Abstract
Background
Solitary fibrous tumor (SFT) and hemangiopericytoma (HPC) are rare mesenchymal tumors in the central nervous system with a high tendency to relapse, having a significant impact on quality of life (QoL). Due to the rarity of intracranial SFT/HPC, the prognostic factors and optimal treatment remain to be elucidated. Meanwhile, quality of life in patients with intracranial SFT/HPC is seldomly concerned. Thus, we aim to survey about the quality of life and underline some aspects demanding concern in intracranial SFT/HPC treatment through summarizing our case series in recent ten years.
Methods
Patients with intracranial SFT/HPC who underwent surgical resection from January 2009 to June 2019 were included in the study. Clinical features, such as age, gender, and resection extent, were collected. The EuroQol Five Dimensions Questionnaire (EQ-5D) was used to assess the patients’ quality of life (QoL). Prognosis factors related to progression-free survival (PFS) and overall survival (OS) were also evaluated.
Results
Thirty-six patients with a mean follow-up period of 61.6 months (range 13–123 months) were included in this study. Sixteen (44.4%) patients achieved gross total resection (GTR). Fourteen patients (38.9%) with tumor progression experienced adjuvant radiotherapy (11.1%) or Gamma Knife surgery (GKS, 27.8%). According to the 2016 WHO classification, there were 6 (16.7%) grade I SFT/HPC, 11 (30.5%) grade II SFT/HPC, and 19 (52.8%) grade III SFT/HPC. The PFS and OS were 29 months (range 4–96 months) and 38 months (range 4–125 months). The median EQ5D-3 L tariff with or without progression was 0.617 (95% CI 0.470–0.756) and 0.939 (95% CI 0.772–0.977) respectively. Gross total resection (GTR, p = 0.024) and grade I SFT/HPC (p = 0.017) were significantly associated with longer PFS. In multivariate analysis, GTR (HR 0.378, 95% CI 0.154–0.927) and adjuvant therapy (HR 0.336, 95% CI 0.118–0.956) result in significantly longer PFS in patients with SFT/HPC.
Conclusions
Patients underwent GTR and adjuvant therapy had longer PFS. Similarly, patients with lower WHO grade had relatively longer PFS. Therefore, GTR is advocated for the treatment of SFT/HPC. And adjuvant therapy such as GKS could be an alternative treatment for patients who underwent STR or with tumor progression. Further, the QoL decreased in patients with tumor progression and metastasis, and more attention is demanded to the QoL of intracranial SFT/HPC patients.
Keywords: Solitary fibrous tumor, Hemangiopericytoma, Quality of life, Prognosis, Central nervous system
Introduction
Solitary fibrous tumors (SFTs) were first reported as local mesenchymal neoplasms in the visceral pleura, but extrathoracic sites are increasingly being recognized then after. Intracranial SFTs are rare and were first described by Carneiro et al. in 1996 [1]. They have been generally considered benign tumors that originated predominantly from thick collagen bands [2]. Hemangiopericytomas (HPCs) are also rare mesenchymal tumors that exhibit similar clinical, radiological, and histological features as SFTs [3]. However, SFTs and HPCs show distinct biological behaviors: SFTs are generally slow-growing indolent tumors with rare recurrence and metastasis after total resection, while HPC grows aggressively with frequent recurrence and metastasis following total resection [3, 4]. Due to their distinct biological behaviors, the combination of intracranial SFTs and HPCs into a single entity was delayed. However, recent pathological findings of their shared genetic etiology prompted the World Health Organization (WHO) to classify the two types of tumors into one combined entity in 2016 [5–8].
Several previous studies explored the prognostic factors of intracranial SFT/HPC, which mostly underlined the extent of resection (EOR) as a major factor for tumor recurrence and metastasis [9–16]. Other factors, including WHO classification [9, 15], adjuvant therapy [16–20], age [21, 22], location, and size of tumor [23] had also been reported that related to patient’s progression-free survival (PFS) and overall survival (OS). However, due to the rarity of intracranial SFT/HPC, little is known about the optimal treatment and prognostic factors, and our current knowledge about SFT/HPC is mainly derived from small case series [4, 9, 11, 14]. Moreover, although it is well-known that the quality of life (QoL) of patients who suffered other intracranial tumors dramatically decreased, especially when tumor recurrence and metastasis occurred, little attention has been paid to the QoL of intracranial SFT/HPC patients [24–26].
