Abstract
Background
The deep sea harbors the majority of the microbial biomass in the ocean and is a key site for organic matter (OM) remineralization and storage in the biosphere. Microbial metabolism in the deep ocean is greatly controlled by the generally depleted but periodically fluctuating supply of OM. Currently, little is known about metabolic potentials of dominant deep-sea microbes to cope with the variable OM inputs, especially for those living in the hadal trenches—the deepest part of the ocean.
Results
In this study, we report the first extensive examination of the metabolic potentials of hadal sediment Chloroflexi, a dominant phylum in hadal trenches and the global deep ocean. In total, 62 metagenome-assembled-genomes (MAGs) were reconstructed from nine metagenomic datasets derived from sediments of the Mariana Trench. These MAGs represent six novel species, four novel genera, one novel family, and one novel order within the classes Anaerolineae and Dehalococcoidia. Fragment recruitment showed that these MAGs are globally distributed in deep-sea waters and surface sediments, and transcriptomic analysis indicated their in situ activities. Metabolic reconstruction showed that hadal Chloroflexi mainly had a heterotrophic lifestyle, with the potential to degrade a wide range of organic carbon, sulfur, and halogenated compounds. Our results revealed for the first time that hadal Chloroflexi harbor pathways for the complete hydrolytic or oxidative degradation of various recalcitrant OM, including aromatic compounds (e.g., benzoate), polyaromatic hydrocarbons (e.g., fluorene), polychlorobiphenyl (e.g., 4-chlorobiphenyl), and organochlorine compounds (e.g., chloroalkanes, chlorocyclohexane). Moreover, these organisms showed the potential to synthesize energy storage compounds (e.g., trehalose) and had regulatory modules to respond to changes in nutrient conditions. These metabolic traits suggest that Chloroflexi may follow a “feast-or-famine” metabolic strategy, i.e., preferentially consume labile OM and store the energy intracellularly under OM-rich conditions, and utilize the stored energy or degrade recalcitrant OM for survival under OM-limited condition.
Conclusion
This study expands the current knowledge on metabolic strategies in deep-ocean Chlorolfexi and highlights their significance in deep-sea carbon, sulfur, and halogen cycles. The metabolic plasticity likely provides Chloroflexi with advantages for survival under variable and heterogenic OM inputs in the deep ocean.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-022-01263-6.
Keywords: Chloroflexi, Metagenome-assembled-genomes, Metabolic potential, Persistent organic pollutant, PAH, PCB, Dehalogenation, Feast-or-famine, Deep ocean, Hadal trenches
Introduction
The deep sea harbors around 75% of the prokaryotic biomass and more than half of the prokaryotic production of the global ocean, and is a key site for organic matter (OM) remineralization and storage in the biosphere [1]. An estimated 1–40% of the photosynthetically fixed carbon in the upper water reach the deep sea [2], which is generally considered oligotrophic in nature [2, 3]. The flux of nutrients varies in intensity and frequency over temporal and spatial scales [4–6], and mass input of particles from surface algal blooms may lead to periodic increases in quantity and bioavailability of OM in the deep ocean [3, 7]. Deep-sea microorganisms therefore have to employ special metabolic strategies to cope with the variable OM conditions to ensure their survival and functioning [3, 7].
Bacteria of the phylum Chloroflexi are dominant members of microbial communities in the global deep ocean [8, 9]. For example, the SAR202 clade of the Chloroflexi on average accounts for > 10%, and in some cases up to 40% of the total prokaryotic community in meso- and bathypelagic water of the Atlantic and Pacific oceans [8, 10–12]. Chloroflexi have also been shown to account for 25.5–41.3% of total 16S rRNA gene sequences in global marine sediments [9, 13, 14]. Currently, the knowledge on the metabolism of deep-sea Chloroflexi mainly relies on metagenomic or single-cell genomic analysis, due to the lacking of cultivated representatives for dominant deep-sea lineages [15–17]. These studies revealed that Chloroflexi from deep-sea waters harbor genes involved in organosulfur compounds degradation [8, 15], sulfite oxidation [8, 15], and metabolism of recalcitrant compounds such as cyclic alkanes and aromatic compounds [15–17]. The analysis of deep-sea Chloroflexi from anoxic subseafloor sediments suggests these bacteria have potential for reductive respiration of organohalogen compounds, and for the fermentation of OM combined with CO2 fixation via the Wood-Ljungdahl pathway [9, 18]. These findings suggest that the Chloroflexi play important roles in biogeochemical cycles of the deep ocean. However, existing studies only covered a few seawater or anoxic subseafloor sites [8, 9, 15–18]. Given the high heterogeneity of the deep-sea habitats [3] and great phylogenetic and functional diversity of Chloroflexi bacteria [9, 17], the current understanding of the metabolisms of deep-sea Chloroflexi is therefore incomplete, and their genomic basis and metabolic strategies to adapt to fluctuations of OM supply (e.g., OM with different recalcitrancy) in the deep ocean are unclear.
The hadal trenches, formed by the subduction of tectonic plates, are the deepest part of the ocean [19]. Multiple sources of OM inputs combined with frequent OM remobilization due to special topographies, tectonic activities, and intra-trench currents, lead to higher heterogeneity and fluctuation of OM in the hadal trenches than those in other deep-sea habitats [19–22]. However, despite the complex OM supply and extreme environmental conditions, such as high pressure, active microbial carbon turnover in hadal sediments has been frequently reported, making the hadal trenches “hot spots” of OM remineralization in the deep ocean [23–25]. Recently, Chloroflexi have been identified as one of the dominant taxa in seawater and sediment of the hadal trenches [15, 26, 27] and were found to primarily belong to novel lineages [27]. In addition, hadal Chloroflexi were not only numerically dominant but also highly transcribed in both hadal seawater and sediments (accounting for up to 36.2% of transcribed prokaryotic 16S rRNA sequences), suggesting high in situ activities [15, 27]. Co-occurrence network analysis further revealed that Chloroflexi lineages were important in mediating the interactive network within hadal microbial communities [26, 27]. To date, only three studies reported the metabolism of hadal Chloroflexi based on 13 MAGs or single amplified genomes (SAGs) recovered from seawaters [15, 17, 28]. These bacteria were shown to encode enzymes to metabolize chitin, dimethyl sulfoxide, aromatic compounds (e.g., phthalate), and osmolytes [15, 28], but the detailed degradation pathways were unclear. Moreover, although these studies shed light on the lifestyles of hadal Chloroflexi, but the limited number of investigated genomes restricted the findings primarily to the pelagic SAR202 group II and III [15, 28]. The metabolic potentials of other dominant and novel lineages of Chloroflexi living in the hadal zone are thus largely unknown.
In this study, we employed a metagenomic approach to fill the knowledge gap on the metabolism of Chloroflexi that are living in surface sediments of the hadal zone. We obtained unique samples from the deepest point of the ocean, the Challenger Deep of the Mariana Trench. Species composition and activity potential of hadal sediment Chloroflexi were revealed using amplicon sequencing of 16S rRNA gene and their transcripts. Representative Chloroflexi MAGs were then retrieved from nine metagenomic datasets, and their phylogeny, distribution, and metabolic potentials were explored. All MAGs were found to belong to novel lineages of Chloroflexi, representing major and widely distributed members of the hadal sediment microorganisms. The recovered Chloroflexi showed the capabilities to degrade a wide range of OM with different levels of recalcitrance. Our analysis also revealed for the first time the presence of hydrolytic and oxidative pathways in deep-sea Chloroflexi for the complete degradation of polyaromatic hydrocarbons (PAHs), polychlorobiphenyl (PCBs), and halogenated organic compounds. Potential metabolic strategies to adapt to fluctuation and heterogeneity of OM in the hadal trenches are proposed based on the metabolic reconstruction.
Results and discussion
Composition and activity of Chloroflexi in sediments of the Challenger Deep
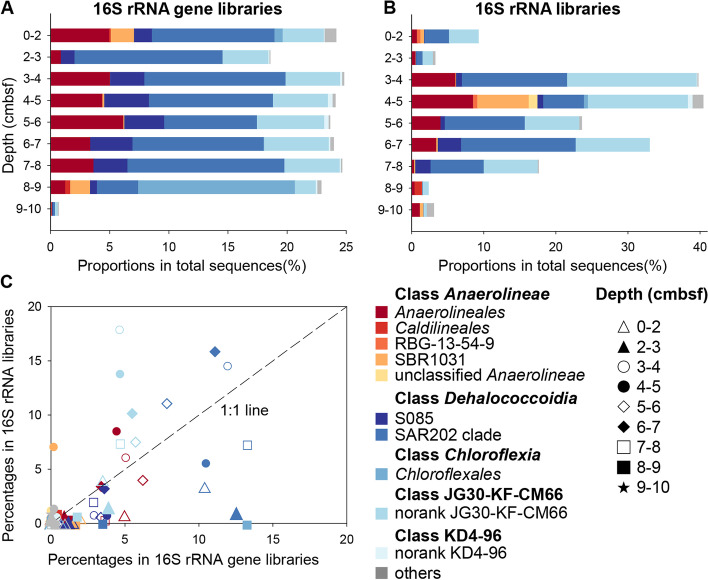
In this study, 16S rRNA genes and their transcripts were sequenced for samples from nine different depths of a sediment core retrieved from the Challenger Deep of the Mariana Trench. The results showed that Chloroflexi accounted on average for 20.9% and 19.1% of the total sequences for the bulk (i.e., 16S rRNA gene) and potentially active (i.e., 16S rRNA) bacterial communities, respectively (Fig. 1A, B, and Additional file 1: Fig. S1). The distribution of the bulk Chloroflexi population was relatively stable and varied between 18.6 and 24.6% of total rRNA gene sequences in the upper 9 cm (Fig. 1A, Additional file 1: Fig. S1). In contrast, the proportion of the transcribed Chloroflexi 16S rRNA sequences varied greatly with depth. Chloroflexi transcripts showed the highest proportion at 3–7 cm below seafloor, accounting for up to 40.6% of the total 16S rRNA sequences (Fig. 1b, Additional file 1: Fig. S1). The bulk and potentially active Chloroflexi populations were mainly composed of members from classes Anaerolineae, Dehalococcoidia, Chloroflexia, JG-KF-CM66, and KD4-96, among which Anaerolineae, Dehalococcoidia, and JG-KF-CM66 were the most dominant and highly transcribed ones (Fig. 1B and C). These results are consistent with previous findings on the microbial composition of hadal trench sediments [26, 27], indicating the general significance of Chloroflexi in maintaining the structure and functions of the hadal biosphere. Compared with deeper sediment layers, Chloroflexi showed relatively lower transcription levels at 0–3 cm, and the possible reasons include (1) The physical/chemical conditions (such as high oxygen levels and organic carbon content) of the surface layers might favor the transcriptional activities of other bacterial taxa, leading to lowered proportions of Chloroflexi in the transcript pool [23, 25]; (2) A fraction of the microbes at these layers might be sourced from shallower habitats such as sediment of the trench slopes due to lateral transportation [19, 21]. These microbes may show low transcriptional activity in hadal trench sediments, due to the extreme environmental conditions, such as high pressure; (3) the potentially higher level of degradation of RNA in the surface layer sediments during sample recovery from the deep trench [27].
