Abstract
The establishing of the first cancer models created a new perspective on the identification and evaluation of new anti-cancer therapies in preclinical studies. Patient-derived xenograft models are created by tumor tissue engraftment. These models accurately represent the biology and heterogeneity of different cancers and recapitulate tumor microenvironment. These features have made it a reliable model along with the development of humanized models. Therefore, they are used in many studies, such as the development of anti-cancer drugs, co-clinical trials, personalized medicine, immunotherapy, and PDX biobanks. This review summarizes patient-derived xenograft models development procedures, drug development applications in various cancers, challenges and limitations.
Keywords: Cancer animal model, PDX, Preclinical study, Humanized model, Avatar model of cancer
Background
The first sparks of cancer models were formed more than 200 years ago when the first report of cancer by environmental factors was presented [1]. In recent century, mice have been widely used in biological research and have made significant contributions to cancer discoveries as a bedrock for cancer models [2] (Fig. 1). Evaluation and classification of cancer models gave scientists a relatively deep insight into the underlying genetic mechanisms of malignancy and cancer progression, and animal models with clinically predictive properties switch candidate drugs to phase 2 clinical trials with confidence from the pre-clinical phase. Therefore, cancer animal model studies focus on the pre-clinical evaluation of the efficacy of biological and chemical agents. However, more than 90% of drugs that successfully pass preclinical studies are ineffective in the human phases. These data suggest that conventional preclinical models such as monolayer cell lines culture or syngeneic and xenograft models are the main reason for the failure of most anti-cancer agents in humans [3, 4].
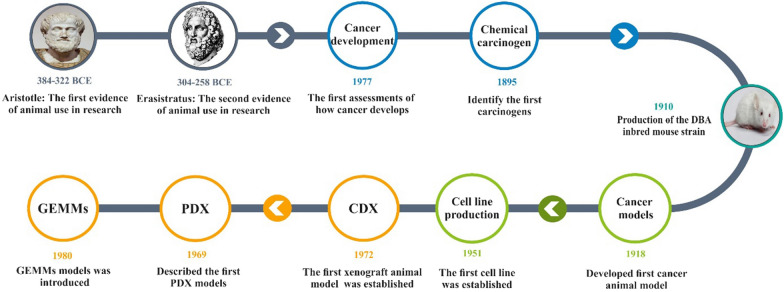
Fig. 1.
Cancer animal models timeline. The timeline shows the first available reports of the use of animal models during the Aristotle and Erasistratus eras, which over time, recognizing how cancer formed in 1777 and identifying carcinogens paved the way for the use of cancerous animal models in 1910 with the development of DBA mice. Finally, in 1918, the first cancer model was developed by Yamagiwa and Ichikawa. Following the development of the first cell line in 1951, the first CDX model was introduced, which greatly contributed to the improvement of cancer science. Furthermore, PDX and GEMMs were introduced in 1969 and 1981, respectively. GEMMs genetically engineered mouse models, PDX patient-derived xenograft, CDX Cell Line-Derived Xenograft model, DBA dilute, brown and non-agouti
Validation of disease model to achieve the best model is essential; three criteria are usually evaluated. Face validity is the first criteria that describe the biology similarity between the human disease and the animal model. The second is target validity which the target agents should have a similar function in the model as in the clinical aspect, and the third is predictive validity which demonstrates that clinically effective therapeutic agents show a similar effect in the disease model [5].
Hence conventional models such as tumor cell lines showed different phenotypes after adaptation to in vitro culture conditions that were frequently distinct between laboratories, thus having a low resemblance to parental tumors. Indeed long-term in vitro cell culture showed alterations in tumor hallmarks caused by epigenetic and genetic changes. Therefore, along with the advantages, these significant limitations lead to low scores of these traditional models from the evaluation criteria of animal models and prevent using these models for drug screening and estimating the pre-clinical efficacy of drugs [6] (Table 1).
Table 1.
Advantages and limitations of different animal models
| Animal models | Advantages | Limitation | Ref. |
|---|---|---|---|
| Chemical carcinogenesis |
Simplicity Assess the cancer process from initiation to metastasis in order Gene analysis in different stages |
Not cover all cancers Tumor rejection by host immune cell Mice lifespan isn't enough for tumor induction Concerns about long-term use of carcinogens |
[117, 118] |
| Syngeneic mouse models |
Immunocompetence Simplicity High engraftment rate |
Non-synonymous mutations Lack of heterogeneity The limited number of cell lines |
[119] |
| GEMMs |
Evaluating drug responses, resistance, and toxicity Allows to answer unique biological questions |
Interspecies differences Random transgenesis Genetic compensation Lethality of some mutations Complexity of disease |
[120] |
| Cell Line-Derived Xenograft model |
Suitable for mechanism studies Rapid growth Evaluate non-targeted cytotoxic agents Available and cheap |
Lack of heterogeneity Lack of immunological agents Lack of tumor micro-environmental |
[77, 121] |
| PDX models |
Retain heterogeneity and mutations Tumor microenvironment Intact endocrine system Metastasis assessment Tumor biobank formation |
Generated in mouse with deficient immunity Different take rates Not suitable for early-stage cancer |
[122, 123] |
| Humanized mice |
Correctly mimics human tumor microenvironment Predictors of drug response in human cancer Creates a natural heterogeneity of tumor cells |
Expensive technically complicated |
[33, 124] |
GEMMs genetically engineered mouse models, PDX patient-derived xenograft, CDX Cell Line-Derived Xenograft model)
Stringent success criteria during the preclinical stages have improved failure rates of the drug development process. The selection of animal models affects the success rate of these stages, which allows for evaluation of the target validity, which can predict the clinical efficacy of specific agents. As a result, the expectation is not to reproduce the human disease with all its complexities in the animal; instead, the model evaluates particular aspects of the disease. When using an animal model, it is crucial to ensure that the chosen model is fit-for-purpose.