Thus, we retrospectively reviewed our department’s intracranial SFT/HPC cases from January 2009 to June 2019 to investigate the clinical characteristics, QoL, and prognosis factors related to PFS and OS.
Patients and methods
From January 2009 to June 2019, patients who underwent surgical resection for intracranial SFT/HPC in West China Hospital, Sichuan University, were identified retrospectively. Two experienced pathologists confirmed the diagnosis of SFT/HPC according to the 2016 WHO classification of tumors of the central nervous system (CNS). Patients without complete information and who refused to be followed were excluded. This study, registered in ChiCTR (ChiCTR2000029718), was approved by the Ethics Committee of West China Hospital and informed consent was obtained from the patient. It adheres to the tenets of the Declaration of Helsinki.
Thirty-nine patients were initially identified for postoperative pathology results of head SFT/HPC. Two patients were excluded for incomplete information and another for the extracranial lesion. Electronic medical record (EMR) was used to collect specific patient information by three individual investigators with final cross-check, including demographic characteristics, location, the largest diameter of tumor (LD), EOR, pathological findings, adjuvant therapies, recurrence and metastasis, and follow-up conditions. The EOR was separately confirmed by two investigators referring to operation records and postoperative magnetic resonance imaging (MRI) findings. Gross total resection (GTR) or subtotal resection (STR) was defined as previously described [2]. Specifically, macroscopically complete tumor resection with or without removal or coagulation of affected dura and underlying bone was classified as GTR. Whereas subtotal tumor resection, decompression, and biopsy were defined as subtotal resection. Progression was defined as recurrence with the lesion reappeared after GTR or the enlargement of residual tumor and metastasis with the extracranial presence of SFT/HPC lesion.
All patients were followed up after hospitalization and evaluated 6 and 12 months after surgery. Then annual examinations were conducted for life. The follow-up period was calculated as the duration from surgery to death or until December 2019 for surviving patients. Follow-up information was obtained from outpatient EMR and telephone interviews. At the last follow-up, the EuroQol Five Dimensions Questionnaire (EQ-5D) was used to evaluate the patient’s QoL through routine medical appointments or telephone interviews. The enrollment and follow-up process is shown in Fig. 1. The EQ-5D tariff was calculated through the time-trade-off method reported in a previous Chinese study [27].
Fig. 1.
The flowchart of enrollment and follow-up. Initially, 39 patients were identified according to postoperative pathology results of SFT/HPC. Then, two patients were excluded for incomplete information and one for the extracranial lesion. Three patients lost response before the last follow-up for QoL. Moreover, eight patients had been dead before the last follow-up. Two patients did not respond to the investigation of QoL at the last follow-up
Statistical analysis
Data analysis was performed by IBM SPSS 25 Software (IBM, New York, USA). Fisher’s exact test and independent-sample T-test were separately used to explore classified and numeric variables. Moreover, the Kruskal-Wallis test was used to analyze the WHO classification among different groups. EQ-5D tariff was tested by using the Mann-Whitney U test. Univariate analysis was conducted with the Kaplan-Meier method for PFS and OS, using the Log-rank test for determining its significance. Multivariate Cox regression analysis was performed using significant factors in univariate analysis. It was considered statistically significant when a p-value was less than 0.05.