Fig. 1.
Composition of the bulk (A) and potentially active (B) Chloroflexi in the hadal sediments at the order level, revealed by 16S rRNA gene and 16S rRNA, respectively. The relative activities of different orders are shown as the ratio between their frequencies in the 16S rRNA library and the 16S rRNA gene library at each sediment depth (C)
MAG reconstruction, genome description, and phylogenomic analysis
A total of 62 Chloroflexi MAGs with completeness > 50% and contamination < 5% were reconstructed from the nine metagenomes covering different sediment layers (Additional file 2: Table S1). These MAGs were further dereplicated at 99% average nucleotide identity (ANI) to yield 17 representatives with an average completeness of 68.56% (51.38–92.99%) and contamination ranged from 0.00 to 3.64% (Table 1 and Additional file 2: Table S1). The genome sizes were estimated to range between 1.85 and 3.90 Mbp, and GC contents were between 58.64 and 69.45% (Table 1). Currently, only thirteen genomes of Chloroflexi have previously been reported from the hadal zone (deeper than 6000 m), and 11 of them were from seawater [15, 17, 28]. Only 2 Chloroflexi MAGs (GCA_004356475.1 and GCA_004356815.1) found in the NCBI database were recovered from hadal sediments, but without any description on their metabolism.
Table 1.
Summary of the 17 representative MAGs retrieved from sediments of the Challenger Deep
| MAGs | Completeness (%) | Contamination (%) | Contig no. | GC% | CDS no. | Estimated genome size (Mbp) | Sequencing depth |
|---|---|---|---|---|---|---|---|
| MT2_13a | 92.99 | 0.00 | 275 | 58.79 | 2237 | 2.74 | 21× |
| MT4_27 | 89.96 | 0.31 | 215 | 65.69 | 1934 | 2.05 | 41× |
| MT6_15 | 87.27 | 3.64 | 168 | 58.64 | 2397 | 2.85 | 28× |
| MT4_14 | 86.30 | 1.98 | 408 | 60.36 | 2572 | 3.05 | 25× |
| MT2_3 | 85.70 | 0.00 | 472 | 59.61 | 3150 | 3.90 | 24× |
| MT6_13 | 84.77 | 0.11 | 425 | 60.34 | 2430 | 2.99 | 29× |
| MT1_55 | 73.57 | 0.00 | 386 | 59.26 | 1683 | 2.33 | 33× |
| MT5_44 | 67.62 | 1.19 | 314 | 65.58 | 1512 | 2.29 | 14× |
| MT5_40 | 66.01 | 0.20 | 359 | 58.76 | 1610 | 2.61 | 18× |
| MT1_49 | 60.51 | 3.08 | 400 | 62.83 | 2008 | 3.18 | 21× |
| MT1_63 | 56.40 | 2.97 | 296 | 59.82 | 1319 | 2.46 | 15× |
| MT4_29 | 54.89 | 2.18 | 365 | 62.58 | 1770 | 3.09 | 15× |
| MT8_34 | 52.62 | 0.00 | 467 | 59.67 | 1199 | 2.16 | 22× |
| MT2_40 | 52.07 | 2.20 | 253 | 69.45 | 1108 | 2.07 | 25× |
| MT6_44 | 51.99 | 0.00 | 486 | 59.57 | 1031 | 1.85 | 23× |
| MT9_49 | 51.53 | 2.38 | 257 | 69.22 | 1189 | 2.16 | 26× |
| MT1_74 | 51.38 | 0.61 | 255 | 65.29 | 1016 | 1.98 | 18× |
aMAGs were named using “site + layer + genome number”, for example MT2_13 means the 13th genome from sediment of 2–3 cm below seafloor from the Mariana Trench
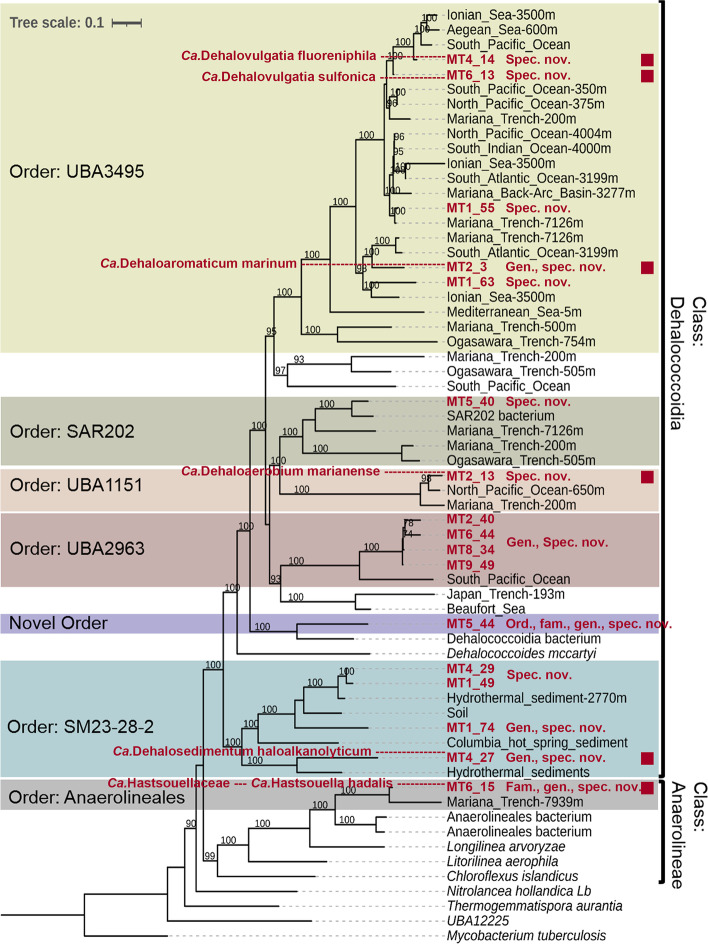
Phylogenomic analysis showed that the retrieved MAGs belonged to the classes Anaerolineae, Dehalococcoidia, and SAR202 (previously classified as a class) (Fig. 2, Additional file 2: Table S2, Additional file 1: Fig. S2). Taxonomic classification was further conducted with GTDB-Tk toolkits [29] (Fig. 2 and Additional file 2: Table S3). The results revealed that these MAGs represent six novel species (MT1_49, MT2_13, MT5_40, MT1_63, MT1_55, MT4_14; relative evolutionary divergence, RED, ranged from 0.89 to 0.99) and four novel genera (MT4_27, MT1_74, MT9_49, MT2_3; RED ranged from 0.76 to 0.89) in the orders SM23-28-2, SAR202 (formerly SAR202 group II), UBA2963 (formerly SAR202 group VII), UBA1151(formerly SAR202 group I), and UBA3495 (formerly SAR202 group III) of the class Dehalococcoidia (Fig. 2, Additional file 2: Table S3 and Additional file 1: Fig. S2). In addition, MT5_44 (RED = 0.52) represents a novel order in the class Dehalococcoidia, and MT6_15 (RED = 0.67) represents a novel family in the order Anaerolineales of the class Anaerolineae (Fig. 2 and Additional file 2: Table S3).
Fig. 2.
Maximum likelihood phylogenomic tree of the 17 selected Chloroflexi MAGs. Genome of Mycobacterium tuberculosis was used as the root. Bootstrap values were calculated based on 100 replicates and the values higher than 90% were indicated at the base of the corresponding node. The colored backgrounds show the genomes belonging to the same order. The taxonomy was determined using GTDB-tk and the novelty of the recovered genomes was determined based on GTDB classification. Red square indicates the MAGs with completeness > 80%
Six MAGs, i.e., MT6_15, MT4_27, MT2_13, MT2_3, MT6_13, and MT4_14, showed completeness higher than 80% and contamination lower than 3.6% (Table 1), and are therefore qualified as type materials according to the criteria defined recently for the taxonomy of uncultivated prokaryotes [30, 31]. Following the guidelines developed by Genomics Standards Consortium [32], Konstantinidis et al. [33], and Chuvochina et al. [34], we propose the names Candidatus Hastsouellaceae (fam. nov.) and Ca. Hastsouella hadalis (genus nov. and species nov.) for MT6_15, Ca. Dehalosedimentum haloalkanolyticum (genus nov. and species nov.) for MT 4_27, Ca. Dehaloaerobium marianense (species nov.) for MT2_13, Ca. Dehaloaromaticum marinum (genus nov., species nov.) for MT2_3, Ca. Dehalovulgatia sulfonica (species nov.) for MT6_13, and Ca. Dehalovulgatia fluoreniphila (species nov.) for MT4_14. Explanations on nomenclature can be found in Additional file 3, and protologues accompanying the proposed names can be found in Table S4.
