Abstract
Objectives
We describe the clinical, mycological, immunological, and genetic characteristics of six HIV-negative patients presenting with invasive cryptococcosis.
Methods
Patients with cryptococcosis without any of the classical risk factors, such as HIV infection, followed at Cayenne Hospital, were prospectively included. An immunologic and genetic assessment was performed.
Results
Five male patients and one female patient, 5 adults and one child, were investigated. All presented a neuromeningeal localization. Cryptococcus neoformans var. gattii and C. neoformans var. grubii were isolated in two and three patients, respectively, whereas one patient could not be investigated. Overall, we did not observe any global leukocyte defect. Two patients were found with high levels of circulating autoantibodies against Granulocyte macrophage-colony stimulating factor (GM-CSF), and none had detectable levels of autoantibodies against Interferon gamma (IFN-γ) Sequencing of STAT1 exons and flanking regions performed for four patients was wild type.
Conclusion
To better understand cryptococcosis in patients with cryptococcosis but otherwise healthy, further explorations are needed with repeated immune checkups and strain virulence studies.
Keywords: cryptococcosis, immunocompetent, STAT1 gene, autoantibodies against GM-CSF, antibodies against IFN-γ, fungal infection
Highlights
Invasive cryptococcosis in otherwise healthy individuals is rare.
This study presents the clinical manifestations and the immune and genetic explorations performed in six of such patients.
Introduction
Cryptococcosis is a life-threatening fungal infection of immunosuppressed patients, well described in HIV-infected patients. More rarely, it occurs in patients without any of the known classical risk factors. The mechanism of the infection of these patients remains unclear, and we could hypothesize that otherwise healthy individuals with cryptococcosis carry a rare inborn error of immunity affecting specifically their immune response to Cryptococcus spp. The particular virulence of certain strains of Cryptococcus could also be involved.
The current classification based on its capsule immunologic and molecular analysis reports three varieties, five serotypes, and eight molecular types (1). Cryptococcus neoformans var. grubii has a worldwide distribution and is mainly responsible for cryptococcosis in patients with acquired immunosuppression (e.g., AIDS), whereas Cryptococcus gattii, mainly found in tropical and subtropical areas, usually strikes otherwise healthy individuals (2–4). In addition, anti-GM-CSF antibodies (5, 6) and primary immune deficiencies have been previously associated with crypotococcosis [STAT1 Gain of Function (GOF) (7, 8), STAT3 deficiency (9, 10), CD40 ligand deficiency (11)].
Few cases of cryptococcosis have been reported in French Guiana, a French overseas territory located on the northeastern coast of South America, supposedly in patients who were otherwise healthy. In the current study, we assessed the clinical, epidemiological, mycological, immunological, and genetic characteristics of patients from French Guiana with invasive cryptococcosis without known underlying causes. A better understanding of these features should help for an earlier and better diagnosis, preventing complicated forms of cryptococcal infections, and should bring new insights into the pathogenesis of the disease.
Materials and Methods
Study Site
The study was performed at the Cayenne Hospital in French Guiana, a French overseas territory of 250,000 inhabitants, located between Brazil and Suriname in the Amazonian region.
Study Design
A prospective analysis was carried out on all consecutive non-HIV patients who were admitted to the Cayenne Hospital from 2011 to 2018 and diagnosed with cryptococcosis.
Case Definition
In accordance with the European Organisation for Research and Treatement of Cancer (ORTC) criteria for invasive fungal infections (12), invasive cryptococcosis was defined by at least one of the following criteria:
i) Histopathologic, cytopathologic, or direct microscopic examination of Cryptococcus obtained by needle aspiration or biopsy from a normally sterile site showing yeast cells.
ii) Recovery of a yeast by culture of a sample obtained by a sterile procedure from a normally sterile site showing a clinical or radiological abnormality consistent with an infectious disease process.
iii) Blood culture that yields yeast and cryptococcal antigen in cerebrospinal fluid (CSF).
iv) Amplification of cryptococcal DNA by PCR combined with DNA sequencing.
Immune Investigation
After inclusion, the patients were evaluated for their immune profile, including the following: i) lymphocyte immunophenotyping, immunoglobulin (IgG, IgA, IgM) levels, complement (CH50, C3, C4) levels, autoimmunity investigation by evaluating the presence of antinuclear antibodies (ANAs), anti-cardiolipin antibodies (ACAs), anti-β2-glycoprotein I antibodies, lupus anticoagulant; ii) the Interleukin (IL)-12/IFN-γ axis exploration, the presence of autoantibodies (auto-Abs) against GM-CSF and IFN-γ; iii) STAT1 exons and flanking intronic regions were sequenced in four patients.