Results
Patient characteristics
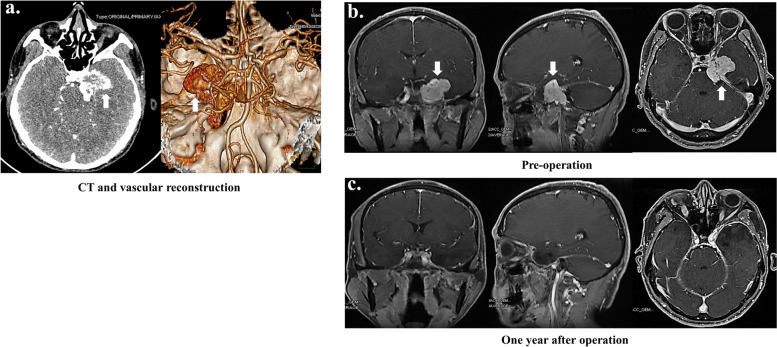
Thirty-six intracranial SFT/HPC patients were included in this study from 2009 to 2019 (Table 1). There were 22 male (61.1%) and 14 female (38.9%) patients, with a median age of 47 years ranging from 13 to 75 years. Twenty-nine tumors (80.5%) were located supratentorial, and seven (19.5%) were infratentorial locations. The maximal tumor diameter ranged from 2.0 cm to 10.0 cm, with a median diameter of 5.0 cm. Sixteen (44.4%) patients underwent GTR. The PFS and OS were 29 (range, 4 to 96) months and 38 (range, 4 to 125) months. Fourteen patients (38.9%) with tumor recurrence or metastasis were further treated with adjuvant radiotherapy (11.1%) or Gamma Knife surgery (GKS, 27.8%). According to the 2016 WHO classification, there were 6 (16.7%) grade I SFT/HPC, 11 (30.5%) grade II SFT/HPC, and 19 (52.8%) grade III SFT/HPC. The mean follow-up period was 61.6 months (range 13–123 months). Among these patients, eight (22.2%) individuals were dead in 4 to 84 months (median, 42 months) after the first operation. Tumor recurrence occurred in 22 (61.1%) patients from 6 to 68 months (median, 25.5 months) after the first surgery. Three (8.33%) patients suffered from extracranial tumor metastases, including the lung (2 patients), ovary (1 patient), and pelvic bone (1 patient). In Fig. 2a, preoperative CT and vascular reconstruction images of typical SFT/HPC patients were shown. Also, preoperative (Fig. 2b) and postoperative (Fig. 2c) enhanced T1WI MRI images were displayed.
Table 1.
Characteristic of 36 patients diagnosed with STF/HPC
| Aspects | Totality |
|---|---|
| Gender (M/F) | 22/14 |
| Age | 47 (13, 75) |
| Tumor location (no. [%]) | |
| Supratentorial | 29 (80.5) |
| Infratentorial | 7 (19.5) |
| Tumor size in cm (no. [%]) | |
| < 5.0 | 17 (47.2) |
| ≥ 5.0 | 19 (52.8) |
| EOR (no. [%]) | |
| GTR | 16 (44.4) |
| STR | 20 (55.6) |
| Adjuvant therapy (no. [%]) | |
| Radiotherapy | 4 (11.1) |
| GKS | 10 (27.8) |
| WHO classification (no. [%]) | |
| Grade I | 6 (16.7) |
| Grade II | 11 (30.5) |
| Grade III | 19 (52.8) |
Fig. 2.
Representative CT and MRI images before and after operation. a Preoperative CT and vascular reconstruction images exhibited the SFT/HPC lesion in the left middle cranial fossa. This irregular high-density mass showing heterogeneous enhancement had unclear boundaries and destroyed the surrounding skull. And this lesion partially enveloped the left internal carotid artery segments C3-C4. b The preoperative enhanced T1WI images were displayed. The mass was located in the left middle cranial fossa, with unclear boundaries and heterogeneous enhancement. The lesion protruded locally into the left pontine corner cistern, partially compressed the left temporal lobe and brainstem and enveloped the C3-C4 segments of the left internal carotid artery. c The enhanced T1WI images of 1-year past operation were exhibited. The area of the original lesion showed postoperative changes without significant enhancement
Quality of life
At the last follow-up, twenty-three patients were tested by EQ5D-3 L. The median EQ5D-3 L tariff of the patients with or without progression were 0.617 (95% CI: 0.470–0.756) and 0.939 (95% CI: 0.772–0.977), respectively. The EQ5D-3 L tariff significantly decreased in patients whose disease progressed (p = 0.002). Further, the dimension for pain or discomfort in patients with tumor recurrence or metastasis (median, 0.092; range, 0–0.236) was significantly higher than non-progression patients (median, 0; range, 0–0.092), with a p-value of 0.049. Moreover, patients with progression obtained higher score (p = 0.026) in dimension of negative emotion (median 0.043, range 0–0.205), compared with non-progression patients (median, 0; range, 0–0.086). Consequently, QoL in intracranial SFT/HPC patients significantly declined when the tumor relapsed. Intracranial patients with tumor recurrence and metastasis were intended to suffer body pain and stay in negative emotion frequently.
