, structural and resident innate immune cells are triggered to express hundreds to thousands of ISGs with pleiotropic antiviral, pro-inflammatory, and immunomodulatory functions. The effects of type I IFNs (IFN-I), produced in bulk by plasmacytoid DCs, epithelial cells, and macrophages, have been widely studied in response to nearly all human respiratory viral infections (influenza, rhinovirus, influenza, etc.). IFN-I directs a pro-inflammatory antiviral signaling paradigm in recruited immune cells (García-Sastre and Biron, 2006; García-Sastre, 2011), which can drive immune pathology if dysregulated. Indeed, a murine-model SARS-CoV infection determined that blocking IFN-I signaling ameliorated lung pathology (Channappanavar et al., 2016). Recent investigations of humans suffering from COVID-19 implicated the cGAS-STING pathway in IFN-I-mediated lung pathology (Di Domizio et al., 2022). Further, IFN-I and III have recently been shown to disrupt lung epithelial cell repair during recovery (Major et al., 2020). However, the role of IFN-I during SARS-CoV-2 (and other virus) responses is complex, as autoantibodies directed against IFNα2 and IFNω were observed in nearly 10% of patients with severe COVID-19 pneumonia but were absent in individuals with mild or asymptomatic infection and uninfected study participants (Bastard et al., 2020). Thus, autoimmune responses against IFN may underlie genetic predisposition to severe COVID-19. Unlike the more ubiquitous IFN-I, IFN-III (IFNλ1–3) are primarily associated with mucosal barriers, especially the lung epithelium, due to more restrictive expression of the IFNλ receptor. Although IFNλ signaling leads to ISG expression in response to virus infection, the gene-expression profiles are more specialized and do not as readily promote the same degree of inflammation as IFN-I (Hemann et al., 2017). Differential activation of interferon-regulatory factor 1 (IRF1) underlies the distinct immune responses triggered by IFN-I and III, resulting in unique gene-expression profiles (Forero et al., 2019). IFNλ signaling also causes translation-independent effects in neutrophils that limit the release of tissue-damaging reactive oxygen and nitrogen species and pro-inflammatory IL1β (Broggi et al., 2017). A 2017 study by Galani et al. determined that low-dose challenge of influenza A virus (IAV) led to containment of viral infection at the mucosal barrier sites in the lung and did not recruit additional pro-inflammatory cells (Galani et al., 2017). However, IFNλ signaling was overwhelmed during high-dose IAV challenge, triggering an IFN-I response, influx of innate responders, and subsequent lung pathology. Others have highlighted the importance of IFNλ signaling in containing influenza to the URT, reducing virus transmission, and directing DC-mediated CD8+ T cell programming (Klinkhammer et al., 2018; Hemann et al., 2019). The effects of IFNλ on CD8+ T cells highlight important links between the innate and adaptive responses and are a growing area of research. Peginterferon lambda-1 has also been tested as a therapeutic in a phase II placebo-controlled randomized trial for the treatment of COVID-19 and was found to be associated with increased odds of viral clearance by day 7 (Feld et al., 2021).
Innate respiratory response: Structural and non-circulating tissue-resident cells
The airway and lung epithelium is a continuous physical and immunologic barrier that forms the interface between the airspace lumen and sub-epithelial respiratory tissue (Figures 1A–1C). Multiple cell types comprise the epithelium and include airway stem, precursor, and intermediate cell types, large secretory goblet and club cells, and ciliated cells. Secretory cells are the main producers of mucin proteins in the airways; however, these cells are also involved in wound healing and immune modulation and responsible for secretion of AMPs (e.g., β-defensins), antimicrobial enzymes (e.g., lysozyme), as well as bacterial protease inhibitors (Geitani et al., 2020; Hewitt and Lloyd, 2021). Ciliated epithelial cells respond directly to luminal stimulation to regulate barrier function, ion and paracellular transport, and permeability through the activities of intercellular junctions (Brune et al., 2015). In the lung parenchyma, AEC2 cells secrete surfactant proteins and granulocyte-monocyte colony-stimulating factor (GM-CSF). GM-CSF has pluripotent effects in the airways, acting as a critical regulator of AM and DC development (Greter et al., 2012; Guilliams et al., 2013; Schneider et al., 2017), mediating granulocyte recruitment during allergic response (Nobs et al., 2019), and helping to maintain gas exchange and survival during infection (Standiford et al., 2012; Schneider et al., 2014; Brown et al., 2017). Inhalation of GM-CSF has even been evaluated as a potential treatment for COVID-19 to stabilize and improve lung function (Lang et al., 2020). In addition to these common cell types, new single-cell RNA sequencing (scRNA-seq) studies have recently identified several additional and rare populations (Treutlein et al., 2014; Madissoon et al., 2019; Deprez et al., 2020; Zaragosi et al., 2020; Hewitt and Lloyd, 2021). These include ionocytes (Montoro et al., 2018; Plasschaert et al., 2018), tuft cells (O’Leary et al., 2019), pulmonary neuroendocrine cells (PNECs) (Branchfield et al., 2016), and hillock cells (Montoro et al., 2018). One of the most important roles of the mucosal epithelium in the lung and airways is in early detection of tissue damage and pathogen insult, and these cells express numerous PRRs and cytokine receptors to survey the luminal landscape for markers of infection, forming the central regulation axis controlling pulmonary inflammation. In a 2018 study, Lehmann et al. profiled the secretome and transcriptome of primary human airway epithelial cells and identified 571 regulated genes, over 20 unique cytokines, and more than 1,000 secreted proteins produced by these cells, highlighting the complex roles for epithelial cells in the airways that help regulate immune responses, exosomal communication, and tissue homeostasis (Lehmann et al., 2018). The aptitude with which epithelial cells communicate with resident and circulatory immune cells, termed the “epithelial-immune axis,” is important for translating external stimuli and damage at mucosal barrier sites to the appropriate immune cells to facilitate an appropriate response. For example, during influenza infection, CXCL16 expression by airway epithelial cells and macrophages engages CXCR6+ CD8+ T resident memory (TRM) cells, recruiting these T cells from the interstitial space rather than circulation, allowing for a more regulated and rapid response to tissue damage and viral infection (Takamura et al., 2019). Retention of CD8+ TRM cells at mucosal sites is moderated by interactions between TRM-expressed ɑE/β7 integrins and E-cadherins on epithelial cells (Higgins et al., 1998). During late stages of infection, major histocompatibility complex (MHC)-II expressed by lung epithelial cells is needed for surface expression of PD-L1, a critical ligand for reducing CD4+ TRM activation and promoting tolerance (Shenoy et al., 2021). Thus, the epithelial cell-immune axis has the capacity to regulate both innate and downstream adaptive responses to respiratory pathogens.
AMs are a unique subset of self-renewing, long-lived, lung-tissue-resident immune cells derived from the fetal liver and seeded early in the perinatal lung by CD116+ precursors (Guilliams et al., 2013; Tan and Krasnow, 2016; Saluzzo et al., 2017; Evren et al., 2022). These cells are found in alveolar spaces where they moderate lipid homeostasis, facilitate gas exchange, and regulate surfactant catabolism (Todd et al., 2016) (Figure 1A). AMs are also important players in the epithelial-immune axis, engaging in continual and reciprocal communication with epithelial cells to modulate cell-to-cell contacts, barrier permeability inflammation, injury, and tissue-repair responses (Westphalen et al., 2014; Bourdonnay et al., 2015; Gibbings et al., 2017). As innate immune cells, AMs are preeminent phagocytes engulfing opsonin-mediated foreign material and apoptotic cell debris from the alveolar space. AMs typically exist in a semi-quiescent state (Gardai et al., 2003; Snelgrove et al., 2008; Yu et al., 2017), maintaining a suppressed immune environment through production of TGFβ and driving Treg cell development (Soroosh et al., 2013; Puttur et al., 2019). However, AMs can also be conscripts of airway resistance, acting as a first line of inflammatory response to infection and insult. Indeed, AMs express numerous surface, vesicular, and cytosolic PRRs, to detect “non-self” signals and relay appropriate immune-response cascades (Janssen et al., 2016) (Figure 2). Once activated, these cells produce pro-inflammatory IL6, TNFɑ, CCL2, and granulocyte-colony stimulating factor (G-CSF) to recruit additional innate immune cells to the site of infection. However, as tissue damage accumulates, phagocytosis of apoptotic cell debris drives AM expression of pro-tolerance and epithelial/ECM repair factors designed to return the alveolar space to a more suppressed, repair-focused state (Gersuk et al., 2006; Landsman and Jung, 2007; Zhang et al., 2008; Lloyd and Snelgrove, 2018). Despite their numerous functions, these cells were once thought to be uniform populations with high degrees of transcriptional plasticity (Mould et al., 2019); however, more recent studies suggest a greater degree of heterogeneity. One such study used scRNA-seq of BAL samples taken from healthy participants and those with cystic fibrosis (CF) and identified four AM superclusters and four “other” AM clusters that may have niche-specific roles (Li et al., 2022). Further unbiased clustering also identified four sub-clusters based on expression of distinct chemokine combinations that selectively recruit different leukocytes and appear to be niche-defined (Li et al., 2022). Others have reported similar diverse populations in the bronchial alveolar lavage fluid (BALF) of healthy subjects (Mould et al., 2021) and sputum samples from CF patients exhibiting differential AM maturity and activation states (Schupp et al., 2020).
ILCs are another non-circulating, self-renewing, tissue-resident innate cell type found in the respiratory tract. These cells share many functional characteristics with traditional CD4 and CD8 T cell subsets (Murphy et al., 2022). However, unlike circulating lymphocytes, ILCs do not express antigen-specific T cell receptors (TCRs), and thus these cells respond via antigen-agnostic detection and production of cytokines. Depending on the ILC subset, these cytokines may be involved in responses to tissue damage, infection, or maintaining airway homeostasis (Simoni et al., 2017). While these cells are transcriptionally plastic and may adopt niche-specific functional roles, in general ILCs are classified into three groups that include ILC1 (T-bet+; Th1-like), ILC2 (GATA3+; Th2-like), and ILC3 (Rorγt+; Th17/22-like) (Mjösberg et al., 2012; Spits et al., 2013; Lund et al., 2017; Steer et al., 2017; Mindt et al., 2018). Cytotoxic NK cells are classified as ILC1 cells (transcriptionally and due to production of IFNγ) but will be discussed separately. ILC1 cells (non-cytotoxic NK cells) represent a small fraction in the lung yet are important mediators of immune surveillance and early response to respiratory pathogens mainly through production of IFNγ and TNFɑ. Indeed, using murine influenza infection models, Vashist et al. demonstrated early (3 days post-infection) production of these cytokines from ILC1s in response to A/H1N1 challenge, while Weizman et al. determined that ILC1 cells produced significantly greater amounts of IFNγ in response to A/PR8/H1N1 than other known producers (NK, CD8 TRM) in the lung (Weizman et al., 2017; Vashist et al., 2018). Group 2 ILCs are central communicators in both peripheral and central airway sites at steady state, converting signals from epithelial cells (IL33, IL25, thymic stromal lymphopoietin) into Th2 (IL5, IL9, IL13), immunoregulatory (IL10), and epithelial repair factor (amphiregulin) relays (Neill et al., 2010). ILC2 cells also engage in further contact-dependent signaling with lymphocytes via PD1 (Th2 cells) and ICOS (ILC2; Treg), can modulate epithelial cell junctions, and have also been shown upregulate MHC-II to present antigen to responding CD4 T cells (Mirchandani et al., 2014; Oliphant et al., 2014). The role of ILC2s during influenza infection, however, is complex and depends on the context of the infecting strain. While IL13+ ILC2s were shown to restore lung function and epithelial integrity via amphiregulin in an A/H1N1 murine lung infection model (Monticelli et al., 2011), others have demonstrated that IL13 produced by ILC2s in response to A/H3N1 enhances pathogenicity by promoting airway hyper-reactivity independently from adaptive responses (Chang et al., 2011). The final group, ILC3s, function like Th17 (IL17) and Th22 (IL22) cells. As with ILC2s, the role of ILC3s during influenza infection is double-edged, with studies suggesting that early IL17 production during A/H1N1 infection (likely from ILC3s) promotes neutrophil recruitment and lung injury (Li et al., 2012) but may also be important for preventing secondary bacterial infections (Robinson et al., 2013; Ardain, Porterfield, et al., 2019). Although studies in humans are limited, ILC3s have been shown to be involved in early clearance of bacterial infections (Gopal et al., 2014; Van Maele et al., 2014; Bayes et al., 2016; Xiong et al., 2016; Treerat et al., 2017; Ardain, Domingo-Gonzalez et al., 2019).
Unconventional and innate-like lymphocytes, including gamma-delta (γδ) TCR-expressing T cells, mucosal-associated invariant T (MAIT) cells, and invariant natural killer T (iNKT) cells also populate the lung and airways and are important for response to respiratory pathogens. γδ T cells provide passive T cell immune responses in the lung and are known to exhibit age- and tissue-specific functionality in humans (Clark and Thomas, 2020). These age-related differences in γδ T cell abundance, functionality, TCR repertoire, and tissue localization may underlie differences in response to respiratory bacterial and viral infections, including tuberculosis, influenza, and human cytomegalovirus (HCMV) (Clark and Thomas, 2020). In a 2018 study, Guo and colleagues explored the immune environment in the lung of neonatal mice infected with IAV and observed that a more tolerant environment was critical to animal survival (Guo et al., 2018). In response to epithelial damage, γδ T cells, through the production of IL17, stimulated secretion of IL33 from epithelial cells, which in turn promoted amphiregulin production from regulatory T and ILC2 cells (Guo et al., 2018). Recent evidence also suggests that γδ T cell numbers are severely reduced in children with COVID-19, though how these cells contribute to disease progression is largely unknown (von Massow et al., 2021).
MAIT cells are the second set of unconventional T cells abundant in the human lung and are defined by expression of a semi-invariant TCR recognizing metabolic derivatives of the riboflavin synthesis pathway presented on MHC-related protein-1 (MR1) (Provine and Klenerman, 2020). MAIT cells provide rapid, antigen-specific immunity against bacterial infections and are important during antiviral response in humans, though this is more likely antigen-agnostic activation mediated by synergistic activities of IL18, IFN-I, IL12, and IL15 (van Wilgenburg et al., 2016; Harker and Lloyd, 2021). During experimental murine influenza infection, MAIT cells were shown to be necessary to protect mice against lethal A/H1N1 challenge (van Wilgenburg et al., 2018). In this model, MAIT cells accumulated early (days 3-5) and were activated to express CD69, CD25, and granzyme B (GzmB) by local cytokines rather than MR1-restricted antigens (van Wilgenburg et al., 2018). Like MAITs, iNKT cells are activated by cytokine responses during viral infection but can also mediate antigen-specific responses to self- and microbial-derived lipids presented to a limited, CD1-restricted TCR repertoire (Harker and Lloyd, 2021). Activation of iNKT cells is important for antiviral responses in the lung, and direct activation of these cells with an intranasal iNKT agonist led to rapid and enhanced protection of piglets from lethal A/H1N1 influenza challenge, suggesting these cells may be a therapeutic target in treatment of severe influenza (Artiaga et al., 2016). However, recent evidence from SARS-CoV-2-infected patients has demonstrated that dysregulation of MAIT and iNKT cells can have detrimental and life-threatening effects. In a 2020 study, Jouan and colleagues found that COVID-19 influences the phenotype and function of these unconventional T cell subsets, observing significant reduction in peripheral levels of iNKT and MAIT (but not γδ T) cells in patients admitted to the ICU and subsequent increase in activated CD69+ iNKT cells in the lung (Jouan et al., 2020). The activation states of MAIT and γδ T cells were predictive of the level of hypoxia patients experienced over the course of infection (Jouan et al., 2020). In a similar study, MAIT cell activation and cytotoxicity, typified by increased expression of CD69, IFNγ, and GzmB, strongly correlated with fatal outcomes in patients with COVID-19 (Flament et al., 2021). The authors also argue that MAIT dysregulation in these patients was likely the result of imbalanced IFN-I and IL18 cytokine secretion by transitional monocyte-macrophages, as macrophages from healthy donors infected with SARS-CoV-2 could drive cytotoxicity in MAIT cells ex vivo (Flament et al., 2021).
Endothelial and mesenchymal cells are the final non-circulating cell types in the airways and are recognized as important and diverse mediators of airway inflammation, cell recruitment, and respiratory responses. Endothelial cells (ECs) form the lining of blood vessels and have long been appreciated in the respiratory system as regulators of gas exchange and ECM remodeling and as physical gatekeepers regulating immune cell influx. However, numerous studies have shown that these cells may play a more direct role in tolerance, immune surveillance (scavenging), active phagocytosis, immune cell recruitment from the periphery, and antigen presentation to conventional (CD4/CD8) and unconventional (iNKT) T cells. Collectively, these EC subsets are referred to as immunomodulatory endothelial cells (IMECs), and their numerous unique functions have been detailed elsewhere (Amersfoort et al., 2022). However, evidence for detrimental functions during antiviral response have also been uncovered. Direct infection of ECs by influenza in animal-challenge models is associated with more severe disease, induction of cytokine storm, and breakdown of barrier functions (Teijaro et al., 2011b; Tundup et al., 2017). In studies of a vascularized lung-on-chip model of COVID-19, Thacker et al. report unproductive infection of ECs by SARS-CoV-2; however, interaction with the virus lead to EC clustering, reduced CD31 expression, loss of barrier integrity, and production of pro-coagulation factors (Thacker et al., 2021), signs of severe disease that may be enhanced in aged individuals (Angelidis et al., 2019). Mesenchymal cells, encompassing fibroblasts, pericytes, smooth muscle, and other stromal cell types, are important physical mediators of lung function, making up the connective tissue in the lung, and are involved in regulating tolerance and resistance responses during infection and lung injury. In healthy states, mesenchymal cells produce growth factors (VEGF, FGF, and GM-CSF) to stimulate cell differentiation; however, these cells can also produce inflammatory cytokines and chemokines and can often be the primary producers IL6 and CCL2, two key factors in establishing resistance. Fibroblasts, along with epithelial and endothelial cells, are responsible for remodeling the ECM, a complex of structural proteins, proteases, and glycosylases that provides structural support in the airways and helps in lung function. During acute pulmonary inflammation, ECM components are degraded by specific proteases expressed by fibroblasts and other structural cells, forming a loose provisional matrix allowing immune cell infiltration, proliferation, and differentiation (Sorokin, 2010; Bonnans et al., 2014; Wight, 2017). If not carefully regulated, ECM remodeling can significantly impact outcomes and potential long-term sequelae in the lung (El Agha et al., 2017). Recent studies have demonstrated the importance of fibroblast-mediated ECM remodeling during acute IAV infection, highlighting a previously underappreciated heterogeneity in this lung cell population. Using single-cell gene-expression profiling of fibroblasts during severe IAV infection in mouse lungs, Boyd et al. describe three distinct fibroblast populations, including traditional ECM-synthesizing and two inflammatory subsets (Boyd et al., 2020). Importantly, this study found that exuberant damage-associated fibroblasts (DRFibs) are responsible for driving lethal immune pathology during viral infection due to expression of ECM-remodeling enzymes. Expression of the matrix metalloprotease ADAMTS4 leads to specific degradation of the ECM component versican, driving production of inflammatory cytokines, an influx of innate and adaptive immune cells, and reduced lung function. Analogous fibroblast populations were also uncovered in human lung biopsies. Levels of ADAMTS4 in the LRT were associated with severe seasonal or avian influenza infection, while DRFibs correlated with acute respiratory failure in deceased donors (Boyd et al., 2020).
Innate respiratory response: Recruited and dynamic recirculating cells
Several other important innate cell types function in the respiratory tract, including cells recruited from the periphery during acute pulmonary inflammation (neutrophils, NK cells, monocytes) as well as more tissue-resident-like cells (DCs, IMs) that require dynamic replacement by hematopoietic cells.