Data Collection
Data were collected from patient medical records: i) general demographic data; ii) laboratory data including biochemistry, hematology, immunology, and microbiology; iii) radiology variables and additional investigations depending on the findings; iv) the clinical and therapeutic management; v) the outcome of the patients.
All patients and/or relatives gave informed written consents.
The study received the agreement of the Committee of Protection of the Persons of the University Paris II on September 6, 2010 and of the AFFSAPS under the number B100712-40.
Results
Description of Cases
During the study period, six patients were included. Clinical, epidemiological, and fungal characteristics are reported in Table 1 . Most were male patients (5/6), with a median (minimum–maximum) age of 23.5 years (4–55 years) at the time of inclusion in the study. They were from various origins; one Creole Haitian male patient, one Creole French Guianese male patient, one Hmong male patient (refugee people from Vietnam war), two Brazilian citizens, and an Amerindian male patient. None of the patients had significant past medical history (cf Table 1 ).
Table 1.
Clinical, epidemiological, fungal characteristics of the 6 Cryptococcosis cases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | MedianIQR | |
|---|---|---|---|---|---|---|---|
| Age (years) | 17 | 15 | 37 | 30 | 55 | 4 | 23.5 (15.5–35.2) |
| Sex | Male | Male | Male | Female | Male | Male | |
| Biotope of the usual residency | Urban | Semi-natural forest | Urban | Urban | Rural | Primary forest | |
| Cultural group | Haitian | Hmong | French Guianese Creole | Brazilian | Brazilian | Amerindian | |
| Medical history | None | None | Meningitis Steatosis Hypothyroiditis Polyglobulia | Thyroid nodules | None | Asthma | |
| Time to diagnosis since the onset of the symptoms (days) | 89 | 16 | 57 | 11 | 3 | 30 | 23 (12.2–50.2) |
| Location of infection | Meningo encephalitis Pulmonary nodule Skin | Meningoencephalitis Hematologic | Meningitis | Meningoencephalitis Cerebral nodules Pulmonary infection | Meningoencephalitis | Meningoencephalitis Pulmonary Nodules | |
| Symptoms | Headache Neck pain Fever | Headache Quadriplegia Blindness Bilateral Hypoacusis Intracranious hypertension | Headaches Vomiting Intracranial hypertension Homonymous hemianopsia | Pulmonary infection Meningitis Intracranious hypertension Blindness Diplopia Scotomas | Fever Headaches Vomiting Intracranious hypertension Confusion Motor deficit Upper right limb Left ptosis | Loss of weigh Cough, Headache, Vomiting, Intracranious hypertension hydrocephalus | |
| Sequelae | None | Blindness Hearing loss Psychomotor retardation | Persistent headaches | Blindness Loss of the sense of smell | Ideomotor slowdown | None | |
| Lumbar punction pressure | Not done | 85 mmHg | Not done | 25 mmHg | 110 mmHg | 49 mmHg | 67 mmHg (43–91)) |
| Brain MRI Abnormalities | Nodular lesions (temporal, frontal, parieto-occipital), Peripheral ring signal Enhancement and Perilesional edema | Periventricular bilateral FLAIR hypersignal | Hyperintensities in the brain’s white matter (supratentorial, cerebellar), ventricular dilatation | Multiple diffuse nodular brain lesions, Perilesional edema | Pachymeningitis Diffuse high-intensity signal | Periventricular hyperintensity, tetra ventricular dilatation | |
| Chest CT abnormalities | Pulmonary nodules of right basal pyramid excavated | None | None | Nodules | None | Pulmonary intraparenchymal cystic formations Parenchymal condensation Excavated nodules | |
| CSF Leukocytes (/mm3) and lymphocyte count (%) | 74 92% | 180 99% | 130 100% | 135 70% | 236 60% | 10 Not realized | 132.5 (88–168.7) 92 (70–99) |
| CSF sugar (mmol/l) Blood sugar (mmol/l) CSF proteins (g/l) | 2,2 5,8 1,5 | 2.1 4.3 0.6 | 0.2 3.9 1.5 | 3,3 3.3 6.5 | 1.9 6.5 6.1 | 1.7 4.5 0.5 | 2.0 (1.7–2.2) 4.4 (4–5.5) 1.5 (0.8–4.9) |
| CSF Antigen titer | 1:10 | 1:100 | 1:120 | 1:10 | 1:100 | Not done | |
| CSF culture and identification | C. gattii | C. neoformans var. grubii | C. neoformans var. grubii | Cryptococcus sp. | C. neoformans var. grubii | C. gattii | 3 C. neoformans var. grubii 2 C. gattii 1 Cryptococcus Sp. |
| Blood antigen titer at diagnosis | 1:20 | 1:100 | 1:100 | 1:10 | 1:1000 | 1:640 | |
| Induction treatment | Amphotericin B + Flucytosine 31 days | Amphothericin B + Flucytosine 31 days | Amphotericin B + Flucytosine 15 days | Amphotericin B + Flucytosine Duration not known | Amphotericin B + Flucytosine 15 days | Amphotericin B + Flucytosine 15 days | |
| Neurosurgery management | No | Yes | No | No | No | Yes | |
| Corticotherapy | Yes | Yes | No | No | No | Yes | |
| Consolidation treatment | Fluconazole 800 mg/day | Fluconazole 800 mg/day + Flucytosine | Fluconazole 800 mg/day | Fluconazole 800 mg/day | Fluconazole 400 mg/day | Fluconazole 12 mg/kg/day |
MRI, magnetic resonance imaging; CT, computed tomography; CSF, cerebrospinal fluid; sp., species; IQR, Interquartile range; CRP, C reactive protein; NK, Natural Killer; BCG, Bacillus Calmette Guerin; IDSA, Infectious Diseases Society America..
