Abstract
Background
It was generally accepted that obesity could increase the morbidity and mortality of surgical patients. However, the influence of body mass index (BMI) on short-term and long-term surgical outcomes of laparoscopic hepatectomy (LH) for patients with liver carcinoma remains unclear. The aim of this study was to evaluate the influence of BMI on surgical outcomes.
Methods
From August 2003 to April 2016, 201 patients with liver carcinoma who underwent LH were enrolled in our study. Based on their BMI in line with the WHO’s definition of obesity for the Asia-Pacific region, patients were divided into three groups: underweight (BMI< 18.5 kg/m2), normal weight (18.5≤BMI< 23 kg/m2), and overweight (BMI≥ 23 kg/m2). Demographics and surgical outcomes of laparoscopic hepatectomy were compared in different BMI stratification. We investigated overall survival and relapse-free survival across the BMI categories.
Results
Of the 201 patients, 23 (11.44%) were underweight, 96 (47.76%) were normal weight, and 82 (40.80%) were overweight. The overall complication rate in the underweight group was much higher than that in the normal weight and overweight groups (p=0.048). Postoperative complications, underweight patients developed grade III or higher Clavien-Dindo classifications (p=0.042). Among the three BMI groups, there were no significant differences in overall and relapse-free survival with Kaplan-Meier analysis (p=0.104 and p=0.190, respectively). On the other hand, gender, age, liver cirrhosis, bile leak, ascites, and Clavien classification (III-IV) were not independent risk factors for overall and relapse-free survival in multivariable Cox proportional hazards models.
Conclusions
BMI status does not affect patients with liver carcinoma long-term surgical outcomes concerned to overall survival and relapse-free survival after laparoscopic hepatectomy. However, being underweight was associated with an increased perioperative complication rate, and perioperative careful monitoring might be required after hepatectomy for underweight with liver carcinoma.
Keywords: Body mass index, Liver carcinoma, Laparoscopic Hepatectomy, Risk factors, Long-term outcome, underweight patients with liver carcinoma.
Introduction
Liver carcinoma is one of the most malignant cancers worldwide and has a particularly high incidence rate in Asian countries. Liver resection is the main method used for the treatment of liver cancer, and the potential advantages of laparoscopy, such as minimally invasive technique, fewer complications, and shorter intraoperative hospital stay, have been identified [1, 2]. Although patients could get an early diagnosis combined with comprehensive treatment nowadays, due to the tumor’s malignant character, the prognosis of liver cancer remains unsatisfactory [3, 4]. Factors such as age, tumor size and tumor number, pathologic TNM stage, and vascular invasion have been identified as factors influencing the prognosis of liver cancer patients [5–7]. Several researchers have found that overweight status increases the risk of hepatocellular carcinoma (HCC) by 17%, and obese status increases the risk of HCC by 90% compared with normal-weight individuals [8, 9]. However, the relationship between weight and laparoscopic hepatectomy prognosis for liver carcinoma remains unclear.
Body mass index (BMI; kg/m2) is a convenient and simple surrogate measure of body fat distribution in clinical settings. The BMI values used to detect overweight and obese status recommended by the World Health Organization (>25 kg/m2) are higher than those suggested by Asian population-based studies (22-25 kg/m2) [10, 11]. The Working Group on Obesity in China has identified that a BMI of 23 is the most sensitive and specific indicator for the overweight status of Chinese people [12]. Therefore, it is useful to evaluate the association between the prognosis of liver carcinoma and overweight defined by this BMI value.
The prognosis of liver cancer remains unsatisfactory. Survival rates at 6, 12, and 24 months after the initial diagnosis have been reported to be 44.1%, 21.7%, and 14.2%, respectively [13]. Many studies have focused on the Child-Pugh score and the a-fetoprotein (AFP) concentration to determine the prognosis of liver cancer [14, 15]. However, the data concerning the influence of underweight and overweight status on this disease have not yet to be defined. The purpose of this study was to assess the effects of weight using BMI on relevant perioperative complications and the overall and relapse-free survival rates in patients with liver carcinoma who underwent laparoscopic hepatectomy.
