Abstract
Background
Wards caring for COVID-19 patients, including intensive care units (ICUs), have an important focus on preventing transmission of SARS-CoV-2 to other patients and healthcare workers.
Aim
To describe an outbreak of carbapenemase-producing Enterobacterales (CPE) in a COVID-19 ICU and to discuss key infection control measures enabling prompt termination of the cluster.
Methods
CPE were isolated from clinical specimens and screening swabs from intensive care patients with COVID-19 disease and from environmental screening. Whole-genome sequencing analysis was instrumental in informing phylogenetic relationships.
Findings
Seven clinical isolates and one environmental carbapenemase-producing Klebsiella pneumoniae isolate – all carrying OXA-48, CTX-M-15 and outer membrane porin mutations in ompK35/ompK36 – were identified with ≤1 single nucleotide polymorphism difference, indicative of clonality. A bundle of infection control interventions including careful adherence with contact precautions and hand hygiene, twice weekly screening for multidrug-resistant organisms, strict antimicrobial stewardship, and enhanced cleaning protocols promptly terminated the outbreak.
Conclusion
Prolonged use of personal protective equipment is common with donning and doffing stations at the ward entrance, leaving healthcare workers prone to reduced hand hygiene practices between patients. Minimizing transmission of pathogens other than SARS-CoV-2 by careful adherence to normal contact precautions including hand hygiene, even during high patient contact manoeuvres, is critical to prevent outbreaks of multidrug-resistant organisms. Appropriate antimicrobial stewardship and screening for multidrug-resistant organisms must also be maintained throughout surge periods to prevent medium-term escalation in antimicrobial resistance rates. Whole-genome sequencing is highly informative for multidrug-resistant Enterobacterales surveillance strategies.
Keywords: Carbapenemase-producing Enterobacterales, Infection prevention and control, Multidrug-resistant organisms, COVID-19
Introduction
Hospitals worldwide have managed intense surges in COVID-19 patients, with intensive care units (ICUs) often the most burdened [1]. Infection prevention and control practices have understandably prioritized the protection of healthcare workers (HCWs) and other patients from transmission of SARS-CoV-2. Personal protective equipment (PPE), utilizing airborne and contact precautions, substantially reduces transmission of SARS-CoV-2 [2].
During significant COVID-19 patient surge periods, entire wards have been utilized to manage patients with prolonged use of PPE and donning and doffing stations placed at ward entrances. In this context, and with an imperative to protect HCWs especially, changing of PPE between patients is not commonplace, and at times was actively discouraged due to worldwide shortages of PPE early in the pandemic. Even the five moments of hand hygiene are more easily overlooked when PPE is worn for prolonged periods and if an entire ward space is considered unclean from a SARS-CoV-2 perspective [3,4]. Additionally, the pandemic environment often involves rapid escalations in inpatient numbers and overstretched healthcare systems, demanding a fluidity in staffing and ward allocations that results in less familiar patient care environments.
In the ICU, this issue is exacerbated by the acuity of the patient cohort and HCW activities inherent in the care of severely unwell patients. Multiple staff members are frequently required to attend to a single patient for manoeuvres such as intubation, line placement, proning and deproning, or the frequent rolling of a patient necessary to ameliorate the risk of pressure injuries which often portend infection. Moreover, movement of HCWs between patients often occurs at speed due to the rapid deterioration of a patient or the multiple demands of a busy unit. In this context, change of gowns and gloves between patients or appropriate hand hygiene can be readily overlooked.
This cross-sectional study of a multidrug-resistant Klebsiella pneumoniae OXA-48/CTX-M-15 cluster, supported by whole-genome sequencing (WGS) analysis, highlights the rapidity of transmission chains in the intensive care environment and the potential for spread through a healthcare network. It also characterizes the challenges and risk factors for such an outbreak in a COVID-19 critical care environment, as well as measures adopted to promptly terminate such a cluster. This study discusses key learning points for infection prevention and control (IPC) priorities in the COVID-19 era.