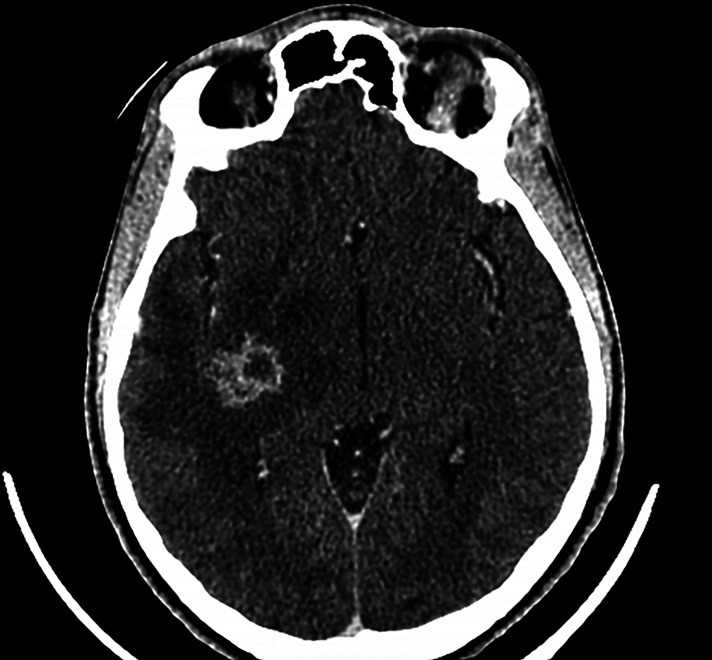
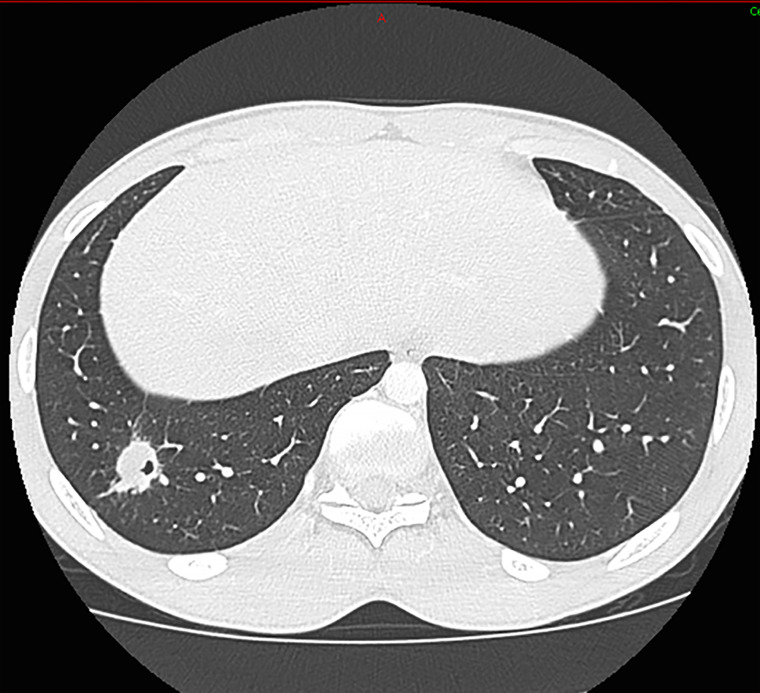
All patients had neurological involvement, mostly meningoencephalitis. One patient was reported with brain cryptococcoma. Another patient suffered from an isolated meningitis without encephalitis. The neurological presentation manifested either as an uncomplicated symptom type (headache or neck stiffness) or in a more severe form as motor deficit, confusion. One patient had vigilance disorders requiring intensive care monitoring. Clinical signs of intracranial hypertension were present for all patients except one. Ophthalmologic disorders such as blindness or diplopia were found in four patients. The median (IQR) CSF opening pressure was 67 mmHg (43–91 mmHg). Median cellularity of CSF was 132.5 (88.0–168.7) leukocytes/mm3 with lymphocytic predominance. Five patients presented abnormalities on their brain imaging (MRI and/or CT scan) when included ( Figure 1 ). Three cases presented, in addition, pulmonary involvement with nodules ( Figure 2 ). Pulmonary symptomatology was often absent, and the lesions were revealed during complimentary tests. Only one patient had a cough.