Methods
Patients and diagnosis
A retrospective cohort study that spanned a 13-year period from August 2003 to April 2016 was performed. A total of 201 patients who underwent laparoscopic hepatectomy for liver carcinoma at our institution were identified. Institutional Review Board approval for this study was obtained from the Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University.
Patients were divided into three groups by BMI according to the WHO’s definition of obesity restricted to the Asia-Pacific region: underweight < 18.5 kg/m2, normal weight 18.5–23 kg/m2, and overweight>23 kg/m2 [16]. BMI was calculated in line with the standardized definition as weight in kilograms divided by height in meters squared, and BMI was recorded on the morning of the surgery.
The diagnosis of liver carcinoma was established using imaging enhanced computed tomography (CT) or magnetic resonance imaging (MRI)) and pathology reports.
Inclusion and exclusion criteria
LH was chosen as the initial therapy for all patients diagnosed with a liver tumor in the present study. The tumor size larger than 10 cm, with tumors invading major vessels, Child-Plug score worse than B, with tumor thrombus in the main portal vein were restricted to the procedure of laparoscopy. Patients who were younger than 18 years old, ASA > IV, or pregnancy were excluded from the study.
Data collection
Surgical technique
Laparoscopic hepatectomy was performed as previously described [17]. Regional occlusion of the left/right inflow and outflow of the liver instead of total hepatic vascular occlusion was used to minimize liver ischemia/reperfusion injury [16]. In the early years of the study (2003–2006), parenchymal transection of the liver was achieved with the LPMOD (Peng’s multifunction operative dissector, SY-IIIB, Hangzhou ShuYou Medical Equipment Co., Ltd, China) technique. Since 2007, most cases have been performed using an ultrasonic aspirator (CUSA; Valleylab, Boulder, Colo). If there were unclear tumor margins, uncontrolled bleeding, embolism, severe adhesion, or other complications, the laparoscopic procedure was changed to open hepatectomy.
Follow-up and analysis
After being discharged from the hospital, all patients were followed up monthly in the first year. The follow-up included physical examinations, a computed tomographic scan or magnetic resonance imaging scan, and alpha-feto-protein (AFP) measurements. If no recurrence was detected, we extended the follow-up time to a quarter. Recurrence was defined as new typical features of a mass on imaging or a rising AFP level. A biopsy was performed if necessary. Survival was defined as the interval from the date of diagnosis of liver cancer to the date of death or the last visit before February 2019.
All patients were followed up in February 2019, and the median follow-up duration was 27 months (range, 2 months).
Data analysis
We used the Shapiro–Wilk test to confirm the normality of data distribution. We presented continuous data as the mean (SD), and we presented categorical data as numbers or percentages. A one-way ANOVA combined with the Bonferroni test for post hoc testing was used in analyzing the continuous data. The chi-square or Fisher’s exact test combined with the Mann–Whitney U test for post hoc testing was used in analyzing the categorical data. The durations of overall survival and relapse-free survival were calculated by Kaplan-Meier analysis, and the results for the subgroups of patients were compared with the log-rank test. A p value < 0.05 was regarded as statistically significant. All statistical analysis was conducted with SPSS version 19.0.
Results
Patient-related variables
Table 1 shows the basic demographics of the patients with the liver disease for the three groups. A total of 201 patients were included in the study. Among them, there were 23 (11.4%) underweight, 96 (47.8%) normal weight, and 82 (40.8%) overweight patients. The overweight group had significant diabetic complications (p<0.01). Patient characteristics, including age, sex, Child-Pugh score, American Society of Anesthesiologists (ASA) score, hypertension, pulmonary, cardiovascular, and cerebrovascular were not significantly different between the three groups, except for liver cirrhosis (p<0.005), were not significantly different between the three groups. But concerning to the cirrhosis, the overweight patients had a significant occurrence(p<0.05).