Distribution of the reconstructed MAGs in hadal sediments and other ecosystems
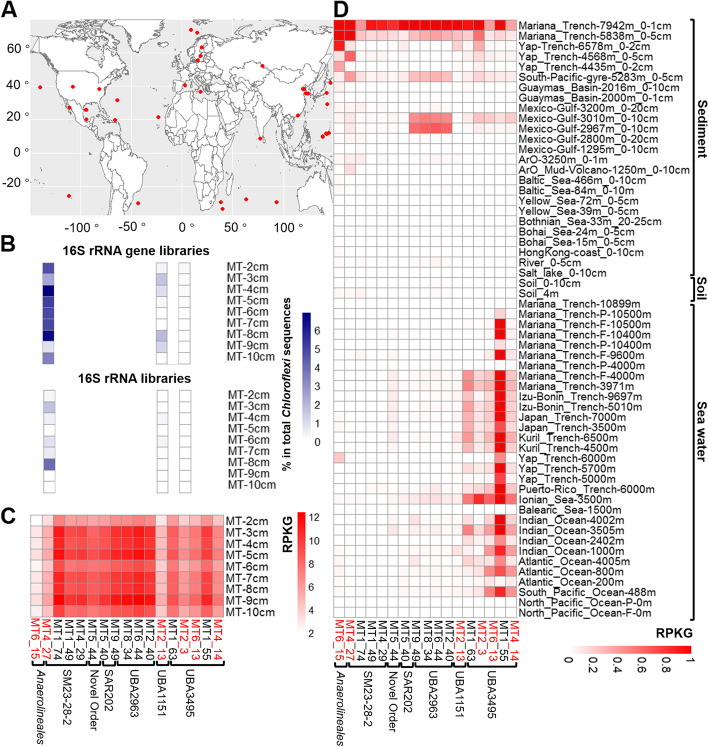
Among the 17 representative MAGs, only MT4_27 was found to contain partial 16S rRNA gene. We further searched all of the 62 MAGs recovered, and found 2 additional MAGs that contain partial 16S rRNA gene, i.e., MT9_9 (represented by MT2_13) and MT8_3 (represented by MT2_3) (Additional file 2: Table S1). These 16S rRNA gene sequences matched with three OTUs from 16S rRNA and 16S rRNA gene libraries constructed in this study (Fig. 3B). The matched OTUs were mainly distributed in the upper 8 cm of the sediment core and together accounted for 4.2–8.5% and 0.1–4.0% of Chloroflexi sequences in the 16S rRNA gene and 16S rRNA libraries, respectively (Fig. 3B). Recruitment of shotgun sequencing reads showed that the 17 representative MAGs were present in all depths (0–10 cm below seafloor) of the sediment core, with MT6_44, MT1_74, MT8_34, MT9_49, and MT2_40 being the most abundant (Fig. 3C). The three MAGs with 16S rRNA gene showed the lowest recruitment values (Fig. 3C). It is therefore reasonable to postulate that the 14 MAGs without 16S rRNA genes might be more abundant in the bulk and active bacterial communities than the three MAGs with 16S rRNA gene (Fig. 3B). These results suggest that the recovered MAGs represent major members of Chloroflexi in the hadal sediment of the Mariana Trench.
Fig. 3.
Distribution of the recovered MAGs in hadal sediments and other natural ecosystems. (A) The sampling sites of the datasets included in the analysis; (B) the relative abundance of the closest matched OTUs in 16S rRNA and 16S rRNA gene libraries of sediments of the Challenger Deep. The MAGs without any value in 16S rRNA or 16S rRNA gene libraries mean no matched OTUs due to a lack of 16S rRNA gene in the corresponding MAG; (C) the reads recruitment of the recovered MAGs in metagenomes from different layers of hadal sediments of the Challenger Deep; and (D) reads recruitments in other natural ecosystems. The names of samples from sediments were shown as “sampling site (region)_water depth of the site_depth below seafloor.” The black dots in the heatmap boxes indicate outlier values. Red-colored names at the bottom indicate the MAGs with completeness > 80%
The global distribution of the recovered MAGs was evaluated by read recruitments against 58 publicly available metagenomes derived from different natural habitats, including seawater and surface sediments from different depths of the open ocean, sediments of mud volcanos, deep-sea oil spilling sites, deep subseafloor, coastal regions, rivers, and salt-lakes, as well as soils (Fig. 3A, D and Additional file 2: Table S5). All of the 17 MAGs showed the highest recruitment values in surface sediments of the Mariana Trench, including the nine samples analyzed in this study (water depth of 10853 m) (Fig. 3C) and two previous samples with depths of 7942 m and 5838m (Fig. 3D and Additional file 2: Table S5), which likely reflect the biogeographic distributions impacted by local environmental selection [8]. The majority of the MAGs (except the order SM23-28-2) have recruited reads from metagenomes derived from sediments and seawaters of the global deep ocean (Fig. 3D and Additional file 2: Table S5), and none of the MAGs was present in seawater or sediments from shallow habitats, including the epi-pelagic zone of the open ocean, coastal regions, river, salt-lake, or soil (Fig. 3D). The results suggest that majority of the recovered MAGs are widespread in seawater and surface sediment of the deep ocean.
However, MAGs from different orders showed apparent preferences on their distributions in different deep-sea habitats. MAGs from the order UBA3495 (formerly SAR202 group III) showed high recruitment values in metagenomes of both deep seawater and sediment (Fig. 3D). The SAR202 group III has previously been shown to be one of the most dominant Chloroflexi in the water column of the global deep ocean [17, 28], and our results highlight the significance of these bacteria in both pelagic and sedimentary habitats of the deep ocean. Expansion of paralogous enzymes, such as flavin-dependent monooxygenases, in SAR202 group III has been suggested to be important for their adaptation to different deep-sea habitats, by diversifying the range of organic molecules that the cells can utilize [16, 17]. In contrast to UBA3495, MAGs from the orders Anaerolineales, SAR202 (formerly SAR202 group II), UBA2963 (former SAR202 group VII), UBA1151(formerly SAR202 group I), and the novel order (MT5_44) showed higher recruit values in metagenomes from deep-sea sediments compared to those from seawater, suggesting their preferential distribution in deep-sea sediment habitats. Interestingly, the MAGs of the order SM23-28-2 (particularly MT1_74) only matched with the reads from sediment metagenomes of the Mariana Trench (Fig. 3D), indicating a potential endemism to the Mariana Trench, which might be a result of long-term adaptation to the special geographic, physical and chemical conditions of the Mariana Trench, such as extreme hydrostatic pressure, tectonic activity, geographic isolation, and nutrient inputs [19, 35].
Metabolic potential for degradation of carbohydrates, fatty acids, proteins, and organosulfur compounds
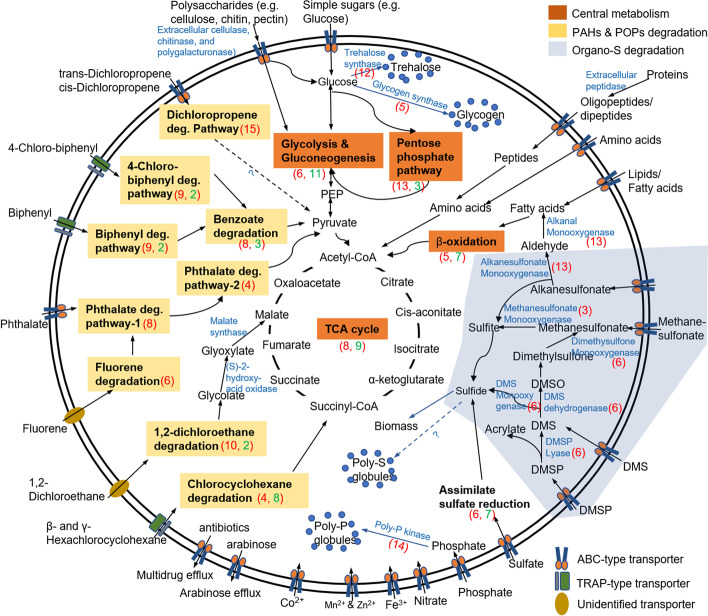
Considering the varied completeness (51.38–92.99%) of the retrieved MAGs, the downstream functional analysis was mainly focused on genes and pathways that were successfully identified and annotated from the MAGs. Discussion on absent genes/pathways was avoided to eliminate possible misleading conclusions due to the incompleteness of the genomes. Genome annotation of the recovered MAGs revealed their potentials for organo-heterotrophic metabolisms and utilization of a wide range of OM (Fig. 4). Gene sets encoding for complete/near-complete pathways or key enzymes in the central carbohydrate metabolism, including glycolysis, tricarboxylic acid cycle (TCA cycle), pentose phosphate pathway, and β-oxidation of fatty acids, were present in all MAGs with genome completeness > 80% (Fig. 4 and Additional file 2: Table S6). These pathways allow the degradation/transformation of simple sugars (e.g., glucose), fatty acids, as well as amino acids. On top of this, genes encoding extracellular cellulases (MT4_29, MT2_13, MT1_55), chitinases (occurred in most MAGs), and polygalacturonases (MT2_13) as well as ABC-type transporters for polysaccharides were also present in the MAGs (Fig. 4 and Additional file 2: Table S7), suggesting the potential to degrade complex polysaccharides, such as cellulose, chitin or pectin. In addition, different types of peptidases as well as ABC-type transporters for amino acids, dipeptides and oligopeptides were found to be present in the Chloroflexi MAGs, indicating their potential to degrade protein detritus (Fig. 4 and Additional file 2: Table S7).