In recent years, some aspects of animal models have been modified to improve the translational value. One of these approaches is to create humanized mouse models. These models are engrafted with human cells and tissues that impart human characteristics to mice and make the model a valuable tool for clinical translation [7]. The humanized mouse models mimic the human immune system; therefore, these models are mainly used to create patient-derived xenograft (PDX) models for cancer studies. In this different generation of animal models, the cancer patient's tissue is transplanted into immunodeficient mice. In subsequent years, studies have evaluated the accuracy of these models in responding to chemotherapy compared to the patient with donated tumor tissue; in most cases, there was a significant correlation between the response to chemotherapy in PDX models and patients [8]. Moreover, PDX evaluation showed significant heterogeneity and faithfully recapitulated the characteristics of their parental tumors on the microscopic, genetic and functional levels [9] (Table 1).
But the increased use of PDX models was postponed until The National Cancer Institute stopped using the (NCI)-60 panel (2016) and introduced the PDX model as a more reliable model [10]. Then it has become popular due to recapitulation clinically relevant. This review intends to further evaluate the PDX model as an applicable and reliable animal model.
PDX models present parental tumor microenvironment structure
In the evaluation of cancer, the surrounding microenvironments should be considered. This microenvironment includes stromal cells, which are composed of Tumor Endothelial Cells (TECs) and Cancer-Associated Fibroblasts (CAFs), Tumor-Associated Macrophages (TAMs) that have a significant effect on cancer progression and metastasis [11, 12]. These cells produce Extra Cellular Matrix (ECM), a network of proteoglycans such as laminin, collagen, and fibronectin that regulates cellular polarization, intracellular signaling and, migration, and creating flexible, stable, and supportive structures for different tissues [13, 14]. ECM compounds are essential in the distribution of drugs in tumor tissue. While the cancer cell lines commonly used to create animal models do not have apparent clinical features of the primary tumor, PDXs are preferred because of preserving tumor microenvironment’s structure. Although the PDX models have some limitations, but its features have primarily eliminated the shortcomings of other cancerous animal models. High tumor heterogeneity, maintaining tumor-stromal, gene expression, and tumor tissue mutations and high predictive value make this model ideal for biomarker evaluation, evaluating cell-based therapies, pre-clinical studies, and use for personalized medicine [8, 15, 16]. These features have made it more popular in recent years and, unlike other models in the Covid-19 pandemic era, have not declined (Fig. 2).
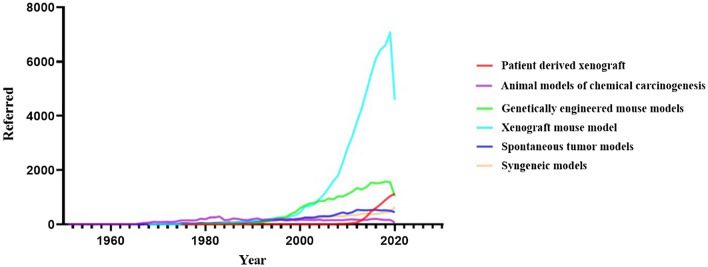
Fig. 2.
Cancer animal models over the years. The animal model of cancer was first introduced in 1918, but the beginning of today’s models was in 1951 with the development of the CDX models, but the development of other models led to competition for a more suitable model with more efficiency. The beginning of the twenty-first century can be considered the beginning of the flourishing of animal models of cancer. CDX model won the competition between the CDX and GEMME models because of its availability and is still the first choice in many studies. But the remarkable thing is that at the beginning of the second decade of the twenty-first century, PDX models have attracted attention and are being used in various studies with considerable speed. Data obtained from the PubMed database. GEMMs genetically engineered mouse models, PDX patient-derived xenograft, CDX Cell Line-Derived Xenograft model)
Methodology to establish PDX models
Generally, these models can be created as orthotopic, heterotopic, and metastatic. In heterotopic models, at the first step, the tumor tissue specimen should be prepared in a cold solution containing Fetal Bovine Serum (FBS), penicillin/streptomycin to increase the success rate of engrafting. The tumor tissue can be transplanted in three approaches; the tumor tissue can be cut into pieces and several 3–5 mm3 pieces, a large amount of tumor or injection of minced tumors can be used for transplantation. The tumor samples are washed three times with the above-mentioned solution before transplantation. Various parts of the mouse can be used as tumor tissue transplantation sites to create PDX models. The tissues can be engrafted heterotopically into the intracapsular fat pad [17, 18], the anterior compartment of the eye [19], under the renal capsule [20], subcutaneously, and orthotopically into the origin of cancerous tissue [21]. Orthotopic transplantation is usually preferred because it has a closer microenvironment to human cancer. But in general, both subcutaneous and orthotopic sites are most commonly used [19].
It should be noted that depending on the type of tumor and the sample area, the concentration and type of antibiotics may change. Small incisions are created on the lower back for subcutaneous transplantation in 4–8-week-old immunodeficient mice, and the tumor tissue sample is placed in each of the surgical areas. The remaining tumor tissues can be stored (− 80 °C for short term and in a liquid nitrogen freezer for long term storage) for genomic and protein evaluations compared with the xenograft tumor. The first generation F1and the subsequent generations are named F2, F3. Although designation can be done in other ways such as (G1, and so on) [22–24]. Reports suggest that the efficiency of nu/nu athymic mice is 75% and that NOD/SCID mice are more commonly used in F1 [25, 26]. The creation of NOD/SCID/IL2rγnull (NSG) mice resulted in higher efficacy of 95–100% in tumors that are difficult to transplant [27] (Fig. 3).