Figure 1.
Brain Computed Tomography-scan with nodular, right insular lesion with cocoon enhancement and peri-lesional edema.
Figure 2.
Pulmonary Computed Tomography–scan with pulmonary nodule of the right basad pyramid excavated.
All patients received induction therapy with amphotericin and flucytosine, 3 received corticosteroid therapy, and 2 had neurosurgical management. Consolidation therapy always included fluconazole. One of the patients also had flucytosine.
Neurological sequelae were common (four out of six patients) ranging from chronic headaches to blindness and deafness.
The serotyping led to the identification of three C. neoformans var. grubii and two C. gattii. The identification of Cryptococcus serotype could not be specified for one patient. The time to diagnosis was longer for C. gattii vs. C. grubii serotypes. One microbiological sampling was performed to isolate Cryptococcus in the lung (case 1) and was positive. Pulmonary lesions were found only for C. gattii species.
Immune Exploration
Immune explorations of the cases are reported in Table 2 . None of the patients presented any remarkable increase of inflammatory markers in their serum with a median (IQR) CRP of 5.8 mg/L (2.7–9.4 mg/L). All patients were HIV and HTLV1 seronegative, and none received any immunosuppressive therapy. Immunoglobulin levels were normal in all patients. All patients had normal global lymphocyte immunophenotyping, except case 2, who showed a transient low NK cell count at the time of infection, which was fully restored a few months after the acute episode.
Table 2.
Immune exploration of the Cryptococcosis cases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| HTLV1 serology | Negative | Negative | Negative | Negative | Negative | Negative |
|
Lymphocyte immunophenotyping
(CD4/CD8/B/NK) |
Normal | NK lymphopenia Re-controlled Normal |
Normal | Normal | Normal | Normal |
| Immunoglobulin (g/g/L) | Normal | Normal | Normal | Normal | Normal | Normal |
|
Study of IL12/Interferon gamma production
(in comparison with a healthy individual) |
Normal | Normal | Normal | Not done | Not done | Normal |
| Anti-GM-CSF antibodies | Positive with positive neutralizing activity |
Negative | Negative | Positive with positive neutralizing activity |
Not done | Negative |
| Anti-IFN γ antibodies | Negative | Negative | Negative | Not done | Not done | Negative |
| STAT1 gene | Not done | Wild type | Wild type | Wild type | Not done | Wild type |
Author Bio: Dr. Jeanne Goupil is an infectious disease specialist. She is currently practicing in the suburbs of Paris. Her research interests include tropical diseases, HIV, and public health.
Anti-nuclear factors were found in only one patient, with titers in the upper limit of normal values (1/80). Circulating anticoagulants were found to be transiently positive in one patient but became negative on a second test. Two patients had anti-cardiolipin antibodies. Complement (C4, CH50, C3) levels were consistently normal. Whole blood activation from four patients with BCG without or with IL-12 or IFN-γ showed normal production of IFN-γ or IL-12, respectively, suggesting a normal IL-12/IFN-γ axis. Two out of five patients tested showed high titers of neutralizing auto-Abs against GM-CSF. All STAT1 exons were sequenced for four patients and were found to be wild type.