Fig. 4.
Overview of the metabolic potentials in the 17 assembled Chloroflexi MAGs. Black arrows show the annotated metabolic pathways and the linkage between different metabolic flows. The pathways with light blue background show the degradation pathways of organosulfur compounds, and those with yellow background show the degradation of different recalcitrant compounds. The red and green values in brackets are numbers of MAGs that encode complete and partial pathways/enzymes, respectively. If only a red value is shown, the associated pathway/enzyme is complete in all of the related MAGs
The hadal sediment Chloroflexi MAGs also had the potential capability to degrade various organosulfur compounds (Fig. 4). Alkanesulfonate monooxygenase coding genes were present in 13 of the 17 recovered MAGs (Figs. 4 and 5 and Additional file 2: Table S7). This enzyme cleaves the carbon-sulfur bonds in a wide range of sulfonated alkanes to produce sulfite and aldehyde [36], with the latter being oxidized to fatty acid by an alkanal monooxygenase, whose coding gene was also present in 13 of the 17 MAGs (Figs. 4 and 5 and Additional file 2: Table S7). In addition, genes encoding homologs of enzymes involved in dimethylsulfide (DMS) (i.e., DMS monooxygenase and DMS dehydrogenase) and methanesulfonate metabolisms (i.e., methanesulfonate monooxygenase) were found in the MAGs (Figs. 4 and 5), and genes encoding the ABC-type sulfonate transporters were also identified (Fig. 4 and Additional file 2: Table S7). These results suggested the potential of hadal sediment Anaerolineae and Dehalococcoidia to utilize multiple organic sulfur compounds as energy, carbon and sulfur sources, a finding that is similar with previous reports on SAR202 clade (primarily SAR202 group III) from deep seawater [8, 28].
Fig. 5.
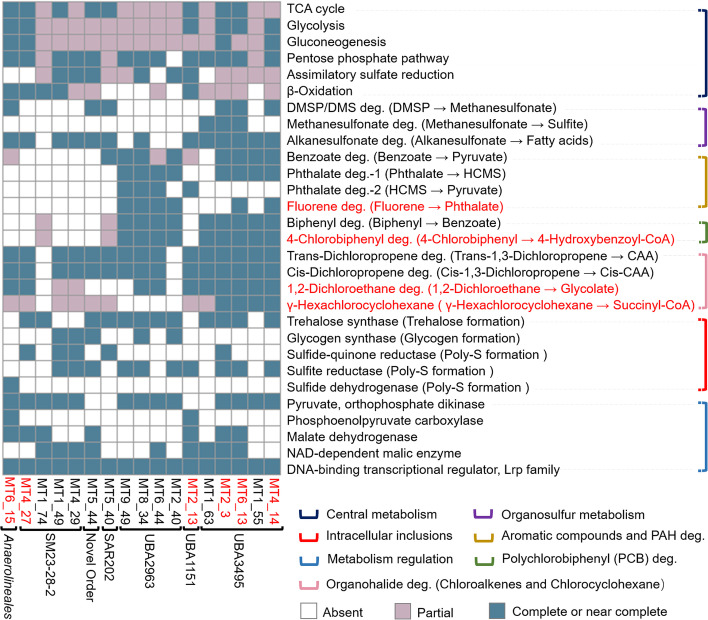
Completeness of the metabolic pathways identified in the Chloroflexi MAGs. “Complete or near complete” indicates pathways that were complete or with one enzyme missing, “partial” indicates pathways with two or more enzymes missing, and “absent” means none of the enzymes in the pathways were identified. The red-colored pathways were further illustrated in Fig. 6 for detailed reaction flows. Red-colored names at the bottom indicate the MAGs with completeness > 80%
Pathways for the degradation of phthalate, benzoate, polyaromatic hydrocarbons and polychlorobiphenyl compounds
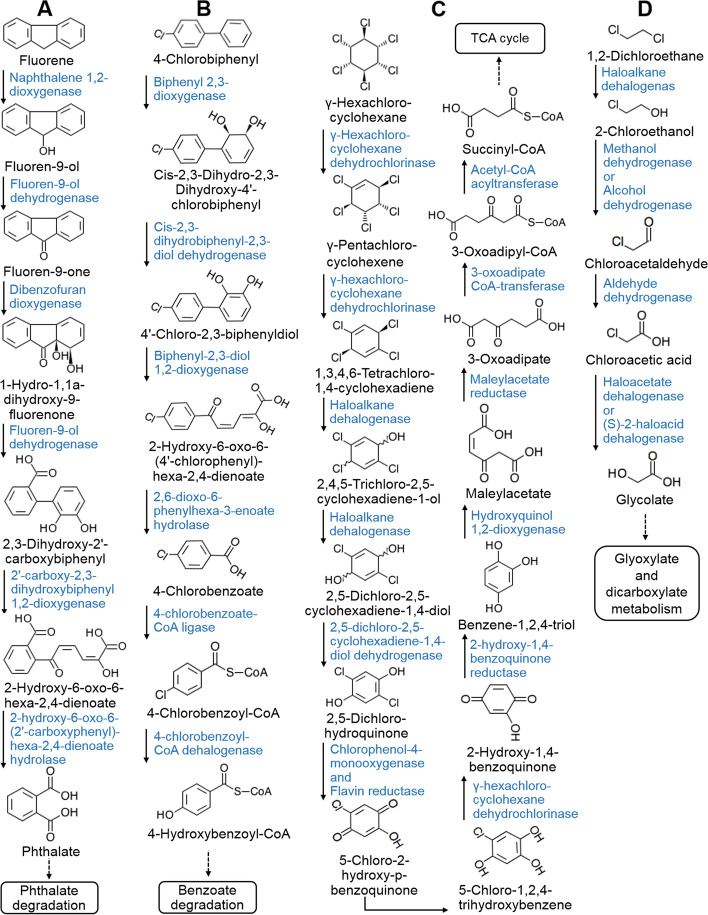
The hadal Chloroflexi MAGs harbored pathways for the degradation of benzoate and phthalate (Figs. 4 and 5). Eight MAGs from the orders SAR202, UBA2963 and UBA3495 contained complete or near complete gene clusters encoding six enzymes for the degradation of benzoate to pyruvate or oxaloacetate (Figs. 4 and 5, Additional file 2: Table S7 and Additional file 1: Fig. S3). Eight MAGs from the orders UBA2963 and UBA3495 contained genes encoding complete or near complete pathways for degradation of phthalate to 4-carboxy-2-hydroxymuconate semialdehyde (HCMS) (Figs. 4 and 5, Additional file 2: Table S7 and Additional file 1: Fig. S4), and four MAGs from UBA2963 and UBA1151 contained genes encoding enzymes that can further degrade HCMS to pyruvate (Fig. 5, Additional file 2: Table S7 and Additional file 1: Fig. S4). As benzoate and phthalate are common intermediates in the degradation of many aromatic compounds, we hypothesized that the recovered MAGs might also be able to degrade substrates with more complex structures. Indeed, complete or near complete pathways for the degradation of polyaromatic hydrocarbons (e.g., fluorene) and polychlorobiphenyls (PCBs, e.g., biphenyl and 4-chlorobiphenyl) were found (Figs. 4 and 5). Six MAGs from the orders UBA2963 and UBA3495 harbored near complete pathways for the transformation of fluorene to phthalate (Figs. 5 and 6 and Additional file 2: Table S7). Nine MAGs from the orders UBA2963 and UBA3495 harbored complete or near complete pathways for the transformation of biphenyl to benzoate (Figs. 4 and 5, Additional file 2: Table S7 and Additional file 1: Fig. S5). In addition, the nine MAGs from the orders UBA2963 and UBA3495 also contained complete or near complete pathways for the degradation of 4-chlorobiphenyl to 4-hydroxy-benzoyl-CoA (Figs. 5 and 6 and Additional file 2: Table S7), which can be further metabolized via benzoate degradation pathway (Fig. 6).
Fig. 6.
The degradation pathways of representative PAHs and POPs identified in hadal sedimentary Chloroflexi MAGs. These pathways were potentially utilized for complete degradation of (A) fluorene, (B) 4-chlorobiphenyl, (C) 1,2-dichloroethane, and (D) γ-hexachlorocyclohexane. The illustrated pathways were found to be complete in at least one MAG recovered in this study
As labile OM is usually readily utilized by microorganisms in the upper water layers, the remaining OM in the deep ocean often includes a variety of structurally complex compounds, such as aromatic compounds [37]. Partial pathways of phthalate degradation (phthalate to protocatechuate), and some enzymes involved in the degradation of benzoate (e.g., catechol 2,3-dioxygeenase) have been reported in SAR202 MAGs/SAGs from seawater of hadal trenches and other deep-sea environments [15–17, 28], and related genes were highly transcribed in situ [15, 28]. Our study advances the existing knowledge by identifying the complete pathways for the degradation of phthalate and benzoate to CO2 by hadal sediment Chloroflexi, and is the first time to show that deep-sea Chloroflexi harbor pathways to completely degrade fluorene, biphenyl and 4- chlorobiphenyl.