Methods
Study design
This is a cross-sectional study of carbapenemase-producing Enterobacterales (CPE) isolates from clinical and environmental specimens, obtained from an ICU caring for patients with SARS-CoV-2 infection over the period September 22nd, 2021 to October 22nd, 2021. The study was conducted during a surge period for the B.1.617.2 (delta) variant of SARS-CoV-2 in Sydney, Australia.
Screening for carbapenemase-producing Enterobacterales
After passive detection of the initial CPE isolates, active patient surveillance was initiated with twice weekly rectal swabs (Amies swab®; Copan, Brescia, Italy) of all inpatients in the ICU as well as the general wards receiving step-down patients from the COVID-19 ICU. Swabs were transported promptly to the laboratory and inoculated within 4 h of collection as described below.
Environmental surface screening was conducted by swabbing of all wet areas and randomly selected computer terminals in the COVID-19 ICU at a single time-point. Air sampling was conducted on to Brilliance ESBL® chromogenic agar plates (Oxoid, Basingstoke, UK) using a Merck Millipore Air Sampler system (MAS-100 NT®; Merck, Darmstadt, Germany) with a total air flow volume of 1000 L (flow rate of 100 L/min for 10 min); adjacent taps were run at maximal speed for the duration of air sampling.
Culture-based methods
All swabs from patients and environmental surfaces were processed for presence of CPE and/or extended-spectrum β-lactamase-producing Enterobacterales. Swabs were inoculated directly on to chromogenic extended-spectrum β-lactamase (ESBL)-selective media (Brilliance ESBL) and incubated at 37 °C for 18–24 h. Brilliance ESBL plates inoculated using the air sampler were incubated directly at 37 °C for 18–24 h. Colonies were identified by matrix-assisted laser desorption/ionization time-of-flight analysis (Bruker Daltonics, Bremen, Germany) and susceptibility testing performed by Vitek 2 (bioMérieux) using Clinical and Laboratory Standards Institute (CLSI) guidelines. Phenotypic confirmation of the presence of ESBLs was also performed as per CLSI Performance Standards for Antimicrobial Susceptibility Testing [5]. Samples were screened for common CPE-related genes (KPC, NDM, VIM, OXA-48, and IMP-1) using Carba-R GeneXpert® (Cepheid) analysis. Extended susceptibility testing was performed by broth microdilution at the reference laboratory (Centre for Infectious Disease & Microbiology Laboratory Services (CIDMLS), ICPMR, NSW Health Pathology and CIDM-Public Health, Westmead Hospital, Australia).
Whole-genome sequencing and analysis
Genome sequencing
Whole-genome sequencing of pure cultures was performed at CIDMLS (seven isolates) and New South Wales Health Pathology – Royal Prince Alfred Hospital laboratory (one clinical isolate). Genomic DNA was extracted using Qiagen DNeasy Blood and Tissue Mini Kit (Qiagen, Hilden, Germany). Sequencing libraries were prepared using DNA Library Prep Kit (Illumina, San Diego, CA, USA) and sequenced on Illumina instruments using either the NextSeq 500 (using NextSeq 500/550 v2 mid output Kit) or the MiSeq (using 150 bp paired-end chemistry).
Primary genome analysis
Sequenced raw reads were subjected to an in-house quality control procedure prior to further analysis using the Nullarbor pipeline (v2.0; https://github.com/tseemann/nullarbor). The relationship between genomes was examined using the single nucleotide polymorphism (SNP) analysis where the SNP was defined as substitutions present in at least 90% of reads with minimum depth coverage of 30. Core genome SNPs (present in all isolates) were obtained using Snippy-core. A cluster was based on the position on the phylogenetic tree with linked isolates identified provided that the SNP distance between isolates was <20 SNPs. The WGS-based multi-locus sequence type (MLST) was inferred from sequencing data using MLST 2.8 from the pipeline. The maximum likelihood tree was generated using FastTree 2.1.9 (http://www.microbesonline.org/fasttree).