Discussion
Cryptococcosis is a well-known fungal infection in French Guiana. A retrospective study, conducted between 1998 and 2008, identified 43 patients with cryptococcosis admitted to hospitals in French Guiana (13). Fourteen cases (32.6%) were not infected with HIV, and of these 14 patients, only 2 (4.7%) had another detected cause of immunosuppression (corticotherapy alone or associated with diabetes mellitus). Whereas the sex ratio (M/F) was equal to 1 in the HIV-negative group, M/F ratio was 2.63 among the 29 HIV-positive patients. Patients of the HIV-negative group were older (51.6 ± 23.9) than those of the HIV-positive group (41.8 ± 12.5). The average incidence of cryptococcosis was estimated at 22.6 cases/million inhabitants/year during the period 1998–2008, about 10 times higher than in metropolitan France (14).
The clinical presentations of our patients were similar to those of cases reported in the literature. Unlike patients with immunodeficiency, cerebral and pulmonary forms are predominant in patients with cryptococcosis but otherwise healthy (13, 15–18). They frequently present as indolent forms of meningitis, and visual symptoms are the most frequent manifestations (13). In rare cases, the neurologic symptomatology can be very noisy and be associated with high and recurrent neurological morbidities, including shunt requirements, serial lumbar punctures, and pressure-related complications (19). The mean time at diagnosis is significantly longer. CSF white blood cell counts are usually higher, and meningeal enhancement on CT scan of the brain is more frequently observed (16, 19). Cryptococcaemia and other extraneural, extrapulmonary (digestive, ganglionic, oropharyngeal, dermatologic, hematologic, or bone) manifestations are much more associated with immunodeficiency and poorer prognosis (18, 20–23).
In this study, we identified three patients with C. neoformans var. grubii and two patients with C. gattii. A French Guianese study showed an epidemiology for Cryptococcus composed mainly with A (77.3%) and B (22.7%); no types C and D were revealed. This differs from a study performed in France, especially for types B and D showing respectively 1.8% and 22.9%. C. neoformans var. gattii usually invades more frequently the brain and pulmonary parenchyma and causes multiple granuloma (24–26). Cryptococcomas can appear in any region of the lung and can have different sizes. Visual alterations are also more frequent in C. gattii (27).
We found anti-GM-CSF auto-Abs in two patients, as previously reported in otherwise healthy patients with cryptococcal meningitis caused by C. gattii (5, 6). One of the patients was infected with C. gattii, whereas we could not test the strain in the other one. These auto-Abs were neutralizing, as shown in vitro, with an abolished STAT5 phosphorylation upon GM-CSF stimulation of control peripheral blood mononuclear cells (PBMCs) in the presence of 10% of patients’ plasma, but not in the presence of 10% of healthy individuals’ plasma, probably inhibiting macrophage function in vivo. We also tested for the presence of anti-IFN-γ auto-Abs, as some patients with cryptococcosis were also reported with such auto-Abs (28–30); none of the 6 patients tested displayed anti-IFN-γ auto-Abs.
In addition, few inborn errors of immunity have been associated with increased susceptibility to cryptococcosis [CD4 lymphopenia (31), X-linked CD40L deficiency (11), STAT3 mutated hyper-IgE syndrome (9, 10)]. Among them, some patients carrying heterozygous STAT1 GOF mutations (7, 8) were found with cryptococcosis (32). However, none of the four patients tested in our cohort carried any rare variants of STAT1. In our studies, no ethnic group was overrepresented such as aborigines in Australia (33) suggesting that genetic factors may be important.
Some limitations should be considered in this study. Due to logistical difficulties in delivering immunoassay and lost to follow-up, the study has missing data. Genetic studies have only screened rare variants of STAT1. A particular virulence of the strain could be evoked (24, 34, 35). Unfortunately, this parameter could not be studied in this study.
Our study highlights the difficulty of determining the causal agent of cryptococcosis in patients. It thus opens up different avenues for consideration. The immune status of the host and a particular virulence of the Cryptococcus strain are the two main hypotheses.