Pathways for hydrolytic degradation of halogenated organic compounds
In this study, we further discovered in hadal sediment Chloroflexi the prevalence of genes encoding haloalkane dehalogenase, haloacetate dehalogenase, and 2-haloacid dehalogenase (Fig. 5 and Additional file 2: Table S7), which catabolize hydrolytic dehalogenation, replacing the halogen atoms in organohalides with hydroxyl groups [38]. These enzymes have a broad specificity and participate in the degradation of multiple halogenated OM [38]. Complete or near-complete pathways for the hydrolytic and oxidative degradation of several chloroalkenes and chlorocyclohexane were further revealed (Figs. 4 and 5). Ten MAGs from the orders Anaerolineales, SM23-28-2, UBA2963, UBA1151, and UBA3495 harbored genes encoding the complete or near-complete pathways for the degradation of 1,2-dichloroethane to glycolate (Figs. 5 and 6 and Additional file 2: Table S7), which can either be further transformed and enter the TCA cycle or be utilized for vitamin B6 biosynthesis. The same pathway also catabolizes the degradation of trans-dichloropropene and cis-dichloropropene to trans-3- and cis-3-chloroacrylic acid, respectively (Figs. 4 and 5, Additional file 2: Table S7 and Additional file 1: Fig. S6). In addition, a pathway for the complete degradation of γ-hexachlorocyclohexane to succinyl-CoA (an intermediate in the TCA cycle) was reconstructed in the Chloroflexi MAGs (Figs. 4 and 5). The entire pathway involves 11 enzymes (Fig. 6 and Additional file 2: Table S7), and complete or near complete sets of genes encoding these enzymes were present in four MAGs from the order UBA3495 (Fig. 5). Eight MAGs from orders Anaerolineales, SM23-28-2, SAR202, UBA1151, UBA3495 and the novel order (MT5_44) also encode for the majority of enzymes for γ-hexachlorocyclohexane degradation, but with 2-6 enzymes missing (Fig. 5 and Additional file 2: Table S7).
Currently, deep-sea Chloroflexi have mainly been implied in reductive dehalogenation [9, 39, 40], a strictly anaerobic process which utilizes halogenated organic compounds as electron acceptor to oxidize hydrogen (or formate) [41]. In contrast, the hydrolytic and oxidative degradation of organohalides are aerobic processes [38]. The genes coding for haloalkane and haloacetate dehalogenases have been previously reported in Chloroflexi genomes from oxic abyssal sediments [42], but our study revealed for the first time the complete or near complete pathways for hydrolytic and oxidative degradation of multiple types of organohalides in hadal Chloroflexi (Figs. 4 and 5). The MAGs in this study were recovered from surface sediments of the Challenger Deep at depth of 0–10 cm below seafloor, which were well oxygenated as revealed by in situ oxygen measurements conducted at the same site [25]. Such an environmental condition well supports the potential of the studied Chloroflexi to degrade organohalides via the annotated pathways. In addition, reads recruitment showed that the majority of the MAGs retrieved in this study were also widely distributed in global deep-sea water and surface sediment (Fig. 3), which highlights the significance of Chloroflexi in carbon and halogen cycling in oxygenic habitats of the deep ocean.
Microbial degradation of persistent organic pollutants in the deepest ocean
The metabolic reconstruction of the recovered MAGs in this study revealed the potential of hadal sediment Chloroflexi for the complete degradation of different types of recalcitrant organic compounds, including PAHs (e.g., fluorene), PCBs (e.g., 4-chlorobiphenyl), haloalkanes (e.g., 1,2-dichloroethane and 1,3-dichloropropene), and chlorocyclohexane (e.g., γ-hexachlorocyclohexane) (Fig. 5 and Additional file 2: Table S7). These findings have important implications for the deep ocean ecosystems, in general, and the hadal trench systems in particular. Many of these compounds are listed as persistent organic pollutants (POPs) by the Stockholm Convention [43] and their presence and accumulation in deep-sea organisms and environments have been widely reported [44, 45]. Recent studies have further revealed that PCBs, microplastics, heavy metals, and halogenated organic pollutants have even accumulated in the deepest trenches of the ocean [46–49], suggesting that the anthropogenic pollutants can be an important part of the OM pool in the hadal trenches. Many types of PAHs and POPs-like compounds can however also be naturally produced via biotic (e.g., biosynthesis via halogenase or haloperoxidase) and abiotic processes (e.g., peroxidative mechanisms, photochemical reactions, volcanic activities) [39, 50, 51], and can be enriched in the deep ocean via the “biological pump” [52]. The capability to metabolize these recalcitrant OM would likely provide Chloroflexi bacteria with survival advantages in nutrient/energy-limited habitats, which might be one of the reasons for their dominance in the sediments of the hadal trenches as observed in this (Fig. 3) and previous studies [26, 27]. On the other hand, close interactive networks have been reported to occur between Chloroflexi lineages and between Chloroflexi and other microbial taxa in the hadal trench sediments [27], which supports the possibility of co-metabolism during the degradation of recalcitrant OM [53]. For example, the degradation of halogenated organic matter by Chloroflexi (i.e., dehalogenation process) may produce semi-labile intermediates serving as substrates for other taxa in the microbial community [41]. The capability to degrade recalcitrant OM and the potential co-metabolism relationships with other microbial taxa might be one of the reasons for previous observations that Chloroflexi lineages play keystone roles in interactive networks of the microbial community in hadal trench sediments [27].
A potentially “feast-or-famine” metabolic strategy in response to fluctuating supply of OM
Deep-sea benthic communities experience feast-or-famine conditions due to the periodical and spatial variations in the input of particles in a generally energy-depleted environment [7]. Deep-sea microbial communities have been shown to respond rapidly to nutrient input, even after long periods of starvation [54]. However, little is known about the genomic basis and potential metabolic strategies of deep-sea microorganisms for such a lifestyle. Our results showed that the hadal Chloroflexi exhibited capabilities of degrading a wide range of organic carbon, sulfur, and halogen compounds (Figs. 4 and 5), including not only labile OM, but also many types of recalcitrant organic compounds (Figs. 5 and 6). In addition, the majority of the MAGs harbor genes encoding alpha, alpha-trehalose synthase, trehalose-6-phosphate synthase and trehalose-phosphate-phosphatase (Fig. 5 and Additional file 2: Table S7), which are key enzymes for the formation of trehalose, a type of intracellular energy storage compound [55]. Some of the MAGs also harbored genes encoding starch (glycogen) synthase and 1,4-alpha-glucan branching enzyme involved in the biosynthesis of glycogen (Fig. 5 and Additional file 2: Table S7) [56], polyphosphate kinase, and phosphate transporter proteins involved in the formation of polyphosphate inclusions (Additional file 2: Table S7) [57], or sulfide-quinone reductase, sulfite reductase, and sulfide dehydrogenase involved in the formation of sulfur globules (Fig. 5 and Additional file 2: Table S7) [56]. Such features are consistent with a “feast-or-famine” metabolic strategy (Fig. 7). During the “feast” condition, such as a pulse input of particulate OM due to diatom bloom in the surface water [7], the bacteria might preferentially uptake and consume labile OM, and excess energy, carbon, and other elements can be stored as intracellular inclusions (Fig. 7). During “famine” condition (i.e., nutrient depleted), the bacteria may enter the “famine” mode to acquire energy from the stored inclusions, and/or from degrading the recalcitrant OM available in the surrounding environments (Fig. 7).
Fig. 7.

The proposed “feast-or-famine” metabolic strategy for hadal sediment Chloroflexi recovered in this study
In support of such a “feast-or-famine” lifestyle, the MAGs also encode modules for regulating metabolism in response to changes in nutrient conditions (Fig. 5). The majority of the MAGs contained genes encoding pyruvate orthophosphate dikinase, malate dehydrogenase, and malic enzyme (Fig. 5), and two of the MAGs also showed potential to encode PEP carboxylase (Fig. 5). These enzymes catalyze the inter-conversions between pyruvate, PEP, oxaloacetate, and malate (Additional file 1: Fig. S7), which interconnect the central carbon metabolic pathways (e.g., the TCA cycle and biosynthesis) and are responsible for regulating carbon fluxes among catabolism, anabolism, and energy supply according to the physiological conditions of the cell (Additional file 1: Fig. S7) [58]. In addition, all of the recovered MAGs harbored the lrp gene (COG1522) encoding the leucine-responsive regulatory protein (LRP) (Fig. 5), which is one of the “feast-or-famine” regulatory proteins that control the expression of more than 30% of bacterial genes in response to changes in nutrient levels [59, 60]. The existence of these regulatory genes suggests the potential of the hadal Chloroflexi to rapidly change the metabolism and physiology under the feast or famine conditions, although the detailed regulation mechanisms can be very complex and are still unknown. Similarly, previous researchers have shown that common marine bacteria (e.g., Vibrio, Alteromonas, Colwellia) from surface seawaters also followed “feast-or-famine” strategy which allows these bacteria to respond rapidly to the changes in nutrient conditions [61]. These findings suggest that the “feast-or-famine” lifestyle might be a common strategy for marine microorganisms to adapt to variable nutrient conditions.
In addition to the changes in nutrient conditions, other environmental factors such as oxygen levels might also trigger the shift between feast and famine metabolic modes of Chloroflexi in surface sediments. For example, input of fresh particles from surface water algal blooms [7, 62] may greatly stimulate the respiration and growth of microorganisms in surface hadal sediment, leading to rapid depletion of dissolved oxygen and the shift from aerobic to anaerobic condition. According to the metabolic potentials annotated (Figs. 4 and 5), the hadal Chloroflexi may consume labile organic carbon (such as simple sugars, amino acids) and store the excess energy intracellularly (e.g., in the form of trehalose, glycogen or poly-P) under aerobic condition and degrade the intracellular energy storage compounds for energy generation under subsequent anaerobic condition. In fact, oxygen-triggered “feast-or-famine” metabolic strategy has been well documented in polyphosphate accumulating organisms (PAOs) from wastewater treatment systems [63, 64], although the detailed mechanisms might be different from those in hadal Chloroflexi (e.g., PAOs usually synthesize polyhydroxyalkanoate under anaerobic condition but no related genes were found in Chloroflexi MAGs).