Resistome analysis
Sequence data were manually examined for major antibiotic resistance genes using Geneious Prime v2020.2.3, with comparison to the NCBI Bacterial Antimicrobial Resistance Reference Gene Database (NCBI Bioproject PRJNA313047) to identify specific resistance gene variants. Characteristics and mobile genetic elements and plasmid markers were inferred from sequencing data using the Plasmid Finder and plasmidMLST services (https://cge.cbs.dtu.dk/services/).
Results and discussion
The ICU included in the current study is a 24-bed facility (two units of 12 beds each), augmented by an additional 10-bed unit during the COVID-19 surge period, situated in a tertiary metropolitan hospital. Each unit was staffed by a staff specialist, registrar and junior medical officer with one nurse allocated per patient at all times and a nursing team leader for oversight. During the COVID-19 surge, regular ICU nursing staff were supported by a small number of redeployed anaesthetic nurses who received specific training in transmission-based precautions, hand hygiene refresher training, PPE donning and doffing, cleaning of reusable equipment, transfer of a COVID-19 patient and fit testing of P2/N95 masks alongside additional ICU responsibilities including management of patients on non-invasive ventilation and high-flow oxygen nasal prongs. Donning of PPE (gown, gloves, P2/N95 mask and eye protection) occurred on entry to the unit with the expectation for change of gloves and hand hygiene between patient contact as per the five moments of hand hygiene principles [3].
Initial passive surveillance revealed four patients in the COVID-19 ICU with CPE-K. pneumoniae isolates of concern over a period of two weeks (Table I ), all from within the same 12-bed unit of the ICU. These included two isolates grown from sputum – one in the context of an acute episode of ventilator-associated pneumonia and one representing endotracheal tube colonization – and two isolated from rectal screening swabs.
Table I.
Characteristics of clinical carbapenemase-producing Enterobacterales (CPE) isolates from COVID-19 ICU
| Characteristic | Patient no. |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Whole-genome sequencing ID | 21-004-0626 | 21-004-0642 | MI21812817 | 21-004-0654 | 21-004-0652 | 21-004-0651 | 21-004-0653 |
| Organism | Klebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae |
| Resistance genotype | OXA-48 | OXA-48 | OXA-48 | OXA-48 | OXA-48 | OXA-48 | OXA-48 |
| CTX-M-15 | CTX-M-15 | CTX-M-15 | CTX-M-15 | CTX-M-15 | CTX-M-15 | CTX-M-15 | |
| aac (3)-IId | aac (3)-IId | aac (3)-IId | aac (3)-IId | aac (3)-IId | aac (3)-IId | aac (3)-IId | |
| Plasmid replicon type | IncL | IncL | IncL | IncL | IncL | IncL | IncL |
| Porin mutations | ompK35/ompK36 | ompK35/ompK36 | ompK35/ompK36 | ompK35/ompK36 | ompK35/ompK36 | ompK35/ompK36 | ompK35/ompK36 |
| Meropenem MIC (mg/L) | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Sample type | Sputum | Sputum | Screen | Screen | Screen | Screen | Screen |
| Days from ICU admission to CPE detection | 24 | 5 | 13 | 6 | 9 | 10 | 5 |
| Prior MRO screen | Negative | Negative | Nil | Negative | Negative | Negative | Nil |
| Bed space common with other CPE patient | Y | Y | Y | Y | Y | Y | Y |
| Bed space common with positive environmental screen | N | Y | N | N | N | N | N |
| Mechanically ventilated | Y | Y | Y | Y | Y | Y | Y |
| Antibiotics received prior to CPE isolation | Piperacillin/tazobactam, flucloxacillin, cefepime | Piperacillin/tazobactam, clindamycin, vancomycin, metronidazole | Piperacillin/tazobactam, amoxicillin/clavulanate, vancomycin | Amoxicillin/clavulanate | Amoxicillin/clavulanate, piperacillin/tazobactam, cefepime | Ceftriaxone, piperacillin/tazobactam | Ceftriaxone, azithromycin |
CPE, carbapenemase-producing Enterobacterales; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MIC, minimum inhibitory concentration; MRO, multidrug-resistant organism; NA, not available; ompK35/ompK36, premature stop codon in the outer membrane porin gene ompK35 and insertion in loop 3 of ompK36.