Neutrophils are the earliest and most abundant immune cell recruited into the lungs and airways during acute pulmonary inflammation (Forbes and Rosenthal, 2014; Wang et al., 2017a; Konrad et al., 2019). Within respiratory tissue spaces, neutrophils act in remediation of pathogens and cellular materials from the airspace through phagocytosis, degranulation, and release of neutrophil extracellular traps (NETs)—web-like chromatin structures comprising cytosolic and granule proteins assembled on decondensed chromatin that trap and dissolve bacterial cells and capture excess cytokines (e.g., CCL5, CXCL12, and CXCL4) in the lung milieu to help modulate inflammation (Brinkmann et al., 2004; Wang et al., 2017a; Papayannopoulos, 2017; Capucetti et al., 2020). The production of NETs, termed NETosis, is a form of programmed neutrophil cell death distinct from necrotic and apoptotic pathways. When dysregulated, NETosis has been shown to exacerbate inflammation leading to microvascular thrombosis and contribute to viral acute respiratory distress syndrome (ARDS) (Yang et al., 2016). Studies in mice have shown that blocking NET formation improved outcomes during A/H1N1 infection (Narasaraju et al., 2011; Sugamata et al., 2012). During human A/H1N1 and A/H7N9 influenza infection, NETs were found to increase the permeability of alveolar epithelial cells leading to enhanced disease, while detection of elevated biomarkers of NETs was found to correlate with severe influenza and, recently, COVID-19 (Zhu et al., 2018; Zuo et al., 2020). During early-stage response, neutrophils potentiate inflammation through production of IL8 and elastase (Nakamura et al., 1992; Takahashi et al., 1993) but also have the capacity to promote tissue-repair mechanisms through expression of matrix metalloproteinases and lipid mediators (González-López et al., 2011; Aguirre et al., 2017; Blázquez-Prieto et al., 2018) and may even dampen acute lung injury through repression of poly (adenosine diphosphate-ribose) polymerase-1 (PARP-1) via transfer of miR-223 to epithelial cells (Neudecker et al., 2017). Neutrophils are also important mediators of adaptive lymphocyte recruitment in the lung and airways in response to both bacterial and viral infections. A 2015 study by Lim and Hyun et al. explored this mechanism in detail using a murine influenza challenge model, reporting not only that early recruitment of neutrophils into the infected trachea is required for CD8 T cell-mediated protection, but also that T cell recruitment involves long-lasting neutrophil “trails” enriched with potent chemotactic factors such as CXCL12 to guide lymphocytes to the site of infection (Lim et al., 2015).
Defined as cytotoxic members of group 1 ILCs, NK cells exhibit antigen-agnostic cytolytic functions through the production of perforin and granzymes and are contributing producers of respiratory IFNγ (Bernink et al., 2013). NK cells are important, but not critical for response to viral infections in the human lung. In response to IAV infection, early production of IFNγ by NK cells limits initial disease severity; however, it may also exacerbate lung pathology and tissue damage during the later stages of influenza infection (Zhou et al., 2013; Abboud et al., 2016). A second subset of NK cells in the lung parenchyma rapidly degranulate, producing GzmB and IFNγ to limit viral spread by direct killing of infected cells (Cooper et al., 2018).
Respiratory or airway macrophages are categorized into three groups: long-lived tissue-resident AMs (discussed above), dynamic recirculating IMs, and recruited peripheral monocytes. Airway macrophages are highly plastic, and although studies are limited, several IM subsets have been recently identified in human lungs by scRNA-seq and transcriptomics (Chakarov et al., 2019; Schyns et al., 2019). Airway macrophages expressing high levels of HIF1α have been called “guardians of tissue repair” (Puttur et al., 2019) and are critical regulators of airway inflammation, barrier integrity, and tissue repair. In general, macrophages act as both phagocytes and surveyors of respiratory infection, expressing high levels of PRRs to detect infections. Macrophages also express surface Fc receptors, which aid in progressive clearance of airway pathogens such as influenza and SARS-CoV-2 (Guilliams et al., 2014; Tay et al., 2019). Airway macrophages also have the capacity to collect, process, and present endogenous and exogenous antigens to lymphocytes, thereby promoting adaptive responses in the lung. IMs populate lung interstitial spaces (Figure 1A) where they act as phagocytes (Fathi et al., 2001; Bedoret et al., 2009), present antigen, and moderate wound repair. Like resident AMs, IMs have numerous immune-modulatory functions and produce IL10, TNFɑ, IL6, and IL1 at steady state (Hoppstädter et al., 2010; Wizemann and Laskin, 2012; Kawano et al., 2016). During acute pulmonary inflammation, circulating monocytes are recruited from the periphery, differentiate into IMs, and are important for reducing inflammation through continued production of IL10 in response to PAMPs. Lastly, macrophages are also implicated in inflammation-mediated lung dysfunction during coronavirus infection. Indeed, an inflammatory monocyte-macrophage (IMM) population was found to significantly contribute to immune-mediated lung pathology during SARS-CoV infection in mice (Channappanavar et al., 2016), while “hyperactivated” inflammatory macrophages contribute to coagulation associated with severe COVID-19 (Merad and Martin, 2020).
Myeloid-derived suppressor cells (MDSCs) are an important monocyte subset recruited from the periphery into the airways during infection (Koushki et al., 2021). MDSCs are heterologous immunosuppressor cells of myeloid origin distinguished in humans by expression of CD34+ CD11b+ CD33+ human leukocyte antigen (HLA)-DR− and categorized as either CD14− CD15+ granulocytic (PMN/G-MDSC) or CD14+ monocytic (M-MDSC) (Bergenfelz and Leandersson, 2020). Depending on the subset, MDSCs are capable of producing arginase-I, reactive oxygen, and nitrogen species and, as their name suggests, are involved in establishing an immune-suppressed local environment through production of IL10 and TGFβ and recruitment of Treg cells (Koushki et al., 2021). MDSC function can have pleiotropic effects on other respiratory immune cells, including downregulation of IL12 signaling in macrophages leading to a suppressive phenotype, reducing NK cell cytotoxic functions and development, and reducing adaptive lymphocyte recruitment and response via stymied DC development (Koushki et al., 2021). MDSCs have also been implicated in promoting respiratory virus disease progression. In a 2021 study of patients with a range of influenza and COVID-19 disease severities, circulating (not respiratory-tissue-associated) M-MDSCs were strongly correlated with severe illness, ICU admission, and poor patient outcomes (Falck-Jones et al., 2021). The authors observed that M-MDSCs sampled from COVID-19 patients suppressed T cell proliferation and IFNγ production through an arginase-I-dependent mechanism, likely leading to suppression and delay of T cell responses during active infection (Falck-Jones et al., 2021). Due to this strong association with severe COVID-19, others have proposed the use of circulating MDSC levels as a biomarker delineating high-risk patients and a possible therapeutic target to reactivate suppressed T cell responses in patients battling severe respiratory viral infection (Rowlands et al., 2021).
Dendritic cells in the airways are long-lived respiratory immune cells that can persist up to 8 weeks presenting antigen (Julia et al., 2002; Ginhoux et al., 2009; Kopf et al., 2014) but over time require replacement from the bone marrow. As professional APCs, DCs sample antigen from the airway lumen through transepithelial projections, then traffic to draining lymph nodes (LNs) in the NALT and BALT to coordinate the training and expansion of naive T cells (Hemann et al., 2016; Kohli et al., 2016) (Figure 1D). Pulmonary DC heterogeneity is a growing area of research, as unique DC subsets have a high degree of importance in coordinating adaptive responses to lung infection and vaccination, as well as acute inflammation. Three groups of pulmonary DCs are widely recognized: plasmacytoid DCs (pDCs) and two monocyte-derived conventional subsets, cDC1 and cDC2. pDCs are rapid and effective mediators of antiviral responses, producing the majority of IFN-I during respiratory viral infections (Rahmatpanah et al., 2018). While all DCs can transport antigen to draining LNs, pDCs are known to be poor T cell primers, and thus the major function of these cells is to establish a pulmonary antiviral state. A recent study by Severa et al. highlights the importance of pDC heterogeneity in COVID-19, reporting distinct pDC phenotypes and IFN-I responses, which differed between asymptomatic and severe SARS-CoV-2 infections (Severa et al., 2021). Asymptomatic COVID-19 was associated with pDC surface expression of PD-L1, antiviral ISGs, and high serum levels of IFN-I, while severe cases were associated with a low frequency of circulating pDCs and increased serum levels of pro-inflammatory cytokines. Further, the authors observed enhanced neuropilin-1-dependent antiviral responses in airway epithelial cells following release of IFN-I from pDCs (Severa et al., 2021). In the alveolar spaces, cDC1s, distinguished by CD103+ expression, sample the alveolar space and interact with epithelial cells via engagement of E-cadherins by CD103. cDC1 cells are the predominant APC involved in antigen trafficking and priming (and cross-priming) of adaptive T cell responses in the draining LNs. Further, like pDCs, these cells are also important mediators of viral response and have been shown to direct CD8 T cell responses during human IAV infection (Hemann et al., 2019). The cDC2 subset is defined by CD103-CD11b+ expression and resides in the lung interstitium (Vroman et al., 2017). These cells have been shown to help establish long-lived memory T cell subsets in the lung following influenza infection in the lung (Kim et al., 2014).
Innate respiratory response: Vaccination and recall
Widespread vaccination remains the best means of preventing severe pathology and transmission of infectious diseases including prominent respiratory pathogens. In principle, an effective vaccine must first trigger innate immune responses sufficient to train adaptive, antigen-specific T and B cell responses, which, depending on the vaccine platform, can elicit systemic or localized immunologic memory (Clem, 2011). Vaccine types are broadly categorized into replicating and non-replicating formulations. Replicating, or live-attenuated (LAV), vaccines mimic a natural infection and therefore trigger classical acute inflammation and immune detection through the natural production of PAMPs. Examples of these vaccines include the live-attenuated influenza vaccine (LAIV) FluMist; measles, mumps, rubella, and varicella (MMRV ProQuad); and the Bacillus Calmette-Guérin for tuberculosis. Protocols have also been proposed for generation of LAVs against coronavirus (Deng et al., 2017, 2019; Wang et al., 2021). Non-replicating vaccines, on the other hand, contain natural pathogen constituents and include subunit (hepatitis B), split-inactivated (influenza), toxoid (tetanus), and conjugate (DTap) vaccine preparations that weakly stimulate local activation at the site of introduction. These vaccine types typically require the addition of an adjuvant, a vaccine component designed to stimulate innate immune responses, discussed below. Innate immune responses following vaccine-generated PAMPs or adjuvants operate in much the same manner as in response to a respiratory pathogen to promote recruitment of DCs, enhance antigen collection, and train adaptive memory responses (Figure 2). Thus, the composition, duration, and strength of cytokine and chemokine production as well as co-stimulatory marker expression from innate APCs following vaccination can significantly affect the quality and quantity of T and B cell responses (Kang and Compans, 2009). Kulkarni and colleagues evaluated the importance of RIG-I, an innate cytosolic PRR detecting viral dsRNA, signaling during influenza vaccination (Kulkarni et al., 2014). After co-administering the RIG-I ligand 5′ppp-dsRNA with influenza vaccine antigens, the authors found that these mice developed robust levels of anti-HA antibodies (exhibiting enhanced antibody affinity maturation), increased germinal center (GC) reactions, increased T follicular helper cell responses and conferred protective immunity against virus challenge (Kulkarni et al., 2014). Interestingly, activation of the RIG-I signaling pathway was also found to significantly reduce the concentration of influenza antigen required for optimal influenza-specific cellular and humoral responses, underscoring the importance of innate immune pathway activation in effective vaccine design (Kulkarni et al., 2014). Further, Kandasamy and colleagues showed that RIG-I signaling was also important for effective polyfunctional T cell responses in mice during influenza infection, which were dependent on downstream production of IFN-I (Kandasamy et al., 2016).
The use of adjuvants in respiratory virus vaccine design has a long history, with numerous safe and effective formulations that have been extensively reviewed (Pulendran et al., 2021). The adjuvants used in human vaccines are diverse and can trigger a range of innate and adaptive immune response types and intensities and, therefore, must be carefully tailored. Three classes of adjuvants are currently approved for use in humans and include aluminum salts (e.g., alum); oil-in-water emulsions, which often combine multiple adjuvant types (e.g., AS03, MF59); and lipid nanoparticles (LNPs; e.g., PEG-based LNP), which were recently approved for use in the Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2) COVID-19 vaccines (Chatzikleanthous et al., 2021; Hou et al., 2021; Teijaro and Farber, 2021). Much like traditional infection, these adjuvants engage innate PRRs to promote immune responses (Figure 2). For example, alum, one of the more classic adjuvants used in influenza vaccine design, engages the NLRP3/ASC1/NLR complex, activating NF-κB signaling and leading to TLR-independent antibody responses that require CD4 T cell help. Conversely, MF59, an emulsion of squalene, Span85, and Tween-80 also used in influenza vaccines, recruits innate immune cells and promotes CD4 T cell-independent anti-influenza antibody production (Ko and Kang, 2018). In the past several years, the SARS-CoV-2 pandemic has necessitated the development and clinical evaluation of multiple vaccine platforms with and without the use of traditional adjuvants. Two examples are the adenoviral vector (AdV)-based DNA vaccines (J&J) and the LNP-mRNA (Moderna; Pfizer/BioNTech) vaccines, which trigger unique innate pathways. The goal of both vaccine types is to transfect and activate DCs to produce the encoded SARS-CoV-2 spike (S) antigen while also presenting peptide epitopes to CD4 and CD8 T cells driving cellular and humoral adaptive responses in parallel. The AdV-based vaccines encapsulate dsDNA coding for the S protein, which is delivered to the nucleus, transcribed into mRNA, and translated. This process inherently produces intermediates that trigger TLR9 and lead to production of IFN-I. In a similar way, mRNA vaccines are carried in a LNP specifically designed for uptake into DCs. While a proportion of delivered mRNA is directly translated into full-length S, exogenous mRNA (via TLR7) and cytosolic mRNA (via MDA5) trigger strong IFN-I production (Figure 2). With both platforms, S protein is produced within DCs and therefore can be processed and presented by MHC-I and MHC-II to engage CD8 and CD4 T cells, respectively (Sprent and King, 2021; Teijaro and Farber, 2021).
Of particular importance in immunization against respiratory pathogens is the site at which the vaccine is administered, whether that be intramuscular, intradermal, epicutaneous, or mucosal. However, designing effective vaccines that stimulate robust and protective immune responses in the respiratory mucosa has been an ongoing challenge. As a result, the majority of vaccines licensed for influenza and SARS-CoV-2, with the exception of the LAIVs, are delivered distally and rely on systemic innate and adaptive immunity, which may not be sufficient for protection at mucosal sites. Indeed, studies of consenting human volunteers experimentally challenged with A/California/2009 (H1N1) influenza were found to be protected from more severe disease by pre-existing mucosal IgA rather than systemic IgG (Gould et al., 2017), suggesting that antibody titers in the periphery are not always robust correlates of protection. Thus, there exists an immunologic disconnect between some current vaccinations delivered systemically and protection afforded at mucosal barriers. Several recent studies have begun to explore the benefits and caveats to local (intranasal) and systemic vaccine delivery. One such group compared routes of immunization with modified vaccinia Ankara (MVA), an approved vaccination against smallpox, and found that MVA viral vectors delivered by epicutaneous scarification protected mice more efficiently (lower dose) against lethal challenge with vaccinia virus than intradermal, subcutaneous, or intramuscular routes (Pan et al., 2021). Intriguingly, immunization by epicutaneous scarification generated CD8+ TRM cells that were as abundant in the lungs and have transcriptional profiles overlapping those of TRM cells generated following direct intratracheal delivery, suggesting that MVA vectors delivered via scarification are promising and efficient vaccine vectors for respiratory pathogens (Pan et al., 2021). The failure of the other non-mucosal MVA vaccine routes to stimulate effective airway immunity is representative of the disconnect between these strategies and robust mucosal immune protection. In a 2018 study, Li et al. also demonstrated that both vaccine route and type of adjuvant impact the phenotype of recruited innate cells (Li et al., 2018). While intranasal (IN) virus vaccination of mice prompted “classic” ILC2 development in the lung, the same immunization delivered intramuscularly resulted in recruitment of “inflammatory” ILC2s, which produced significantly more IL13 than IN-vaccine-derived ILC2s. IL13 production was responsible for influencing IFNγ, IL22, and IL17A expression in ILC1 and ILC3s and plays a critical role in shaping APC recruitment, activation, and subsequent B and T cell immunity (Li et al., 2018).
Direct IN vaccination against influenza has only been successfully achieved with LAIV, though these replicating vaccines cannot be delivered to all demographics, highlighting the need for improved split, protein, or subunit IN vaccination. Dong et al. recently demonstrated that the poor immunogenicity of IN-delivered protein-based influenza vaccines could be overcome by delivery of hemagglutinin (HA) within graphene oxide polyethyleneimine (GP) nanoparticles (Dong et al., 2021). Un-adjuvanted HA/GP nanoparticles induced innate immunity at comparable levels to CpG in mice and conferred protection against homo- and heterosubtypic influenza infection in mice (Dong et al., 2021). In another recent study investigating improved IN vaccine strategies, Varma and colleagues designed and tested a hydrogel laden with cGAMP adjuvant and a broadly active HA antigen developed using the computationally optimized broadly reactive antigen (COBRA) approach (Varma et al., 2022). The hydrogel was designed to increase the mucoadhesion and retention (>6 days) of the cGAMP adjuvant and, upon IN vaccination in a prime-boost schedule, resulted in significantly higher IgG and IgA levels when compared to more traditional methods (Varma et al., 2022). Due to the success of the LNP-based mRNA vaccines, ongoing studies into aerosolized LNP-based IN vaccines are also being conducted (Zhang et al., 2020).
While these mucosal vaccine approaches are promising, there is still significant ground to cover before any are licensed for administration in humans. The current lot of systemic vaccines are highly effective at reducing overall severe disease during respiratory infection; however, a drawback is that certain respiratory pathogens may still replicate in the airways for a time before IgG-mediated neutralization, thus affording space for transmission events. This is an important consideration with respect to the “goal” of vaccination, and tradeoffs are often made between reducing transmission events and reducing symptom severity. However, this may not be as significant an issue with mRNA vaccine platforms. In a 2021 study, Mades, Chellamathu, and Kojima et al. observed significant IgG titers in saliva and oral mucosa 15 days after the first dose of either Moderna or Pfizer/BioNTech COVID-19 vaccines (Mades et al., 2021). Other groups have also shown successful IN protection against SARS-CoV-2 in mice immunized with an AdV-based vaccine promoting both mucosal and systemic IgA production (Hassan et al., 2020). Further, a recent preprint describes broad mucosal protection against SARS-CoV-2 elicited following un-adjuvanted IN spike booster vaccination, providing new incentives to invest in a possible hybrid (systemic and mucosal) vaccine approach to close the gap between systemic and mucosal responses (Mao et al., 2022).
Innate respiratory response: Trained immunity
The exposure of the innate immune system to pathogens within the context of infection or immunization can lead to development of a specialized innate memory-like state termed trained immunity. Trained immunity is characterized by antigen-agnostic increases in innate immune responsiveness against secondary infection, which can provide broad protection against heterotypic infections (Netea et al., 2020). Trained immunity is distinct from tolerance, as trained immune cells undergo programmatic “priming” resulting in enhanced effector functions, while with tolerance, programmatic changes result in suppressed functions (Chumakov et al., 2021) (Figures 3D–3F). During trained immunity, these programmatic changes are coordinated along a metabolic-epigenetic axis. During the first challenge, PRRs are engaged and trigger the upregulation of metabolic pathways including glycolysis, TCA cycle, and fatty acid metabolism, the products of which alter the epigenetic landscape of chromatin in gene regions important for innate immune responses. While trained immunity typically lasts 6 months to 5 years, recent studies on epigenetic and metabolic profiling suggest these effects could be trans-generational (Netea et al., 2020). Several specific pathways underlying these adaptive-like innate responses have been described for monocytes, DCs, NKs, and fibroblasts and have been reviewed elsewhere (Netea et al., 2016, 2020).