Table 1.
Patient Demographics with liver cancer stratified by BMI status
| Variables | Underweight (N=23) | Normal weight (N=96) N=201 |
Overweight (N=82) | p value |
|---|---|---|---|---|
| Age (years)a | 55.96±18.78 | 57.08±12.11 | 58.17±12.49 | 0.736 |
| Gender | ||||
| Male | 14 (60.9) | 75 (78.1) | 62 (75.6) | 0.226 |
| Female | 9 (39.1) | 21 (21.9) | 20 (24.4) | |
| ASA score | ||||
| 1–2 | 20 (87.0) | 83 (86.5) | 69 (84.1) | 0.891 |
| 3–4 | 3 (13.0) | 13 (13.5) | 13 (15.9) | |
| Diabetes mellitus | 2 (8.7) | 2 (2.1) | 15 (18.3) | <0.01* |
| Hypertension | 1 (4.3) | 8 (8.3) | 10 (12.2) | 0.46 |
| Pulmonary comorbidity | 1 (4.3) | 11 (11.5) | 3 (3.7) | 0.45 |
| Cardiovascular | 1 (4.3) | 2 (2.1) | 3 (3.7) | 0.76 |
| Cerebrovascular | 1 (4.3) | 1 (1.0) | 0 (0) | 0.18 |
| Child-Pugh score | ||||
| Class A | 22 (95.7) | 86 (89.6) | 71 (86.6) | 0.457 |
| Class B | 1 (4.3) | 10 (10.4) | 11 (13.4) | |
| Liver cirrhosis | 3 (15) | 48 (100) | 32 (54) | 0.005* |
| Album g/dl | 3.86±0.49 | 4.04±0.50 | 3.98±0.59 | 0.385 |
ASA American Society of Anesthesiologists, *p<0.05
Tumor-related variables
Table 2 displays the oncological status, including tumor size, tumor number, tumor stage, the extent of live resection, and UICC. The oncological status was comparable in the three BMI groups.
Table 2.
Oncological status stratified by BMI status
| Variables | Underweight N=23 |
Normal weight N=96 |
Overweight N=82 |
p value |
|---|---|---|---|---|
| Tumor size | 4.04±2.43 | 3.91±2.03 | 3.53±1.94 | 0.377 |
| Tumor number | 1.43±1.04 | 1.23±0.59 | 1.21±0.60 | 0.330 |
| Tumor | ||||
| Cholangiocarcinoma | 0 (0) | 6 (6.3) | 3 (3.7) | 0.389 |
| Hepatocellular carcinoma | 18 (78.3) | 77 (80.2) | 68 (83.0) | |
| Adenocarcinoma | 3 (13.0) | 5 (5.2) | 2 (2.4) | |
| Metastatic hepatic | 2 (8.7) | 8 (8.3) | 9 (11.0) | |
| UICC | ||||
| Stage I | 8 (34.8%) | 34 (35.4%) | 27 (32.9%) | 0.822 |
| Stage II | 7 (30.4%) | 46 (47.9%) | 38 (46.3%) | |
| Stage IIIA | 2 (8.7%) | 10 (10.4%) | 13 (15.9%) | |
| Stage IIIB | 4 (17.4%) | 5 (5.2%) | 4 (4.9%) | |
| Stage IIIC | 2 (8.7%) | 1 (1.0%) | 0 (0) | |
| Extent of live resection | ||||
| Wedge | 4 (17.4) | 17 (17.7) | 22 (26.8) | 0.555 |
| Segmentectomy | 15 (65.2) | 59 (61.5) | 48 (58.5) | |
| Hemihepatectomy | 4 (17.4) | 20 (20.8) | 12 (14.6) | |
UICC Union for International Cancer Control, *p<0.05
Short-term outcomes
Table 3 shows short-term outcomes stratified by BMI status. The conversion rates were 13.0%, 14.6%, and 14.6% for the three BMI groups. The mean postoperative length of hospital stay for the three groups was 9.87±4.36 days, 10.10±5.88 days, and 9.82±5.27 days, p= 0.938, respectively. Interestingly, the overall complication rate in the underweight group was much higher than that in the normal weight and overweight groups (47.82% vs 21.88% vs 17.10%, respectively, p=0.048). Regarding postoperative complications, underweight patients developed grade III or higher Clavien-Dindo classifications more easily than patients in the other two groups (p=0.042). The underweight group had significantly higher comorbidity rates (p=0.048) of bile leak and ascites (p=0.037 and p=0.032, respectively) than the normal weight group. Other variables, including estimated blood loss and blood transfusion, as well as postoperative complications, intraabdominal sepsis, surgical site infection, pneumonia, bleeding, and chemotherapy.