These cases were identified following a period of reduced regularity and frequency of multidrug-resistant organism (MRO) screening in the ICU, driven by intense workloads in both the ICU and the laboratory where COVID-19 testing demands were high. Furthermore, acutely unwell patients with COVID-19 pneumonitis within the unit had been managed with increasingly broad-spectrum antibiotics which almost certainly contributed to selection for resistant organisms in this setting. This was in part due to the high demand on infectious diseases staff (who form a key part of the regular antimicrobial stewardship service) in providing COVID-19-related clinical support, and diversion of the focus of multi-disciplinary clinical meetings from primarily stewardship to COVID-19-related clinical priorities. In addition, IPC resources and staff were consumed with COVID-19-related activities over this period, particularly in managing COVID-19 contact-tracing programmes involving inpatients and staff, and with responding to a high volume of COVID-19-related IPC queries. As such, formal hand hygiene audits were suspended during this period and the frequency of educational IPC visits to the ICU and other wards was markedly reduced. Though there was an expectation for changing gloves between patients, this was not formally audited and lapses in this practice were anecdotally reported in retrospect.
Infection prevention and control interventions were carefully targeted to try to eradicate the CPE cluster from the ICU. Patients with known CPE colonization were isolated in single rooms. Changing of gowns and gloves was mandated before and after contact with patients identified to be carrying an MRO (including CPEs). Donning and doffing stations were placed at the room entrances of such patients and adherence enforced by the ICU staff. Education of ICU staff was prioritized regarding strict adherence to contact precautions, including the ‘five moments of hand hygiene’. Use of alcohol on gloved hands was permitted for non-MRO-colonized patients where change of gloves may have compromised healthcare worker or patient safety [6]. MRO surveillance using rectal and nasal swabs was reinitiated twice weekly in the ICU and weekly in the two COVID-19 step-down wards that received recovering patients from the ICU. The nurse unit managers took responsibility for ensuring compliance with screening which was validated by the microbiologist. Bleach cleaning was enhanced in all wet areas of the COVID-19 ICU daily for two weeks.
Antimicrobial stewardship was enhanced within the ICU. Specific elements of this intervention included a focus on antimicrobial stewardship decision-making via twice-weekly multidisciplinary meetings between the ICU staff and infectious diseases and microbiology clinicians, and preferential use of narrow-spectrum antibiotics with restricted access to carbapenems unless microbiologically indicated (e.g. invasive disease with demonstrated presence of an ESBL-producing organism, with approval by an infectious diseases physician).
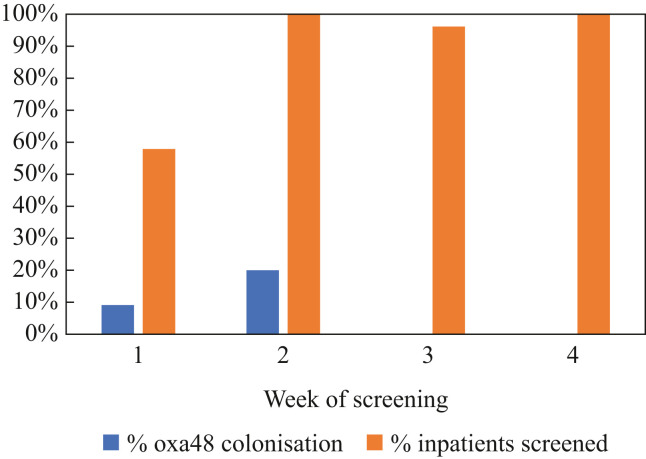
After instituting regular twice weekly screening swabs of all intensive care patients for MROs, an additional three K. pneumoniae CPE clinical isolates (Table I) were identified in the subsequent week from the same unit with none identified in the following two weeks (from a total of 70 screening episodes) (Figure 1 ), nor in the subsequent two-month period (data not shown). No additional cases were identified in the two COVID-19 step-down wards in the two weeks following this outbreak (from a total of 30 screening episodes).
