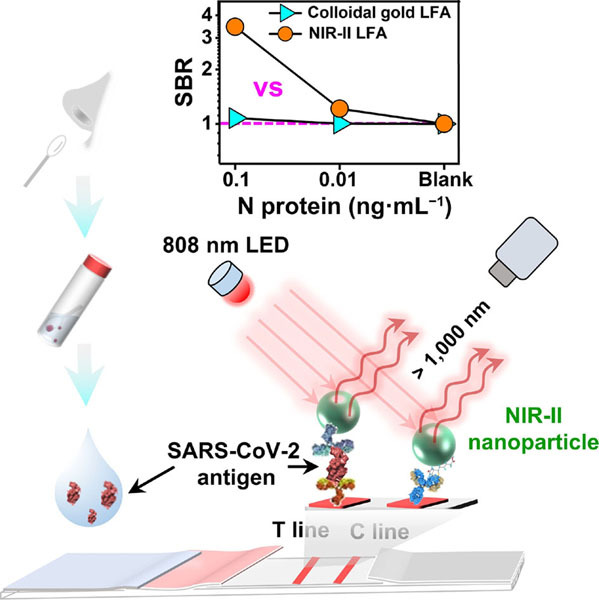
Abstract
Early detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is an efficient way to prevent the spread of coronavirus disease 2019 (COVID-19). Detecting SARS-CoV-2 antigen can be rapid and convenient, but it is still challenging to develop highly sensitive methods for effective diagnosis. Herein, a lateral flow assay (LFA) based on fluorescent nanoparticles emitting in the second near-infrared (NIR-II) window is developed for sensitive detection of SARS-CoV-2 antigen. Benefiting from the NIR-II fluorescence with high penetration and low autofluorescence, such NIR-II based LFA allows enhanced signal-to-background ratio, and the limit of detection is down to 0.01 ng·mL−1 of SARS-CoV-2 antigen. In the clinical swab sample tests, the NIR-II LFA outperforms the colloidal gold LFA with higher overall percent agreement with the polymerase chain reaction test. The clinical samples with low antigen concentrations (∼ 0.015−∼ 0.068 ng·mL−1) can be successfully detected by the NIR-II LFA, but fail for the colloidal gold LFA. The NIR-II LFA can provide a promising platform for highly sensitive, rapid, and cost-effective method for early diagnosis and mass screening of SARS-CoV-2 infection.
Electronic Supplementary Material
Supplementary material (the operation procedure and cost of the materials needed of NIR-II lateral flow assays, the dynamic light scattering spectrum of the NIR-II nanoparticles, the components and testing principle, optimization of main parameters pertaining to the LFA performance, the colloidal gold LFA strip, the fluorescence intensity distribution curves and the T/C values of the strips for clinical samples by NIR-II LFA, and results of clinical swab samples detected by colloidal gold LFA) is available in the online version of this article at 10.1007/s12274-022-4351-1.
Keywords: coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), antigen detection, the second near-infrared (NIR-II) fluorophores, lateral flow assay
Electronic Supplementary Material
Sensitively detecting antigen of SARS-CoV-2 by NIR-II fluorescent nanoparticles
Acknowledgements
This work is supported by Guangdong Provincial Department of Science and Technology-key research and development project (No. 2020B1111160003), Shenzhen Science and Technology Innovation Commission technology breakthrough project (No. JSGG20191231141403880), Shenzhen San-Ming Project (No. SZSM201809085), and Shenzhen Science and Technology Innovation Commission general project (No. JCYJ20180504165657443). Also, we are grateful to the Shenzhen Center for Disease Control and Prevention for providing and detecting the clinical samples.
Footnotes
Ruibin Hu, Tao Liao, and Yan Ren contributed equally to this work.
Contributor Information
Qihui Lin, Email: lhjkzx@szlhq.gov.cn.
Guoxin Wang, Email: wanggx@wwhsbio.com.
Yongye Liang, Email: liangyy@sustech.edu.cn.
References
- [1].Wang C, Horby P W, Hayden F G, Gao G F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhu N, Zhang D Y, Wang W L, Li X W, Yang B, Song J D, Zhao X, Huang B Y, Shi W F, Lu R J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. Coronavirus Disease (COVID-19) [Online]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed Feb, 2022).