Table 3.
Short-term outcomes stratified by BMI status
| Variables | Underweight | Normal weight | Overweight | p value |
|---|---|---|---|---|
| Estimated blood loss (ml, mean ± SD) | 635.65±605.51 | 468.48±760.08 | 426.46±634.45 | 0.444 |
| Blood transfusion | 178.26±285.97 | 198.13±493.80 | 152.56±368.28 | 0.777 |
| Conversion | 3 (13.0) | 14 (14.6) | 12 (14.6) | 0.980 |
| Operative time (min, mean ± SD) | 177.04±110.10 | 171.24±104.10 | 159.93±79.50 | 0.641 |
| Postoperative length of stay (D) | 9.87±4.36 | 10.10±5.88 | 9.82±5.27 | 0.938 |
| Complication | 11 (47.8) | 21 (21.9) | 14 (17.1) | 0.048* |
| Bile leak | 5 (21.7) | 6 (6.3) | 4 (4.9) | 0.037* |
| Introabodominal sepsis | 1 (4.3) | 4 (4.2) | 3 (3.7) | 0.432 |
| Surgical site infection | 0 (0) | 3 (3.1) | 1 (1.2) | 0.737 |
| Ascites | 3 (13.0) | 4 (4.2) | 0 (0) | 0.032* |
| Pneumonia | 2 (8.7) | 3 (3.1) | 3 (3.7) | 0.758 |
| Bleeding | 0 (0) | 1 (1.0) | 3 (3.7) | 0.647 |
| Clavien classification | ||||
| I-II | 5 (21.7) | 18 (18.8) | 15 (18.3) | 0.732 |
| III–IV | 2 (8.7) | 3 (3.1) | 1 (1.2) | 0.042* |
| Chemotherapy | 7 (30.4) | 38 (39.6) | 38/82 (46.3) | 0.442 |
*p<0.05
Long-term outcomes
The median follow-up durations were 26 months, 30 months, and 28 months in the underweight, normal weight, and overweight groups, respectively. Kaplan-Meier survival curve analysis of overall survival rate and relapse-free survival rate in patients stratified by the BMI are shown in Figs. 1 and 2. Overall and relapse-free survival showed no significance among the three groups (p=0.104 and p=0.190, respectively). The variables that have important clinical significance were incorporated into the multivariate analysis. The results revealed that gender, age, liver cirrhosis, bile leak, ascites, and Clavien classification (III-IV) were not independent risk factors for overall and relapse-free survival. So maybe preoperative nutritional supplementation could improve outcomes, as was done in the ERAS setting.
Fig. 1.