Figure 1.
Proportion of carbapenemase-producing Enterobacterales (CPE)-positive cases and surveillance coverage in the COVID-19 intensive care unit by week of sampling.
Environmental screening of all wet locations (comprising 19 swabs) and randomly selected computer terminals (three swabs) in the COVID-19 ICU detected a carbapenemase-producing K. pneumoniae from the sink of one bed space where a CPE-positive patient was nursed. Other environmental screening swabs were negative for CPEs. Air sampling at seven locations across the ICU detected no CPE isolates.
Phenotypic testing revealed similar resistance patterns in all the isolates. Resistance to meropenem was universal with a minimum inhibitory concentration (MIC) for meropenem of >32 mg/L in all clinical and environmental isolates, which was higher than anticipated with the OXA-48 resistance mechanism alone [7]. One clinical isolate displayed heteroresistance with a meropenem MIC range of 0.012 to >32 mg/L, a phenomenon well described for OXA-48-producing Enterobacterales [8]. Isolates also showed phenotypic resistance to ampicillin, amoxicillin/clavulanate, piperacillin/tazobactam, ceftriaxone, ceftazidime, cefepime, aztreonam, ciprofloxacin and trimethoprim but remained susceptible to ceftazidime/avibactam (MIC ranging from 0.25 to 1.5 mg/L) and amikacin (MIC ≤2 mg/L), significantly constraining treatment options. Most were resistant to gentamicin, though one clinical isolate showed an MIC in the susceptible range (≤1 mg/L). All isolates tested positive for blaOXA-48 on the Carba-R GeneXpert® assay (Cepheid, Sunnyvale, CA, USA).
Whole-genome sequencing was conducted on the seven clinical isolates and the one environmental isolate. All were determined by inferred MLST to be ST16 strains of K. pneumoniae. All isolates were found to carry a plasmid closely related to other IncL blaOXA-48 plasmids, in which blaOXA-48 is inserted in the tir gene [9,10]. This was accompanied by an extended-spectrum β-lactamase gene (blaCTX-M-15) and an aminoglycoside resistance gene (aac (3)-IId). A premature stop codon was also identified in the outer membrane porin gene ompK35 in all isolates, in addition to a single amino acid aspartate insertion in loop 3 of ompK36, the combination of which would be expected to contribute to carbapenem resistance [11]. The clinical isolate core genomes were all within a single nucleotide polymorphism (SNP) of one another, suggesting a high likelihood of clonality. The single environmental isolate was also closely related to the clinical isolates (0–1 SNP), indicating a high probability of environmental contamination or transmission.
This study reveals several critical COVID-19-enabled IPC issues that collectively facilitated a clinical and environmental CPE K. pneumoniae outbreak in a COVID-19 ICU. The COVID-19 surge atmosphere within a critical care ward with extended use of common PPE and overstretched healthcare system resources created a perfect storm for transmission of MROs.
The outbreak was rapidly terminated using routine IPC ‘best practice’ measures, including enhanced education regarding adherence to hand hygiene and contact precautions between patients, isolation of patients colonized with CPEs, pragmatic use of alcohol on gloved hands when necessary, regular patient MRO screening, enhanced cleaning practices and strict antimicrobial stewardship. This was a package intervention and, as such, it was not possible to tease apart the contributions of each component; however, the composite effect was striking in promptly eliminating CPE carriage from the unit.