Long-term trained immunity and heterologous protection against subsequent infections are also afforded by immunization with whole-organism or replicating LAVs. Numerous studies have reported and characterized the development of trained immunity following Bacillus Calmette-Guérin (BCG), measles, smallpox, and oral polio vaccination. The initiation, functional immune consequences, and innate “priming” observed in myeloid, NK, and DC populations following immunization have been extensively defined and reflect those observed with post-infection trained immunity (Netea et al., 2016). Interestingly, several groups have shown that trained immunity arising from vaccination with LAVs may reduce susceptibility and severity of SARS-CoV-2 infection. Evidence from Rivas et al. suggests that healthcare workers recently vaccinated with Bacillus Calmette-Guérin had reduced SARS-CoV-2 seropositivity and, if infected, reduced symptoms and better outcomes, comparatively (Rivas et al., 2021). Additional studies from the Netherlands (Moorlag et al., 2020) and United Arab Emirates (Amirlak et al., 2021) corroborate this evidence, finding improved COVID-19 outcomes with recent Bacillus Calmette-Guérin vaccination. Thus, the robust and long-term humoral and T cell correlates of protection afforded to us by vaccination against SARS-CoV-2 and newly emerging variants (Tarke et al., 2022) may synergize with trained innate immunity to more rapidly clear SARS-CoV-2 infections and reduce symptoms.
Adaptive respiratory immune response: Priming antigen-specific immunity
While antigen-agnostic innate immune responses are triggered within minutes to hours following detection of extracellular or cytosolic PAMPs derived from infection or vaccination, the adaptive immune response is highly antigen specific and takes several days to weeks to develop (Figure 3). These responses include CD8+ T cells, which are responsible for direct killing of infected cells in the lung, CD4 T cells, which drive additional activation of innate and adaptive cytolytic immunity (Th1) or B cell responses (Th2), and B cells, which are responsible for production of antibodies to neutralize pathogens or promote additional function responses in innate cells. Other regulatory T cell subsets also function in the respiratory tract including γδ T cells and Treg cells and work to return the airways to a more tolerant space. Following an initial antigen-specificity training phase, long-lived adaptive responses also form, with long-lived memory T and B cells establishing residency in lung tissue niches. Critically, antigens produced by subsequent infections that are recognized by long-lived memory T and B cells prompt a much more rapid (hours to days) adaptive response to clear infections, oftentimes in parallel with innate responses—a basic set of principles underlying nearly all current vaccination strategies.
Priming adaptive, antigen-specific immune responses during active respiratory infection or post-vaccination occurs in draining LNs housed in the NALT and BALT (Figures 1D, 3C, and 3I). In addition to the draining LNs, evidence suggests that T cells are also primed and expanded in the bone marrow and spleen during intermediate and late stages of respiratory infection. An example of these T cell training and response dynamics was demonstrated by Wu et al. using a combination of mathematical models of multicompartment CD8 T cell trafficking and murine influenza (A/X31/H3N2) infection (Wu et al., 2011). Recently, Jenkins et al. described a pathway for murine mDCs, which traffic from the lungs of influenza-infected mice to draining LNs and can re-enter circulation and localize in the spleen (Jenkins et al., 2021). Here, these mDCs activate CD8 T cell responses, which, intriguingly, were found to be longer lived than traditional LN-primed CD8 T cells. While it remains unknown how conserved this mechanism is and if this type of T cell priming occurs in humans, it is clear that differential activation and training of T cell responses during respiratory lung infection is exciting as new functional activities are described for T cells primed in unique niches.
Like T cells, antigen exposed B cells also migrate within the LNs and accumulate at the T-B cell zone border and form antigen-specific cognate pairs with activated CD4+ Th2 cells. A subset of these B cells differentiate into short-lived plasma cells (Schwickert et al., 2011; Shulman et al., 2013), while others enter specialized B cell maturation regions in the LNs termed GCs (Coffey et al., 2009; Pereira et al., 2010) (Figures 1D and 3I-III). B cells expressing high-affinity immunoglobulin variants are selected for clonal expansion and differentiation into long-lived plasma or memory B cell subsets by T follicular helper (Tfh) cells, shown to be necessary for sustained GC reactions and development of long-lived memory B cells (Crotty, 2011; Qi, 2016). Recent studies sampling draining LN sites from participants immunized with Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine demonstrated that antigen presentation in GCs occurs over months and is likely a result of sustained and robust Tfh cell responses (Turner et al., 2020, 2021; Kim et al., 2022; Mudd et al., 2022). In another study, expanded public TCRs from circulating Tfh (cTfh) clonotypes were found in patients recovering from mild COVID-19 symptoms (Lu et al., 2021). The corresponding SARS-CoV-2 spike epitope was conserved across variants, presented by multiple HLAs. The SARS-CoV-2 epitope also activated these public cTfh clonotypes in both patients with mild symptoms and healthy donors, suggesting a more universal nature, which may be an important component of vaccines specifically eliciting T cell responses (Lu et al., 2021). Sustained antigen presentation, however, is not conserved following all vaccinations or vaccine types. It has long been appreciated that while infection by influenza virus induces robust and long-lived humoral responses, seasonal influenza vaccination strategies have largely failed in this regard. Devarajan and colleagues speculated that this could be due to differences in antigen availability and duration of Tfh responses in the LNs between influenza infection and vaccination with inactivated vaccines (Devarajan et al., 2022). Their 2022 study found that strong influenza-induced Tfh responses require CD4 effectors to continually recognize local antigens and receive signals from ongoing infection (Devarajan et al., 2022). GC reactions stalled with inactivated vaccination but could be prolonged with the administration of LAIV. This apparent requirement for continued antigen presence on the maintenance of GC reactions is likely an evolutionary tolerance feature to prevent over-activation and development of autoimmunity. Further, several studies have also described generation of T cell-independent antibody responses, suggesting a sort of immunologic redundancy in the development of these critical humoral responses. One such study showed that Tfh cells, enhanced by IL2 signaling early in life, mediated high-affinity antibody production required for protection against RSV; however, these cells were dispensable during primary influenza infection and recall responses (Pyle et al., 2021). This suggests that, in certain circumstances such as influenza re-infection, TRM cells and Tfh/T cell-independent antibody responses may be sufficient for protection and compensate for a lack of robust Tfh activity in GCs during recall responses (Lee et al., 2005; Wu et al., 2014; Miyauchi et al., 2016).
Once trained, effector T and B cells exit the LN via efferent lymphatics and re-enter circulation. In response to a chemotactic gradient produced by airway endothelial and innate immune cells, these lymphocytes traffic back to the site of infection where they accumulate in the airway parenchyma and mediate various antigen-specific functions described below.
Adaptive respiratory immune response: T lymphocytes
T cells play critical roles in the immune response to respiratory infection through multiple functional activities including innate and adaptive immune cell activation, environment-dependent pro-inflammatory and immune-modulatory cytokine production, antigen-specific killing of infected cells, training humoral responses, and long-term immune memory, surveillance, and re-activation potential. T cell responses in the lung can be temporally divided into the effector responses mounted during acute infection and long-lived memory T cells maintained in the lungs and circulation thereafter.
Short-lived, cytotoxic CD8+ T cells recognize class I MHC-bound peptide (pMHC) and mediate targeted killing of infected cells through secretion of cytolytic enzymes. Dysregulation of these cells during infection can lead to lung damage, as was observed in a recent preprint by Guo et al., demonstrating that overproduction of CXCR3 drives CD8+ T cell-dependent lung pathology during influenza infection (Guo et al., 2022). In other contexts, CD8+ T cells are also important producers of anti-inflammatory cytokines such as IL10. Additional cytotoxic contributions are made by CD4+ T cell subsets derived from naive and central memory clones that acquire cytotoxic functions (Teijaro et al., 2011a; Strutt et al., 2013). Traditional CD4+ T cell responses can be divided into several “helper” lineages depending on the cytokine environment a naive T cell is exposed to. Th1 are potent responders to intracellular lung infection, activate resident macrophages, and secrete IFNγ and IL2. Th2 cells produce numerous cytokines, including IL4, IL5, IL9, IL10, and IL13, to regulate inflammation in the airways. Tfh cells specialize in B cell training in LNs and help establish humoral responses to infection. A newly described lung-resident CD4, termed T resident helper (TRH) cells, were identified following influenza infection in mice and function similarly to both TRM and Tfh cells, but operate locally to promote humoral responses in the lung (Schattgen and Thomas, 2021; Son et al., 2021; Swarnalekha et al., 2021). CD4+/FOXP3+ Tregs, recruited during late stages of pulmonary inflammation, are important mediators of airway tolerance through production of IL10 and TGFβ (Chen and Kolls, 2013). Tregs also promote tissue repair through secretion of amphiregulin, inducing differentiation of type I and type II pneumocytes and blocking fibrocyte recruitment and proliferation (D’Alessio et al., 2009).
During the later stages of respiratory immune responses, long-lived memory T cells begin to develop and emigrate to various T cell compartments over decades (Thome et al., 2014). Following both infection and vaccination, subsets of CD4+ and CD8+ T cells in the airways transition into CD69+ CD103+, long-lived, non-circulating resident adaptive immune cells termed TRM cells. These cells are stable in mucosal sites throughout life (Masopust and Soerens, 2019; Szabo et al., 2019) and are phenotypically and transcriptionally distinct from other T effector memory populations expressing a core transcriptional signature that includes upregulation of integrin and chemokine receptors, increased immunomodulatory signatures, and reduced tissue egress markers (Purwar et al., 2011; Hombrink et al., 2016; Mackay et al., 2016; Kumar et al., 2017; Oja et al., 2017). As resident adaptive immune cells, TRM cells have numerous functions in the lung and airways. In mice, CD4+ TRM cells dominate immune surveillance in the airways and adopt shared transcriptional, phenotypic, and functional properties with CD8+ T cells, initiating memory recall responses through localized cytokine expression and activation of mucosal cellular immunity (Beura et al., 2019). TRM cells have been shown to be important for mediating post-vaccination and post-infection cross-protection against heterosubtypic influenza strains (Turner et al., 2013; Wu et al., 2014; Kumar et al., 2018). Importantly, Zens et al. report that TRM cells were only observed in airway mucosa following IN immunization with LAIV, but not with split-inactivated preparations, suggesting that the development of these cells is highly dependent on strong mucosal immune responses (Zens et al., 2016).
T cell responses in the lung have been an important area of research during the SARS-CoV-2 pandemic and have been reviewed extensively (Grifoni et al., 2020; Bertoletti et al., 2021; Kedzierska and Thomas, 2022). In a 2021 study, Grau-Expósito et al. identified associations of both peripheral and lung-resident virus-specific T cells with clinical outcomes during acute infection (Grau-Expósito et al., 2021). Severity was associated with increased IFNγ and IL4 production and apoptosis in T cells, while SARS-CoV-2-specific T cells from non-hospitalized participants had increased expression of CCR7, IL10, IL12p70, and degranulation markers. These authors also show that SARS-CoV-2-specific TRM cells are established in the airways for at least 10 months post-infection (Grau-Expósito et al., 2021). Interestingly, Niessl and colleagues determined that 32% of donors across all age groups sampled prior to the outbreak of SARS-CoV-2 had pre-existing tonsillar populations of CD8+ TRM cells that cross-reacted with SARS-CoV-2 antigens (Niessl et al., 2021). Further, a pre-existing population of T cells with cross-reactivity to SARS-CoV-2 replication-transcription complex (RTC) antigens was also identified among healthcare workers with high exposure risk yet remained consistently PCR- and seronegative, showed no clinical signs of disease, and had indicators of abortive SARS-CoV-2 infection (Swadling et al., 2021). These data suggest that pre-existing multispecific memory T cell responses are likely drawing on previous exposures to seasonal coronaviruses and may be important determinants of SARS-CoV-2 disease progression. Other groups have shown that de novo TRM responses initiated following SARS-CoV-2 infection are associated with reduced severity. Indeed, Szabo et al. used scRNA-seq and flow cytometry to generate high-dimensional immune profiles of COVID-19 in patients across age and severity groups (Szabo et al., 2021). This group found that young participants with asymptomatic disease had an increased TRM profile compared to older participants with more severe disease. Severity, inflammation, and persistence were associated with lower T cell responses and enhanced inflammatory monocyte signatures, highlighting the importance of T cell responses (Szabo et al., 2021). Further, using longitudinal sampling of a small set of patients across the entire course of COVID-19 disease progression, Tan et al. found early induction of SARS-CoV-2-specific T cells associated with mild disease and viral clearance, which they argue could be a prognostic factor in assigning disease severity risk (Tan et al., 2021).
The specificity component of the adaptive T cell response is ascribed to the surface-expressed TCR, which engages the pMHC. The TCR repertoire refers to the composition and TCR clonal diversity generated within a population of T cells responding to a particular pathogen. Studies have shown that TCRs associating with the same antigen tend to have similar amino acid sequences within the major peptide recognition loop (Dash et al., 2017) and, further, that this similarity can be used as a measure to determine overall TCR diversity within a repertoire. Building from these concepts, several groups have found that polyfunctional T cell responses with high TCR repertoire diversity correlate with more favorable respiratory disease outcomes, especially during viral infections (Kedzierska et al., 2006; van Gisbergen et al., 2011; Wang et al., 2012; Wilkinson et al., 2012; Sridhar et al., 2013; Cukalac et al., 2014; Pizzolla et al., 2018; Sant et al., 2018; Koutsakos et al., 2019). In one such study, authors captured and sequenced the polyclonal TCR repertoire of recently activated CD8+ T cells responding to A/H7N9 influenza infection, comparing the repertoires of recovered adult patients to those with fatal outcomes, and found significant variation in diversity over time between patient groups (Wang et al., 2018). The authors conclude that patient recovery was associated with the early clonal expansion of a few optimal, and likely antigen-specific, T cell clones, while fatal outcomes were characterized by a delay in CD8+ clonal expansion and diversity (Wang et al., 2018). Investigating TCR diversity has also been an important avenue in understanding the T cell responses during SARS-CoV-2 infection and following vaccination. In a recent study, Minervina and Pogorelyy et al. sought to determine the effects of diverse and repeated antigen exposures on SARS-CoV-2 memory T cell subsets within a cohort of adults receiving two doses of the Pfizer BNT162b2 mRNA COVID-19 vaccine, natural infection, or breakthrough infection (Minervina et al., 2022). Using antigen-specific scRNA and scTCR sequencing of SARS-CoV-2-specific T cells, the authors determined that the order of antigen exposure determined the relative distribution of SARS-CoV-2-spike- and non-spike-specific responses. While post-infection vaccination led to the largest expansion of spike-specific and effector T cells, TCR repertoires analyzed from breakthrough participants comprised mainly non-spike responses. Importantly, all antigen exposure types elicited diverse TCR repertoires without repertoire narrowing from repeat exposures, suggesting that the current, multiple-dose and booster mRNA vaccine regimens are not negatively impacting our ability to adequately respond to diverse SARS-CoV-2 variants of concern (VOCs) (Minervina et al., 2022). Thus, understanding changes in TCR repertoire diversity following infection or vaccination is essential for determining adaptive immune efficiency and T cell response potency and is a viable metric for evaluating vaccine efficacy and post-infection response.
Adaptive respiratory immune response: B lymphocytes
The other arm of the adaptive immune response in the airways comprise B cell and humoral (antibody) responses. During primary infection or vaccine introduction, short-lived plasma cells secrete low-affinity IgG and IgA in tissue sites while GC-trained, affinity-matured plasma cells localize late during infection to secrete high-affinity Ig. IgA is more ubiquitous in the respiratory mucosa; however, both IgA and IgG have been shown to clear invading respiratory pathogens through opsonophagocytic activation and direct or cross-neutralization of homosubtypic and heterosubtypic influenza strains (Rangel-Moreno et al., 2008; Onodera et al., 2012; Adachi et al., 2015; Allie et al., 2018; Barker et al., 2021). The Fc portion of various IgG subtypes can also trigger cellular effector functions that drive antibody-mediated immune functions. In humans, a striking amount of heterogeneity has been observed in Fc domain structural repertoires (Wang et al., 2015; Wang et al., 2017b), which is of particular relevance in the context of influenza virus infection and vaccination. Anti-HA IgG effector functions can modulate influenza vaccine efficacy (Wang et al., 2015; Maamary et al., 2017) as well as the protective potential of broadly reactive anti-hemagglutinin (HA) and anti-neuraminidase (NA) IgGs (DiLillo et al., 2014, 2016). Recent serosurvey studies in adults have shown that influenza vaccination induces specific modulations in post-translational modifications of the Fc, resulting in diverse glycoform profiles, and in IgG subclass distributions among vaccine-elicited IgGs (Wang et al., 2015). These results suggest that anti-HA effector IgG functional profiles can be regulated by vaccination in adults. During SARS-CoV-2 infection, a form of immunologic imprinting has also been described in association with the so-called “hybrid” immunity generated following exposure to infection and subsequent vaccination. Here, Rodda et al. determined that previously infected individuals generated more SARS-CoV-2-specific memory B cells, neutralizing antibodies, and a distinctive population of S-specific CD4 T cells expressing IFNγ and IL10 after vaccination than naïve-vaccinated individuals (Rodda et al., 2022). Interestingly, additional vaccinations did not elicit these same CD4 T cell profiles, suggesting that immune imprinting may underlie the unique immune signatures associated with hybrid immunity to SARS-CoV-2 (Rodda et al., 2022).
During the resolution phase of acute pulmonary inflammation, subsets of B cells differentiate into resting memory cells or, like their T cell counterparts, take up residence in the respiratory tract as tissue-resident memory B (BRM) cells. In a 2012 study, Onodera et al. first characterized BRM populations as virus-binding B cells that accumulated and persisted in the lungs following murine influenza infection (Onodera et al., 2012). Indeed, human lungs are known to be enriched with antigen-specific BRM cells (Barker et al., 2021) that are phenotypically and functionally distinct from other long-lived memory B cells. In a 2018 study, Allie et al. determined that IgM+ BRM cells in the lung required antigen for development and early engagement of CD40L expressed by T cells, and these BRM cells were ultimately responsible for early isotype-switched plasma cell (IgA and IgG) responses during secondary influenza challenge (Allie et al., 2018). Importantly, only influenza antigen-specific BRM cells were activated, highlighting antigen-specific responses at mucosal sites (Allie et al., 2018). A recent study by Poon and colleagues found direct evidence for SARS-CoV-2 antigen-specific CD69+ BRM formation and retention in human lungs and LN, which exhibit a profile distinct from that of circulating memory B cells (Poon et al., 2021). It remains to be seen if these cells form following intramuscular immunization against SARS-CoV-2; however, the presence of these cells following infection is encouraging evidence for robust, long-term mucosal protection against lineage-proximal SARS-CoV-2 infection. Together, airway BRM populations participate in protective humoral responses to pulmonary infections with known pathogens and are important mediators of airway immune surveillance.
Immune pathology associated with dysregulated inflammation
The global burden of morbidity and mortality associated with dysregulated immune responses in the respiratory tract is vast. These inflammatory conditions are prompted by various triggers and may lead to airway tissue damage, fibrosis, and long-term sequelae resulting in reduced capacity of the lungs to effectively mediate gas exchange. In addition to acute respiratory distress syndrome discussed below, chronic obstructive pulmonary disease (COPD), asthma, and allergy responses are also pervasive, impacting hundreds of millions of people each year; however, these important immune-mediated pulmonary complications fall outside the scope of this review.