- [4].Huang C L, Wang Y M, Li X W, Ren L L, Zhao J P, Hu Y, Zhang L, Fan G H, Xu J Y, Gu X Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hu B, Guo H, Zhou P, Shi Z L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Corman V M, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu D K, Bleicker T, Brunink S, Schneider J, Schmidt M L, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jin Y H, Cai L, Cheng Z S, Cheng H, Deng T, Fan Y P, Fang C, Huang D, Huang L Q, Huang Q, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu G Q, Rusling J F. COVID-19 antibody tests and their limitations. ACS Sens. 2021;6:593–612. doi: 10.1021/acssensors.0c02621. [DOI] [PubMed] [Google Scholar]
- [9].Gillot C, Douxfils J, Cadrobbi J, Laffineur K, Dogné J M, Elsen M, Eucher C, Melchionda S, Modaffarri É, Tré-Hardy M, et al. An original ELISA-based multiplex method for the simultaneous detection of 5 SARS-CoV-2 IgG antibodies directed against different antigens. J. Clin. Med. 2020;9:3752. doi: 10.3390/jcm9113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frumence E, Lebeau G, Viranaicken W, Dobi A, Vagner D, Lalarizo Rakoto M, Sandenon Seteyen A L, Giry C, Septembre-Malaterre A, Raffray L, et al. Robust and low-cost ELISA based on IgG-Fc tagged recombinant proteins to screen for anti-SARS-CoV-2 antibodies. J. Immunol. Methods. 2021;495:113082. doi: 10.1016/j.jim.2021.113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tan C W, Chia W N, Qin X J, Liu P, Chen M I C, Tiu C, Hu Z L, Chen V C W, Young B E, Sia W R, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- [12].Soleimani R, Khourssaji M, Gruson D, Rodriguez-Villalobos H, Berghmans M, Belkhir L, Yombi J C, Kabamba-Mukadi B. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID-19 patients. J. Med. Virol. 2021;93:1465–1477. doi: 10.1002/jmv.26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Infantino M, Grossi V, Lari B, Bambi R, Perri A, Manneschi M, Terenzi G, Liotti I, Ciotta G, Taddei C, et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: An Italian experience. J. Med. Virol. 2020;92:1671–1675. doi: 10.1002/jmv.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020;58:1081–1088. doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- [15].Zhang C, Zheng T T, Wang H, Chen W, Huang X Y, Liang J Q, Qiu L P, Han D, Tan W H. Rapid one-pot detection of SARS-CoV-2 based on a lateral flow assay in clinical samples. Anal. Chem. 2021;93:3325–3330. doi: 10.1021/acs.analchem.0c05059. [DOI] [PubMed] [Google Scholar]
- [16].Chen R, Ren C P, Liu M, Ge X P, Qu M S, Zhou X B, Liang M F, Liu Y, Li F Y. Early detection of SARS-CoV-2 seroconversion in humans with aggregation-induced near-infrared emission nanoparticle-labeled lateral flow immunoassay. ACS Nano. 2021;15:8996–9004. doi: 10.1021/acsnano.1c01932. [DOI] [PubMed] [Google Scholar]
- [17].Wang D M, He S G, Wang X H, Yan Y Q, Liu J Z, Wu S M, Liu S G, Lei Y, Chen M, Li L, et al. Rapid lateral flow immunoassay for the fluorescence detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020;4:1150–1158. doi: 10.1038/s41551-020-00655-z. [DOI] [PubMed] [Google Scholar]
- [18].Wondfo, 2019-nCoV Antibody Test (Lateral Flow Method) [online]. https://en.wondfo.com.cn/pt/index77.html (accessed Feb 1, 2022).
- [19].Li Z T, Yi Y X, Luo X M, Xiong N, Liu Y, Li S Q, Sun R L, Wang Y Q, Hu B C, Chen W, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ravi N, Cortade D L, Ng E, Wang S X. Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020;165:112454. doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu L Z, Li D Y, Ramadan S, Li Y B, Klein N. Facile biosensors for rapid detection of COVID-19. Biosens. Bioelectron. 2020;170:112673. doi: 10.1016/j.bios.2020.112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lou B, Li T D, Zheng S F, Su Y Y, Li Z Y, Liu W, Yu F, Ge S X, Zou Q D, Yuan Q, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 2020;56:2000763. doi: 10.1183/13993003.00763-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hou H Y, Wang T, Zhang B, Luo Y, Mao L, Wang F, Wu S J, Sun Z Y. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin. Transl. Immunol. 2020;9:e01136. doi: 10.1002/cti2.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lauer S A, Grantz K H, Bi Q F, Jones F K, Zheng Q L, Meredith H R, Azman A S, Reich N G, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou J, Li C, Liu X J, Chiu M C, Zhao X Y, Wang D, Wei Y X, Lee A, Zhang A J, Chu H, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- [26].Hui K P Y, Cheung M C, Perera R A P M, Ng K C, Bui C H T, Ho J C W, Ng M M T, Kuok D I T, Shih K C, Tsao S W, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020;5:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection [Online]. https://www.who.in//publication//i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays.