The use of WGS in this study enabled detailed analysis of the relatedness of the K. pneumoniae isolates to inform transmission pathways and led to a better understanding of resistance patterns. The utility of WGS in resolving IPC issues down to the SNP and plasmid level cannot be overstated and, in well-resourced settings, should be prioritized in future infection control strategies [12].
Given the known broad host range and promiscuity of the IncL plasmid, it is likely that this plasmid existed in other Enterobacterales within the institution and/or community before detection of this cluster, but had not manifested a phenotype that was detectable on standard screens [13]. OXA-48 isolates are notorious for evading detection due to carbapenem MICs which often sit below clinical breakpoints [14]. Enhanced plasmid mobility by tir gene interruption, enabling increased conjugation across a wide host range, is likely to have further contributed to the promiscuous spread of this plasmid [10].
The finding of outer membrane porin defects in these isolates was almost certainly pivotal to the detection of this outbreak. Such porin mutations, including the single amino acid aspartate insertion in loop 3 of ompK36 seen in this cluster, represent a well-established phenomenon in K. pneumoniae populations [11,15]. However, it was the overlay of this IncL plasmid acquisition into an organism with an established porin defect that augmented the phenotype of the OXA-48 to beyond the clinical resistance breakpoint.
This finding highlights the potential loopholes in phenotype-based MRO screening approaches using standard platforms. Dedicated screening for CPEs using low-concentration meropenem screening breakpoints (0.25 mg/L) and/or genotypic methods should be prioritized to facilitate sensitive detection of CPEs that may otherwise evade recognition [16]. If not detected at an early stage, MROs may progressively acquire additional resistance genes through horizontal plasmid transfer and dissemination of genes of concern, leading to marked clinical consequences across a range of settings including critical care environments.
This cautionary tale should highlight to other COVID-19 inpatient units, especially in critical care areas, the importance of cognizance to non-SARS-CoV-2 pathogens in IPC frameworks alongside the imperative to prevent transmission of SARS-CoV-2 itself. Adherence to the five moments of hand hygiene and appropriate contact precautions between patients, as well as regular MRO screening, is necessary to prevent transmission of MROs. Furthermore, as in other settings, antimicrobial stewardship must be prioritized to minimize selective pressures for MROs in the COVID-19 context.
The risks of CPE spread within a critical care unit are immense, both for the individual patient and for the wider community. Acute infection with MROs often requires treatment with antibiotics that carry high toxicity and/or lower efficacy, due to severe limitations on the repertoire of efficacious antibiotics, with detrimental effects on individual patient morbidity and mortality. Use of broad-spectrum or ‘last-line’ agents in turn drives escalating antimicrobial resistance at the community level as organisms progressively acquire additional resistance elements. The ICU environment is particularly prone to this effect due to the high patient acuity, frequent instrumentation and prosthetic line use, vulnerability to acute haemodynamic deteriorations triggering broad-spectrum sepsis management, and high rates of antibiotic usage in general.
In conclusion, prioritizing IPC measures is vital to prevent selection for, and spread of, MROs within the critical care environment, including in COVID-19 units. Such measures align with World Health Organization mandates to optimize infection control approaches, including hand hygiene, as pivotal tools in the arsenal against escalating antimicrobial resistance rates [17].
Conflict of interest statement
None declared.
Funding sources
None.
Acknowledgements
The authors are grateful to the laboratory scientists of the New South Wales Health Pathology – Nepean, New South Wales Health Pathology – ICPMR, New South Wales Health Pathology – Royal Prince Alfred Hospital and St Vincent's Pathology Service (SydPath) for their technical assistance, Dr M. Jayawardena for assistance with broth microdilution assays, and Dr E. Martinez for bioinformatic analysis.