ARDS is a hypoxic pulmonary syndrome and one of the most common pathologic outcomes of dysregulated inflammation in the lungs and airways. One global study of >29,000 patients observed that 10% of all patients admitted to the ICU and 23% of patients requiring mechanical ventilation were diagnosed with ARDS, with mortality rates reaching 46% in severe cases (Bellani et al., 2016). Patient survival was associated with long-term cognitive and musculoskeletal sequelae, underscoring the destructive nature of this inflammatory disease (Herridge et al., 2011, 2016). ARDS is commonly triggered by pneumonia arising from viral, bacterial, fungal, or opportunistic infection, and oftentimes, two or more agents co-present. ARDS has also been observed due to indirect means including non-pulmonary sepsis, substantial burn injury, or medical interventions (Thompson et al., 2017). The disease progresses through three phases. The exudative phase in the initial injury response is triggered by alveolar epithelial damage in the lung. Resident AMs sense this damage and produce inflammatory mediators recruiting neutrophils, monocytes, and effector T cells. Inflammation and tissue damage lead to barrier disruption, accumulation of alveolar fluids, and microvascular damage—all exacerbated by the additional mechanical stress of labored breathing. While the mechanisms behind aberrant fluid influx and accumulation are not fully understood, one study in mice found that dysregulated pulmonary fluid homeostasis during respiratory IAV infection was mediated by reduced function in epithelial sodium channels (ENaC) and CF transmembrane regulators (CFTR) in the alveoli (Leroy et al., 2006; Brand et al., 2018). Typical biomarkers of ARDS include systemic inflammation cytokines IL6 and IL8, elevated protein C (dysregulated coagulation), and markers of epithelial and endothelial damage such as SP-D and angiopoietin. In the proliferative phase, there is a shift from type I (“resistance”: CD4 Th1, neutrophils, classical macrophages) to type II (“repair”: TNFα, IL4, IL5, IL9, and IL13) immune responses. Fibroblasts expand and a provisional ECM is formed to restore structure to the alveoli and clear the edema. Type II immunity is normally associated with tissue repair but may also initiate fibrosis, the third phase of ARDS (Gieseck et al., 2017). During the fibrosis phase, epithelial cell damage and release of DAMPs prevents tissue repair, leading to fibrosis. Recent studies of human and murine influenza infection have also identified damage-associated fibroblast populations, which may exacerbate inflammation through expression of ECM proteases and degradation of versican (Boyd et al., 2020). Large genomic studies have been performed to identify genes associated with development of ARDS, as not all patients with risk factors progress to acute respiratory distress. Associations with ARDS were found with IL6, IL10, TNFα, VEGF, ACE, and 30 additional genes comprising responses to inflammation and endothelial or epithelial injury (Meyer and Christie, 2013).
Understanding the role that dysregulated inflammation and ARDS play in the pathology of multiple respiratory disease states is critical to prevention, intervention, and mitigation of adverse events. The reported associations between ACE and ARDS are particularly interesting in the context of coronavirus infection, as ACE2, a homolog of ACE that functions as a negative regulator of the renin-angiotensin system (RAS) and can protect against severe lung failure (Imai et al., 2005), serves as the cellular receptor for both SARS-CoV and SARS-CoV-2, viral agents known to cause significant lung damage and acute respiratory distress, which suggests that viral disruption of RAS may also directly contribute to ARDS (Li et al., 2003; Gu and Korteweg, 2007; Harrison et al., 2020; Hoffmann et al., 2020). Indeed, during SARS-CoV infection, lung injury can be experimentally attenuated by blocking the RAS pathway, which may be a possible therapeutic target (Imai et al., 2005; Kuba et al., 2005). More recently, a mechanism by which ACE2 downregulation leads to dysregulation in the renin-angiotensin-aldosterone system (RAAS), inflammation, and ARDS during SARS-CoV-2 infection was described. Briefly, virion attachment and budding reduces ACE2 at the cell surface, causing decreased cleavage of angiotensin I/II and a subsequent increase in vascular permeability and vasoconstriction. Acute inflammation is a secondary consequence of SARS-CoV-2-mediated epithelial damage triggering platelet accumulation and localized release of NETs, clotting, and coagulation factors, which have recently been shown to cleave spike and increase entry of SARS-CoV-2 (Kastenhuber et al., 2022). Together, inflammation and clotting lead to the significant vascular and pulmonary pathophysiology associated with COVID-19 (Gupta et al., 2020; Merad and Martin, 2020). In addition to these physiologic disruptions to the RAAS, several groups provide compelling evidence that SARS-CoV-2 infection in the alveolar space causes an inflammatory pneumonia immunologically distinct from other types of ARDS. Using high-dimensional cellular and transcriptional profiling of BALF sampled from patients with COVID-19, Grant et al. identified a unique enrichment of inflammatory monocytes and T cells (Grant et al., 2021). SARS-CoV-2-infected AMs released chemotactic factors to recruit IFNγ-producing T cells, which in turn enhanced pro-inflammatory transcriptional profiles in the AMs (Grant et al., 2021). In a similar study, Liao and colleagues also uncovered an association between COVID-19 severity and inflammatory monocytes and, further, characterized disease-associated CD8 T cell infiltrates in the BALF as less expanded, more proliferative, and more heterogeneous than CD8 T cells sampled from moderate COVID-19 cases (Liao et al., 2020). Cytokine storm syndrome (CSS) is often used to describe the dysregulation and positive feedback of pro-inflammatory cytokines mediating immunopathology in the lung during infections with SARS-CoV-2 or other respiratory pathogens (Huang et al., 2020; Karki and Kanneganti, 2021). Investigators treating mice with TNFα and IFNγ observed synergistic activities between these two cytokines leading to PANoptosis, an inflammatory cell death pathway that perpetuates and phenocopies the CSS observed in patients with COVID-19 (Karki et al., 2021). However, development of true CSS may be more limited in human SARS-CoV-2 infection than initially expected—restricted to a subset of severe cases. A 2020 study comparing influenza and SARS-CoV-2 infections in hospitalized patients found that patients with COVID-19 exhibited lower overall cytokine levels than patients with influenza. While increased levels of IL6, G-CSF, IL1RA, and MCP1 predicted poor outcomes in COVID-19, levels of these pro-inflammatory factors were not significantly higher than patients with influenza (Mudd et al., 2020). Further, suppression of IFN-I signaling among patients with COVID-19 was observed by single-cell gene-expression analysis, leading the authors to conclude that patients with COVID-19 are less inflamed than patients infected with influenza (Mudd et al., 2020). Previous studies of large adult human cohorts found that severe influenza infection was characterized by increased numbers of CD16+ monocytes and virus-specific CD8+ T cells, reduced Th2 and hematopoiesis markers, and increased pro-inflammatory, chemotactic cytokines and tissue repair factors without apparent CSS (Wong et al., 2018). This suggests that additional factors, possibly health-associated, age-associated, or genetic, may be precursors to CSS observed in severe COVID-19.
As discussed above, robust adaptive humoral and T cell responses often help to balance innate inflammation during later stages of respiratory infection. However, in the case of COVID-19, these adaptive responses have also been shown exacerbate disease severity and contribute to post-acute sequelae of SARS-CoV-2 (PASC), referred to colloquially as “long COVID.” Several groups have begun investigating the long-term consequences and mechanistic basis of acute and PASC. Recent studies by Vijayakumar, Boustani, Ogger, Papadaki and colleagues showed significant dysregulation of lung immune-proteomic profiles in SARS-CoV-2 patients for at least 6 months after hospital discharge (Vijayakumar et al., 2022). Although these profiles resolved over a year’s time, significant markers of continued cellular apoptosis, tissue repair, and epithelial injury were reported, suggesting long-term consequences of acute SARS-CoV-2 infection (Vijayakumar et al., 2022). Development of sub-clinical autoantibodies may also underlie PASC (Bastard et al., 2020; Su et al., 2022), and a distinctive IgM and IgG3 signature has been proposed as a predictor of PASC development (Cervia et al., 2022). To study these effects, a recent preprint has proposed a model of PASC lung disease (using mouse-adapted SARS-CoV-2 MA10) for targeted therapeutic and antiviral identification and assessment (Dinnon et al., 2022). Further, several groups have been working to generate two-dimensional (human bronchoalveolar organoid) and three-dimensional (human alveolar stem cell) ex vivo model systems to investigate SARS-CoV-2 cellular replication characteristics and pathogenesis (Youk et al., 2020; Lamers et al., 2021).
Biologic factors impacting innate and adaptive immune responses in the respiratory tract
As discussed throughout this review, there is enormous complexity among the cellular and humoral immune responses within the airways following infection and vaccination, which requires a delicate balance between resistance and tolerance. The regulation, potency, and duration of these innate and adaptive facets, however, can be significantly impacted by underlying biological factors including age (Wu et al., 2021), biological sex (Ursin and Klein, 2021), race/ethnicity (Prata Menezes et al., 2021), host genetics (Patarčić et al., 2015; Casanova and Abel, 2021), pregnancy (Sappenfield et al., 2013; Liong et al., 2020), host microbiome (de Steenhuijsen Piters, Sanders and Bogaert, 2015; de Steenhuijsen Piters, Binkowska and Bogaert, 2020), chronic underlying conditions (Glezen et al., 2000; Honce and Schultz-Cherry, 2019), and many more (Mettelman and Thomas, 2021). As the lung is a kaleidoscope of heterogeneous cell subsets, each playing a specific and necessary role, it is critical to understand how these biological factors alter aspects of the pulmonary immune response. Here, we discuss a few pivotal studies to underscore the importance of considering these factors when studying airway immune responses.
Age is a well-known biological factor that dictates the immune response to respiratory infections. Importantly, the risk conferred by age is not consistent across all infections. Dynamics of certain respiratory viruses, like influenza, are complex, with a more bimodal distribution of severity affecting both the very young and the aged populations. Interestingly, SARS-CoV-2 targeted mostly the aged population and not the young (Preston et al., 2021), causing a significant burden of morbidity and mortality in the elderly (Pijls et al., 2021; Wu et al., 2021). Despite these being the general trends of age-dependent severity, there are exceptions, including the rare population of children infected by SARS-CoV-2 developing multisystem inflammatory syndrome in children (MIS-C) and other long-term consequences of mild pediatric infection. Unfortunately, these age-dependent mechanisms during infection are not fully explained, and there exists an almost complete void in our understanding of how immunity is established in infants and how immune repertoires evolve with each successive encounter. Recently, it was demonstrated that the antibody-mediated protection to A/H1N1pdm and A/H3N2 influenza strains significantly differed between children and adults, with protection against influenza illness correlating with antibodies to epitopes in the HA head in children but other sites of influenza in adults (Ranjeva et al., 2019). This difference in protection is due to antibodies reactivating most strongly to portions of the influenza virus that have been previously detected even during exposure to new strains, termed antigenic imprinting (Davenport et al., 1953, 1955; Cobey and Hensley, 2017; Henry et al., 2018), and responding most strongly to influenza strains that circulated during the individual’s childhood, termed antigenic seniority (Lessler et al., 2012), though both of these mechanisms require dedicated studies for further investigation. The study of antigenic imprinting is crucial to improving vaccination strategies to viruses like influenza virus and the pandemic SARS-CoV-2 virus (Feldstein et al., 2021; Wheatley et al., 2021; Mao et al., 2022). Many studies have begun to tease apart how exposures (both infection and vaccination) drive the success of a child’s immune response to pathogens, but there is so much left to be determined.
Many studies have investigated age-dependent differences in immune response to respiratory viruses and collectively describe variations in inflammatory states and mucosal T cell responses at sites of infection (Oshansky et al., 2014; Guo et al., 2018; Wong et al., 2021). These differences have been extensively reviewed (Chen et al., 2020; Bajaj et al., 2021; Mettelman and Thomas, 2021). Briefly, one study comparing the immune response to SARS-CoV-2 infection in children and adults demonstrated that children have significantly fewer T cells responding to SARS-CoV-2 infection, particularly IFNγ-producing CD8+ and CD4+ T cells, fewer inflammatory monocytes, and more activated Tfh responses (Cohen et al., 2021). Based on this study, children were characterized as having less immune activation at baseline compared to adults.
At the other end of the age spectrum is the aging and elderly population, which is known to be at high risk of more severe infection to multiple respiratory pathogens (van Wilgenburg et al., 2018) due to mechanisms like immunosenescence and inflammaging (Franceschi et al., 2000; Chen et al., 2020; Pietrobon et al., 2020; Fulop et al., 2021). Numerous studies have highlighted the potential mechanisms by which aging affects our ability to properly respond to infection and vaccination (Lanzer et al., 2014; Chen et al., 2020), with many groups working to improve upon current vaccination strategies for these high-risk populations. Several groups have accumulated evidence of respiratory virus-induced cellular senescence on immune subsets including normal human lung fibroblasts and epithelial cells, which are integral components of the host response to viral infection in the airways (Kohli et al., 2021). Some factors have been identified that contribute to differences in immune response specifically in aged populations, including reduced MHC-I and TAP (viral clearance) and reduced MX1 and IFITM1 (antiviral) in nasal epithelium as an example (Chason et al., 2018). One such study found that, in the aged population, a predictor of successful vaccine-induced humoral immune response was pre-existing SARS-CoV-2 cross-reactive CD4+ T cells (Meyer-Arndt et al., 2022). It has also been found that IL17A and IFNγ concentrations were inversely related to age during an acute phase of SARS-CoV-2 infection, indicating a difference in the immune response in aged adults (Pierce et al., 2020). Additionally, in the context of SARS-CoV-2, gene-expression markers of senescence (CDKN2A and senescence-associated secretory phenotypes [SASP] factor genes) were upregulated in pulmonary cells in patients with severe COVID-19 (Tsuji et al., 2022). In order to characterize and compare the lung-specific immune response to influenza and SARS-CoV-2 in aged individuals, another study utilized lung tissue from deceased donors 22–68 years old (Nguyen et al., 2021). Mononuclear cells from the lung tissues were infected with multiple influenza strains and SARS-CoV-2 and then assessed for correlations in the frequency of immune cell populations with age. Of the immune subsets, only CD8+ T cells significantly declined in the elderly (7.5% of total cells when >50 years of age compared to approximately 21% of total cells in donors <50 years old), and most of these cells were antigen-experienced cells (CD45RO+). This negative association of memory CD8+ T cells is a lung-specific trait, as it was not observed in the periphery, and further phenotyping of these cells indicated that the TRM cells (CD69+ CD103+) were also negatively associated with age (25% of total cells when >50 years of age compared to approximately 48% of total cells in donors <50 years old). T cell activation after infection with influenza virus, measured by HLA-DR and CD69, and secretion of pro-inflammatory cytokines (GM-CSF, IFNγ, and IFNɑ) were also negatively correlated with age. Importantly, Nguyen and colleagues determined that the source of IFNγ was the memory CD8+ T cells, indicating that with age, your IFNγ-producing memory CD8+ T cells in the lung wane. Using HLA-restricted donors, they were able to assay influenza-specific CD8+ T cells, and the CD103+ CD69+ influenza-specific CD8+ TRM cells were again significantly reduced with age. After infection with SARS-CoV-2 (with all donors being naive to SARS-CoV-2), there was no upregulation of activation markers on any T cell subset, and when measuring secreted immune factors of these SARS-CoV-2-infected cells, there was no induction of these pro-inflammatory cytokines, regardless of age. These findings confirm the ability of SARS-CoV-2 to evade IFN responses, which has been well studied (Kim and Shin, 2021), allowing unhampered viral replication and leading to more severe illness.
Genetics is an important yet understudied factor affecting our immune response to pathogens. Much of what we do know stems from studies of primarily European populations (Sharma and Palaniappan, 2021), which does not allow for the ultimate goal of biomarkers for prevention and treatment across diverse populations. Thus, there is a deficiency in both resources and an understanding of the effects of genetics on diverse groups. However, new studies have begun to address these gaps, accounting for a diversity of genetic backgrounds in biomedical research.
One example highlighting the necessity of accounting for ancestry when studying disease associations and their functional mechanism of severe influenza and COVID-19 is the genetic associations of IFN Induced Transmembrane Protein 3 (IFITM3) gene. IFITM3 has been well studied, with concrete evidence of its importance in limiting infection of numerous respiratory viruses (Brass et al., 2009; Feeley et al., 2011; Londrigan et al., 2020), including influenza virus and SARS-CoV-2 (Zani et al., 2021). Multiple studies have identified risk variants in IFITM3 that confer risk of severe illness, but these have been found to be ancestry dependent. Two variants, rs12252 (Everitt et al., 2012) and rs34481144 (Allen et al., 2017), have been the best studied IFITM3 variants and highlight the population-specific nature of these associations. Generally, the coding variant rs12252 has been associated with severe influenza illness in populations of Asian descent, while the promoter variant rs34481144 is associated with severe influenza illness in European based populations, reflective of their allele frequencies (AFs). The rs34481144 SNP is an expression quantitative trait locus (eQTL) for IFITM3 in numerous human cohorts with the risk allele (A) having lower expression of IFITM3. Further, rs34481144 was found to be a protein quantitative locus (pQTL) with lower IFITM3 in individuals with the risk genotype (AA), and the SNP modulates promoter activity as well as binding of transcription factors. A previous study in a mouse model concluded that Ifitm3 is important for the survival of tissue-resident CD8+ T cells upon secondary infection, and, interestingly, frequency and number of CD8+ T cells in the airways of patients with naturally acquired influenza virus infection were correlated with the genotype of rs34481144, with fewer CD8+ T cells in individuals carrying the risk allele. Interestingly, these variants have already been assessed for utility in COVID-19 risk assessment (Cuesta-Llavona et al., 2021; Mohammed et al., 2021), and these IFITM3 variants seem likely candidate biomarkers, though again, further study across populations will be needed for validation.
As with influenza, we are also seeing more studies on population-specific genetic variants in COVID-19 severity, which have been well reviewed (Velavan et al., 2021; Ferreira de Araújo et al., 2022). One genetic association with COVID-19 severity of note is a variant that directly modulates the immune response in the airways to SARS-CoV-2 infection (Downes et al., 2021). Specifically, GWASs of severe COVID-19 illness (The Severe Covid-19 GWAS group, 2020; Pairo-Castineira et al., 2021; Shelton et al., 2021) have detected multiple loci of interest across multiple populations, including a Neanderthal locus at 3p21.31 (Zeberg and Pääbo, 2020), which comprises multiple genes of potential effect on illness progression and was found to double the risk of respiratory failure in COVID-19 cases. In a thoughtful study to determine the mechanism of the detected GWAS signal, Downes et al. used a suite of computational and molecular tools to discover the causal variant and airways-specific mechanism of severity of COVID-19 controlled by the presence of this mutation (Downes et al., 2021). The lead GWAS SNP (rs11385942), which doubled the risk of respiratory failure in these populations, was found to be in linkage disequilibrium (LD) with the causal, non-coding SNP rs17713054, which is rare in most populations but is common in South Asian populations. They found that the risk allele created an active enhancer with better binding of a lung-prevalent transcription factor, CEBP/B. The SNP is an eQTL for LZTFL1 in the lung, with the risk allele associated with higher LZTFL1 expression. Micro Capture C (MCC) determined that the enhancer specifically interacted with the promoter of LZTFL1. This study was able to identify the causal variant of this genetic association of significant effect size, which was a gain-of-function enhancer SNP interacting with increasing expression of LZTFL1, specifically in lung ciliated epithelial cells. The Mendelian disease of LZTFL1 is a ciliopathy, indicating a potential consequence of this risk allele on mucociliary clearance in the lung. Lastly, the authors discuss and describe a role of LZTFL1 in reducing or delaying the epithelial-to-mesenchymal (EMT) responses to SARS-CoV-2 infection. These data highlight a population-specific risk allele, which likely contributed to the high disease severity and mortality in Asian populations by directly modulating lung-specific mechanisms of mucociliary clearance and/or EMT. The proposed mechanism involving EMT is under debate, as other groups have shown an opposing role of EMT in severe SARS-CoV-2 infection, illustrating a pathogenic role, thus requiring more investigation into the implications of this variant and the careful balance of the EMT pathway in a potentially protective role and pathogenic role in establishing fibrosis, known to be a hallmark of severe COVID-19. These data illustrate the ability of researchers to quickly come together with large population genetics studies to find novel biomarkers for existing and emerging diseases. Other initiatives including the COVID-19 Host Genetics Initiative and GenOMICC (Genetics of Mortality in Critical Care) study are illuminating examples of scientists coming together across multiple studies to discover associated variants, which can allow for a better understanding of the disease, discovery of biomarkers of severity, and potential development of novel treatments and therapeutics (COVID-19 Host Genetics Initiative; Kousathanas et al., 2022).
Outstanding questions
Using these examples and the studies described throughout this review, we highlight the complexity of what is known about the intricate facets of the immune response within the airways. Despite tremendous efforts to characterize and understand the airways during infection and vaccination, there remains a vast amount of work to be done to fill in the gaps in our knowledge.