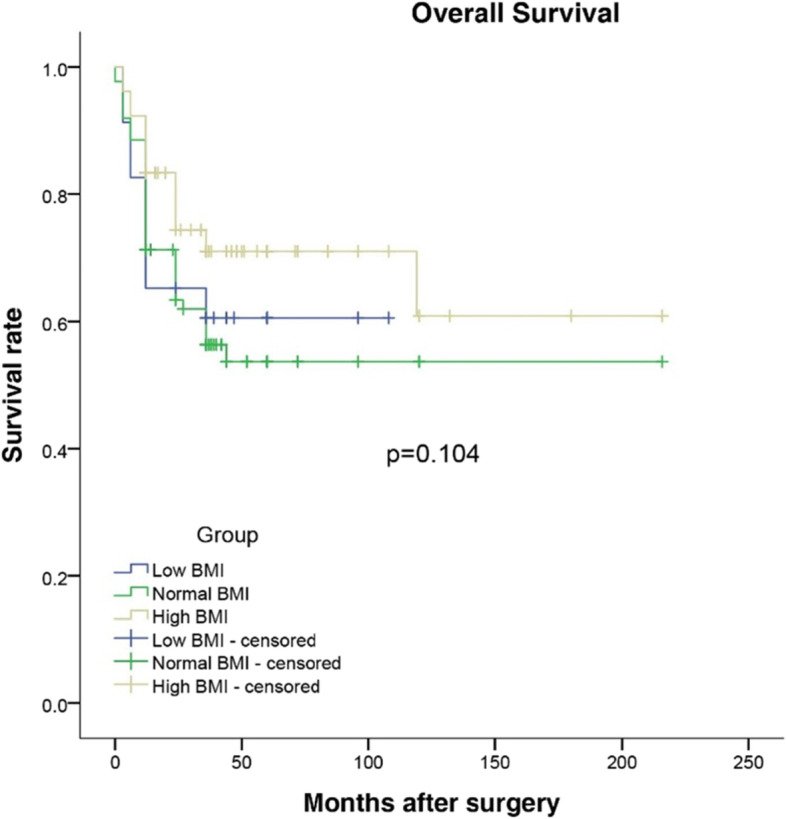
Overall survival after laparoscopic hepatectomy for patients with liver cancer stratified by BMI status
Fig. 2.
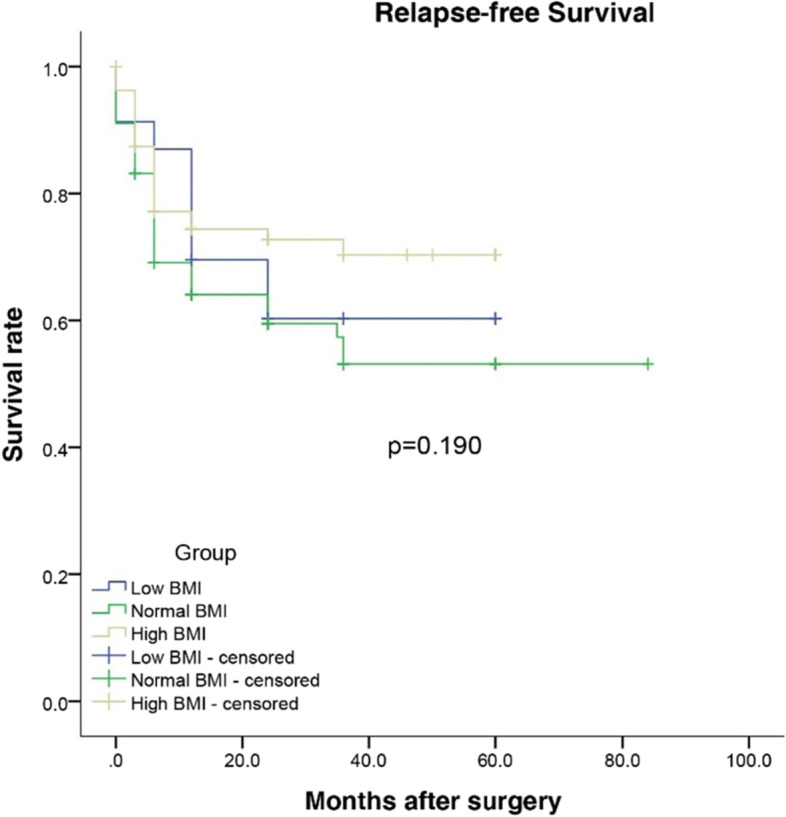
Relapse-free survival after laparoscopic hepatectomy for patients with liver cancer stratified by BMI status
Discussion
Laparoscopic hepatectomy has become a feasible option for patients with liver malignancy. As it is a new technique, researchers have given more attention to perioperative and long-term outcomes. Although being overweight and obese does not preclude laparoscopy [16], little is known about the influence of recipient BMI on long-term outcomes, especially the recurrence rate and the overall survival rate after LH for HCC. In the present study, we observed that patients with elevated BMI accounted for 40.8%. General surgeons will encounter more overweight and obese patients with liver cancer in the future [18]. Therefore, it is important to fully understand the effect of elevated BMI on these patients. We carried out this retrospective analysis using our institutional database and showed that (i) being overweight did not increase the conversion rate, (ii) being underweight was associated with an increased number of complications and more severe complications, and (iii) being overweight does not affect patients with liver carcinoma surgical outcomes after laparoscopic hepatectomy. It was interesting to find that being overweight is not an independent risk factor for overall and relapse-free survival for liver carcinoma patients.
Many studies have demonstrated that increased BMI increases the laparoscopic conversion rate and prolongs the operative time [19, 20]; thus, surgeons tend to be reluctant to perform laparoscopic surgery for overweight patients. However, there were no differences in the conversion rate and operative time across the three groups in our study, which was consistent with Troisi et al. [21]. This may be because all operations were performed by the same experienced surgeon team who had finished training and was capable of removing liver tumors using laparoscopic techniques, and even more, there were no patients with BMI greater than 40 in the present study. Meanwhile, not