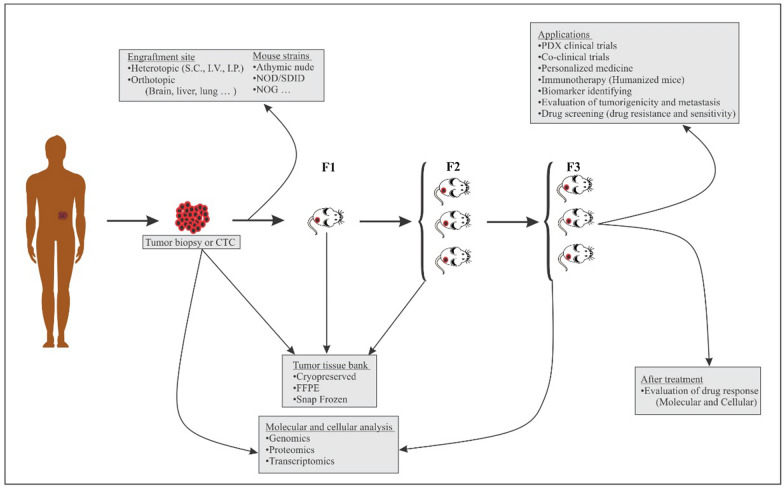
Fig. 3.
The process of creating PDX models and their applications. The generations are named F1, F2, F3, etc. Different cancer tissues can be used to create PDX models (orthotopic or heterotopic engraftment), as well as different hosts with varying degrees of immune deficiency. Tumor biopsy and generations F1 and F2 can be sampled for tissue bank, and tumor biopsy and F3 can be used for Genomics, Proteomics, and Transcriptomics analyzes. The F3 generation of these models can be used in various studies. CTC circulating tumor cell, S.C subcutaneously, I.V intravenous, I.P intraperitoneal, FFPE formalin-fixed paraffin-embedded
Xenograft tumors are assessed at least twice a week by a vernier caliper to measure tumor length and width. The time required to reach cancer depends on type of cancer, the location of the transplant, and the recipient strain. It takes an average of 2–4 months, and the transplant has not been successful if no tumor is detected over 6 months. When the size of the tumors reaches 1–2 cm3, we can begin the tumor passage. Finally, the third generation (F3 or G3) can be used for drug treatment and commonly employed in studies. However, genetics and histology (rather than merely the number of passages) should be the main determinants of PDX derivation from the patient’s tumor [28–30].
In recent years, the development of PDX models in humanized mice has been considered, in particular form in humanized PDX models, human immune system and human tumor tissue both together will be engrafted to the model and then human immune system reconstitute in an immunodeficient mice along with patient tumor engraftment [31].
There are three main classes of humanized mice; (A) human gene transgenic mice that are modified by specific human gene expression; (B) humanized organ mice which in this model the mice carrying a specific organ of human such as human hepatocyte infusion; (C) humanized immune system mice which are established in immunodeficient mice and human cells will reconstitute the mice immune system which describe briefly in Table 2 [32, 33].
Table 2.
Establishment of humanized immune system mouse models
| Humanized establishment method | Mouse strain | Rout of administration | Advantage | Limitation |
|---|---|---|---|---|
| PBMC engraftment | NOD-SCID mice | Intravenous injection of PBMC (5–10 × 106), the engraftment consists of T cells | Cost effective, simple establishment pattern suitable model for T-cell-related immune research | Lack of necessary cytokines in order to B and NK cell in vivo proliferation, GVHD development makes a short period for experiment |
| Human HSC engraftment (CD34+) from BM, UCB, FL, MBP | NOD-SCID, NSG | Intravenous injection of 1 × 105 HSCs, when the count of human CD45+ > 25% in peripheral blood the model is established | More complete immune reconstitution, GVHD rarely occurs | Long period of model establishment, maturation of human T cells in murine thymus makes human T cell restricted to mouse H2 |
| Human BLT (bone marrow, liver, thymus) model | NOD-SCID, NSG | Intravenous injection of CD34+ HSC (0.5–1 × 106) from human bone marrow, implantation of human fetal liver and thymus in to mouse sub renal capsule when the count of humanCD45+ > 25% in peripheral blood the model is established | Maturation of T cells in autologous human thymus, human T cell restricted to human HLA, highest immune reconstitution; B, T, macrophages and dendritic cells. long term maintenance of model | GVHD development due to mouse DCs, positive and negative selection processes of human T cells; although lighter GVHD than PBMC humanized model. Engraftments should carry from same donor, complex technique and ethical problems |
Applications of PDX models in cancer research
PDX clinical trial
PDX Clinical Trials (PCTs) are substantial for clinical decision-making before human clinical trials and the development of anti-cancer agents. PCT is referred to as “phase II type clinical trial-like models”. In 2015, Gao et al. designed a high-throughput in vivo drug screening method, “1 × 1 × 1”, which intended one animal per model per treatment using a large number of PDX models. However, two or three animals per model per treatment (2 × 1 × 1 or 3 × 1 × 1) PDX clinical trials because of more representative of generalizable drug response have recently become more common [34] (Fig. 4A).