One of the most important challenges we currently face is reducing morbidity and mortality during respiratory infections. Understanding how individual diversity (demographics, risk factors, biologic and genetic variation) contributes to disease susceptibility, disease severity, and vaccine effectiveness is highly important, as these diseases impact all populations. As such, a pressing area of ongoing and future research is to define mechanisms of immune imprinting across demographics, especially within immunologic naive pediatric populations where new antigen exposures are frequent. This type of research has broad implications surrounding vaccination strategies and understanding how life-long immune responses are determined at the earliest stages of life. As others have previously argued (Henry et al., 2018), to investigate the impact of original antigenic sin, immune imprinting, and/or antigenic seniority in the additive effects of exposures during childhood on our immune response to respiratory infections, we need birth cohorts that follow infants during their development to determine how our immune system responds to exposure by infection, vaccination, and any combination of those exposures. Understanding the immunologic consequences and mechanistic drivers behind these immune conditioning events could provide the information needed to develop better vaccination strategies at a young age without creating a bias in the development or trajectory of the immune response. Further, proper vaccine development and vaccination strategies will reduce the current vaccine inefficiencies and improve protection in the general population and, most importantly, the high-risk population.
At the cellular and physiologic levels, new sampling and scRNA-seq approaches have opened lines of investigation and provided insights into the previously underappreciated complexity and heterogeneity within the respiratory tract, especially across different demographics and disease states. In addition to the diversity identified within traditional immune compartments, other studies highlight heterogeneity among specialized pulmonary fibroblast, epithelial and endothelial, and structural populations, arguing for continued research into the genesis of these baseline and activation phenotypes. Several additional unanswered questions related to these studies are outlined in Hewitt and Lloyd, 2021.
The necessity of safe, effective, and widely available vaccinations cannot be understated; therefore, continued development and improvement of vaccinations, including mucosal and IN preparations, should be prioritized. Despite significant efforts in producing vaccine adjuvants to enhance the immune response, new strategies are needed for identification and testing of adjuvants in humans for a more streamlined approach (Pulendran et al., 2021). This is of particular importance for vaccination against respiratory pathogens in that there may be an immunologic disconnection between vaccinations delivered systemically and robust mucosal immune responses. Developing and comparing systemic and direct mucosal vaccination is a subject of intensive research and debate surrounding the inherent “goal” of vaccination, whether that be reduction of transmission, reduction of severe disease, or both. During the SARS-CoV-2 pandemic, it has become apparent that both humoral and T cell-mediated immune memory are protective and underlie unique contributions to responses against VOCs, thus conversations on whether future vaccinations should include a T cell-specific component are ongoing. Further, initiatives like the SARS-CoV-2 Assessment of Viral Evolution (SAVE) program have been critical for assessing risk of emerging VOCs, their transmission potential, and importantly, the effects these variants have on vaccine-mediated immunity (DeGrace et al., 2022).
Another underappreciated mechanism of respiratory health during infections is interactions with the respiratory microbiome. The human respiratory tract has a rich commensal microbiome; however, it also houses opportunistic and pathogenic microbes poised to exacerbate airway dysfunction, cause primary and secondary pneumonias, contribute to asthma, and initiate ARDS, with effects differing significantly across demographics. The microbiome has also been implicated in viral transmission and viral disease progression, including influenza and SARS-CoV-2 infection (Hurst et al., 2022). Understanding the intricate balance of numerous features of the respiratory immune response is of utmost importance for improving preventative measures, screening tools, and treatment options across the diverse human population. This topic fits into the larger conversation surrounding the role played by the baseline mucosal immune environment—comprising commensal bacteria; structural, resident, and circulating innate immune cells; and their various secreted immune factors—in determining the thresholds for respiratory resistance and tolerance (Abt et al., 2012; Tsang et al., 2014, 2020). ARDS is a prime example of why balance between these responses is important yet remains a broad diagnostic classification that may have multiple distinct underlying etiologies and treatment paths. The atypical ARDS presentation observed during SARS-CoV-2 infection suggests we do not fully understand this important disease state. Certainly, continued research is needed to delineate triggers, pathways, and therapeutic intervention points for acute pulmonary diseases, many of which may relate to balance between pulmonary tolerance and resistance.
The respiratory tract is a complex system critical to human health, engaging with our external environment and providing a first line of defense against pulmonary insults. In this review, we have outlined, in broad strokes, the mucosal immune responses to infection and vaccination in the respiratory tract and provided examples from two key respiratory viral pathogens. The field has grown substantially in the past several years, with the advent of new technologies, new lines of inquiry, and new viral adversaries driving innovative and collaborative research projects. While we have a great understanding of the respiratory system and its components, the next stage of pulmonary research must engage the massive challenge of biologic, genetic, and demographic diversity that underlies each individual along with the implications that these have on our respiratory health, vaccine efficacy, and response to respiratory pathogens. Together, these investigations will vastly improve human respiratory health for all groups of people.
Acknowledgments
This publication was supported by ALSAC at St. Jude Children’s Research Hospital (SJCRH), the Center for Influenza Vaccine Research for High-Risk Populations (CIVR-HRP) contract number 75N93019C00052 (P.G.T.), the SJCRH Center of Excellence for Influenza Research and Surveillance (P.G.T.), HHSN272201400006C, National Institute of Allergy and Infectious Diseases (NIAID) award 3U01AI144616-02S1 (P.G.T.), and Ruth L. Kirschstein National Research Service Award (NRSA) Individual Postdoctoral Fellowship (NRSA-NIAID) award number F32AI157296 (R.C.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
P.G.T. has consulted or received honorarium and/or travel support from Illumina, JNJ, and 10X. P.G.T. serves on the Scientific Advisory Board of ImmunoScape and CytoAgents.
References
- Abboud G., Tahiliani V., Desai P., Varkoly K., Driver J., Hutchinson T.E., Salek-Ardakani S. Natural killer cells and innate interferon gamma participate in the host defense against respiratory vaccinia virus infection. J. Virol. 2016;90:129–141. doi: 10.1128/JVI.01894-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt M.C., Osborne L., Monticelli L., Doering T., Alenghat T., Sonnenberg G., Paley M., Antenus M., Williams K., Erikson J., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/J.IMMUNI.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y., Onodera T., Yamada Y., Daio R., Tsuiji M., Inoue T., Kobayashi K., Kurosaki T., Ato M., Takahashi Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J. Exp. Med. 2015;212:1709–1723. doi: 10.1084/JEM.20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Agha E., Moiseenko A., Kheirollahi V., De Langhe S., Crnkovic S., Kwapiszewska G., Szibor M., Kosanovic D., Schwind F., Schermuly R.T., et al. Two-way conversion between Lipogenic and Myogenic fibroblastic phenotypes Marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261–273.e3. doi: 10.1016/J.STEM.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A., Blazquez-Prieto J., Amado-Rodriguez L., Lopez-Alonso I., Batalla-Solis E., Gonzalez-Lopez A., Sanchez-Perez M., Mayoral-Garcia C., Gutierrez-Fernandez A., Albaiceta G.M. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J. Mol. Med. 2017;95:487–497. doi: 10.1007/s00109-017-1510-z. [DOI] [PubMed] [Google Scholar]
- Allen E.K., Randolph A.G., Bhangale T., Dogra P., Ohlson M., Oshansky C.M., Zamora A.E., Shannon J.P., Finkelstein D., Dressen A., et al. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat. Med. 2017;23:975–983. doi: 10.1038/nm.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allie S.R., Bradley J.E., Mudunuru U., Schultz M.D., Graf B.A., Lund F.E., Randall T.D. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2018:97–108. doi: 10.1038/s41590-018-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R., Sportiello M., Kozlovski S., Kumar A., Reilly E.C., Zarbock A., Garbi N., Topham D.J. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nature Reviews Immunology. 2021;21:49–64. doi: 10.1038/s41577-020-00470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amersfoort J., Eelen G., Carmeliet P. Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol. 2022;2022:1–13. doi: 10.1038/s41577-022-00694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirlak I., Haddad R., Hardy J.D., Khaled N.S., Chung M.H., Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum. Vaccin. Immunother. 2021;17:3913–3915. doi: 10.1080/21645515.2021.1956228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidis I., Simon L.M., Fernandez I.E., Strunz M., Mayr C.H., Greiffo F.R., Tsitsiridis G., Ansari M., Graf E., Strom T.M., et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019:1–17. doi: 10.1038/s41467-019-08831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardain A., Domingo-Gonzalez R., Das S., Kazer S.W., Howard N.C., Singh A., Ahmed M., Nhamoyebonde S., Rangel-Moreno J., Ogongo P., et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature. 2019;570:528–532. doi: 10.1038/s41586-019-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardain A., Porterfield J.Z., Kløverpris H.N., Leslie A. Type 3 ILCs in Lung Disease. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardain A., Marakalala M.J., Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2020:245–256. doi: 10.1111/imm.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiaga B.L., Yang G., Hutchinson T.E., Loeb J.C., Richt J.A., Lednicky J.A., Salek-Ardakani S., Driver J.P. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Scientific Rep. 2016;6:37999–38010. doi: 10.1038/srep37999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 2021;11:1793. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K.A., Etesami N.S., Shenoy A.T., Arafa E.I., de Ana C.L., Smith N.M., Martin I.M., Goltry W.N., Barron A.M., Browning J.L., et al. Lung-resident memory B cells protect against bacterial pneumonia. J. Clin. Invest. 2021;131 doi: 10.1172/JCI141810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Beziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/SCIENCE.ABD4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes H.K., Ritchie N.D., Evans T.J. Interleukin-17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infect. Immun. 2016;84:3507–3516. doi: 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoret D., Wallemacq H., Marichal T., Desmet C., Quesada Calvo F., Henry E., Closset R., Dewals B., Thielen C., Gustin P., et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. for the LUNG SAFE Investigators and the ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA - J. Am. Med. Assoc. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Bergenfelz C., Leandersson K. The generation and identity of human myeloid-derived suppressor cells. Front. Oncol. 2020;10:109. doi: 10.3389/fonc.2020.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink J.H., Peters C.P., Munneke M., te Velde A.A., Meijer S.L., Weijer K., Hreggvidsdottir H.S., Heinsbroek S.E., Legrand N., Buskens C.J., et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- Bertoletti A., Le Bert N., Qui M., Tan A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol. Immunol. 2021;18:2307–2312. doi: 10.1038/s41423-021-00743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura L.K., Fares-Frederickson N.J., Steinert E.M., Scott M.C., Thompson E.A., Fraser K.A., Schenkel J.M., Vezys V., Masopust D. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019;216:1214–1229. doi: 10.1084/JEM.20181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez-Prieto J., Lopez-Alonso I., Huidobro C., Albaiceta G.M. The emerging role of neutrophils in repair after acute lung injury. Am. J. Respir. Cell Mol. Biol. 2018;59:289–294. doi: 10.1165/RCMB.2018-0101PS/. [DOI] [PubMed] [Google Scholar]
- Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdonnay E., Zaslona Z., Penke L.R.K., Speth J.M., Schneider D.J., Przybranowski S., Swanson J.A., Mancuso P., Freeman C.M., Curtis J.L., Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 2015;212:729–742. doi: 10.1084/JEM.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D.F., PALISI Pediatric Intensive Care Influenza PICFLU Investigators. Allen E.K., Randolph A.G., Guo XzJ., Weng Y., Sanders C.J., Bajracharya R., Lee N.K., Guy C.S., Vogel P., et al. Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature. 2020;587:466–471. doi: 10.1038/s41586-020-2877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchfield K., Nantie L., Verheyden J.M., Sui P., Wienhold M.D., Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand J.D., Lazrak A., Trombley J.E., Shei R.J., Adewale A.T., Tipper J.L., Yu Z., Ashtekar A.R., Rowe S.M., Matalon S., Harrod K.S. Influenza-mediated reduction of lung epithelial ion channel activity leads to dysregulated pulmonary fluid homeostasis. JCI Insight. 2018;3 doi: 10.1172/JCI.INSIGHT.123467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass A.L., Huang I.C., Benita Y., John S.P., Krishnan M.N., Feeley E.M., Ryan B.J., Weyer J.L., van der Weyden L., Fikrig E., et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, west nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science (New York, N.Y.) 2004;303:1532–1535. doi: 10.1126/SCIENCE.1092385. [DOI] [PubMed] [Google Scholar]
- Broggi A., Tan Y., Granucci F., Zanoni I. IFN-λ suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat. Immunol. 2017;18:1084–1093. doi: 10.1038/ni.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.L., Sequeira R.P., Clarke T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017;8:1512–1611. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., et al. Pulmonary epithelial barrier function: some new players and mechanisms. Am. J. Physiol. - Lung Cell Mol. Physiol. 2015:L731–L745. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capucetti A., Albano F., Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front. Immunol. 2020;11:1259. doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Lethal infectious diseases as Inborn Errors of immunity: toward a synthesis of the Germ and genetic Theories. Annu. Rev. Pathol. Mech. Dis. 2021:23–50. doi: 10.1146/annurev-pathol-031920-101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervia C., Zurbuchen Y., Taeschler P., Ballouz T., Menges D., Hasler S., Adamo S., Raeber M.E., Bachli E., Rudiger A., et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 2022;13:446. doi: 10.1038/s41467-021-27797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov S., Lim H.Y., Tan L., Lim S.Y., See P., Lum J., Zhang X.M., Foo S., Nakamizo S., Duan K., et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363:eaau0964. doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N.J., Smith D.E., DeKruyff R.H., Umetsu D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A., Vijay R., Mack M., Zhao J., Meyerholz D., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chason K.D., Jaspers I., Parker J., Sellers S., Brighton L.E., Hunsucker S.A., Armistead P.M., Fischer W.A. Age-associated changes in the respiratory epithelial response to influenza infection. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2018;73:1643–1650. doi: 10.1093/gerona/gly126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzikleanthous D., O’Hagan D.T., Adamo R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: toward Multicomponent vaccines. Mol. Pharmaceutics. 2021:2867–2888. doi: 10.1021/acs.molpharmaceut.1c00447. [DOI] [PubMed] [Google Scholar]
- Chen J., Kelley W.J., Goldstein D.R. Role of aging and the immune response to respiratory viral infections: potential implications for COVID-19. J. Immunol. 2020;205:313–320. doi: 10.4049/JIMMUNOL.2000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Kolls J.K. T cell–mediated host immune defenses in the lung. Annu. Rev. Immunol. 2013;31:605–633. doi: 10.1146/ANNUREV-IMMUNOL-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov K., Avidan M.S., Benn C.S., Bertozzi S.M., Blatt L., Chang A.Y., Jamison D.T., Khader S.A., Kottilil S., Netea M.G., et al. Old vaccines for new infections: Exploiting innate immunity to control COVID-19 and prevent future pandemics. Proc. Natl. Acad. Sci. United States America. 2021;118 doi: 10.1073/pnas.2101718118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B.L., Thomas P.G. A cell for the ages: human γδ T cells across the Lifespan. Int. J. Mol. Sci. 2020;21:8903–8918. doi: 10.3390/IJMS21238903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem A.S. Fundamentals of vaccine Immunology. J. Glob. Infect. Dis. 2011;3:73. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobey S., Hensley S.E. Immune history and influenza virus susceptibility. Curr. Opin. Virol. 2017:105–111. doi: 10.1016/j.coviro.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey F., Alabyev B., Manser T. Initial clonal expansion of germinal center B cells takes place at the Perimeter of Follicles. Immunity. 2009;30:599–609. doi: 10.1016/J.IMMUNI.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.A., Li A.P.Y., Hachim A., Hui D.S.C., Kwan M.Y.W., Tsang O.T.Y., Chiu S.S., Chan W.H., Yau Y.S., Kavian N., et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 2021;12:4678. doi: 10.1038/s41467-021-24938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G.E., Ostridge K., Khakoo S.I., Wilkinson T.M.A., Staples K.J. Human CD49a+ lung natural killer cell cytotoxicity in response to influenza A virus. Front. Immunol. 2018;9:1671. doi: 10.3389/fimmu.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crainiciuc G., Palomino-Segura M., Molina-Moreno M., Sicilia J., Aragones D.G., Li J.L.Y., Madurga R., Adrover J.M., Aroca-Crevillen A., Martin-Salamanca S., et al. Behavioural immune landscapes of inflammation. Nature. 2022;601:415–421. doi: 10.1038/s41586-021-04263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Vol. 29. 2011. pp. 621–663. (Follicular helper CD4 T cells (TFH)). [DOI] [PubMed] [Google Scholar]
- Cuesta-Llavona E., Albaiceta G.M., Garcia-Clemente M., Duarte-Herrera I.D., Amado-Rodriguez L., Hermida-Valverde T., Enriquez-Rodriguez A.I., Hernandez-Gonzalez C., Melon S., Alvarez-Arguelles M.E., et al. Association between the interferon-induced transmembrane protein 3 gene (IFITM3) rs34481144/rs12252 haplotypes and COVID-19. Curr. Res. Virol. Sci. 2021;2:100016. doi: 10.1016/j.crviro.2021.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukalac T., Chadderton J., Handel A., Doherty P.C., Turner S.J., Thomas P.G., La Gruta N.L. Reproducible selection of high avidity CD8+ T-cell clones following secondary acute virus infection. Proc. Natl. Acad. Sci. United States America. 2014;111:1485–1490. doi: 10.1073/pnas.1323736111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio F.R., Tsushima K., Aggarwal N.R., West E.E., Willett M.H., Britos M.F., Pipeling M.R., Brower R.G., Tuder R.M., McDyer J.F., King L.S. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P., Fiore-Gartland A.J., Hertz T., Wang G.C., Sharma S., Souquette A., Crawford J.C., Clemens E.B., Nguyen T.H.O., Kedzierska K., et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport F.M., Hennessy A., Stuart-Harris C., Francis T. Epidemiology of influenza; comparative serological observations in England and the United States. Lancet (London, England) 1955;266:469–474. doi: 10.1016/S0140-6736(55)93328-6. [DOI] [PubMed] [Google Scholar]
- Davenport F.M., Hennessy A.V., Francis T., With the Technical Assistance of Phyllis Fabisch Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J. Exp. Med. 1953;98:641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrace M.M., Ghedin E., Frieman M.B., Krammer F., Grifoni A., Alisoltani A., Alter G., Amara R.R., Baric R.S., Barouch D.H., et al. Defining the risk of SARS-CoV-2 variants on immune protection. Nature. 2022;31:2022. doi: 10.1038/s41586-022-04690-5. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveris C.S., Webster E.R., Banik S.M., Boxer S.G., Bertozzi C.R. Membrane-tethered mucin-like polypeptides sterically inhibit binding and slow fusion kinetics of influenza A virus. Proc. Natl. Acad. Sci. United States America. 2020;117:12643–12650. doi: 10.1073/PNAS.1921962117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Hackbart M., Mettelman R.C., O’Brien A., Mielech A.M., Yi G., Kao C.C., Baker S.C. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc. Natl. Acad. Sci. 2017;114:E4251–E4260. doi: 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., van Geelen A., Buckley A.C., O’Brien A., Pillatzki A., Lager K.M., Faaberg K.S., Baker S.C. Coronavirus Endoribonuclease activity in Porcine Epidemic Diarrhea virus suppresses type I and type III interferon responses. J. Virol. 2019;93 doi: 10.1128/jvi.02000-18. JVI-02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez M., Zaragosi L.E., Truchi M., Becavin C., Ruiz Garcia S., Arguel M.J., Plaisant M., Magnone V., Lebrigand K., Abelanet S., et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 2020;202:1636–1645. doi: 10.1164/rccm.201911-2199OC. [DOI] [PubMed] [Google Scholar]