in line with previous studies, which found that overweight patients had worse outcomes than their leaner counterparts [22], our results showed that overweight patients had a lower complication rate (17.10%) compared to underweight (47.82%) and normal-weight patients (21.88%) and that decreased BMI values were a risk factor for a higher incidence of postoperative complications. We also found that severe complications of grades III to IV according to the Clavien-Dindo classification were much more frequent in the underweight group than in the normal weight and overweight group. One cause may be that overweight patients are protected by adequate fat storage, better nutrition, and systemic insulin resistance which underweight people do not have [23]. Meanwhile, patients with a lower BMI preoperatively are more likely to indicate excessive nutritional consumption and malnutrition resulting from the more aggressive tumor [24–26].
To our knowledge, little research has investigated the association between BMI and prognosis in patients undergoing LH with liver cancer. Daniel reported that overweight status had better oncologic outcomes following hepatectomy in HCC patients [27], but the endpoint of Daniel’s study was 3 months. The follow-up duration was relatively short, and more data were needed on the overall survival rate as an important therapeutic measure of liver cancer if treatment intensity and life expectancy were to be judged [15]. Chronic disorders such as cardiovascular disease, hypertension, and diabetes, which are closely related to obesity, increase the risk of physical disability and mortality rates in the long run [28]. It has been reported that nearly 70% of deaths related to high BMIs are due to cardiovascular disease, and over 60% of those deaths occur among obese patients [29]. Our study aimed to verify the long-term outcome of liver carcinoma patients who underwent LH, and our results suggested that BMI status had no impact on either overall or relapse-free survival in univariate and multivariate analysis. Therefore, the influence of BMI on survival for liver carcinoma patients who underwent LH remains controversial; thus, multicenter and large sample studies were future needed.