Univariate analysis of the factors related to tumor PFS and OS
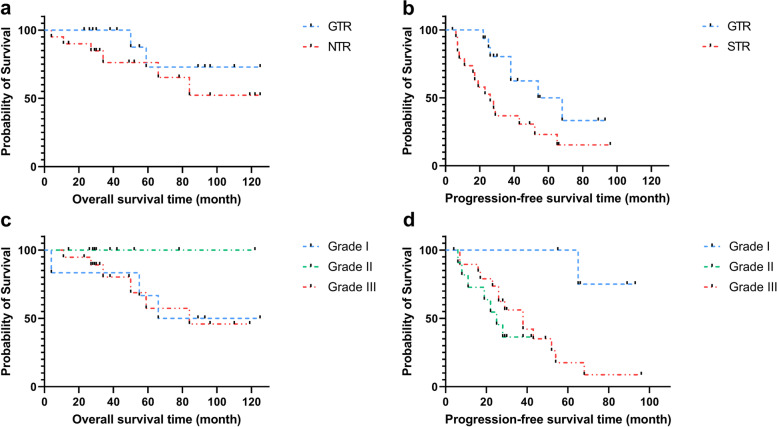
Several prognosis factors were detected for disease progression, involving LD (< 5.0 cm and ≥ 5.0 cm), EOR, adjuvant therapy, and WHO grade. Consequently, EOR and WHO grade displayed statistical significance (Table 2). Patients who underwent GTR obtained longer PFS (60.7 vs. 36.7 months, p = 0.024) than those who underwent STR patients. There is no significant difference in OS between the GTR and STR groups (92.7 vs. 88.6 months, p = 0.270) (Fig. 3). Moreover, patients with Grade I SFT/HPC had longer PFS (86.0 months) than those with Grade II (26.1 months) and Grade III (40.5 months) (p = 0.017).
Table 2.
Univariate analysis for EOR, adjuvant therapy, and WHO classification
| Overall survival | Progression-free survival | |||
|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | |
| LD (cm) | 0.553 | 0.164 | ||
| < 5.0 | 91.52 | (65.30, 117.73) | 61.31 | (40.24, 82.37) |
| ≥ 5.0 | 70.23 | (53.28, 87.18) | 42.04 | (27.29,56.80) |
| EORa | 0.270 | 0.024 | ||
| GTR | 92.68 | (72.65, 112.72) | 60.66 | (44.89, 76.44) |
| STR | 88.57 | (65.59, 111.55) | 36.73 | (22.60, 50.85) |
| Adjuvant therapy | 0.057 | 0.06 | ||
| With AT | 99.98 | (83.45, 116.53) | 51.46 | (39.09, 63.84) |
| Without AT | 30.38 | (21.14, 39.61) | 22.14 | (10.33, 33.95) |
| WHO gradea | 0.254 | 0.017 | ||
| Grade I | 92.54 | (54.61, 130.48) | 86.00 | (74.12, 97.88) |
| Grade II | – | – | 26.09 | (18.04, 34.14) |
| Grade III | 81.81 | (69.58, 103.04) | 40.46 | (28.46, 52.46) |
aEOR and WTO grade were statistically significant in PFS
Fig. 3.
Kaplan-Meier survival curves display over all-survival and progression-free survival with different EOR and WHO grade. Figures exhibited EOR (a, b) and WHO grade (c, d)-related overall-survival and progression-free survival. EOR and WHO grade in PFS were statistically significant with p-value equaling 0.024 and 0.017. GTR, gross total resection; STR, subtotal resection
Multivariate analysis of the factors related to PFS
According to the outcomes of univariate analysis, EOR, adjuvant therapy, and WHO classification were used for multivariate analysis because of the limited sample sizes of this study. As shown in Table 3, EOR (HR: 0.378; 95% CI: 0.154, 0.927) and adjuvant therapy (HR: 0.336; 95% CI: 0.118, 0.956) were statistically significant for patients’ PFS with SFT/HPC.
Table 3.
Multivariate analysis for EOR, adjuvant therapy, and WHO classification of PFS
| Aspects | p-value | HR | 95% CI of OR |
|---|---|---|---|
| Adjuvant therapya | 0.041 | 0.336 | (0.118, 0.956) |
| EORa | 0.034 | 0.378 | (0.154, 0.927) |
| WHO grade | 0.096 | 1.663 | (0.914, 3.025) |
aEOR and adjuvant therapy were statistically significant in multivariate analysis
Discussion
This study first investigated the QoL of patients with intracranial SFT/HPC and found that the EQ5D-3 L index value decreased significantly in patients with progression. Moreover, we further explored several prognosis factors related to the patient’s PFS and OS. The multivariate analyses suggested EOR and adjuvant therapy influenced patient PFS and OS in intracranial SFT/HPC.