References
- 1.Bruyneel A., Gallani M.C., Tack J., d’Hondt A., Canipel S., Franck S., et al. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intens Crit Care Nurs. 2021;62:102967. doi: 10.1016/j.iccn.2020.102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook T.M., El-Boghdadly K., Brown J., Pickering A.E. The safety of anaesthetists and intensivists during the first COVID-19 surge supports extension of use of airborne protection PPE to ward staff. Clin Med (Lond) 2021;21:e137. doi: 10.7861/clinmed.2020-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sax H., Allegranzi B., Uçkay I., Larson E., Boyce J., Pittet D. ‘My five moments for hand hygiene’: a user-centred design approach to understand, train, monitor and report hand hygiene. J Hosp Infect. 2007;67:9–21. doi: 10.1016/j.jhin.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Mędrzycka-Dąbrowska W., Lange S., Zorena K., Dąbrowski S., Ozga D., Tomaszek L. Carbapenem-resistant Klebsiella pneumoniae infections in ICU COVID-19 patients – a scoping review. J Clin Med. 2021;10:2067. doi: 10.3390/jcm10102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute . 29th edn. CLSI; Wayne, PA: 2020. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 6.Shless J., Crider Y., Pitchik H., Qazi A., Styczynski A., LeMesurier R., et al. Evaluation of the effects of repeated disinfection on medical exam gloves: Part 1. Changes in physical integrity. J Occup Environ Hyg. 2022;19:102–110. doi: 10.1080/15459624.2021.2015072. [DOI] [PubMed] [Google Scholar]
- 7.Al-Abdely H.M., Midgley C.M., Alkhamis A.M., Abedi G.R., Lu X., Binder A.M., et al. Middle East respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg Infect Dis. 2019;25:753–766. doi: 10.3201/eid2504.181595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Camacho E., Paño-Pardo J.R., Sotillo A., Elías-López C., Martínez-Martínez L., Gómez-Gil R., et al. Meropenem heteroresistance in clinical isolates of OXA-48-producing Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2019;93:162–166. doi: 10.1016/j.diagmicrobio.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Poirel L., Bonnin R.A., Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012;56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potron A., Poirel L., Nordmann P. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother. 2014;58:467–471. doi: 10.1128/AAC.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajardo-Lubián A., Ben Zakour N.L., Agyekum A., Qi Q., Iredell J.R. Host adaptation and convergent evolution increases antibiotic resistance without loss of virulence in a major human pathogen. PLoS Pathogens. 2019;15 doi: 10.1371/journal.ppat.1007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock S.J., Parkhill J., Brown N.M. Changing the paradigm for hospital outbreak detection by leading with genomic surveillance of nosocomial pathogens. Microbiology (Reading) 2018;164:1213–1219. doi: 10.1099/mic.0.000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozwandowicz M., Brouwer M.S.M., Fischer J., Wagenaar J.A., Gonzalez-Zorn B., Guerra B., et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 14.Oueslati S., Nordmann P., Poirel L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother. 2015;70:1059–1063. doi: 10.1093/jac/dku524. [DOI] [PubMed] [Google Scholar]
- 15.Wong J.L.C., Romano M., Kerry L.E., Kwong H.S., Low W.W., Brett S.J., et al. OmpK36-mediated carbapenem resistance attenuates ST258 Klebsiella pneumoniae in vivo. Nature Commun. 2019;10:3957. doi: 10.1038/s41467-019-11756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattouh R., Tijet N., McGeer A., Poutanen S.M., Melano R.G., Patel S.N. What is the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence settings? Antimicrob Agents Chemother. 2015;60:1556–1559. doi: 10.1128/AAC.02304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartari E., Abbas M., Pires D., de Kraker M.E.A., Pittet D., World Health Organization SAVE LIVES Clean Your Hands global campaign – ‘Fight antibiotic resistance – it’s in your hands. Clin Microbiol Infect. 2017;23:596–598. doi: 10.1016/j.cmi.2017.04.021. [DOI] [PubMed] [Google Scholar]