Conclusions
This study provides an extensive exploration of the metabolic potential of novel and dominant Chloroflexi lineages retrieved from the hadal sediments of the Mariana Trench. The results demonstrated a high metabolic plasticity of the hadal sediment Chloroflexi, including the complete pathways for hydrolytic or oxidative degradation of recalcitrant OM such as PAHs, PCBs, and organohalides. Our findings expand the current understanding on metabolic capabilities of deep-sea Chloroflexi, and highlight their significance on carbon, sulfur, and halogen cycling in the deep ocean. The metabolic plasticity, the capability to form intracellular storage inclusions, as well as the regulatory modules to respond to nutrient conditions discovered in the MAGs support the notion that the hadal sediment Chloroflexi employ a “feast-or-famine” metabolic strategy. Such a metabolic strategy allows the bacteria to fulfill energy and nutrient requirement via degradation of different substrates according to the nutrient conditions, and regulate the cell activities (e.g., growth, motility) accordingly, providing advantages for their adaptation to the variable OM conditions in the hadal trenches and other deep-sea habitats. This study therefore provides new perspectives on the metabolism and adaptation strategies of Chloroflexi in deep-sea environments.
Material and methods
Site description and sampling
Sediment samples were obtained from the Challenger Deep of the Mariana Trench (site MT, 11.4037 °N, 142.3630 °E, water depth of 10853 m) during the cruise from December 2016 to January 2017 by the MV Zhangjian. Samples were collected using a box corer attached to a Hadal Lander [25]. Details of the sampling procedure were given in Liu et al. [27]. After recovering onboard, the sediment samples were immediately subsampled using sterile plastic corers and stored at – 80 °C on board.
Amplicon sequencing analysis on 16S rRNA and rRNA gene diversity
Sediment cores were thawed on ice and were depth fractioned to 0–2-, 2–3-, 3–4-, 4–5-, 5–6-, 6–7-, 7–8-, 8–9- and 9–10-cm subsamples. Total DNA and RNA were co-extracted from triplicate 1-g sediments of each depth fraction using the PowerSoil Total RNA Isolation Kit and DNA Elution Accessory Kit (MoBio Lab, USA) following the manufacturer’s instructions. The RNA samples were treated with DNase I and cDNAs were synthesized using the GoScriptTM Reverse Transcription System (Promega, USA) with random primers. DNA and cDNA samples were amplified with a barcoded primer set 338F/806R targeting the V3–V4 hypervariable regions of bacteria [65]. Detailed procedure for DNA/RNA coextraction and PCR were given in Liu et al. [27]. PCR products from each sample were purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluorTM-ST (Promega, USA). Purified amplicons from different samples were pooled in equimolar ratio and subjected to paired-end sequencing (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) in Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The procedure of the sequence processing was the same as Liu et al. [27]. Briefly, the raw reads were demultiplexed, quality filtered, and assembled, followed by the identification and removal of chimeric sequences. Operational taxonomic units (OTUs) were obtained using UPARSE (v. 7.1) at a 97% similarity cutoff, and their taxonomy was assigned by RDP Classifier against the SILVA 16S rRNA database (SSU138) with a confidence threshold of 70%. The final OTU table contained 6199–70461 sequences (with a mean of 37603) for different samples (Additional file 2: Table S8).
Metagenomic sequencing and genome reconstruction
Total genomic DNA was extracted from 10–20 g of sediments from each depth fraction, using the FastDNA® SPIN Kit for Soil (MP Biomedicals, USA). DNA fragment libraries were prepared by shearing genomic DNA from each sample and were then subjected to metagenomic sequencing in the BGI group (Shenzhen, China) using the BGIseq 500 platform, which generated 238.5 Gb of raw reads for the nine metagenomes (23.1–36.8 Gb for individual metagenome, with an average value of 26.5 Gb).
Metagenomic reads from each sample were quality filtered using Trimmomatic v. 0.38 [66] with parameters specified as “LEADING:30 TRAILING:30 CROP:90 HEADCROP:10 SLIDINGWINDOW:4:25 MINLEN:50,” and were separately assembled with IDBA_UD v. 1.1.3 (k-mer range 50–80, step 15) [67]. The quality-filtered reads were mapped back to the assemblies using Bowtie2 (v. 2.3.4.1) [68], and coverage was determined according to the mapping results with the jgi_summarize_bam_contig_depths script [68]. Metagenome binning was conducted for assemblies longer than 2500 bp using MetaBAT v. 2.12.1 [69] and CONCOCT v. 1.0.0 [70] with default parameters, and were subsequently refined using Binning_refiner v. 1.2 [71]. Quality of the MAGs was assessed by CheckM v. 1.1.2 using the lineage_wf workflow [72], and only MAGs with completeness > 50% and contamination < 5% were kept for further analysis. Redundant bins were subsequently dereplicated using dRep v. 2.3.2 [73] at 99% average nucleotide identity (ANI) (all other parameters were set to the default), and MAG with the highest quality was selected from each cluster for downstream analysis. The genome size was estimated by dividing the size of the MAG by its estimated completeness.
Phylogenomic analysis and taxonomic classification
A phylogenomic tree was constructed for the MAGs and 1872 Chloroflexi genomes retrieved from the NCBI, JGI and National Genomics Data Center (NGDC) databases (downloaded in April, 2020), using the 43 universal single-copy genes (SCGs) used by CheckM [72]. Protein sequences of the SCGs were identified using HMMER v. 3.1b2 [74] with default parameters, individually aligned with MAFFT v. 7.467 [75] and then concatenated. Phylogenomic tree was constructed based on the alignment using FastTree2 v. 2.1.11 [76], with a JTT model, a gamma approximation and 100 bootstrap replicates.
The closest genomes of each MAG were determined based on their placements in the phylogenomic tree. A final maximum-likelihood phylogenomic tree was then constructed using the MAGs recovered in this study, their closest relatives, all Chloroflexi genomes previously reported from trenches, and representative genomes of all known classes of Chloroflexi, with Mycobacterium tuberculosis (GCA_000195955.2) as outgroup. The phylogenomic tree was visualized using iTOL [77]. Detailed taxonomic classification of the MAGs was determined using GTDB-Tk, which is based on the phylogenetically calibrated Genome Taxonomy Database (GTDB) [29]. The novelty of the MAGs was determined in GTDB-tk based on a combination of their placement in the GTDB reference tree, their RED values, and their average nucleotide identity (ANI) to reference genomes [29, 78]. Briefly, classifications were primarily determined by the placement of the genomes in the GTDB reference tree; if the placement of a genome is ambiguous, the RED value is used for further classification based on well-established standards for each taxonomy level in GTDB; and the assignment of a genome to an existing species was based on the ANI value to reference genomes with a threshold of 95% [29, 78, 79].
Relative abundance estimation and global distribution
To estimate relative abundance and distribution of recovered MAGs in the sampled hadal sediments, 16S rRNA gene of the MAGs was predicted using barrnap v. 0.9 with default parameters (https://github.com/tseemann/barrnap) and searched with BLASTN [80] against the 16S rRNA and 16S rRNA gene libraries constructed in this study. Only the BLASTN hits with identity > 97%, alignment length > 300 bp, and e-value < 1e−5 were considered, and the closest matched OTUs were selected. If multiple OTUs were identified as the closest matches for a MAG, the most abundant OTU was selected. The closest-match OTUs were then extracted and their relative abundance in the 16S rRNA and 16S rRNA gene libraries from different layers of the sediment were examined.
The distribution of MAGs in hadal sediments was also estimated via reads recruitment as described by Mehrshad et al. [8]. Briefly, rRNA gene sequences in MAGs were first masked to avoid bias in recruitment results. Recruitments were performed using BLASTN, and hits were filtered with a length cut-off of 50 bp, an identity cut-off of 95%, and an e-value cut-off of 1e−5 [8]. Qualified hits were used to compute the RPKG (reads recruited per kilobase of genome per gigabase of metagenome) values, which reflect a normalized abundance allowing the comparison across different genomes and metagenomes. Reads recruitment was also applied against 58 publicly available metagenomes derived from microbial communities in seawater and surface sediments from epi-, meso-, bathyl-, and hadal zones of the open ocean, and those from sediments of mud volcano, deep-sea oil spilling sites, deep subseafloor, coastal regions, river, and salt-lake, as well as two metagenomes from soil (Additional file 2: Table S5), to estimate the global distribution of recovered MAGs.
Gene annotation and metabolic reconstruction
Coding sequences in the MAGs were predicted using Prodigal v. 2.6.3 with default setting [81]. Functional annotation was performed by using BlastKOALA against the KEGG database with default parameters [82], by running similarity searches with BLASTP against the Cluster of Orthologous Groups (COG, December 2014 release) with an e-value cut-off of 0.001 [83], as well as by running PROKKA under the “-- metagenome” mode and with kingdom specified as “Bacteria” [84]. Carbohydrate active enzymes were identified using dbCAN 1.0 (default setting) against the CAZy database (version 07312019) [85]. Missing enzymes for pathways of interest were further searched by running tBLASTn against relevant reference sequences in the NCBI database. A tBLASTn hit with an e-value ≤ e−5, sequence identity ≥ 30%, and a percent alignment length ≥ 30% was considered a potential homolog [86]. The tBLASTn hits were also confirmed by the BLASTp search against the RefSeq protein database [86].
Supplementary Information
Additional file 1. Supplementary figures.
Additional file 2. Supplementary tables.
Additional file 3. Taxonomy names proposed for the six MAGs qualified as type material.
Acknowledgements
The authors thank Prof. Weicheng Cui, Prof. Yunping Xu, Dr. Binbin Pan and their scientific and technological teams, as well as crews of the R/V Zhangjian for cruise organization, technological support, and assistance on sampling. We also thank Dr. Shan Zhang from the University of New South Wales, Australia, for her advice in the naming of the new species.