- Devarajan P., Vong A.M., Castonguay C.H., Kugler-Umana O., Bautista B.L., Jones M.C., Kelly K.A., Xia J., Swain S.L. Strong influenza-induced TFH generation requires CD4 effectors to recognize antigen locally and receive signals from continuing infection. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/PNAS.2111064119. e2111064119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S., Kanneganti T.-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo D.J., Tan G.S., Palese P., Ravetch J.V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcR interactions for protection against influenza virus in vivo. Nat. Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo D.J., Palese P., Wilson P.C., Ravetch J.V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 2016;126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K.H., Leist S.R., Okuda K., Dang H., Fritch E.J., Gully K.L., De la Cruz G., Evangelista M.D., Asakura T., Gilmore R.C., et al. A model of persistent post SARS-CoV-2 induced lung disease for target identification and testing of therapeutic strategies. bioRxiv. 2022 doi: 10.1101/2022.02.15.480515. Preprint at. [DOI] [Google Scholar]
- Di Domizio J., Gulen M.F., Saidoune F., Thacker V.V., Yatim A., Sharma K., Nass T., Guenova E., Schaller M., Conrad C., et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature Preprint at bioRxiv. 2022;603:145–151. doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Wang Y., Gonzalez G.X., Ma Y., Song Y., Wang S., Kang S.M., Compans R.W., Wang B.Z. Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo-and heterologous strains. Proc. Natl. Acad. Sci. United States America. 2021;118 doi: 10.1073/pnas.2024998118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMC2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes D.J., Cross A.R., Hua P., Roberts N., Schwessinger R., Cutler A.J., Munis A.M., Brown J., Mielczarek O., de Andrea C.E., et al. COvid-19 Multi-omics Blood ATlas COMBAT Consortium Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat. Genet. 2021;53:1606–1615. doi: 10.1038/s41588-021-00955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt A.R., The GenISIS Investigators. Clare S., Pertel T., John S.P., Wash R.S., Smith S.E., Chin C.R., Feeley E.M., Sims J.S., Adams D.J., et al. The MOSAIC Investigators IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren E., Ringqvist E., Doisne J.M., Thaller A., Sleiers N., Flavell R.A., Di Santo J.P., Willinger T. CD116+ fetal precursors migrate to the perinatal lung and give rise to human alveolar macrophages. J. Exp. Med. 2022;219 doi: 10.1084/jem.20210987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Jones S., Vangeti S., Yu M., Falck-Jones R., Cagigi A., Badolati I., Osterberg B., Lautenbach M.J., Ahlberg E., Lin A., et al. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. J. Clin. Invest. 2021;131 doi: 10.1172/JCI144734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi M., Johansson A., Lundborg M., Orre L., Skold C., Camner P. Functional and Morphological differences between human alveolar and interstitial macrophages. Exp. Mol. Pathol. 2001;70:77–82. doi: 10.1006/EXMP.2000.2344. [DOI] [PubMed] [Google Scholar]
- Feeley E.M., Sims J.S., John S.P., Chin C.R., Pertel T., Chen L.M., Gaiha G.D., Ryan B.J., Donis R.O., Elledge S.J., Brass A.L. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7:e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld J.J., Kandel C., Biondi M.J., Kozak R.A., Zahoor M.A., Lemieux C., Borgia S.M., Boggild A.K., Powis J., McCready J., et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021;9:498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein L.R., Tenforde M.W., Friedman K.G., Newhams M., Rose E.B., Dapul H., Soma V.L., Maddux A.B., Mourani P.M., Bowens C., et al. Characteristics and outcomes of US children and Adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA - J. Am. Med. Assoc. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira de Araújo J.L., Menezes D., Saraiva-Duarte J.M., Ferreira L., Aguiar R., Souza R. Systematic review of host genetic association with Covid-19 prognosis and susceptibility: what have we learned in 2020? Rev. Med. Virol. 2022;32 doi: 10.1002/RMV.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament H., Rouland M., Beaudoin L., Toubal A., Bertrand L., Lebourgeois S., Rousseau C., Soulard P., Gouda Z., Cagninacci L., et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 2021;22:322–335. doi: 10.1038/s41590-021-00870-z. [DOI] [PubMed] [Google Scholar]
- Flerlage T., Boyd D.F., Meliopoulos V., Thomas P.G., Schultz-Cherry S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021;19:425–441. doi: 10.1038/s41579-021-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S.J., Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- Forero A., Ozarkar S., Li H., Lee C.H., Hemann E.A., Nadjsombati M.S., Hendricks M.R., So L., Green R., Roy C.N., et al. Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III. Interferons. Immunity. 2019;51 doi: 10.1016/j.immuni.2019.07.007. 451–464.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Pawelec G., Khalil A., Cohen A.A., Hirokawa K., Witkowski J.M., Franceschi C. Immunology of aging: the birth of inflammaging. Clin. Rev. Allergy Immunol. 2021;2021:1–14. doi: 10.1007/S12016-021-08899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani I.E., Triantafyllia V., Eleminiadou E.E., Koltsida O., Stavropoulos A., Manioudaki M., Thanos D., Doyle S.E., Kotenko S.V., Thanopoulou K., Andreakos E. Interferon-λ mediates non-redundant Front-line antiviral protection against influenza virus infection without Compromising host Fitness. Immunity. 2017;46:875–890.e6. doi: 10.1016/j.immuni.2017.04.025. [DOI] [PubMed] [Google Scholar]
- García-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162:12–18. doi: 10.1016/j.virusres.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in détente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gardai S.J., Xiao Y.Q., Dickinson M., Nick J.A., Voelker D.R., Greene K.E., Henson P.M. By binding SIRPα or Calreticulin/CD91, lung Collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/S0092-8674(03)00758-X. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitani R., Moubareck C.A., Xu Z., Karam Sarkis D., Touqui L. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis. Front. Immunol. 2020;11:1198. doi: 10.3389/fimmu.2020.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersuk G.M., Underhill D.M., Zhu L., Marr K.A. Dectin-1 and TLRs Permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 2006;176:3717–3724. doi: 10.4049/JIMMUNOL.176.6.3717. [DOI] [PubMed] [Google Scholar]
- Gibbings S.L., Thomas S.M., Atif S.M., McCubbrey A.L., Desch A.N., Danhorn T., Leach S.M., Bratton D.L., Henson P.M., Janssen W.J., Jakubzick C.V. Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cel. Mol. Biol. 2017;57:66–76. doi: 10.1165/RCMB.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseck R.L., Wilson M.S., Wynn T.A. Vol. 18. Nature Publishing Group; 2017. (Type 2 Immunity in Tissue Repair and Fibrosis). [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/JEM.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen K.P.J.M., Klarenbeek P., Kragten N., Unger P.P., Nieuwenhuis M., Wensveen F., ten Brinke A., Tak P., Eldering E., Nolte M., van Lier R. The costimulatory molecule CD27 maintains clonally diverse CD8+ T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Glezen W.P., Greenberg S.B., Atmar R.L., Piedra P.A., Couch R.B. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- González-López A., Astudillo A., Garcia-Prieto E., Fernandez-Garcia M.S., Lopez-Vazquez A., Batalla-Solis E., Taboada F., Fueyo A., Albaiceta G.M. Inflammation and matrix remodeling during repair of ventilator-induced lung injury. Am. J. Physiol. - Lung Cell Mol. Physiol. 2011;301:500–509. doi: 10.1152/AJPLUNG.00010.2011. [DOI] [PubMed] [Google Scholar]
- Gopal R., Monin L., Slight S., Uche U., Blanchard E., A. Fallert Junecko B., Ramos-Payan R., Stallings C.L., Reinhart T.A., Kolls J.K., et al. Unexpected role for IL-17 in protective immunity against Hypervirulent Mycobacterium tuberculosis HN878 infection. PLOS Pathog. 2014;10:e1004099. doi: 10.1371/JOURNAL.PPAT.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V.M.W., Francis J.N., Anderson K.J., Georges B., Cope A.V., Tregoning J.S. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R.A., The NU SCRIPT Study Investigators. Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., Abbott D.A., Donnelly H.K., Donayre A., Goldberg I.A., et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590:635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Expósito J., Sanchez-Gaona N., Massana N., Suppi M., Astorga-Gamaza A., Perea D., Rosado J., Falco A., Kirkegaard C., Torrella A., et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021;12:3010–3017. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M., Helft J., Chow A., Hashimoto D., Mortha A., Agudo-Cantero J., Bogunovic M., Gautier E., Miller J., Leboeuf M., et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/J.IMMUNI.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/AJPATH.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/JEM.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Bruhns P., Saeys Y., Hammad H., Lambrecht B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- Guo K., Yombo D.J.K., Xu J., Wang Z., Schmit T., Tripathi J., Hur J., Sun J., Olszewski M.A., Khan N. The chemokine receptor CXCR3 promotes CD8+ T cell-dependent lung pathology during influenza pathogenesis. bioRxiv. 2022 doi: 10.1101/2022.02.14.480379. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.z.J., Dash P., Crawford J.C., Allen E.K., Zamora A.E., Boyd D.F., Duan S., Bajracharya R., Awad W.A., Apiwattanakul N., et al. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity. 2018;49:531–544.e6. doi: 10.1016/j.immuni.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S., Dujardin H.C., Bakocevic N., Fleige H., Danzer H., Willenzon S., Suezer Y., Hämmerling G., Garbi N., Sutter G., Worbs T., Förster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker J.A., Lloyd C.M. Overlapping and distinct features of viral and allergen immunity in the human lung. Immunity. 2021:617–631. doi: 10.1016/j.immuni.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/J.IT.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184.e13. doi: 10.1016/J.CELL.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann E.A., Sjaastad L.E., Langlois R.A., Legge K.L. Plasmacytoid dendritic cells require direct infection to sustain the pulmonary influenza A virus-specific CD8 T cell response. J. Virol. 2016;90:2830–2837. doi: 10.1128/JVI.02546-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann E.A., Green R., Turnbull J.B., Langlois R.A., Savan R., Gale M. Interferon-λ modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat. Immunol. 2019;20:1035–1045. doi: 10.1038/s41590-019-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann E.A., Gale M., Savan R. Interferon lambda genetics and biology in regulation of viral control. Front. Immunol. 2017;8:1707. doi: 10.3389/fimmu.2017.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C., Palm A.E., Krammer F., Wilson P.C. From Original Antigenic Sin to the Universal Influenza Virus Vaccine. Trends Immunol. 2018;39:70–79. doi: 10.1016/j.it.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge M.S., Tansey C.M., Matte A., Tomlinson G., Diaz-Granados N., Cooper A., Guest C.B., Mazer C.D., Mehta S., Stewart T.E., et al. Functional Disability 5 Years after acute respiratory distress syndrome. New Engl. J. Med. 2011;364:1293–1304. doi: 10.1056/NEJMOA1011802. [DOI] [PubMed] [Google Scholar]
- Herridge M.S., Moss M., Hough C.L., Hopkins R.O., Rice T.W., Bienvenu O.J., Azoulay E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42:725–738. doi: 10.1007/S00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- Hewitt R.J., Lloyd C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021:347–362. doi: 10.1038/s41577-020-00477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.M.G., Mandlebrot D.A., Shaw S.K., Russell G.J., Murphy E.A., Chen Y.T., Nelson W.J., Parker C.M., Brenner M.B. Direct and regulated interaction of integrin αEβ7 with E-Cadherin. J. Cell Biol. 1998;140:197–210. doi: 10.1083/JCB.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.W.S., Prabhu N., Betts R.J., Ge M.Q., Dai X., Hutchinson P.E., Lew F.C., Wong K.L., Hanson B.J., Macary P.A., Kemeny D.M. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol. 2011;187:6011–6021. doi: 10.4049/JIMMUNOL.1100987. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/J.CELL.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P.G., Strickland D.H., Wikström M.E., Jahnsen F.L. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Hombrink P., Helbig C., Backer R.A., Piet B., Oja A.E., Stark R., Brasser G., Jongejan A., Jonkers R.E., Nota B., et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 2016;17:1467–1478. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- Honce R., Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppstädter J., Diesel B., Zarbock R., Breinig T., Monz D., Koch M., Meyerhans A., Gortner L., Lehr C.M., Huwer H., Kiemer A.K. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010;11:124–215. doi: 10.1186/1465-9921-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries D.C., O’Connor R.A., Larocque D., Chabaud-Riou M., Dhaliwal K., Pavot V. Pulmonary-resident memory lymphocytes: pivotal Orchestrators of local immunity against respiratory infections. Front. Immunol. 2021;12:3817. doi: 10.3389/fimmu.2021.738955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J.H., McCumber A.W., Aquino J.N., Rodriguez J., Heston S.M., Lugo D.J., Rotta A.T., Turner N.A., Pfeiffer T.S., Gurley T.C., et al. Age-related changes in the nasopharyngeal microbiome are associated with SARS-CoV-2 infection and symptoms among children, adolescents, and young adults. Clinical Infectious Diseases. 2022 doi: 10.1093/cid/ciac184. ciac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen W.J., Stefanski A.L., Bochner B.S., Evans C.M. Control of lung defence by mucins and macrophages: ancient defence mechanisms with modern functions. Eur. Respir. J. 2016;48:1201–1214. doi: 10.1183/13993003.00120-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.M., Bachus H., Botta D., Schultz M.D., Rosenberg A.F., León B., Ballesteros-Tato A. Lung dendritic cells migrate to the spleen to prime long-lived TCF1hi memory CD8+ T cell precursors after influenza infection. Sci. Immunol. 2021;6:eabg6895. doi: 10.1126/sciimmunol.abg6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouan Y., Guillon A., Gonzalez L., Perez Y., Boisseau C., Ehrmann S., Ferreira M., Daix T., Jeannet R., Francois B., et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia V., Hessel E.M., Malherbe L., Glaichenhaus N., O'Garra A., Coffman R.L. A restricted subset of dendritic cells captures Airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/S1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/J.JHIN.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M., Suryawanshi A., Tundup S., Perez J.T., Schmolke M., Manicassamy S., Manicassamy B. RIG-I signaling is critical for efficient polyfunctional T cell responses during influenza virus infection. PLOS Pathog. 2016;12:e1005754. doi: 10.1371/JOURNAL.PPAT.1005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.M., Compans R.W. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Mol. Cells. 2009;27:5–14. doi: 10.1007/S10059-009-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.S., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine Shock syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/J.CELL.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Kanneganti T.D. The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunology. 2021;42:681–705. doi: 10.1016/J.IT.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber E.R., Mercadante M., Nilsson-Payant B., Johnson J.L., Jaimes J.A., Muecksch F., Weisblum Y., Bram Y., Chandar V., Whittaker G.R., et al. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. eLife. 2022;11:77444. doi: 10.7554/ELIFE.77444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Kayama H., Nakama T., Hashimoto T., Umemoto E., Takeda K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int. Immunol. 2016;28:489–501. doi: 10.1093/INTIMM/DXW012. [DOI] [PubMed] [Google Scholar]
- Kedzierska K., Day E.B., Pi J., Heard S.B., Doherty P.C., Turner S.J., Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or Private TCR usage. J. Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- Kedzierska K., Thomas P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022;3:100562. doi: 10.1016/J.XCRM.2022.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.S., Braciale T.J. Respiratory Dendritic Cell Subsets Differ in Their Capacity to Support the Induction of Virus-Specific Cytotoxic CD8+ T Cell Responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.S., Gorski S., Hahn S., Murphy K.M., Braciale T. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40:400–413. doi: 10.1016/J.IMMUNI.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Zhou J.Q., Sturtz A.J., Horvath S.C., Schmitz A.J., Lei T., Kalaidina E., Thapa M., Alsoussi W.B., Haile A., et al. Germinal centre-driven maturation of B cell response to SARS-CoV-2 vaccination. Nature Preprint at bioRxiv. 2022 doi: 10.1101/2021.10.31.466651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.M., Shin E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021:750–760. doi: 10.1038/s12276-021-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhammer J., Schnepf D., Ye L., Schwaderlapp M., Gad H.H., Hartmann R., Garcin D., Mahlakoiv T., Staeheli P. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife. 2018;7 doi: 10.7554/elife.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L., Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem. Cel. Biol. 2018;150:661–676. doi: 10.1007/S00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E.J., Kang S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018;14:3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J., Veenstra I., Demaria M. The struggle of a good friend getting old: cellular senescence in viral responses and therapy. EMBO Rep. 2021;22 doi: 10.15252/EMBR.202052243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli K., Janssen A., Förster R. Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur. J. Immunol. 2016;46:2659–2668. doi: 10.1002/EJI.201646359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad F.M., et al. How adhesion molecule patterns change while neutrophils traffic through the lung during inflammation. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/1208086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Schneider C., Nobs S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2014;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- Kousathanas A., Pairo-Castineira E., Rawlik K., Stuckey A., Odhams C.A., Walker S., Russell C.D., Malinauskas T., Wu Y., Millar J., et al. Whole genome sequencing reveals host factors underlying critical Covid-19. Nature. 2022;7:2022. doi: 10.1038/s41586-022-04576-6. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushki K., Salemi M., Miri S.M., Arjeini Y., Keshavarz M., Ghaemi A. Role of myeloid-derived suppressor cells in viral respiratory infections; Hints for discovering therapeutic targets for COVID-19. Biomed. Pharmacother. 2021;144:112346. doi: 10.1016/j.biopha.2021.112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos M., Illing P.T., Nguyen T.H.O., Mifsud N.A., Crawford J.C., Rizzetto S., Eltahla A.A., Clemens E.B., Sant S., Chua B.Y., et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- Kratzel A., Steiner S., Todt D., V'kovski P., Brueggemann Y., Steinmann J., Steinmann E., Thiel V., Pfaender S. Temperature-dependent surface stability of SARS-CoV-2. J. Infect. 2020;81:452–482. doi: 10.1016/J.JINF.2020.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R.R., Rasheed M.A.U., Bhaumik S.K., Ranjan P., Cao W., Davis C., Marisetti K., Thomas S., Gangappa S., Sambhara S., Murali-Krishna K. Activation of the RIG-I pathway during influenza vaccination enhances the germinal center reaction, promotes T follicular helper cell induction, and provides a dose-sparing effect and protective immunity. J. Virol. 2014;88:13990–14001. doi: 10.1128/JVI.02273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J., Senda T., Sun X., Ho S.H., Lerner H., et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/J.CELREP.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B.V., Kratchmarov R., Miron M., Carpenter D.J., Senda T., Lerner H., Friedman A., Reiner S.L., Farber D.L. Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight. 2018;3 doi: 10.1172/JCI.INSIGHT.123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Kometani K., Ise W. Memory B cells. Nat. Rev. Immunol. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., Hammad H. Lung Dendritic Cells in Respiratory Viral Infection and Asthma: From Protection to Immunopathology. Annual Review of Immunology. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- Lamers M.M., Vaart J., Knoops K., Riesebosch S., Breugem T.I., Mykytyn A.Z., Beumer J., Schipper D., Bezstarosti K., Koopman C.D., et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021;40:e105912. doi: 10.15252/EMBJ.2020105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L., Jung S. Lung macrophages serve as Obligatory intermediate between blood monocytes and alveolar macrophages. J. Immunol. 2007;179:3488–3494. doi: 10.4049/JIMMUNOL.179.6.3488. [DOI] [PubMed] [Google Scholar]