- [28].Kim H Y, Lee J H, Kim M J, Park S C, Choi M, Lee W, Ku K B, Kim B T, Changkyun Park E, Kim H G, et al. Development of a SARS-CoV-2-specific biosensor for antigen detection using scFv-Fc fusion proteins. Biosens. Bioelectron. 2021;175:112868. doi: 10.1016/j.bios.2020.112868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee J H, Choi M, Jung Y, Lee S K, Lee C S, Kim J, Kim J, Kim N H, Kim B T, Kim H G. A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2) Biosens. Bioelectron. 2021;171:112715. doi: 10.1016/j.bios.2020.112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang L Y, Fang X N, Liu X B, Ou H C, Zhang H Y, Wang J J, Li Q, Cheng H Y, Zhang W Y, Luo Z F. Discovery of sandwich type COVID-19 nucleocapsid protein DNA aptamers. Chem. Commun. 2020;57:10235–10238. doi: 10.1039/D0CC03993D. [DOI] [PubMed] [Google Scholar]
- [31].Lin Q Y, Wen D H, Wu J, Liu L L, Wu W J, Fang X E, Kong J L. Microfluidic immunoassays for sensitive and simultaneous detection of IgG/IgM/antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020;92:9454–9458. doi: 10.1021/acs.analchem.0c01635. [DOI] [PubMed] [Google Scholar]
- [32].Guo J C, Chen S Q, Tian S L, Liu K, Ni J, Zhao M, Kang Y J, Ma X, Guo J H. 5G-enabled ultra-sensitive fluorescence sensor for proactive prognosis of COVID-19. Biosens. Bioelectron. 2021;181:113160. doi: 10.1016/j.bios.2021.113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grant B D, Anderson C E, Williford J R, Alonzo L F, Glukhova V A, Boyle D S, Weigl B H, Nichols K P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020;92:11305–11309. doi: 10.1021/acs.analchem.0c01975. [DOI] [PubMed] [Google Scholar]
- [34].Liu D, Ju C H, Han C, Shi R, Chen X H, Duan D M, Yan J H, Yan X Y. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;173:112817. doi: 10.1016/j.bios.2020.112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Antaris A L, Chen H, Cheng K, Sun Y, Hong G S, Qu C R, Diao S, Deng Z X, Hu X M, Zhang B, et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016;15:235–242. doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- [36].Hong G S, Diao S, Chang J L, Antaris A L, Chen C X, Zhang B, Zhao S, Atochin D N, Huang P L, Andreasson K I, et al. Through-skull fluorescence imaging of the brain in a new nearinfrared window. Nat. Photonics. 2014;5:723–730. doi: 10.1038/nphoton.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Welsher K, Liu Z, Sherlock S P, Robinson J T, Chen Z, Daranciang D, Dai H J. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009;4:773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kenry, Duan Y K, Liu B. Recent advances of optical imaging in the second near-infrared window. Adv. Mater. 2018;30:1802394. doi: 10.1002/adma.201802394. [DOI] [PubMed] [Google Scholar]
- [39].Chang, B. S.; Li, D. F.; Ren, Y.; Qu, C. R.; Shi, X. J.; Liu, R. Q.; Liu, H. G.; Tian, J.; Hu, Z. H.; Sun, T. L. et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng., in press, 10.1038/s41551-021-00773-2. [DOI] [PubMed]
- [40].Zhu S J, Tian R, Antaris A L, Chen X Y, Dai H J. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 2019;31:e1900321. doi: 10.1002/adma.201900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hong G S, Lee J C, Robinson J T, Raaz U, Xie L M, Huang N F, Cooke J P, Dai H J. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang C Y, Zhou L, Du K, Zhang Y, Wang J, Chen L J, Lyu Y N, Li J, Liu H, Huo J L, et al. Foundation and clinical evaluation of a new method for detecting SARS-CoV-2 antigen by fluorescent microsphere immunochromatography. Front. Cell Infect. Microbiol. 2020;10:553837. doi: 10.3389/fcimb.2020.553837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang C W, Yang X S, Zheng S, Cheng X D, Xiao R, Li Q J, Wang W Q, Liu X X, Wang S Q. Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza a virus. Sens. Actuators B Chem. 2021;345:130372. doi: 10.1016/j.snb.2021.130372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitively detecting antigen of SARS-CoV-2 by NIR-II fluorescent nanoparticles