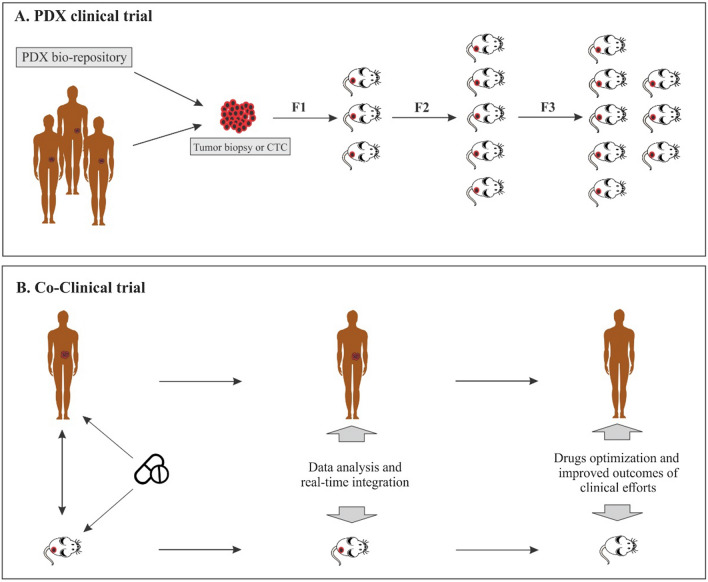
Fig. 4.
PDX clinical trial and Co-clinical trial. A In models that follow the PDX clinical trial approach, a large number of PDXs originate from several patients or samples of a bio-repository. Each model tests a specific drug regimen, and the information obtained is evaluated. B Co-clinical trials were developed to achieve precision medicine. In fact, the mouse trials are performed in parallel with the human trials, and then in real-time, the information obtained from the mouse study is transferred to the human study and integrated. This leads to the most effective clinical outcome
PDX models have become a powerful tool for evaluating drug efficacy and drug sensitivity, also known as PDX clinical trials. As shown in Table 3, several studies have tested the response rate of different drugs to different cancers in PDX models. These studies have shown that the response rate of PDX models to the drug is correlated with clinical outcomes [35]. In fact, before a human clinical trial, PDX clinical trials are critical to evaluate anti-cancer therapies. Accordingly, these cancer model features have led research centers and pharmaceutical companies to develop PDX repositories. The Novartis Institute of Biomedical Research has launched the PDX library. In this project, Novartis has created more than 1000 Avatar mice of various cancerous tissues, technically, there is no difference between Avatar and PDX, but an Avatar is mainly used in cases where the goal is personalized medicine; they use the predictive capacity of this model for particular patients, in contrast, PDXs are more commonly used in preclinical studies of drug evaluation and co-clinical trials. Accordingly, research centers and large pharmaceutical companies seek to establish a PDX repository, to be used in the development of preclinical studies on anti-cancer drugs [36]. Europeans have established a consortium called EurOPDX to store PDXs, which currently holds more than 1500 PDXs samples [37, 38]. Since 2017, the NCI has also been developing a national repository of Patient-Derived Models (PDMs), including PDX models. The overall goal of NCI is to establish a long-term storage site for at least 1000 PDX models so that researchers have sufficient biological and clinical diversity to conduct their studies [39, 40].
Table 3.
Cancer PDX models in therapeutic approaches
| Tumor histotype | PDX model | Treatment/molecular alterations | Response rate (RR) | Ref. |
|---|---|---|---|---|
| Breast cancer | Orthotopic | Docetaxel, 5-fluorouracil, trastuzumab | 71% | [125] |
| Docetaxel, doxorubicin, trastuzumab + Lap | 100% | [126] | ||
| Colorectal cancer | Heterotopic | Cetuximab, panitumumab | 100% | [80] |
| Heterotopic | WT KRAS | 100% responded to cetuximab | [127] | |
| Heterotopic | Oxaliplatin | 92% | [79] | |
| Ovarian cancer | Heterotopic | Cisplatin | 81% | [128] |
| Heterotopic | Doxorubicin, cyclophosphamide, 5-fu | 0–27% | [42] | |
| Gastric cancer | Heterotopic | Regorafenib | 96% | [129] |
| Non-small cell lung cancer | Heterotopic | EGFR mutated | 10% responded to gefitinib | [130] |
| Pancreatic ductal adenocarcinoma (PDAC) | Heterotopic | Gemcitabine | 17% | [131] |
Cancer biology
This model has helped increase our understanding of the response of tumor cells to drugs, which leads to effective treatment strategies. Other applications of such models include creating PDX resistance to treatment, in which the consecutive administration of a particular drug can lead to the production of drug-resistant PDX models. These resistant tumors are more consistent with cancerous cells than cell lines, which can be used to trace drug resistance biomarkers and investigate drug resistance mechanisms [41]. For example, ovarian cancer exposed to long-term cisplatin induces resistance to this substance, Similar to clinical conditions. These models are used to find new anti-cancer agents, to evaluate suitable drugs for patients with platinum resistance [42]. Intratumoral heterogeneity of primary tumors in patients is recapitulated by PDX tumors, increasing evidence indicating that tumors have a distinct subset called Cancer Stem Cells (CSCs). Another advantage of this model is the maintenance of CSCs. These cells have been purified and characterized by several types of PDX tumors through specific surface markers, which can be used for drug screening and discovery studies [43].
Co-clinical trials
PDX models have accelerated the development of medications and the phases of clinical trials. Currently, the prediction value of PDX models is used in co-clinical trials. Phases 1/2 of the clinical trials take more than 5 years. Due to limitations in analysis and data integration, co-clinical trials have been suggested at this time (mouse hospitals are manifested), whereby new drug therapies are performed on experimental tumor models concurrently with clinical trials. Finally, the pre-clinical and clinical data are aggregated [44, 45]. The approach of the co-clinical trial using the PDX model facilitates the prioritization of optimal medications, facilitates rapid classification of respondents, determines biomarkers, and detects resistance mechanisms [44]. This format of trials has been or is being conducted for some cancers, such as Sarcoma [46], and Solid tumors [47] (Fig. 4B).