- Lang F.M., Lee K.M.C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer K.G., Johnson L.L., Woodland D.L., Blackman M.A. Impact of ageing on the response and repertoire of influenza virus-specific CD4 T cells. Immun. Ageing. 2014;11:9. doi: 10.1186/1742-4933-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.O., Rangel-Moreno J., Moyron-Quiroz J.E., Hartson L., Makris M., Sprague F., Lund F.E., Randall T.D. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Muller M.M., Klassert T.E., Driesch D., Stock M., Heinrich A., Conrad T., Moore C., Schier U.K., Guthke R., Slevogt H. Differential regulation of the transcriptomic and secretomic landscape of sensor and effector functions of human airway epithelial cells. Mucosal Immunol. 2018;11:627–642. doi: 10.1038/mi.2017.100. [DOI] [PubMed] [Google Scholar]
- Leroy C., Prive A., Bourret J.C., Berthiaume Y., Ferraro P., Brochiero E. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am. J. Physiol. - Lung Cell Mol. Physiol. 2006;291:1207–1219. doi: 10.1152/ajplung.00376.2005. [DOI] [PubMed] [Google Scholar]
- Lessler J., Riley S., Read J.M., Wang S., Zhu H., Smith G.J.D., Guan Y., Jiang C.Q., Cummings D.A.T. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012;8:e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yang P., Sun Y., Li T., Wang C., Wang Z., Zou Z., Yan Y., Wang W., Wang C., et al. IL-17 response mediates acute lung injury induced by the 2009 Pandemic Influenza A (H1N1) Virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kolling F.W., Aridgides D., Mellinger D., Ashare A., Jakubzick C.V. ScRNA-seq Expression of APOC2 and IFI27 Identifies Four Alveolar Macrophage Superclusters in Cystic Fibrosis and Healthy BALF. bioRxiv. 2022 doi: 10.1101/2022.01.30.478325. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jackson R.J., Ranasinghe C. Vaccination route can significantly alter the innate lymphoid cell subsets: a feedback between IL-13 and IFN-γ. npj Vaccin. 2018;3:10–19. doi: 10.1038/s41541-018-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Lim K., Hyun Y.M., Lambert-Emo K., Capece T., Bae S., Miller R., Topham D.J., Kim M. Neutrophil trails guide influenza specific CD8+ T cells in the airways. Science. 2015;349 doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong S., Oseghale O., To E.E., Brassington K., Erlich J.R., Luong R., Liong F., Brooks R., Martin C., O’Toole S., et al. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc. Natl. Acad. Sci. United States America. 2020;117:24964–24973. doi: 10.1073/PNAS.2006905117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li T., Deng Y., Liu S., Zhang D., Li H., Wang X., Jia L., Han J., Bei Z., et al. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021;107:105–107. doi: 10.1016/J.JHIN.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C.M., Snelgrove R.J. Type 2 immunity: expanding our view. Sci. Immunol. 2018;3 doi: 10.1126/SCIIMMUNOL.AAT1604. [DOI] [PubMed] [Google Scholar]
- Londrigan S.L., Wakim L.M., Smith J., Haverkate A.J., Brooks A.G., Reading P.C. IFITM3 and type I interferons are important for the control of influenza A virus replication in murine macrophages. Virology. 2020;540:17–22. doi: 10.1016/j.virol.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Lu X., Hosono Y., Nagae M., Ishizuka S., Ishikawa E., Motooka D., Ozaki Y., Sax N., Maeda Y., Kato Y., et al. Identification of conserved sars-cov-2 spike epitopes that expand public ctfh clonotypes in mild covid-19 patients. J. Exp. Med. 2021;218 doi: 10.1084/jem.20211327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S.J., Portillo A., Cavagnero K., Baum R.E., Naji L.H., Badrani J.H., Mehta A., Croft M., Broide D.H., Doherty T.A. Leukotriene C4 potentiates IL-33-induced group 2 innate lymphoid cell activation and lung inflammation. J. Immunol. 2017;199:1096–1104. doi: 10.4049/JIMMUNOL.1601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamary J., Wang T.T., Tan G.S., Palese P., Ravetch J.V. Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc. Natl. Acad. Sci. United States America. 2017;114:10172–10177. doi: 10.1073/pnas.1707950114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay L.K., Minnich M., Kragten N.A.M., Liao Y., Nota B., Seillet C., Zaid A., Man K., Preston S., Freestone D., et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/SCIENCE.AAD2035/. [DOI] [PubMed] [Google Scholar]
- Mades A., Chellamathu P., Kojima N., Lopez L., MacMullan M.A., Denny N., Angel A.N., Santacruz M., Casian J.G., Brobeck M., et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Scientific Rep. 2021;11:24448–24456. doi: 10.1038/s41598-021-03931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madissoon E., Wilbrey-Clark A., Miragaia R.J., Saeb-Parsy K., Mahbubani K.T., Georgakopoulos N., Harding P., Polanski K., Huang N., Nowicki-Osuch K., et al. ScRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biol. 2019;21:1–16. doi: 10.1186/s13059-019-1906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele L., Carnoy C., Cayet D., Ivanov S., Porte R., Deruy E., Chabalgoity J.A., Renauld J.C., Eberl G., Benecke A.G., et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J. Infect. Dis. 2014;210:493–503. doi: 10.1093/INFDIS/JIU106. [DOI] [PubMed] [Google Scholar]
- Major J., Crotta S., Llorian M., McCabe T.M., Gad H.H., Priestnall S.L., Hartmann R., Wack A. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369:712–717. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T., Israelow B., Suberi A., Zhou L., Reschke M., Peña-Hernández M.A., Dong H., Homer R.J., Saltzman W.M., Iwasaki A. Unadjuvanted intranasal spike vaccine booster elicits robust protective mucosal immunity against sarbecoviruses. bioRxiv. 2022 doi: 10.1101/2022.01.24.477597. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Soerens A.G. Tissue-resident T cells and other resident leukocytes. 2019;37:521–546. doi: 10.1146/annurev-immunol-042617-053214-IMMUNOL-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Massow G., Oh S., Lam A., Gustafsson K. Gamma delta T cells and their Involvement in COVID-19 virus infections. Front. Immunol. 2021;12:4428. doi: 10.3389/fimmu.2021.741218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense Strategy. Science. 2012;335:936–941. doi: 10.1126/SCIENCE.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettelman R.C., Thomas P.G. Human susceptibility to influenza infection and severe disease. Cold Spring Harbor Perspect. Med. 2021;11:a038711. doi: 10.1101/cshperspect.a038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N.J., Christie J.D. Genetic heterogeneity and risk of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2013;34:459–474. doi: 10.1055/s-0033-1351121. [DOI] [PubMed] [Google Scholar]
- Meyer-Arndt L., Schwarz T., Loyal L., Henze L., Kruse B., Dingeldey M., Gurcan K., Uyar-Aydin Z., Muller M.A., Drosten C., et al. Cutting Edge: serum but not mucosal antibody responses are associated with pre-existing SARS-CoV-2 spike cross-reactive CD4+ T cells following BNT162b2 vaccination in the elderly. J. Immunol. 2022;208:1001–1005. doi: 10.4049/JIMMUNOL.2100990. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt B.C., Fritz J.H., Duerr C.U. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Frontiers in Immunology. 2018:840. doi: 10.3389/fimmu.2018.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervina A.A., Pogorelyy M.V., Kirk A.M., Crawford J.C., Allen E.K., Chou C.H., Mettelman R.C., Allison K.J., Lin C.Y., Brice D.C., et al. the SJTRC Study Team SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat. Immunol. 2022;2022:1–10. doi: 10.1038/s41590-022-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani A.S., Besnard A.G., Yip E., Scott C., Bain C.C., Cerovic V., Salmond R.J., Liew F.Y. Type 2 innate lymphoid cells drive CD4 + Th2 cell responses. J. Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- Miyauchi K., Sugimoto-Ishige A., Harada Y., Adachi Y., Usami Y., Kaji T., Inoue K., Hasegawa H., Watanabe T., Hijikata A., et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 2016;17:1447–1458. doi: 10.1038/ni.3563. [DOI] [PubMed] [Google Scholar]
- Mjösberg J., Bernink J., Golebski K., Karrich J., Peters C., Blom B., te Velde A., Fokkens W., van Drunen C., Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mohammed F.S., Farooqi Y.N., Mohammed S. The interferon-induced transmembrane protein 3 -rs12252 allele may predict COVID-19 severity among ethnic Minorities. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.692254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu B., Otmishi P., Jani P., Walker J., Sarmiento X., Guardiola J., Saad M., Yu J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009;2:1–11. doi: 10.2147/JIR.S4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltedo B., Li W., Yount J.S., Moran T.M. Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection. PLOS Pathogens. 2011;7:e1002345. doi: 10.1371/journal.ppat.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli L.A., Sonnenberg G.F., Abt M.C., Alenghat T., Ziegler C.G.K., Doering T.A., Angelosanto J.M., Laidlaw B.J., Yang C.Y., Sathaliyawala T., et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro D.T., Haber A.L., Biton M., Vinarsky V., Lin B., Birket S.E., Yuan F., Chen S., Leung H.M., Villoria J., et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S.J.C.F.M., Rodriguez-Rosales Y.A., Gillard J., Fanucchi S., Theunissen K., Novakovic B., de Bont C.M., Negishi Y., Fok E.T., Kalafati L., et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 2020;33:108387. doi: 10.1016/J.CELREP.2020.108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould K.J., Jackson N.D., Henson P.M., Seibold M., Janssen W.J. Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI insight. 2019;4 doi: 10.1172/JCI.INSIGHT.126556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould K.J., Moore C.M., McManus S.A., McCubbrey A.L., McClendon J.D., Griesmer C.L., Henson P.M., Janssen W.J. Airspace macrophages and monocytes exist in transcriptionally distinct subsets in healthy adults. Am. J. Respir. Crit. Care Med. 2021;203:946–956. doi: 10.1164/RCCM.202005-1989OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyron-Quiroz J.E., Rangel-Moreno J., Kusser K., Hartson L., Sprague F., Goodrich S., Woodland D.L., Lund F.E., Randall T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Mudd P.A., Crawford J.C., Turner J.S., Souquette A., Reynolds D., Bender D., Bosanquet J.P., Anand N.J., Striker D.A., Martin R.S., et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd P.A., Minervina A.A., Pogorelyy M.V., Turner J.S., Kim W., Kalaidina E., Petersen J., Schmitz A.J., Lei T., Haile A., et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185:603–613.e15. doi: 10.1016/j.cell.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J.M., Ngai L., Mortha A., Crome S.Q. Tissue-dependent Adaptations and functions of innate lymphoid cells. Front. Immunol. 2022;13:810. doi: 10.3389/FIMMU.2022.836999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N.G., Crystal R.G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 1992;89:1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., van Rooijen N., Chow V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/J.AJPATH.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan N., Taytard J., Duquesnoy P., Thouvenin G., Corvol H., Amselem S., Clement A. Surfactant protein A: a key player in lung homeostasis. Int. J. Biochem. Cel. Biol. 2016;81:151–155. doi: 10.1016/J.BIOCEL.2016.11.003. [DOI] [PubMed] [Google Scholar]
- NCIRD . Centers for Disease Control and Prevention (US); 2020. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments, CDC COVID-19 Science Briefs. [PubMed] [Google Scholar]
- Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K.A., Bucks C., Kane C.M., Fallon P.G., Pannell R., et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O'Neill L.A., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L., van der Meer J., Mhlanga M.M., Mulder W., et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudecker V., Brodsky K.S., Clambey E.T., Schmidt E.P., Packard T.A., Davenport B., Standiford T.J., Weng T., Fletcher A.A., Barthel L., et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci. Translational Med. 2017;9 doi: 10.1126/scitranslmed.aah5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H.O., McAuley J.L., Kim Y., Zheng M.Z., Gherardin N.A., Godfrey D.I., Purcell D.F., Sullivan L.C., Westall G.P., Reading P.C., et al. Influenza, but not SARS-CoV-2, infection induces a rapid interferon response that wanes with age and diminished tissue-resident memory CD8+ T cells. Clin. Translational Immunol. 2021;10 doi: 10.1002/cti2.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessl J., Sekine T., Lange J., Konya V., Forkel M., Maric J., Rao A., Mazzurana L., Kokkinou E., Weigel W., et al. Identification of resident memory CD8+ T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci. Immunol. 2021;6 doi: 10.1126/SCIIMMUNOL.ABK0894/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobs S.P. What Makes the lung unique – tissue-specific immunity in the respiratory tract. Eur. Med. J. 2020:80–90. doi: 10.33590/emj/20-00089. [DOI] [Google Scholar]
- Nobs S.P., Kayhan M., Kopf M. GM-CSF intrinsically controls eosinophil accumulation in the setting of allergic airway inflammation. J. Allergy Clin. Immunol. 2019;143:1513–1524.e2. doi: 10.1016/J.JACI.2018.08.044. [DOI] [PubMed] [Google Scholar]
- Oja A.E., Piet B., Helbig C., Stark R., van der Zwan D., Blaauwgeers H., Remmerswaal E.B.M., Amsen D., Jonkers R.E., Moerland P.D., et al. Trigger-happy resident memory CD4+ T cells inhabit the human lungs. Mucosal Immunol. 2017;11:654–667. doi: 10.1038/mi.2017.94. [DOI] [PubMed] [Google Scholar]
- Okada T., Miller M.J., Parker I., Krummel M.F., Neighbors M., Hartley S.B., O'Garra A., Cahalan M.D., Cyster J.G. Antigen-Engaged B Cells Undergo Chemotaxis toward the T Zone and Form Motile Conjugates with Helper T Cells. PLOS Biology. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary C.E., Schneider C., Locksley R.M. Tuft cells-systemically Dispersed sensory epithelia Integrating immune and neural Circuitry. Annu. Rev. Immunol. 2019;37:47–72. doi: 10.1146/annurev-immunol-042718-041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant C.J., Hwang Y., Walker J., Salimi M., Wong S., Brewer J., Englezakis A., Barlow J., Hams E., Scanlon S., et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Takahashi Y., Yokoi Y., Ato M., Kodama Y., Hachimura S., Kurosaki T., Kobayashi K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. United States America. 2012;109:2485–2490. doi: 10.1073/PNAS.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshansky C.M., Gartland A.J., Wong S.S., Jeevan T., Wang D., Roddam P.L., Caniza M.A., Hertz T., DeVincenzo J.P., Webby R.J., Thomas P.G. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care Med. 2014;189:449–462. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., Walker S., Parkinson N., Fourman M.H., Russell C.D., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2020;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- Palm A.-K.E., Henry C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Liu L., Tian T., Zhao J., Park C.O., Lofftus S.Y., Stingley C.A., Yan Y., Mei S., Liu X., Kupper T.S. Epicutaneous immunization with modified vaccinia Ankara viral vectors generates superior T cell immunity against a respiratory viral challenge. npj Vaccin. 2021;6:1–9. doi: 10.1038/s41541-020-00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- Patarčić I., Gelemanovic A., Kirin M., Kolcic I., Theodoratou E., Baillie K.J., de Jong M.D., Rudan I., Campbell H., Polasek O. The role of host genetic factors in respiratory tract infectious diseases: systematic review, meta-analyses and field synopsis. Scientific Rep. 2015;5:16119. doi: 10.1038/srep16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.P., Kelly L.M., Cyster J.G. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol. 2010;22:413–419. doi: 10.1093/INTIMM/DXQ047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C.A., Preston-Hurlburt P., Dai Y., Aschner C.B., Cheshenko N., Galen B., Garforth S.J., Herrera N.G., Jangra R.K., Morano N.C., et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and inflammaging: risk factors of severe COVID-19 in older people. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijls B.G., Jolani S., Atherley A., Derckx R.T., Dijkstra J.I.R., Franssen G.H.L., Hendriks S., Richters A., Venemans-Jellema A., Zalpuri S., Zeegers M.P. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11:e044640. doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolla A., Nguyen T.H., Sant S., Jaffar J., Loudovaris T., Mannering S.I., Thomas P.G., Westall G.P., Kedzierska K., Wakim L.M. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 2018;128:721–733. doi: 10.1172/JCI96957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert L.W., Zilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., Klein A.M., Jaffe A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M.M.L., Rybkina K., Kato Y., Kubota M., Matsumoto R., Bloom N.I., Zhang Z., Hastie K.M., Grifoni A., Weiskopf D., et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021;6:9105. doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata Menezes N., Malone J., Lyons C., Cadet K., Dean L., Millett G., Baral S. Racial and ethnic disparities in viral acute respiratory infections in the United States: protocol of a systematic review. Syst. Rev. 2021;10:196. doi: 10.1186/s13643-021-01749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston L.E., Chevinsky J.R., Kompaniyets L., Lavery A.M., Kimball A., Boehmer T.K., Goodman A.B. Characteristics and disease severity of US children and Adolescents diagnosed with COVID-19. JAMA Netw. Open. 2021;4:e215298. doi: 10.1001/jamanetworkopen.2021.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine N.M., Klenerman P. MAIT cells in health and disease. Annu. Rev. Immunol. 2020;38:203–228. doi: 10.1146/ANNUREV-IMMUNOL-080719-015428. [DOI] [PubMed] [Google Scholar]
- Pulendran B.S., Arunachalam P., O’Hagan D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021:454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R., Campbell J., Murphy G., Richards W.G., Clark R.A., Kupper T.S. Resident Memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttur F., Gregory L.G., Lloyd C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell Biol. 2019;97:246–257. doi: 10.1111/imcb.12235. [DOI] [PubMed] [Google Scholar]
- Pyle C.J., Labeur-Iurman L., Groves H.T., Puttur F., Lloyd C.M., Tregoning J.S., Harker J.A. Enhanced IL-2 in early life limits the development of Tfh and protective antiviral immunity. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H. T follicular helper cells in space-time. Nat. Rev. Immunol. 2016;16:612–625. doi: 10.1038/nri.2016.94. [DOI] [PubMed] [Google Scholar]
- Rahmatpanah F., Agrawal S., Jaiswal N., Nguyen H.M., McClelland M., Agrawal A. Airway epithelial cells prime plasmacytoid dendritic cells to respond to pathogens via secretion of growth factors. Mucosal Immunol. 2018;12:77–84. doi: 10.1038/s41385-018-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J., Carragher D.M., Misra R.S., Kusser K., Hartson L., Moquin A., Lund F.E., Randall T.D. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J. Immunol. 2008;180:454–463. doi: 10.4049/JIMMUNOL.180.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjeva S., Subramanian R., Fang V.J., Leung G.M., Ip D.K.M., Perera R.A.P.M., Peiris J.S.M., Cowling B.J., Cobey S. Age-specific differences in the dynamics of protective immunity to influenza. Nat. Commun. 2019;10:1660. doi: 10.1038/s41467-019-09652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S.J., Franki S.N., Pierce R.H., Dimitrova S., Koteliansky V., Sprague A.G., Doherty P., de Fougerolles A.R., Topham D.J. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Ripperger T.J., Bhattacharya D. Transcriptional and Metabolic control of memory B cells and plasma cells. Annu. Rev. Immunol. 2021;39:345–368. doi: 10.1146/annurev-immunol-093019-125603. [DOI] [PubMed] [Google Scholar]
- Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., Van Eyk J.E., Cheng S., Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Invest. 2021;131 doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K.M., Choi S.M., McHugh K.J., Mandalapu S., Enelow R.I., Kolls J.K., Alcorn J.F. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1β production in mice. J. Immunol. 2013;191:5153–5159. doi: 10.4049/jimmunol.1301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.B., Morawski P.A., Pruner K.B., Fahning M.L., Howard C.A., Franko N., Logue J., Eggenberger J., Stokes C., Golez I., et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell Preprint at bioRxiv. 2022 doi: 10.1016/j.cell.2022.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands M., Segal F., Hartl D. 12, myeloid-derived suppressor cells as a potential biomarker and therapeutic target in COVID-19. Front. Immunol. 2021 doi: 10.3389/fimmu.2021.697405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluzzo S., Gorki A.D., Rana B.M., Martins R., Scanlon S., Starkl P., Lakovits K., Hladik A., Korosec A., Sharif O., et al. First-breath-induced type 2 pathways Shape the lung immune environment. Cell Rep. 2017;18:1893–1905. doi: 10.1016/J.CELREP.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant S., Grzelak L., Wang Z., Pizzolla A., Koutsakos M., Crowe J., Loudovaris T., Mannering S.I., Westall G.P., Wakim L.M., et al. Single-cell approach to influenza-specific CD8+ T cell receptor repertoires across different age groups, tissues, and following influenza virus infection. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappenfield E., Jamieson D.J., Kourtis A.P. Pregnancy and susceptibility to infectious diseases. Infect. Dis. Obstet. Gynecol. 2013 doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule P., Trauet J., Dutriez V., Lekeux V., Dessaint J.P., Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech. Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Schattgen S.A., Thomas P.G. TRH cells, helpers making an impact in their local community. Sci. Immunol. 2021;6 doi: 10.1126/SCIIMMUNOL.ABF2886. [DOI] [PubMed] [Google Scholar]