Moreover, as reported, postoperative complications had a negative impact on tumor recurrence and the long-term survival rate, especially for severe postoperative complications [30]. In our study, we again verified that postoperative complications might have an effect on the prognosis of liver cancer patients. We found that underweight patients suffered more complications. One reason is that the surgical trauma and tissue damage of the complications could result in immune suppression and, in turn increase the possibility of immune escape and tumor progression [31, 32]. Second, a large number of cytotoxic mediators from the inflammatory response caused by the infected complications could provide a microenvironment for the growth and invasion of tumor cells and further promote the development of tumor recurrence [33]. At the same time, minimally invasive laparoscopic surgery could avoid complications such as poor wound healing and pulmonary infection caused by a long incision, and it yielded more advantages for overweight patients.
This study has some limitations. First, it was a retrospective, non-randomized survey that could not been avoided selection bias. Due to few patients in the underweight group, type II error is likely to be the cause of stage III tumor in low-weight patients. Second, this was a single-center study, and there is still no standard treatment for liver cancer. The results in our center might have been influenced by our surgical strategy. Third, we only used BMI to assess overweight, which was inadequate for assessing the abdominal adiposity of Asian people.
Despite these limitations, our study helps to clarify that LH for the treatment of overweight patients with hepatocellular carcinoma is feasible and safe. These results need to be verified by larger prospective and randomized studies.
Conclusion
Our data showed that BMI status does not affect patients with liver carcinoma long-term surgical outcomes concerned to overall survive and relapse-free survival after laparoscopic hepatectomy. Patients can be scheduled for surgery normally, regardless overweight and underweight. However, perioperative careful monitoring might be required for
Acknowledgements
The authors would like to thank LiQingQuan for assistance with the statistical analysis. The first two authors contributed equally to the study.
Abbreviations
- BMI
Body mass index
- LH
Laparoscopic hepatectomy
- ASA
American Society of Anesthesiologists
- LPMOD
Peng’s multifunction operative dissector
- CT
Enhanced computed tomography
- MRI
Magnetic resonance imaging
- AFP
Alpha-feto-protein
- HCC
Hepatocellular carcinoma
Authors’ contributions
ZL performed the statistical analysis and drafted the manuscript. WJG and YX conducted the study and managed the database. KJX and ZX participated in its design and drafted the manuscript. The authors have approved the final manuscript.
Funding
This work was supported by funds from the Zhejiang Province Natural Science Foundation (LY19H030011) and Public Health Bureau of Zhejiang Province (2016KYA152).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the institutional Ethical Board of Sir Run Run Shaw Hospital of Zhejiang University. The need for informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci. 2015;22:342–352. doi: 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28:950–960. doi: 10.1007/s00464-013-3254-3. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:39–45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford MJ, Arnold M, Bardot A, Ferlay J, De P. Comparison of liver cancer incidence and survival by subtypes across seven high-income countries. Int J Cancer. 2021;149(12):2020–2031. doi: 10.1002/ijc.33767. [DOI] [PubMed] [Google Scholar]
- 5.Esnaola NF, Mirza N, Lauwers GY, Ikai I, Regimbeau JM, Belghiti J, Yamaoka Y, Curley SA, Ellis LM, Nagorney DM, Vauthey JN. Comparison of clinicopathologic characteristics and outcomes after resection in patients with hepatocellular carcinoma treated in the United States, France, and Japan. Ann Surg. 2003;238:711–719. doi: 10.1097/01.sla.0000094436.34556.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, Abdalla EK. Tumour size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 7.Sakuraoka Y, Kubota K, Tanaka G, et al. Is left-sided involvement of hepatocellular carcinoma an important preoperative predictive factor of poor outcome? World J Surg Oncol. 2020;18(1):1–9. doi: 10.1186/s12957-020-02100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 9.Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, Serraino D, Franceschi S, Talamini R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 2009;20:353–357. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 10.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu LN, Miaskowski C, Wang JS, Chen SC, Chen ML. Accuracy of body mass index to determine obesity in women with breast cancer: an observational study of Taiwanese sample. Int J Nurs Stud. 2010;47:994–1000. doi: 10.1016/j.ijnurstu.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Tong G, Tong W, Lu L, Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health. 2011;13(11):35. doi: 10.1186/1471-2458-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global data on body mass index. BMI classification. http://apps.who.int/bmi/index.jsp (Accessed 3 December 2009).