Personalized medicine
PDX enables the growth of a tumor from any patient in an in vivo system called “Avatar models” or “mirror model”. Avatar is mainly used in personalized medicine approaches. They use the predictive capacity of this model for a particular patient. This model allows for the evaluation of drug sensitivity and precision drug efficacy and eliminates the cost of non-targeted therapy by providing a personalized regimen [48]. For example, Kopetz et al. established an Avatar model of colorectal cancer with BRAF mutation, which by using vemurafenib as an oral BRAF inhibitor, drug resistance was observed due to KRAS (Kirsten Rat Sarcoma Virus) and NRAS (Neuroblastoma RAS Viral Oncogene Homolog) mutations at low allele frequency in this tumor subset [49]. Each patient can have an equivalent animal, assuming that the animal model (PDX) has a similar function in the patient. Additionally, the acquisition of tumor profiles at different times with different therapies by PDX models allows for the evaluation and understanding of various molecular factors, signaling pathways, the flow of tumor growth metabolism, and molecular changes leading to metastases and drug resistance [48].
Immunotherapy
In recent years, immunotherapy has achieved widespread success against various malignancies, so the limitations of the xenograft cell line-based model and Genetically Engineered Mouse Model (GEMM) have led researchers to become more inclined towards PDX models by reconstitution human immunity in PDX models and to create humanized models, scientists will be able to evaluate immunotherapies. In this approach, the correlation between the hematopoietic system and the tumor of the same patient is necessary. Humanized PDX models by reconstituting the human immune system and tumor growth enable the investigator to study tumor biology and immune system functionality. Zhao et al. have been developed humanized PDX model by type I human leukocyte antigen matched human immune system in NOD-SCID Il2rg−/− (NSG) mice as an immuno-oncology model to study immunotherapy approaches which both therapeutic and side effects of pembrolizumab and ipilimumab had been investigated in this model [50]. Immune check point investigation in gastric cancer humanized avatar model by engrafting the human tumor and PBMC injection showed delayed tumor growth by using anti-check point monoclonal antibodies [51]. These Humanized models have been used to evaluate many targeted therapies such as anti-PD1/PDL-1, CTLA-4, and other checkpoint inhibitors [52–54].
In 2010, the autologous CAR-T effect on PDX models of the same patient was evaluated for the first time [55]. In another study, PDX models of three hepatocellular carcinoma patients were developed, all of which retained the primary tumor characteristics, CAR-T inhibited tumor growth in the first group, and the tumor was eliminated in the other two groups [56]. In a 2017 study, the pancreatic cancer PDX models were used to evaluate anti-mesothelin CAR-T cells, which effectively inhibited the tumor [57]. Another application of PDX models in cell therapy is assessing various aspects of CAR-T cell treatment and biology. One of these aspects is the interaction between CAR-T and other immune cells such as Tregs and Myeloid-Derived Suppressor Cells (MDSCs) in the tumor microenvironment. These immune-inhibiting cells could be one of the causes of the negative results of some CAR-T trials. In the presence of these immune-inhibiting cells, PDX models will show more accurate and acceptable results, eventually, models with Patient immune systems such as humanized PDX models have received more attention from immunotherapy researchers [58]. Humanized PDX models are future tools in personalized medicine and will support clinical decisions. In an avatar (hIL2-NOG mice) of human melanoma patients, anti-PD-1 (Programmed Cell death Protein 1) antibody response and tumor-infiltrating T cells supported the clinical decision for immunotherapy [59]. However, the humanized immune-PDX models still need to be more validated. Still, they can eventually be a suitable approach for personalized medicine and clinical decision, especially for novel cancer immunotherapies (Fig. 5).
Fig. 5.
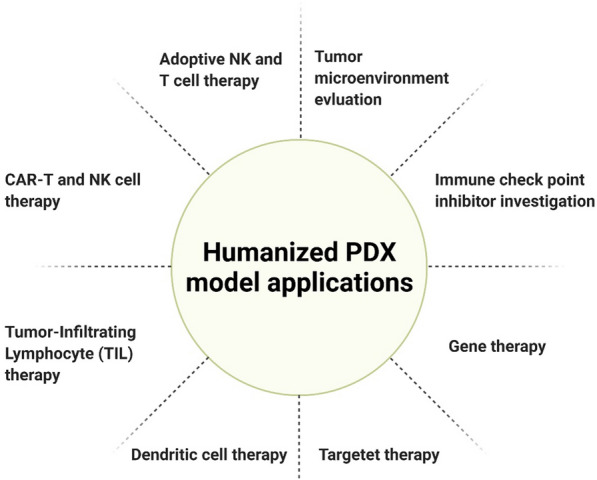
Humanized PDX model applications in immunotherapy
In xenograft models used in immunotherapy, the type of immunodeficient mice should be considered. As shown in Table 4, NOD/SCID /IL2rγnull (NSG) mice suitable for immunotherapy, these mice lack the production of cytokines such as IL-2, IL-4, IL-7, IL-9, IL-15 and, IL-21, which cause the loss of immune cells B, T, and NK, so these rodents are essential for transplantation of primary human tumors, while other immunocompromised mice are suitable for the development of cancer cell line-derived models [60, 61].
Table 4.