- Schneider C., Nobs S.P., Heer A.K., Kurrer M., Klinke G., van Rooijen N., Vogel J., Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10:e1004053. doi: 10.1371/JOURNAL.PPAT.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Nobs S.P., Heer A.K., Hirsch E., Penninger J., Siggs O.M., Kopf M. Frontline Science: Coincidental null mutation of Csf2rα in a colony of PI3Kγ-/- mice causes alveolar macrophage deficiency and fatal respiratory viral infection. J. Leukoc. Biol. 2017;101:367–376. doi: 10.1189/JLB.4HI0316-157R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp J.C., Khanal S., Gomez J.L., Sauler M., Adams T.S., Chupp G.L., Yan X., Poli S., Zhao Y., Montgomery R.R., et al. Single-cell transcriptional Archetypes of airway inflammation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2020;202:1419–1429. doi: 10.1164/RCCM.202004-0991OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert T.A., Victora G.D., Fooksman D.R., Kamphorst A.O., Mugnier M.R., Gitlin A.D., Dustin M.L., Nussenzweig M.C. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schyns J., Bai Q., Ruscitti C., Radermecker C., De Schepper S., Chakarov S., Farnir F., Pirottin D., Ginhoux F., Boeckxstaens G., et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun. 2019;10:3964–4016. doi: 10.1038/s41467-019-11843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G.C. Viruses and interferons. Annu. Rev. Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Severa M., Diotti R.A., Etna M.P., Rizzo F., Fiore S., Ricci D., Iannetta M., Sinigaglia A., Lodi A., Mancini N., et al. Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021;17:e1009878. doi: 10.1371/JOURNAL.PPAT.1009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Palaniappan L. improving diversity in medical research,” nature reviews disease primers. Nat. Res. 2021;7:74. doi: 10.1038/s41572-021-00316-8. [DOI] [PubMed] [Google Scholar]
- Shelton J.F., Shastri A.J., Ye C., Weldon C.H., Filshtein-Sonmez T., Coker D., Symons A., Esparza-Gordillo J., Chubb A., Fitch A., et al. The 23andMe COVID-19 Team Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat. Genet. 2021;53:801–808. doi: 10.1038/s41588-021-00854-7. [DOI] [PubMed] [Google Scholar]
- Shenoy A.T., Lyon De Ana C., Arafa E.I., Salwig I., Barker K.A., Korkmaz F.T., Ramanujan A., Etesami N.S., Soucy A.M., Martin I.M.C., et al. Antigen presentation by lung epithelial cells directs CD4+ TRM cell function and regulates barrier immunity. Nat. Commun. 2021;12:5834–5916. doi: 10.1038/s41467-021-26045-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z., Gitlin A.D., Targ S., Jankovic M., Pasqual G., Nussenzweig M.C., Victora G.D. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni Y., Fehlings M., Kloverpris H.N., McGovern N., Koo S.L., Loh C.Y., Lim S., Kurioka A., Fergusson J.R., Tang C.L., et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017;46:148–161. doi: 10.1016/J.IMMUNI.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove R.J., Goulding J., Didierlaurent A.M., Lyonga D., Vekaria S., Edwards L., Gwyer E., Sedgwick J.D., Barclay A.N., Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- Son Y.M., Cheon I.S., Wu Y., Li C., Wang Z., Gao X., Chen Y., Takahashi Y., Fu Y.X., Dent A.L., et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G.L. Surfactant protein D in respiratory and non-respiratory diseases. Front. Med. 2018;5:1. doi: 10.3389/FMED.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Soroosh P., Doherty T.A., Duan W., Mehta A.K., Choi H., Adams Y.F., Mikulski Z., Khorram N., Rosenthal P., Broide D.H., Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013;210:775–788. doi: 10.1084/JEM.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Sprent J., King C. COVID-19 vaccine side effects: the positives about feeling bad. Sci. Immunol. 2021;6:9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S., Begom S., Bermingham A., Hoschler K., Adamson W., Carman W., Bean T., Barclay W., Deeks J.J., Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- Standiford L.R., Standiford T.J., Newstead M.J., Zeng X., Ballinger M.N., Kovach M.A., Reka A.K., Bhan U. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am. J. physiologyLung Cell. Mol. Physiol. 2012;302:L447–L454. doi: 10.1152/AJPLUNG.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Steenhuijsen Piters W.A.A., Binkowska J., Bogaert D. Early life microbiota and respiratory tract infections. Cell Host and Microbe. 2020:223–232. doi: 10.1016/j.chom.2020.07.004. [DOI] [PubMed] [Google Scholar]
- de Steenhuijsen Piters W.A.A., Sanders E.A.M., Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philosophical Trans. R. Soc. B: Biol. Sci. R. Soc. Lond. 2015 doi: 10.1098/rstb.2014.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C.A., Martinez-Gonzalez I., Ghaedi M., Allinger P., Matha L., Takei F. Group 2 innate lymphoid cell activation in the neonatal lung drives type 2 immunity and allergen sensitization. J. Allergy Clin. Immunol. 2017;140:593–595.e3. doi: 10.1016/J.JACI.2016.12.984. [DOI] [PubMed] [Google Scholar]
- Strutt T.M., McKinstry K.K., Marshall N.B., Vong A.M., Dutton R.W., Swain S.L. Multipronged CD4+ T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunological Rev. 2013;255:149–164. doi: 10.1111/IMR.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamata R., Dobashi H., Nagao T., Yamamoto Ki, Nakajima N., Sato Y., Aratani Y., Oshima M., Sata T., Kobayashi K., et al. Contribution of neutrophil-derived myeloperoxidase in the early phase of fulminant acute respiratory distress syndrome induced by influenza virus infection. Microbiol. Immunol. 2012;56:171–182. doi: 10.1111/J.1348-0421.2011.00424.X. [DOI] [PubMed] [Google Scholar]
- Swadling L., Diniz M.O., Schmidt N.M., Amin O.E., Chandran A., Shaw E., Pade C., Gibbons J.M., Le Bert N., Tan A.T., et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2021;601 doi: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarnalekha N., Schreiner D., Litzler L.C., Iftikhar S., Kirchmeier D., Kunzli M., Son Y.M., Sun J., Moreira E.A., King C.G. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021;6:6808. doi: 10.1126/SCIIMMUNOL.ABB6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo P.A., Dogra P., Gray J.I., Wells S.B., Connors T.J., Weisberg S.P., Krupska I., Matsumoto R., Poon M.M., Idzikowski E., et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity. 2021;54:797–814.e6. doi: 10.1016/J.IMMUNI.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo P.A., Miron M., Farber D.L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 2019;4:9673. doi: 10.1126/sciimmunol.aas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi G.W., Andrews D.F., Lilly M.B., Singer J.W., Alderson M.R. Effect of granulocyte-macrophage colony-stimulating factor and interleukin-3 on interleukin-8 production by human neutrophils and monocytes. Blood. 1993;81:357–364. doi: 10.1182/BLOOD.V81.2.357.357. [DOI] [PubMed] [Google Scholar]
- Takamura S., Kato S., Motozono C., Shimaoka T., Ueha S., Matsuo K., Miyauchi K., Masumoto T., Katsushima A., Nakayama T., et al. Interstitial-resident memory CD8+ T cells sustain frontline epithelial memory in the lung. J. Exp. Med. 2019;216:2736–2747. doi: 10.1084/JEM.20190557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., Zhuang Y., Tham C.Y., Chia A., Smith G.J., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/J.CELREP.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.Y.S., Krasnow M.A. Developmental origin of lung macrophage diversity. Development (Cambridge) 2016;143:1318–1327. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.X., Esterbauer R., Vanderven H.A., Juno J.A., Kent S.J., Wheatley A.K. Inducible Bronchus-Associated Lymphoid Tissues (iBALT) Serve as Sites of B Cell Selection and Maturation Following Influenza Infection in Mice. Front. Immunol. 2019;10:611. doi: 10.3389/fimmu.2019.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., Bloom N.I., Goodwin B., Phillips E., Mallal S., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859.e11. doi: 10.1016/J.CELL.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Wiehe K., Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front. Immunol. 2019;10:332. doi: 10.3389/FIMMU.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Turner D., Pham Q., Wherry E.J., Lefrancois L., Farber D.L. Cutting Edge: tissue-Retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/JIMMUNOL.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D., Roberts E., Scott F., Martinborough E., Peach R., Oldstone M., Rosen H. Endothelial cells are central Orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/J.CELL.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker V.V., Sharma K., Dhar N., Mancini G., Sordet-Dessimoz J., McKinney J.D. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. 2021;22 doi: 10.15252/EMBR.202152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Severe Covid-19 GWAS Group Genomewide association study of severe covid-19 with respiratory failure. New Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/nejmoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J.J.C., Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T., Kato T., Lerner H., Shen Y., Farber D. Spatial Map of human T cell Compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/J.CELL.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.T., Chambers R.C., Liu K.D. In: Drazen J.M., editor. Vol. 377. 2017. Acute respiratory distress syndrome; pp. 562–572. [DOI] [PubMed] [Google Scholar]
- Todd E.M., Zhou J.Y., Szasz T.P., Deady L.E., D’Angelo J.A., Cheung M.D., Kim A.H.J., Morley S.C. Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli. Blood. 2016;128:2785–2796. doi: 10.1182/BLOOD-2016-03-705962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treerat P., Prince O., Cruz-Lagunas A., Munoz-Torrico M., Salazar-Lezama M.A., Selman M., Fallert-Junecko B., Reinhardt T.A., Alcorn J.F., Kaushal D., et al. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 2017;10:1069–1081. doi: 10.1038/mi.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B., Brownfield D.G., Wu A.R., Neff N.F., Mantalas G.L., Espinoza F.H., Desai T.J., Krasnow M.A., Quake S.R. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J.S., Schwartzberg P., Kotliarov Y., Biancotto A., Xie Z., Germain R., Wang E., Olnes M., Narayanan M., Golding H., et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157:499–513. doi: 10.1016/j.cell.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T.K., Lee K.H., Foxman B., Balmaseda A., Gresh L., Sanchez N., Ojeda S., Lopez R., Yang Y., Kuan G., Gordon A. Association between the respiratory microbiome and susceptibility to influenza virus infection. Clin. Infect. Dis. 2020;71:1195–1203. doi: 10.1093/CID/CIZ968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Minami S., Hashimoto R., Konishi Y., Suzuki T., Kondo T., Sasai M., Torii S., Ono C., Shichinohe S., et al. SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response. Nat. Aging. 2022;2:115–124. doi: 10.1038/s43587-022-00170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundup S., Kandasamy M., Perez J.T., Mena N., Steel J., Nagy T., Albrecht R.A., Manicassamy B. Endothelial cell tropism is a determinant of H5N1 pathogenesis in mammalian species. PLOS Pathog. 2017;13:e1006270. doi: 10.1371/JOURNAL.PPAT.1006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D.L., Bickham K.L., Thome J.J., Kim C.Y., D'Ovidio F., Wherry E.J., Farber D.L. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2013;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.S., Zhou J.Q., Han J., Schmitz A.J., Rizk A.A., Alsoussi W.B., Lei T., Amor M., McIntire K.M., Meade P., et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature. 2020;586:127–132. doi: 10.1038/s41586-020-2711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R.L., Klein S.L. Sex differences in respiratory viral pathogenesis and treatments. Annu. Rev. Virol. 2021;8:393–414. doi: 10.1146/annurev-virology-091919-092720. [DOI] [PubMed] [Google Scholar]
- Varma D.M., Batty C.J., Stiepel R.T., Graham-Gurysh E.G., Roque J.A., 3rd, Pena E.S., Hasan Zahid M.S., Qiu K., Anselmo A., Hill D.B., et al. Development of an intranasal Gel for the delivery of a broadly acting subunit influenza vaccine. ACS Biomater. Sci. Eng. 2022:2c00015. doi: 10.1021/ACSBIOMATERIALS.2C00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist N., Trittel S., Ebensen T., Chambers B.J., Guzman C.A., Riese P. Influenza-activated ILC1s contribute to antiviral immunity Partially influenced by differential GITR expression. Front. Immunol. 2018;9 doi: 10.3389/FIMMU.2018.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Pallerla S.R., Ruter J., Augustin Y., Kremsner P.G., Krishna S., Meyer C.G. Host genetic factors determining COVID-19 susceptibility and severity. eBioMedicine. 2021;72:103629. doi: 10.1016/J.EBIOM.2021.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar B., Boustani K., Ogger P.P., Papadaki A., Tonkin J., Orton C.M., Ghai P., Suveizdyte K., Hewitt R.J., Desai S.R., et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55:542–556.e5. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman H., Hendriks R.W., Kool M. Dendritic cell subsets in asthma: Impaired Tolerance or exaggerated inflammation? Front. Immunol. 2017;8:941. doi: 10.3389/fimmu.2017.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.C., Dash P., McCullers J.A., Doherty P.C., Thomas P.G. T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci. Translational Med. 2012;4 doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hossain M., Thanabalasuriar A., Gunzer M., Meininger C., Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 2017;358:111–116. doi: 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- Wang T.T., Maamary J., Tan G., Bournazos S., Davis C., Krammer F., Schlesinger S., Palese P., Ahmed R., Ravetch J. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell. 2015;162:160–169. doi: 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Sewatanon J., Memoli M.J., Wrammert J., Bournazos S., Bhaumik S.K., Pinsky B.A., Chokephaibulkit K., Onlamoon N., Pattanapanyasat K., et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science (New York, N.Y.) 2017;355:395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang C., Song Y., Coleman J.R., Stawowczyk M., Tafrova J., Tasker S., Boltz D., Baker R., Garcia L., et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. United States America. 2021;118 doi: 10.1073/PNAS.2102775118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhu L., Nguyen T.H.O., Wan Y., Sant S., Quinones-Parra S.M., Crawford J.C., Eltahla A.A., Rizzetto S., Bull R.A., et al. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat. Commun. 2018;9:824–912. doi: 10.1038/s41467-018-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala C.L., et al. ACS Central Science; 2022. Mucins Inhibit Coronavirus Infection in a Glycan-dependent Manner; p. 1c01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford W.T., Ghio A.J., Wright J.R. Complement-mediated host defense in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L790–L798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- Weizman O. El, Adams N.M., Schuster I.S., Krishna C., Pritykin Y., Lau C., Degli-Esposti M.A., Leslie C.S., Sun J.C., O’Sullivan T.E. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808.e12. doi: 10.1016/J.CELL.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen K., Gusarova G.A., Islam M.N., Subramanian M., Cohen T.S., Prince A.S., Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley A.K., et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021:956–959. doi: 10.1016/j.it.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T.N. Provisional matrix: a role for versican and hyaluronan. Matrix Biol. 2017;60–61:38–56. doi: 10.1016/J.MATBIO.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., STOP-HCV consortium. Scherwitzl I., Hutchinson E.C., Leng T., Kurioka A., Kulicke C., de Lara C., Cole S., Vasanawathana S., Limpitikul W., et al. MAIT cells are activated during human viral infections. Nat. Commun. 2016;7:11653–11711. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., Loh L., Chen Z., et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat Commun. 2018;9:4706. doi: 10.1038/s41467-018-07207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T.M., Li C.K.F., Chui C.S.C., Huang A.K.Y., Perkins M., Liebner J.C., Lambkin-Williams R., Gilbert A., Oxford J., Nicholas B., et al. Preexisting influenza-specific CD4 + T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- Wizemann T.M., Laskin D.L. Enhanced phagocytosis, chemotaxis, and production of reactive oxygen intermediates by interstitial lung macrophages following acute endotoxemia. 1994;11:358–365. doi: 10.1165/AJRCMB.11.3.8086172. [DOI] [PubMed] [Google Scholar]
- Wong S.S., Oshansky C.M., Guo X.Z.J., Ralston J., Wood T., Seeds R., Newbern C., Waite B., Reynolds G., Widdowson M.A., et al. for the SHIVERS Investigation Team Severe influenza is characterized by prolonged immune activation: results from the SHIVERS cohort study. J. Infect. Dis. 2018;217:245–256. doi: 10.1093/infdis/jix571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.-S., Oshansky C.M., Guo X.Z.J., Ralston J., Wood T., Reynolds G.E., Seeds R., Jelley L., Waite B., Jeevan T., et al. Activated CD4+ T cells and CD14hiCD16+ monocytes correlate with antibody response following influenza virus infection in humans. Cell Rep. Med. 2021;2:100237. doi: 10.1016/j.xcrm.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbs T., Hammerschmidt S., Förster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO Global Health Estimates; 2020. The Top 10 Causes of Death. [Google Scholar]
- Wu H., Kumar A., Miao H., Holden-Wiltse J., Mosmann T.R., Livingstone A.M., Belz G.T., Perelson A.S., Zand M.S., Topham D.J. Modeling of influenza-specific CD8+ T cells during the primary response Indicates that the spleen is a major source of effectors. J. Immunol. 2011;187:4474–4482. doi: 10.4049/JIMMUNOL.1101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hu Y., Lee Y.T., Bouchard K.R., Benechet A., Khanna K., Cauley L.S. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014;95:215–224. doi: 10.1189/JLB.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Goplen N.P., Sun J. Aging and respiratory viral infection: from acute morbidity to chronic sequelae. Cell Biosci. 2021 doi: 10.1186/s13578-021-00624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Keith J., Samilo D., Carter R., Leiner I., Pamer E. Innate lymphocyte/Ly6Chi monocyte Crosstalk promotes Klebsiella pneumoniae clearance. Cell. 2016;165:679–689. doi: 10.1016/J.CELL.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Biermann M.H., Brauner J.M., Liu Y., Zhao Y., Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front. Immunol. 2016;7:302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youk J., Kim T., Evans K.V., Jeong Y.I., Hur Y., Hong S.P., Kim J.H., Yi K., Kim S.Y., Na K.J., et al. Three-dimensional human alveolar stem cell Culture models reveal infection response to SARS-CoV-2. Cell Stem Cell. 2020;27:905–919.e10. doi: 10.1016/J.STEM.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Buttgereit A., Lelios I., Utz S.G., Cansever D., Becher B., Greter M. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–912.e4. doi: 10.1016/J.IMMUNI.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Zani A., et al. Interferon-induced transmembrane protein 3 (IFITM3) limits lethality of SARS-CoV-2 in mice. bioRxiv. 2021 doi: 10.1101/2021.12.22.473914. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragosi L.E., Deprez M., Barbry P. Using single-cell RNA sequencing to unravel cell lineage relationships in the respiratory tract. Biochem. Soc. Trans. 2020:327–336. doi: 10.1042/BST20191010. [DOI] [PubMed] [Google Scholar]
- Zeberg H., Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587:610–612. doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- Zens K.D., Chen J.K., Farber D.L. Vaccine-generated lung tissue–resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1 doi: 10.1172/JCI.INSIGHT.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Leal J., Soto M.R., Smyth H.D.C., Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of Experiments. Pharmaceutics. 2020;12:1042. doi: 10.3390/PHARMACEUTICS12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., He F., Li P., Hardwidge P.R., Li N., Peng Y. The Role of Innate Immunity in Pulmonary Infections. Biomed. Res. Int. 2021;2021:6646071. doi: 10.1155/2021/6646071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.J., QU Jm, SHAO Cz, ZHANG J., HE Lx, YUAN Zh. Aspergillus fumigatus conidia upregulates NOD2 protein expression both in vitro and in vivo1. Acta Pharmacologica Sinica. 2008;29:1202–1208. doi: 10.1111/J.1745-7254.2008.00860.X. [DOI] [PubMed] [Google Scholar]
- Zhou G., Juang S.W.W., Kane K.P. NK cells exacerbate the pathology of influenza virus infection in mice. Eur. J. Immunol. 2013;43:929–938. doi: 10.1002/EJI.201242620. [DOI] [PubMed] [Google Scholar]
- Zhu L., Liu L., Zhang Y., Pu L., Liu J., Li X., Chen Z., Hao Y., Wang B., Han J., et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J. Infect. Dis. 2018;217:428–437. doi: 10.1093/INFDIS/JIX475. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C.N., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/JCI.INSIGHT.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]