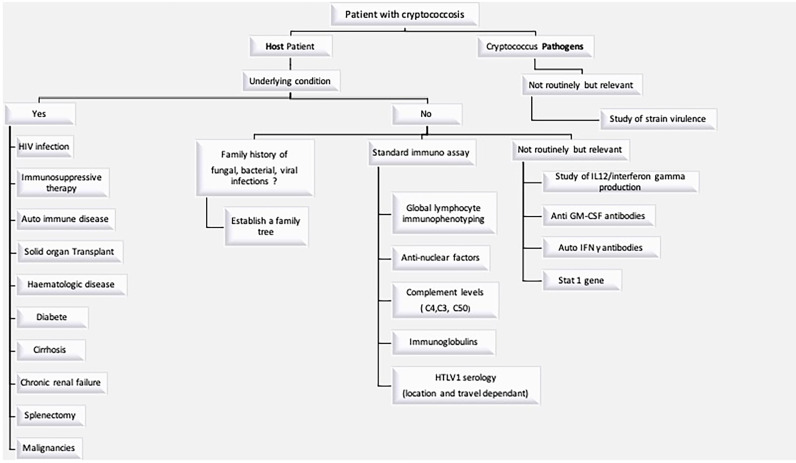
The immune status of the host is a key issue, since, as notified in IDSA Guidelines (25), treatment depends on it. A comprehensive immune and genetic exploration, in our opinion, is the first step in answering the various questions. To our knowledge, the present study is the first that proposes a standardized and detailed immunological assessment for so-called “immunocompetent” patients suffering from cryptococcal disease ( Figure 3 ). It seems unclear whether these patients have phenotypic or genetic deficits. Genetic analyses are not easy to carry out routinely but must be integrated into research programs. It could also be assumed that cryptococcosis is the cause of immunosuppression. In case 2, a dosage of lymphocyte NK was abnormal during the infection. A control was performed a few months later with normal proportion of lymphocyte NK. This suggests that Cryptococcus infection can suppress the immune system, and its elimination contributes to the reestablishment of an immune equilibrium. French Guiana is known for its specificities in terms of tropical infections, and we could evoke the virulence of a specific strain in our patients. Further in vivo investigation is essential to understand the basic mechanism of virulence of C. gattii and C. grubii especially in tropical areas where epidemiology is different from the other areas (36–40).
Figure 3.
Investigation to be performed in a patient with cryptococcosis.
Conclusion
This study describes the clinical, biological, immunological, and genetic characteristics of six non-HIV patients in French Guiana suffering from cryptococcosis. Clinical presentations can be devious, and they highlight the particularities of this infection according to the gattii or grubii serotype. Cryptococcosis is a potentially emerging disease. Two out of the six patients tested had high titers of neutralizing auto-Abs against GM-CSF, and this consequent percentage deserves further studies on these antibodies. None of the four patients tested carry rare variants of STAT1, the only candidate gene tested yet. Studying patients with cryptococcosis but otherwise healthy should help to progressively decipher the crucial physiopathological mechanisms underlying this disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Committee of Protection of the Persons of the University Paris II on 2010-06-09 and of the AFFSAPS under the number B100712-40. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Conceptualization: JGdB and MD. Formal analysis: JGdB. Investigation: JGdB, LE, FH, MM, FL, PA, DB, AP, CA, and OL. Methodology: JGdB and MD. Supervision: MD. Writing—original draft: JGdB. Writing—review and editing: JGdB, LE, FH, MM, PA, DB, CA, FD, NE, AP, FL, and MD. All authors have read and approved the final article.
Funding
The work was funded by the French National Research Agency (ANR) under the “Investments for the future” program (ANR-10-IAHU-01), the ANR-FNS LTh-MSMD-CMCD (ANR-18-CE93-0008-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), and the National Institute of Allergy and Infectious Diseases of the NIH (grant no. R01AI127564).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Desnos-Ollivier M, Patel S, Raoux-Barbot D, Heitman J, Dromer F. Cryptococcosis Serotypes Impact Outcome and Provide Evidence of Cryptococcus Neoformans Speciation. mBio (2015) 6(3):4. doi: 10.1128/mBio.00311-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Vroey C, Gatti F. Cryptococcus Neoformans Var. Gattii Vanbreuseghem and Takashio, 1970. Mycoses (1989) 32(12):675. doi: 10.1111/j.1439-0507.1989.tb02200.x [DOI] [PubMed] [Google Scholar]
- 3. Dixit A, Carroll SF, Qureshi ST. Cryptococcus Gattii: An Emerging Cause of Fungal Disease in North America. Interdiscip Perspect Infect Dis (2009) 2009:840452. doi: 10.1155/2009/840452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorrell TC. Cryptococcus Neoformans Variety Gattii. Med Mycol (2001) 39(2):155−68. doi: 10.1080/714031012 [DOI] [PubMed] [Google Scholar]