Types of immunocompromised mice that can be used to develop cancer models
| Mice generations | Immune defections | Features | Ref. |
|---|---|---|---|
| Athymic nude mice | Homozygous mutations in the Foxn1 gene (encoding the Forkhead box N1 transcription factor) | Defects in hair follicles and more importantly in the thymus and cause cells of B and T to lose their function. But antigen-presenting cells (APCs), macrophages, and NK cells are active in these mice | [19, 132] |
| Rag1/Rag2 mice | Rag1/2 recombinase defects | B and T cells in these mice lose normal performance due to the loss of function of the recombinase, such as athymic nude. These mice are particularly used to evaluate DNA damaging therapies because Rag knockout mice have a high tolerance for DNA damage | [19, 133] |
| SCID mice | Mutations in the Prkdc gene (protein kinase, DNA activated, catalytic polypeptide) | B and T cell leakage in these mice causes cellular immunity. Commonly, other mutations are used in combination with SCID mutations. The possibility of the death of these mice is very high due to spontaneous T-cell lymphomas | [134, 135] |
| SCID/Beige mice | Combination of Beige mutation (Bg) with the SCID mutation | To defects in B- and T-cells, Bg mutation causes defects in lysosomes and the function of NK cells, but on the other hand, this mutation causes a threefold increase in macrophages compared to the Wild Balb/C | [136, 137] |
| NOD/SCID mice | NOD (non-obese diabetic) mutation along with SCID | Directly affects cellular immunity by interfering with the performance of NK, APC, and macrophage cells | [138, 139] |
| NOG and NSG mice | NOD/SCID mice with IL2 receptor gamma truncation/disruption mutations | The incidence of lymphoma in NSG mice is lower than in NOD/SCID mice, and they have longer life spans, making them grow up to engraft more human tumors. These mice are suitable for the transplantation of low-growth tumors | [140, 141] |
| NRG mice | In NOG mice, Rag1 mutation replaced the SCID mutation | NRG has been used for intra-oral injection for PDX development | [140] |
PDX model achievements for five common cancers
The PDX model has been developed for various cancers over the years and has used for tumor evaluation and drug screening (Table 5). Here’s the application of this model is reviewed in five common cancers worldwide.
Table 5.
Five common PDX models properties
| Origin | Tumor take rate | Tumor latency | FDA approved protein biomarkers | Application of biomarkers | ECM (Matrigel) | Ref. |
|---|---|---|---|---|---|---|
| NSCLC | 24%; 25/102 | ≥ 5 months | EGFR1, MMP7, CA6, KIT, CRP, C9, and SERPINA3 (not FDA approved) | Diagnosis | – | [28, 142–144] |
| Gastric cancer | 15%; 35/232 | 3 months | Mast/stem cell growth factor receptor (SCFR)/c-Kit | Diagnosis, treatment selection | Yes | [145] |
| Colorectal cancer | 63%; 54/85 | 2 months | Carcinoembryonic antigen (CEA), fibrin, fibrinogen degradation product DR-70 |
Disease monitoring, treatment response, progression Disease monitoring, diagnostics |
Yes | [73, 76] |
| Breast cancer | 15%; 20/130 | 4–5 months |
CA 27, 29-CA 15-3 Estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER/Neu) |
Monitoring disease, treatment response Prognosis, treatment selection |
– | [146, 147] |
| Prostate cancer | 10–20%; 21/261 | 4–12 months | Prostate-specific antigen (PSA) and p63 |
Disease monitoring, diagnostics Differential diagnosis |
– | [87, 146] |
Lung cancer
PDX of Non-Small-Cell Lung Carcinoma (NSCLC) by imitating the molecular pattern of mutations and histopathological characteristics had been reported on 127 stable models by Wang et al. [62]. In the following, they showed that these emulator models are as same as their donors in gene expression profiling [62]. One hundred surgically resected specimens had been used to develop the PDX model of NSCLC by Ilie and colleagues. Among them, 35 models were established, and anything the team expects from these models was met, similar to tumor specimen [63]. These models have been used to evaluate common drug resistance such as docetaxel, cisplatin and can also be used to identify therapeutic predictive biomarkers [28, 64]. Lung tumor tissue samples were implanted under the sub-renal capsule in immunodeficient mice at a ˃ 95% rate [65].
Lung cancer patients who are at greater risk of recurrence after surgery are predictable, Due to the success of the PDX model’s engraftment rate. Similarly, in a study of 63 of 157 tissue samples of lung cancer transplanted to mice, they were compared with the clinical outcomes of patients, which were predicted to have a lower survival rate than others in a multivariate analysis [25].
Breast cancer
PDX models provide a robust basis to study this fertile soil. First, cancer-related female death is not a single disease but a network of mutations’ which may lead to the development of new target therapies that, in turn, a preclinical in vivo model is needed [66]. The first transplantation of mammary tumor lines had been established in 1903, till 2003 that the efficient engraftment of human tumor tissue had been done in immunodeficient mice, CDX models of HER2+ (Epidermal growth factor Receptor 2) models, GEMM model of the BRCA1 (Breast Cancer Gene 1) mutation, and spontaneous mammary adenocarcinoma in transgenic mice were the most available in vivo models to study targeted therapies [67].
Initially, models of hormone-dependent cancers had many challenges. But over time and further research into the orthotopic transplantation of breast cancer tissue in the mammary fat pads of immunodeficient mice, estrogen supplementation has achieved some favorable engraftment rates [68, 69] (Table 2). The different parts of tumor tissue had been reported to use for Xenograft model establishment; primary breast, pleural effusion, ascites, and metastatic sites like the ovarian, brain, Nodal, skin, and soft tissue. Statistically Controversial metastatic site was found between the PDX model and human tumor; bone and brain metastasis are more frequent in patients but lymph nodes and lung in PDXs [18]. Triple-negative (ER-negative, PR-negative, and HER2-negative) tumor tissues have higher engraftment rates due to the aggressiveness of this type of cancer [69].