Quality of life plays an important role in long-term surviving patients, but little attention has been paid to patients with intracranial SFT/HPC. EQ-5D is a valid and convenient implementation to survey QoL of patients who suffered from the tumor with satisfactory reliability and validity [28, 29]. Unsurprisingly, the overall QoL of patients with progression deteriorated significantly, suggesting more attention was demanded in managing intracranial SFT/HPC patients. Specifically, patients with recurrence or metastasis were burdened with much more pain and pressure, in contrast with seldom pain management and psychological support under present treatment.
In accord with previous studies, our results also demonstrated that patients with STR are more likely to suffer from tumor recurrence or metastasis. Concretely, Zeng et al. had reported that the recurrence rate and metastasis rate were respectively 59.09% (13/22) and 50.0% (3/6) in none GTR patients with a GTR rate of 74.13% (43/58) [3]. Furthermore, in other case series, the GTR rates varied from 46.7 to 86.9%, and the rate of recurrence or metastasis was 77.8 to 85.71% in none GTR patients [9, 11, 30, 31]. For the long-term follow-up, patients who obtained GTR were observed with longer PFS and OS [3, 11]. Our results analogously exhibited that the patients who performed GTR got longer PFS.
Concerning WHO classification in CNS, most researchers considered the classification as a crucial prognostic factor [2, 9, 15]. Tumor with higher-grade and nuclear atypia was more likely to relapse, but some studies demonstrated that “benign-looking” WHO grade I SFT/HPC could recur malignant degeneration [32]. Other pathologic classifications could be utilized to identify prognosis factors for intracranial SFT/HPC patients [15]. Our results showed that the WHO classification was significant for PFS in univariate analysis, while it was not statistically significant for OS and both in multivariate analysis, similar to the previous publication [9]. The limited sample size probably caused counterintuitive results.
Patients with other postoperative treatments, such as external beam radiotherapy and Gamma Knife, probably had a better prognosis [3, 11, 14, 19, 20]. Other studies showed radiotherapy could significant promote OS (HR: 0.02; 95% CI: 0.00–0.31) and cause-specific survival (HR: 0.02; 95% CI: 0.00–0.45) in multivariate analysis [19]. Our results exhibited similar results that adjuvant therapy could extend PFS. Therefore, adjuvant therapy enabled extended PFS and OS, which could be used as remedial measures when GTR could not perform and metastasis occurred.
Despite the retrospective nature, we used several methods to avoid recalling bias, including catching information from medical records by different researchers. Moreover, we can see that SFT/HPC is a rare disease, so most current studies contain 10 to 64 patients, encountering the problem of a small sample size like us. Some studies reported relatively larger samples but ran a comparatively long period, which made it difficult to maintain the comparability in the cohort, such as the bias due to the update of treatment. Also, as a single-center study, we had to recognize that selection bias was inevitable. However, patients in this study were from several provinces in China, which relatively ameliorated this influence. In the further study, we could use some quantitative synthesis methods or set up multicenter cooperation to figure out this problem.
Conclusions
Intracranial SFT/HPC are rare mesenchymal tumors with a high relapse tendency, shortening the OS and decreasing the QoL of patients, particularly those with tumor progression and metastasis. Several factors are relevant to the prognosis, such as EOR, adjuvant therapy, and WHO grade. GTR without causing new neurological deficits is advocated for the treatment of SFT/HPC. And adjuvant therapy such as GKS could be an alternative treatment for patients who underwent STR or with tumor progression.
Acknowledgements
We would like to express our gratitude to all patients and their family who take part in this research.
Abbreviations
- CNS
Central nervous system
- EQ-5D
EuroQol Five Dimensions Questionnaire
- EMR
Electronic medical record
- EOR
Extent of resection
- GKS
Gamma knife surgery
- GTR
Gross total resection
- HPC
Hemangiopericytoma
- LD
Largest diameter
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PFS
Progression-free survival
- QoL
Quality of life
- SFT
Solitary fibrous tumor
- STR
Subtotal resection
- WHO
World Health Organization
Authors’ contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by YY, HY, and LL. Illustrations were generated by YY and SLY. SJ and PZZ supervised the study concept and revised the manuscript for intellectual content. The first draft of the manuscript was written by YY and HY, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study is supported by Sichuan Province Science and Technology Support Program (2022YFS0322) and the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2019HXFH018).