Authors’ contributions
RL and JF designed the work and supervised the entire procedures of the experiment, data analysis, and manuscript writing. XW, JW, TJ, ZW, WW, HZ, CY, and ZS conducted experiments. WS, XW, LW, and JD conducted bioinformatic analysis. JC, TT, and YW provided ideas for data analysis and interpretation. RL, XW, WS, and JF wrote the manuscript. All authors provided comments, edited and approved the manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC0310600), the National Natural Science Foundation of China (Grant No. 91951210, 41773069, 41906134), the Shanghai Natural Science Foundation (20ZR1423700), and a R&D project registered as ANP 21005-4, “PROBIO-DEEP - Survey of potential impacts caused by oil and gas exploration on deep-sea marine holobionts and selection of potential bioindicators and bioremediation processes for these ecosystems” sponsored by Shell Brasil under the ANP R&D levy as “Compromisso de Investimentos com Pesquisa e Desenvolvimento.”
Availability of data and materials
The sequences of 16S rRNA and 16S rRNA gene libraries, the metagenomic raw reads of the Mariana Trench sediments, as well as all metagenome-assembled Chloroflexi genomes of this study are available in the NCBI database and can be accessed under project ID PRJNA692099.
Declarations
Ethics approval and consent to participate
Ethics approval was not required for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rulong Liu, Email: rlliu@shou.edu.cn.
Jiasong Fang, Email: jsfang@shou.edu.cn.
References
- 1.Arístegui J, Gasol JM, Duarte CM, Herndld GJ. Microbial oceanography of the dark ocean’s pelagic realm. Limnol Oceanogr. 2009;54:1501–1529. [Google Scholar]
- 2.Herndl GJ, Reinthaler T. Microbial control of the dark end of the biological pump. Nat Geosci. 2013;6:718–724. doi: 10.1038/ngeo1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jørgensen BB, Boetius A. Feast and famine-microbial life in the deep-sea bed. Nat Rev Microbiol. 2007;5:770–781. doi: 10.1038/nrmicro1745. [DOI] [PubMed] [Google Scholar]
- 4.Conte MH, Ralph N, Ross EH. Seasonal and interannual variability in deep ocean particle fluxes at the Oceanic Flux Program (OFP)/Bermuda Atlantic Time Series (BATS) site in the western sargasso sea near Bermuda. Deep Sea Res Part II Top Stud Oceanogr. 2001;48:1471–1505. [Google Scholar]
- 5.Arístegui J, Montero MF. Temporal and spatial changes in plankton respiration and biomass in the Canary Islands region: the effect of mesoscale variability. J Mar Syst. 2005;54:65–82. [Google Scholar]
- 6.Smith KL, Jr, Ruhl HA, Huffard CL, Messié M, Kahru M. Episodic organic carbon fluxes from surface ocean to abyssal depths during long-term monitoring in NE Pacific. Proc Natl Acad Sci U S A. 2018;115:12235–12240. doi: 10.1073/pnas.1814559115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabowski E, Letelier RM, Laws EA, Karl DM. Coupling carbon and energy fluxes in the North Pacific Subtropical Gyre. Nat Commun. 2019;10:1895. doi: 10.1038/s41467-019-09772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrshad M, Rodriguez-Valera F, Amoozegar MA, López-García P, Ghai R. The enigmatic SAR202 cluster up close: shedding light on a globally distributed dark ocean lineage involved in sulfur cycling. ISME J. 2018;12:655–668. doi: 10.1038/s41396-017-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fincker M, Huber JA, Orphan VJ, Rappé MS, Teske A, Spormann AM. Metabolic strategies of marine subseafloor Chloroflexi inferred from genome reconstructions. Environ Microbiol. 2020;22:3188–3204. doi: 10.1111/1462-2920.15061. [DOI] [PubMed] [Google Scholar]
- 10.Morris RM, Rappé MS, Urbach E, Connon SA, Rappe MS, Giovannoni SJ. Prevalence of the Chloroflexi-related SAR202 bacterioplankton cluster throughout the mesopelagic zone and deep ocean. Appl Environ Microbiol. 2004;70:2836–2842. doi: 10.1128/AEM.70.5.2836-2842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schattenhofer M, Fuchs BM, Amann R, Zubkov MV, Tarran GA, Pernthaler J. Latitudinal distribution of prokaryotic pico- plankton populations in the Atlantic Ocean. Environ Microbiol. 2009;11:2078–2093. doi: 10.1111/j.1462-2920.2009.01929.x. [DOI] [PubMed] [Google Scholar]
- 12.Varela MM, Van Aken HM, Herndl GJ. Abundance and activity of chloroflexi-type SAR202 bacterioplankton in the meso- and bathypelagic waters of the (sub)tropical Atlantic. Environ Microbiol. 2008;10:1903–1911. doi: 10.1111/j.1462-2920.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 13.Parkes RJ, Cragg B, Roussel E, Webster G, Weightman A, Sass H. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere:geosphere interactions. Mar Geol. 2014;352:409–425. [Google Scholar]
- 14.Hoshino T, Doi H, Uramoto GI, Wörmer L, Adhikari RR, Xiao N, et al. Global diversity of microbial communities in marine sediment. Proc Natl Acad Sci U S A. 2020;117:27587–27597. doi: 10.1073/pnas.1919139117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao ZM, Huang JM, Cui GJ, Li WL, Li J, Wei ZF, et al. In situ meta-omic insights into the community compositions and ecological roles of hadal microbes in the Mariana Trench. Environ Microbiol. 2019;21:4092–4108. doi: 10.1111/1462-2920.14759. [DOI] [PubMed] [Google Scholar]
- 16.Landry Z, Swan BK, Herndl GJ, Stepanauskas R, Giovannoni SJ. SAR202 Genomes from the dark ocean predict pathways for the oxidation of recalcitrant dissolved organic matter. mBio. 2017;8:e00413–e00417. doi: 10.1128/mBio.00413-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saw JHW, Nunoura T, Hirai M, Takaki Y, Parsons R, Michelsen M, et al. Pangenomics analysis reveals diversification of enzyme families and niche specialization in globally abundant SAR202 bacteria. mBio. 2020;11:e02975–e02919. doi: 10.1128/mBio.02975-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sewell HL, Kaster AK, Spormann AM. Homoacetogenesis in deep-sea Chloroflexi, as inferred by single-cell genomics, provides a link to reductive dehalogenation in terrestrial Dehalococcoidetes. mBio. 2017;8:e02022–e02017. doi: 10.1128/mBio.02022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson AJ. The hadal zone: life in the deepest oceans. Cambridge: Cambridge University Press; 2015. [Google Scholar]
- 20.Ichino MC, Clark MR, Drazen JC, Jamieson A, Jones DOB, Martin AP, et al. The distribution of benthic biomass in hadal trenches: a modelling approach to investigate the effect of vertical and lateral organic matter transport to the seafloor. Deep Sea Res Part I Oceanogr Res Pap. 2015;100:21–33. [Google Scholar]
- 21.Liu R, Wang L, Wei Y, Fang J. The hadal biosphere: recent insights and new directions. Deep Sea Res Part II Top Stud Oceanogr. 2018;155:11–18. [Google Scholar]
- 22.Ikehara K, Usami K, Kanamatsu T. Repeated occurrence of surface-sediment remobilization along the landward slope of the Japan Trench by great earthquakes. Earth Planets Space. 2020;72:1–9. [Google Scholar]
- 23.Glud RN, Wenzhofer F, Middelboe M, Oguri K, Turnewitsch R, Canfield DE, et al. High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth. Nat Geosci. 2013;6:284–288. [Google Scholar]
- 24.Wenzhöfer F, Oguri K, Middelboe M, Turnewitsch R, Toyofuku T, Kitazato H, et al. Benthic carbon mineralization in hadal trenches: assessment by in situ O2 microprofile measurements. Deep Sea Res Part I Oceanogr Res Pap. 2016;116:276–286. [Google Scholar]
- 25.Luo M, Glud RN, Pan B, Wenzhöfer F, Xu Y, Lin G, et al. Benthic carbon mineralization in hadal trenches: Insights from in-situ determination of benthic oxygen consumption. Geophys Res Lett. 2018;45:2752–2760. [Google Scholar]
- 26.Hiraoka S, Hirai M, Matsui Y, Makabe A, Minegishi H, Tsuda M, et al. Microbial community and geochemical analyses of trans-trench sediments for understanding the roles of hadal environments. ISME J. 2020;14:740–756. doi: 10.1038/s41396-019-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Wang Z, Wang L, Li Z, Fang J, Wei X, et al. Bulk and active sediment prokaryotic communities in the Mariana and Mussau trenches. Front Microbiol. 2020;11:1521. doi: 10.3389/fmicb.2020.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei ZF, Li WL, Huang JM, Wang Y. Metagenomic studies of SAR202 bacteria at the full-ocean depth in the Mariana Trench. Deep Sea Res Part I Oceanogr Res Pap. 2020;165:103396. [Google Scholar]
- 29.Chaumeil PA, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–1927. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks DH, Chuvochina M, Chaumeil PA, Rinke C, Mussig AJ, Hugenholtz P. A complete domain-to-species taxonomy for bacteria and archaea. Nat Biotechnol. 2020;38:1079–1086. doi: 10.1038/s41587-020-0501-8. [DOI] [PubMed] [Google Scholar]