Colorectal cancer
Various studies have shown that PDX models maintain heterogeneity of the Colorectal Cancer (CRC) primary tumor [70, 71]. The results of drug evaluations are most similar to the clinical settings. The rate of engraftment is dependent on the immunodeficient mice strain and the method of delivery. Tumor tissue is usually transplanted heterotopically into the subcutaneous or sub-renal site [72, 73]. However, several studies have isolated tumor tissue cells by enzymatic digestion and used them to develop this model [74, 75]. In other research, orthotopically, scientists developed CRC PDX models with endogenous metastases that can migrate to the lungs and liver similar to a patient’s primary tumor. Subcutaneous engraftment does not metastasize to other organs [74, 76, 77]. PDX models with NOD/SCID mice have the highest engraftment rate in this cancer [9]. PDX CRC models maintain important gene mutations such as KRAS, BRAF (v-Raf murine sarcoma viral oncogene homolog B), PIK3CA (Phosphatidylinositol-4, 5-bisphosphate 3-kinase), gene expression, copy number changes, and microsatellite instability of the primary tumor [78, 79]. Almost all studies on preserving the stromal structure, histological differentiation, and histopathological subtypes by these models are in agreement [74, 76]. PDX model of colorectal cancer in the evaluation of response rates of drugs such as cetuximab and other systemic chemotherapeutic agents [76, 79, 80] as well as the cell line production [81, 82], drug and biomarker discovery [83, 84], further understanding of tumor biology [83] and colosphere production [85] have had broad applications.
Prostate cancer
Prostate cancer PDX models have been introduced as models with molecular diversity and cellular heterogeneity that have similar histology compared to primary tumors [86, 87]. Prostate cancer PDX models can be used to evaluate the efficacy of anti-cancer drugs [88]. However, the transplantation rate of human prostate cancer was low, and only advanced and high-growth cancerous tissue was successfully transplanted [89]. The sub renal capsule (SRC) site has a high transplantation rate because it has highly vascularized potential than the subcutaneous site for primary human prostate cancer; however, orthotropic models have shown the best expression of androgen receptor and Prostate specific antigen (PSA) [90, 91]. Another approach recently considered to increase the rate of transplantation is the use of CTCs, which have been used to produce various cancers, including the PDX prostate model. In this type of PDX model, there is no need for surgery to remove the tumor tissue [92]. The prostate tumor take rate is low 10–20% and requires Supplementing mice with a specific dose of exogenous androgens [87]. Different PDX models of prostate cancer for performing pre-clinical studies and evaluating biological processes such as identification of pluripotent stem cells [93], angiogenesis [94], response to androgen ablation therapies [95], and Chromosomal abnormalities [96] have been created over the years.
Gastric cancer
Gastric cancer cell lines and animal models derived cell lines were used for drug screening; although these models had advantages, most importantly, the low predictive value of these models for clinical outcomes makes them inappropriate [97, 98]. The primary way to overcome these limitations is to use PDX that allows the transplantation of a patient’s tumor tissue into immunocompromised mice to form a population of mice with a tumor of the same molecular pattern. Suitable for evaluating targeted and personalized therapies [37, 80]. The most important achievement of an ideal preclinical model is preserving the genetic and histological features of the patient’s tumor, which are kept in the PDX model of gastric cancer for several generations [99–101]. Various factors have contributed to increasing the success rate of PDX modeling, including engraftment site [28, 37] using Matrigel [102] and type of immunocompromised mice [37, 103]. However, the diffuse type of gastric cancer is reported to have a low engraftment rate due to insufficient tumor cells [104]. Epstein bar virus (EBV)-related B-cell lymphomas infect approximately 68% of PDX tumor models [102, 105]. These are due to the presence of EBV in the patient's primary transplanted B cells, which are activated in the body of immunocompromised mice and cause lymphoma instead of the tumor [106]. PDX models have helped to improve gastric cancer therapies in recent years. Because of their in vivo benefits, and have been used in numerous pre-clinical trials to evaluate treatment response and identify biomarkers [38].
Any challenges?
It seems animal models are still more available and also reliable with known challenges. Avatar or PDX models have high predictive power and are widely used in drug screening. Still, the long-time tumor latency (4–6 months) is perhaps the most prominent weakness from a personalized medicine perspective. Another limitation of these models is the different take rates in various cancers and hosts. For example, as discussed above, developing breast cancer models is more challenging than other cancers [17, 107]. PDX models are a valuable tool in oncology research and drug evaluation, but they are costly and require equipped laboratories. Another challenge we face in these models is to create an acceptable metastatic model. Usually, two approaches are used to develop metastasis. First, the tumor is grafted heterotopically or orthotopically, but in the second approach, patient-derived tumor cells are injected into mice through the tail vein. Both methods have limitations besides advantages. For example, in the first approach, spontaneous metastasis takes a long time. The rate of metastasis is low, on the other hand, by injecting tumor cells, despite the increase in metastasis rate. Still, we are dealing with an unrealistic heterogeneity, and in fact, most cells are trapped in the lungs rather than metastasizing to the lungs [108].
PDX models are also not suitable for early-stage cancers because of their low take rate. Due to the host with immunodeficiency, it is impossible to evaluate the effect of the immune system on the tumor [22]. This limitation makes it impossible to assess the relationship between the therapeutics cells and the host immune cells (such as Tregs and MDSCs) in cell-based therapies [60]. However, with the advent of Humanized models, this limitation has been partially removed.