- 14.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi CK. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J. Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 16.Xin Y, Yu H, Fang X, Yu X, Yu H, Fang XM. The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiology. 2016;16(29). 10.1186/s12871-016-0194-1. [DOI] [PMC free article] [PubMed]
- 17.Cai XJ, Li ZY, Zhang YL, Yu H, Liang X, Jin RN, Luo F. Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc. 2014;28(4):1334–1341. doi: 10.1007/s00464-013-3333-5. [DOI] [PubMed] [Google Scholar]
- 18.Barakat O, Skolkin MD, Toombs BD, et al. Major liver resection for hepatocellular carcinoma in the morbidly obese: a proposed strategy to improve outcome. World J Surg Oncol. 2008;6(1):1–5. doi: 10.1186/1477-7819-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Wu L, Li X, Wu X, Li B. Outcome of laparoscopic colorectal surgery in obese and nonobese patients: a meta-analysis. Surg. Endosc. 2012;26(3):783–789. doi: 10.1007/s00464-011-1952-2. [DOI] [PubMed] [Google Scholar]
- 20.Duan XF, Tang P, Shang XB, Jiang HJ, Zhao Q, Yu ZT. High body mass index worsens survival in patients with esophageal squamous cell carcinoma after esophagectomy. Dig Surg. 2017;34:319–327. doi: 10.1159/000453044. [DOI] [PubMed] [Google Scholar]
- 21.Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB. 2014;16(1):75–82. doi: 10.1111/hpb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathur AK, Ghaferi AA, Osborne NH, Pawlik TM, Campbell DA, Englesbe MJ, Welling TH. Body mass index and adverse perioperative outcomes following hepatic resection. J. Gastrointest. Surg. 2010;14:1285–1291. doi: 10.1007/s11605-010-1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rui R, Lou J, Zou L, Zhong R, Wang J, Xia D, Wang Q, Li H, Wu J, Lu X, Li C, Liu L, Xia J, Xu H. Excess body mass index and risk of liver cancer: a nonlinear dose-response meta-analysis of prospective studies. PLoS ONE. 2012;7(9):e44522. doi: 10.1371/journal.pone.0044522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. Analysis of the impact of the body mass index in patients with gastric carcinoma. Surg. Today. 2012;42(10):945–949. doi: 10.1007/s00595-012-0183-z. [DOI] [PubMed] [Google Scholar]
- 25.Horii N, Sawda Y, Kumamoto T, et al. Impact of intramuscular adipose tissue content on short-and long-term outcomes of hepatectomy for colorectal liver metastasis: a retrospective analysis. World J Surg Oncol. 2020;18(1):1–10. doi: 10.1186/s12957-020-01836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Xing H, Liu D, et al. Negative impact of low body mass index on liver cirrhosis patients with hepatocellular carcinoma. World J Surg Oncol. 2015;13(1):1–6. doi: 10.1186/1477-7819-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heise D, Bednarsch J, Kroh A, et al. Laparoscopic hepatectomy reduces postoperative complications and hospital stay in overweight and obese patients. World J Gastrointest Surg. 2021;13(1):19–29. doi: 10.4240/wjgs.v13.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health, United States, 2015 . With special feature on racial and ethnic health disparities. Hyattsville: National Center for Health Statistics (US); 2016. p. 461. [PubMed] [Google Scholar]
- 29.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada H, Fukagawa T, Haga Y, Oba K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg. 2017;1:11–23. doi: 10.1002/ags3.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Sierzega M, Kolodziejczyk P, Kulig J. Polish Gastric Cancer Study Group. Impact of anastomotic leakage on long- term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035–1042. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request