- 31.Murray AE, Freudenstein J, Gribaldo S, Hatzenpichler R, Hugenholtz P, Kämpfer P, et al. Roadmap for naming uncultivated Archaea and Bacteria. Nat Microbiol. 2020;5:987–994. doi: 10.1038/s41564-020-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowers RM, Kyrpides NC, Stepanauskas R, Harmon-smith M, Doud D, Jarett J, et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat Biotechnol. 2017;35:725–731. doi: 10.1038/nbt.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstantinidis KT, Rosselló-Móra R, Amann R. Uncultivated microbes in need of their own taxonomy. ISME J. 2017;11:2399–2406. doi: 10.1038/ismej.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuvochina M, Rinke C, Parks DH, Rappé MS, Tyson GW, Yilmaz P, et al. The importance of designating type material for uncultured taxa. Syst Appl Microbiol. 2019;42:15–21. doi: 10.1016/j.syapm.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Peoples LM, Grammatopoulou E, Pombrol M, Xu X, Osuntokun O, Blanton J, et al. Microbial community diversity within sediments from two geographically separated hadal trenches. Front Microbiol. 2019;10:347. doi: 10.3389/fmicb.2019.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis HR. Mechanism for sulfur acquisition by the alkanesulfonate monooxygenase system. Bioorg Chem. 2011;39:178–184. doi: 10.1016/j.bioorg.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Fatayer S, Coppola AI, Schulz F, Walker BD, Broek TA, Meyer G, et al. Direct visualization of individual aromatic compound structures in low molecular weight marine dissolved organic carbon. Geophys Res Lett. 2018;45:5590–5598. [Google Scholar]
- 38.Ang TF, Maiangwa J, Salleh AB, Normi YM, Leow TC. Dehalogenases: from improved performance to potential microbial dehalogenation applications. Molecules. 2018;23:1100. doi: 10.3390/molecules23051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atashgahi S, Häggblom MM, Smidt H. Organohalide respiration in pristine environments: implications for the natural halogen cycle. Environ Microbiol. 2018;20:934–948. doi: 10.1111/1462-2920.14016. [DOI] [PubMed] [Google Scholar]
- 40.Futagami T, Morono Y, Terada T, Kaksonen AH, Inagaki F. Distribution of dehalogenation activity in subseafloor sediments of the Nankai Trough subduction zone. Philos Trans R Soc Lond Ser B Biol Sci. 2013;368:20120249. doi: 10.1098/rstb.2012.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Sanford R, Yan J, Chen G, Cápiro NL, Li X, et al. Roles of organohalide-respiring Dehalococcoidia in carbon cycling. mSystems. 2020;5:e00757–e00719. doi: 10.1128/mSystems.00757-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuillemin A, Kerrigan Z, D’Hondt S, Orsi WD. Exploring the abundance, metabolic potential and gene expression of subseafloor Chloroflexi in million-year-old oxic and anoxic abyssal clay. FEMS Microbiol Ecol. 2020;96:fiaa223. doi: 10.1093/femsec/fiaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagen PE, Walls MP. The Stockholm Convention on persistent organic pollutants. Nat Res Environ. 2005;19:49–52. [Google Scholar]
- 44.Barrett J, Chase Z, Zhang J, Holl MMB, Willis K, Williams A, et al. Microplastic pollution in deep-sea sediments from the Great Australian Bight. Front Mar Sci. 2020;7:576170. [Google Scholar]
- 45.Koenig S, Huertas D, Fernández P. Legacy and emergent persistent organic pollutants (POPs) in NW Mediterranean deep-sea organisms. Sci Total Environ. 2013;443:358–366. doi: 10.1016/j.scitotenv.2012.10.111. [DOI] [PubMed] [Google Scholar]
- 46.Cui J, Yu Z, Mi M, He L, Sha Z, Yao P, et al. Occurrence of halogenated organic pollutants in hadal trenches of the Western Pacific Ocean. Environ Sci Technol. 2020;54:15821–15828. doi: 10.1021/acs.est.0c04995. [DOI] [PubMed] [Google Scholar]
- 47.Jamieson AJ, Malkocs T, Piertney SB, Fujii T, Zhang Z. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat Ecol Evol. 2017;1:51. doi: 10.1038/s41559-016-0051. [DOI] [PubMed] [Google Scholar]
- 48.Liu M, Xiao W, Zhang Q, Shi L, Wang X, Xu Y. Methylmercury bioaccumulation in deepest ocean fauna: implications for ocean mercury biotransport through food webs. Environ Sci Technol Lett. 2020;7:469–476. [Google Scholar]
- 49.Peng G, Bellerby R, Zhang F, Sun X, Li D. The ocean’s ultimate trashcan: hadal trenches as major depositories for plastic pollution. Water Res. 2020;168:115121. doi: 10.1016/j.watres.2019.115121. [DOI] [PubMed] [Google Scholar]
- 50.Simoneit BR. Hydrothermal petroleum. In: Wilkes H, editor. Hydrocarbons, oils and lipids: diversity, origin, chemistry and fate. Springer; 2018. pp. 1–35. [Google Scholar]
- 51.Wang W, Li Z, Zeng L, Dong C, Shao Z. The oxidation of hydrocarbons by diverse heterotrophic and mixotrophic bacteria that inhabit deep-sea hydrothermal ecosystems. ISME J. 2020;14:1994–2006. doi: 10.1038/s41396-020-0662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leri AC, Mayer LM, Thornton KR, Northrup PA, Dunigan MR, Ness KJ, et al. A marine sink for chlorine in natural organic matter. Nat Geosci. 2015;8:620–624. [Google Scholar]
- 53.Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W, et al. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev. 2008;32:927–955. doi: 10.1111/j.1574-6976.2008.00127.x. [DOI] [PubMed] [Google Scholar]
- 54.Sebastián M, Estrany M, Ruiz-González C, Forn I, Sala MM, Gasol JM, et al. High growth potential of long-term starved deep ocean opportunistic heterotrophic bacteria. Front Microbiol. 2019;10:760. doi: 10.3389/fmicb.2019.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanaporn M, Titball RW. Trehalose and bacterial virulence. Virulence. 2020;11:1192–1202. doi: 10.1080/21505594.2020.1809326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahl C, Prange A. Bacterial sulfur globules: occurrence, structure and metabolism. In: Shively JM, editor. Inclusions in prokaryotes. Springer; 2006. p. 21. [Google Scholar]
- 57.Achbergerová L, Nahálka J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb Cell Fact. 2011;10:63. doi: 10.1186/1475-2859-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauer U, Eikmanns BJ. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev. 2005;29:765–794. doi: 10.1016/j.femsre.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama K, Ishijima SA, Clowney L, Koike H, Aramaki H, Tanaka C, et al. Feast/famine regulatory proteins (FFRPs): Escherichia coli Lrp, AsnC and related archaeal transcription factors. FEMS Microbiol Rev. 2006;30:89–108. doi: 10.1111/j.1574-6976.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 60.Kroner GM, Wolfe MB, Freddolino PL. Escherichia coli Lrp regulates one-third of the genome via direct, cooperative, and indirect routes. J Bacteriol. 2019;201:e00411–e00418. doi: 10.1128/jb.00411-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eilers H, Pernthaler J, Amann R. Succession of pelagic marine bacteria during enrichment: a close look at cultivation-induced shifts. Appl Environ Microbiol. 2000;66:4634–4640. doi: 10.1128/aem.66.11.4634-4640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chow CH, Cheah W, Tai JH. A rare and extensive summer bloom enhanced by ocean eddies in the oligotrophic western North Pacific Subtropical Gyre. Sci Rep. 2017;7(1):6199. doi: 10.1038/s41598-017-06584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarayre C, Nguyen HT, Brognaux A, Delepierre A, De Clercq L, Charlier R, et al. Characterisation of phosphate accumulating organisms and techniques for polyphosphate detection: a review. Sensors (Basel). 2016;16(6):797. doi: 10.3390/s16060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saia SM, Carrick HJ, Buda AR, Regan JM, Walter MT. Critical review of polyphosphate and polyphosphate accumulating organisms for agricultural water quality management. Environ Sci Technol. 2021;55(5):2722–2742. doi: 10.1021/acs.est.0c03566. [DOI] [PubMed] [Google Scholar]
- 65.Liu R, Wang L, Liu Q, Wang Z, Li Z, Fang J, et al. Depth-resolved distribution of particle-attached and free-living bacterial communities in the water column of the New Britain Trench. Front Microbiol. 2018;9:625. doi: 10.3389/fmicb.2018.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng Y, Leung HC, Yiu SM, Chin FY. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–1428. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 68.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang DD, Froula J, Egan R, Wang Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ. 2015;3:e1165. doi: 10.7717/peerj.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alneberg J, Bjarnason BS, de Bruijn I, Schirmer M, Quick J, Ijaz UZ, et al. Binning metagenomic contigs by coverage and composition. Nat Methods. 2014;11:1144–1146. doi: 10.1038/nmeth.3103. [DOI] [PubMed] [Google Scholar]
- 71.Song WZ, Thomas T. Binning_refiner: improving genome bins through the combination of different binning programs. Bioinformatics. 2017;33:1873–1875. doi: 10.1093/bioinformatics/btx086. [DOI] [PubMed] [Google Scholar]
- 72.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eddy SR. A new generation of homology search tools based on probabilistic inference. Genome Inform. 2009;23:205–211. [PubMed] [Google Scholar]
- 75.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36(10):996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 79.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 85.Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:445. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng Y, Chen X, Madsen AM, Zervas A, Nielsen TK, Andrei AS, et al. Potential rhodopsin- and bacteriochlorophyll-based dual phototrophy in a high Arctic glacier. mBio. 2020;11:e02641–e02620. doi: 10.1128/mBio.02641-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary figures.
Additional file 2. Supplementary tables.
Additional file 3. Taxonomy names proposed for the six MAGs qualified as type material.
Data Availability Statement
The sequences of 16S rRNA and 16S rRNA gene libraries, the metagenomic raw reads of the Mariana Trench sediments, as well as all metagenome-assembled Chloroflexi genomes of this study are available in the NCBI database and can be accessed under project ID PRJNA692099.