Availability of data and materials
The datasets of the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study, registered in ChiCTR (ChiCTR2000029718), was approved by the Ethics Committee of West China Hospital, and informed consent was obtained from the patient. It adheres to the tenets of the Declaration of Helsinki.
Consent for publication
There is no specific individual information and this item is not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Yu and Yu Hu contributed equally in this work as the co-first authors.
References
- 1.Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106(2):217–224. doi: 10.1093/ajcp/106.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Kim BS, Kim Y, Kong DS, Nam DH, Lee JI, Suh YL, Seol HJ. Clinical outcomes of intracranial solitary fibrous tumor and hemangiopericytoma: analysis according to the 2016 WHO classification of central nervous system tumors. J Neurosurg. 2018;129(6):1384–1396. doi: 10.3171/2017.7.JNS171226. [DOI] [PubMed] [Google Scholar]
- 3.Zeng L, Wang Y, Wang Y, Han L, Niu H, Zhang M, Ke C, Chen J, Lei T. Analyses of prognosis-related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors. J Neurooncol. 2017;131(1):153–161. doi: 10.1007/s11060-016-2282-y. [DOI] [PubMed] [Google Scholar]
- 4.Kinslow CJ, Bruce SS, Rae AI, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Sonabend AM, Wang TJC. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol. 2018;138(1):173–182. doi: 10.1007/s11060-018-2787-7. [DOI] [PubMed] [Google Scholar]
- 5.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 6.Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184(4):1209–1218. doi: 10.1016/j.ajpath.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(2):180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta neuropathologica. 2013;125(5):651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zhao K, Han L, Jiao L, Liu W, Xu Y, Niu H, Ke C, Shu K, Lei T. Solitary fibrous tumor/hemangiopericytoma of spinal cord: a retrospective single-center study of 16 cases. World Neurosurg. 2019;123:e629–e638. doi: 10.1016/j.wneu.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Park JH, Kim YI, Jeun SS. Treatment strategy of intracranial hemangiopericytoma. Brain Tumor Res Treat. 2015;3(2):68–74. doi: 10.14791/btrt.2015.3.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, Barani IJ, Berger MS, McDermott MW, Parsa AT. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118(6):1628–1636. doi: 10.1002/cncr.26411. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Vargas PM, Thenier-Villa JL, Sanroman Alvarez P, Serantes Combo A, Calero Felix L, Galarraga Campoverde RA, Azevedo Gonzalez E, Martin-Gallego A, Martinez-Rolan R, de la Lama Zaragoza A, et al. Hemangiopericytoma/solitary fibrous tumor in the central nervous system. experience with surgery and radiotherapy as a complementary treatment: a 10-year analysis of a heterogeneous series in a single tertiary center. Neurocirugia (Astur) 2020;31(1):14–23. doi: 10.1016/j.neucir.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Champeaux C, Rousseau P, Devaux B, Nataf F, Tauziede-Espariat A. Solitary fibrous tumours and haemangiopericytoma of the meninges. A retrospective study for outcome and prognostic factor assessment. Neurochirurgie. 2018;64(1):37–43. doi: 10.1016/j.neuchi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Zeng XW, Wu JS, Dou YF, Wang Y, Zhong P, Xu R, Jiang CC, Wang XQ. Solitary fibrous tumor of the central nervous system: a clinicopathologic study of 24 cases. Acta neurochirurgica. 2012;154(2):237–248. doi: 10.1007/s00701-011-1160-9. [DOI] [PubMed] [Google Scholar]
- 15.Macagno N, Vogels R, Appay R, Colin C, Mokhtari K, Küsters B, Wesseling P, Figarella-Branger D, Flucke U, Bouvier C. Grading of meningeal solitary fibrous tumors/hemangiopericytomas: analysis of the prognostic value of the Marseille Grading System in a cohort of 132 patients. Brain Pathol. 2019;29(1):18–27. doi: 10.1111/bpa.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melone AG, D'Elia A, Santoro F, Salvati M, Delfini R, Cantore G, Santoro A. Intracranial hemangiopericytoma--our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. 2014;81(3-4):556–562. doi: 10.1016/j.wneu.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishna R, Rostomily R, Sekhar L, Rockhill J, Ferreira M. Hemangiopericytoma: radical resection remains the cornerstone of therapy. J Clin Neurosci. 2014;21(4):612–615. doi: 10.1016/j.jocn.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Jia Q, Zhou Z, Zhang D, Yang J, Liu C, Wang T, Wu Z, Yang C, Wei H, Zhao J, et al. Surgical management of spinal solitary fibrous tumor/hemangiopericytoma: a case series of 20 patients. Eur Spine J. 2018;27(4):891–901. doi: 10.1007/s00586-017-5376-0. [DOI] [PubMed] [Google Scholar]