- 5. Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF Autoantibodies in Patients With Cryptococcal Meningitis. J Immunol Baltim Md 1950 (2013) 190(8):3959−66. doi: 10.4049/jimmunol.1202526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saijo T, Chen J, Chen SC-A, Rosen LB, Yi J, Sorrell TC, et al. Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies Are a Risk Factor for Central Nervous System Infection by Cryptococcus Gattii in Otherwise Immunocompetent Patients. mBio (2014) 5(2):e00912–00914. doi: 10.1128/mBio.00912-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FL. STAT1 Signaling Within Macrophages Is Required for Antifungal Activity Against Cryptococcus Neoformans. Infect Immun (2015) 83(12):4513−27. doi: 10.1128/IAI.00935-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang W, Chen X, Gao G, Xing S, Zhou L, Tang X, et al. Clinical Relevance of Gain- and Loss-Of-Function Germline Mutations in STAT1: A Systematic Review. Front Immunol 11 mars (2021) 12. doi: 10.3389/fimmu.2021.654406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandesris M-O, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal Dominant STAT3 Deficiency and Hyper-IgE Syndrome: Molecular, Cellular, and Clinical Features From a French National Survey. Med (Baltimore) (2012) 91(4):e1−19. doi: 10.1097/MD.0b013e31825f95b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odio CD, Milligan KL, McGowan K, Rudman Spergel AK, Bishop R, Boris L, et al. Endemic Mycoses in Patients With STAT3 Mutated Hyperimmunoglobulin E (Job’s) Syndrome. J Allergy Clin Immunol (2015) 136(5):1411–3.e2. doi: 10.1016/j.jaci.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-Linked Hyper-IgM Syndrome: Clinical and Immunologic Features of 79 Patients. Med (Baltimore) (2003) 82(6):373−84. doi: 10.1097/01.md.0000100046.06009.b0 [DOI] [PubMed] [Google Scholar]
- 12. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis Off Publ Infect Dis Soc Am (2020) 71(6):1367−76. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Debourgogne A, Iriart X, Blanchet D, Veron V, Boukhari R, Nacher M, et al. Characteristics and Specificities of Cryptococcus Infections in French Guiana, 1998-2008. Med Mycol (2011) 49(8):864−71. doi: 10.3109/13693786.2011.584198 [DOI] [PubMed] [Google Scholar]
- 14. Gangneux JP. An Estimation of Burden of Serious Fungal Infections in France. NCBI; (2016). Available at: https://www-ncbi-nlm-nih-gov.gate2.inist.fr/pubmed/?term=An+estimation+of+burden+of+serious+fungal+infections+in+France. [DOI] [PubMed] [Google Scholar]
- 15. Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O. French Cryptococcosis Study Group. Determinants of Disease Presentation and Outcome During Cryptococcosis: The CryptoA/D Study. PloS Med (2007) 4(2):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pappas PG. Cryptococcal Infections in Non-HIV-Infected Patients. Trans Am Clin Climatol Assoc (2013) 124:61−79. doi: 10.1371/journal.pmed.0040021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jongwutiwes U, Sungkanuparph S, Kiertiburanakul S. Comparison of Clinical Features and Survival Between Cryptococcosis in Human Immunodeficiency Virus (HIV)-Positive and HIV-Negative Patients. Jpn J Infect Dis (2008) 61(2):111−5. [PubMed] [Google Scholar]
- 18. Lui G, Lee N, Ip M, Choi KW, Tso YK, Lam E, et al. Cryptococcosis in Apparently Immunocompetent Patients. QJM Mon J Assoc Phys (2006) 99(3):143−51. doi: 10.1093/qjmed/hcl014 [DOI] [PubMed] [Google Scholar]
- 19. Marr KA, Sun Y, Spec A, Lu N, Panackal A, Bennett J, et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in HIV-Negative People in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am (2019) 70(2):252–61. doi: 10.1093/cid/ciz193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, et al. Cryptococcosis in Human Immunodeficiency Virus-Negative Patients in the Era of Effective Azole Therapy. Clin Infect Dis Off Publ Infect Dis Soc Am (2001) 33(5):690−9. doi: 10.1086/322597 [DOI] [PubMed] [Google Scholar]
- 21. Speed B, Dunt D. Clinical and Host Differences Between Infections With the Two Varieties of Cryptococcus Neoformans. Clin Infect Dis (1995) 21(1):28−34. doi: 10.1093/clinids/21.1.28 [DOI] [PubMed] [Google Scholar]
- 22. Hoang LMN, Maguire JA, Doyle P, Fyfe M, Roscoe DL. Cryptococcus Neoformans Infections at Vancouver Hospital and Health Sciences Centre (1997-2002): Epidemiology, Microbiology and Histopathology. J Med Microbiol (2004) 53(Pt 9):935−40. doi: 10.1099/jmm.0.05427-0 [DOI] [PubMed] [Google Scholar]