- 19.Ghia AJ, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, Brown PD. Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery. 2013;72(2):203–209. doi: 10.1227/NEU.0b013e31827b9e68. [DOI] [PubMed] [Google Scholar]
- 20.González-Vargas PM, Thenier-Villa JL, Sanromán Álvarez P, Serantes Combo A, Calero Félix L, Galárraga Campoverde RA, Azevedo González E, Martín-Gallego Á, Martínez-Rolan R, de la Lama Zaragoza A, et al. Hemangiopericytoma/solitary fibrous tumor in the central nervous system. Experience with surgery and radiotherapy as a complementary treatment: a 10-year analysis of a heterogeneous series in a single tertiary center. Neurocirugia (English Edition) 2020;31(1):14–23. doi: 10.1016/j.neucie.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI, Bruce JN. The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a Surveillance, Epidemiology, and End Results analysis. J Neurosurg. 2014;120(2):300–308. doi: 10.3171/2013.10.JNS13113. [DOI] [PubMed] [Google Scholar]
- 22.Hall WA, Ali AN, Gullett N, Crocker I, Landry JC, Shu HK, Prabhu R, Curran W. Comparing central nervous system (CNS) and extra-CNS hemangiopericytomas in the Surveillance, Epidemiology, and End Results program: analysis of 655 patients and review of current literature. Cancer. 2012;118(21):5331–5338. doi: 10.1002/cncr.27511. [DOI] [PubMed] [Google Scholar]
- 23.Boyett D, Kinslow CJ, Bruce SS, Sonabend AM, Rae AI, McKhann GM, Sisti MB, Bruce JN, Cheng SK, Wang TJC. Spinal location is prognostic of survival for solitary-fibrous tumor/hemangiopericytoma of the central nervous system. J Neurooncol. 2019;143(3):457–464. doi: 10.1007/s11060-019-03177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewes C, Sagberg LM, Jakola AS, Solheim O. Perioperative and postoperative quality of life in patients with glioma-a longitudinal cohort study. World Neurosurg. 2018;117:e465–e474. doi: 10.1016/j.wneu.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 25.Northcott PA, Robinson GW, Kratz CP, Mabbott DJ, Pomeroy SL, Clifford SC, Rutkowski S, Ellison DW, Malkin D, Taylor MD, et al. Medulloblastoma. Nat Rev Dis Prim. 2019;5(1):11. doi: 10.1038/s41572-019-0063-6. [DOI] [PubMed] [Google Scholar]
- 26.Alexander BM, Cloughesy TF. Adult Glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 27.Liu GG, Wu H, Li M, Gao C, Luo N. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17(5):597–604. doi: 10.1016/j.jval.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25(5):365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan JP, Grills I, Chiang VL, Dong H, Berg A, Warnick RE, Kondziolka D, Kavanagh B. Quality of life outcomes for brain metastasis patients treated with stereotactic radiosurgery: pre-procedural predictive factors from a prospective national registry. J Neurosurg. 2018;131(6):1848–1854. doi: 10.3171/2018.8.JNS181599. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Xiao CW, Wang T, Wu JS, Jiang CC, Qian J, Wei CH, Wang XQ. Orbital solitary fibrous tumor: a clinicopathologic study of ten cases with long-term follow-up. Acta neurochirurgica. 2012;154(2):249–255. doi: 10.1007/s00701-011-1254-4. [DOI] [PubMed] [Google Scholar]
- 31.Champeaux C, Rousseau P, Devaux B, Nataf F, Tauziède-Espariat A. Solitary fibrous tumours and haemangiopericytoma of the meninges. A retrospective study for outcome and prognostic factor assessment. Neuro-Chirurgie. 2018;64(1):37–43. doi: 10.1016/j.neuchi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Apra C, Mokhtari K, Cornu P, Peyre M, Kalamarides M. Intracranial solitary fibrous tumors/hemangiopericytomas: first report of malignant progression. J Neurosurg. 2018;128(6):1719–1724. doi: 10.3171/2017.1.JNS162593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request.