- 23. Jean S-S, Fang C-T, Shau W-Y, Chen Y-C, Chang S-C, Hsueh P-R, et al. Cryptococcaemia: Clinical Features and Prognostic Factors. QJM Mon J Assoc Phys (2002) 95(8):511−8. doi: 10.1093/qjmed/95.8.511 [DOI] [PubMed] [Google Scholar]
- 24. Chen SC-A, Meyer W, Sorrell TC. Cryptococcus Gattii Infections. Clin Microbiol Rev (2014) 27(4):980−1024. doi: 10.1128/CMR.00126-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am (2010) 50(3):291−322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voelz K, May RC. Cryptococcal Interactions With the Host Immune System. Eukaryot Cell (2010) 9(6):835−46. doi: 10.1128/EC.00039-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu L-P, Wu J-Q, Xu B, Ou X-T, Zhang Q-Q, Weng X-H. Cryptococcal Meningitis in Non-HIV-Infected Patients in a Chinese Tertiary Care Hospital, 1997–2007. Med Mycol (2010) 48(4):570−9. doi: 10.3109/13693780903437876 [DOI] [PubMed] [Google Scholar]
- 28. Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to Interferon-Gamma Associated With Adult-Onset Immunodeficiency in Non-HIV Individuals in Northern Thailand. PloS One (2013) 8(9):1–6. doi: 10.1371/journal.pone.0076371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rujirachun P, Sangwongwanich J, Chayakulkeeree M. Triple Infection With Cryptococcus, Varicella-Zoster Virus, and Mycobacterium Abscessus in a Patient With Anti-Interferon-Gamma Autoantibodies: A Case Report. BMC Infect Dis (2020) 20(1):232. doi: 10.1186/s12879-020-4949-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-Onset Immunodeficiency in Thailand and Taiwan. N Engl J Med (2012) 367(8):725−34. doi: 10.1056/NEJMoa1111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zonios DI, Falloon J, Huang C-Y, Chaitt D, Bennett JE. Cryptococcosis and Idiopathic CD4 Lymphocytopenia. Med (Baltimore) (2007) 86(2):78−92. doi: 10.1097/md.0b013e31803b52f5 [DOI] [PubMed] [Google Scholar]
- 32. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 Gain-of-Function Mutations Underlie an Unexpectedly Broad Clinical Phenotype. Blood (2016) 127(25):3154−64. doi: 10.1182/blood-2015-11-679902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and Host- and Variety-Dependent Characteristics of Infection Due to Cryptococcus Neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis Off Publ Infect Dis Soc Am (2000) 31(2):499−508. doi: 10.1086/313992 [DOI] [PubMed] [Google Scholar]
- 34. Herkert PF, Hagen F, Pinheiro RL, Muro MD, Meis JF, Queiroz-Telles F. Ecoepidemiology of Cryptococcus Gattii in Developing Countries. J Fungi (2017) 3(4):62. doi: 10.3390/jof3040062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Favalessa OC, Lázera M dos S, Wanke B, Trilles L, Takahara DT, Tadano T, et al. Fatal Cryptococcus Gattii Genotype AFLP6/VGII Infection in a HIV-Negative Patient: Case Report and a Literature Review. Mycoses (2014) 57(10):639−43. doi: 10.1111/myc.12210 [DOI] [PubMed] [Google Scholar]
- 36. Escandón P, Lizarazo J, Agudelo CI, Castañeda E. Cryptococcosis in Colombia: Compilation and Analysis of Data From Laboratory-Based Surveillance. J Fungi Basel Switz (2018) 4(1):8. doi: 10.3390/jof4010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Epelboin L, Nacher M, Mahamat A, Pommier de Santi V, Berlioz-Arthaud A, Eldin C, et al. Q Fever in French Guiana: Tip of the Iceberg or Epidemiological Exception? PloS Negl Trop Dis (2016) 10(5):e0004598. doi: 10.1371/journal.pntd.0004598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mosnier E, Martin N, Razakandrainibe R, Dalle F, Roux G, Buteux A, et al. Cryptosporidiosis Outbreak in Immunocompetent Children From a Remote Area of French Guiana. Am J Trop Med Hyg (2018) 98(6):1727−32. doi: 10.4269/ajtmh.17-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eldin C, Mahamat A, Demar M, Abboud P, Djossou F, Raoult D. Q Fever in French Guiana. Am J Trop Med Hyg (2014) 91(4):771−6. doi: 10.4269/ajtmh.14-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blaizot R, Nabet C, Blanchet D, Martin E, Mercier A, Dardé M-L, et al. Pediatric Amazonian Toxoplasmosis Caused by Atypical Strains in French Guiana, 2002-2017. Pediatr Infect Dis J (2019) 38(3):e39−42. doi: 10.1097/INF.0000000000002130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.