Furthermore, there are epigenetic alterations of tumors among hierarchical generations, new mutations, and angiogenesis system of the host, which feds the esurient tumor cells are limitations. As Todd Glub, head of the cancer program at the Broad Institute, and his colleagues analyzed, the median of 12% of the genome had been affected by the fourth passage [109]. Of course, genetic alterations or chromosome abrasions are inevitable, but more efforts should be made to investigate how much these alterations are functional and will affect the outcome. However, the PDX model is essential option for tumor simulation. These limitations can be overcome by introducing a new generation of immunodeficient mice or efficient methods such as orthotopic cancer models and Humanized mice; humanized mice are models with little or without immune system which are injected with human stem cells derived from cord blood or fetal tissue. In these models human immune system included T cells, B cells and other cells will be created and enables scientist to explore human immune system function directly, but again the angiogenesis system will remain unresolved; as the vascular tissues of the tumor will be replaced by mouse angiogenesis system gradually during different passages [110]. Patient-derived tumor organoids (PDTOs) have been suggested as preclinical models that demonstrated higher success rate and ease of use, however they will not be able to preserve tumor heterogeneity. It seems more opportunities will be obtained in precision oncology era by integration of PDX and PDTO models [111].
When using PDX models, it should be noted that a transplanted tumor can trigger an immune response against the host that is called Graft Versus Host Diseases (GVHD), which can be delayed by using NSG mice without Major Histocompatibility Complex-I (MHC-I) [71, 112]. Another challenge that can be mentioned is Tumor Infiltration Lymphocyte (TILs) in the primary tumor, which may transform the tumor into lymphoma [113]. Post-Transplant Lymphoproliferative Disorder (PTLD) can be another challenge that may arise in creating PDX models. Choi et al., showed that NOG mice had a higher rate of EBV-related B-cell lymphoma development owing to their immunodeficiency. In contrast, lymphoma did not survive in nude mice, which could be due to Natural Killer cells in these mice [114]. If tumor tissue is transplanted to nude mice in the first generation, we will not have lymphoma in subsequent generations, even if using mice with maximal immune deficiency such as NOG, NSG [115, 116]. Rituximab can also be used as a treatment for lymphoma to inhibit its growth in PDX models without affecting the rate of cancer engraftment [36].
In recent years, many attempts have been made to provide in vitro models, including organoids and microfluidics, which will reduce the use of animals according to the principle of reduction from Replacement, Reduction, and Refinement (3R) rules. However, they have a long way to go to become a reliable model. PDX model centers are required to heed the notices and rules of ethics committees and animal rights organizations. They have introduced laws and regulations, which will not impede the development of PDX models but it will bring pacification to the animals.
Conclusion
PDX models are among the most reliable and standard models in preclinical studies about a century after the first tumor model. Although these models, like other models, have limitations, they have many applications in the development of anti-cancer drugs, cancer biology, co-clinical trials, personalized medicine, and immunotherapy because of maintaining primary tumor characteristics. Therefore, many countries and institutes have begun to create PDX biobanks, and PDX libraries and pharmaceutical companies spend a lot of money to produce this source.
Acknowledgements
Not applicable.
Abbreviations
- PDX
Patient derived xenograft
- TECs
Tumor endothelial cells
- CAFs
Cancer-associated fibroblasts
- TAMs
Tumor-associated macrophages
- ECM
Extra Cellular Matrix
- FBS
Fetal bovine serum
- PCTs
PDX clinical trials
- CSCs
Cancer stem cells
- GEMM
Genetically engineered mouse model
- MDSCs
Myeloid-derived suppressor cells
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- CAR-T
Chimeric antigen receptor
- NSCLC
Non-small-cell lung carcinoma
- Her2
Epidermal growth factor receptor 2
- CRC
Colorectal cancer
- KRAS
Kirsten rat sarcoma virus
- BRAF
V-Raf murine sarcoma viral oncogene homolog B
- PIK3CA
Phosphatidylinositol-4, 5-bisphosphate 3-kinase
- SRC
The sub renal capsule
- CTC
Circulating tumor cells
- PSA
Prostate specific antigen
- MHC
Major histocompatibility complex
- EBV
Epstein bar virus
- GVHD
Graft versus host diseases
- TILs
Tumor infiltration lymphocyte
- PTLD
Post-transplant lymphoproliferative disorder
- 3Rs
Replacement, Reduction, and Refinement
Author contributions
SA and ZG have written the introduction, methodology, and some applications of PDX models (PDX clinical trial, Cancer biology, personalized medicine). SM and MS figures and tables and introduced the PDX models development. MAA and KB evaluated PDX in various cancer types. SM have written some of the applications of PDX models (co-clinical trials, immunotherapy), challenges and conclusions. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shahrokh Abdolahi and Zeinab Ghazvinian contributed equally to this work
References
- 1.Pott P. Chirurgical observations relative to the cataract, the polypus of the nose, the cancer of the scrotum, the different kinds of ruptures, and the mortification of the toes and feet, by Percivall Pott, FRS Surgeon to St. Bartholomew’s-Hospital. Printed, by TJ Carnegy, For L. Hawes, W. Clarke, and R. Collins, in Pater-noster Row; 1775.
- 2.Hedrich HJ. The house mouse as a laboratory model: a historical perspective. The Laboratory Mouse. Burlington: Elsevier Science; 2012. p. 868. [Google Scholar]
- 3.Johnson J, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis LM, Fidler IJ. Finding the tumor copycat: therapy fails, patients don’t. Nat Med. 2010;16(9):974–975. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- 5.Rezaee R, Abdollahi M. The importance of translatability in drug discovery. Expert Opin Drug Discov. 2017;12(3):237–239. doi: 10.1080/17460441.2017.1281245. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson L, Kirk R. High drug attrition rates—where are we going wrong? Nat Rev Clin Oncol. 2011;8(4):189–190. doi: 10.1038/nrclinonc.2011.34. [DOI] [PubMed] [Google Scholar]
- 7.Ajith A, et al. Humanized mouse model as a novel approach in the assessment of human allogeneic responses in organ transplantation. Front Immunol. 2021;12:2301. doi: 10.3389/fimmu.2021.687715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izumchenko E, et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28(10):2595–2605. doi: 10.1093/annonc/mdx416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown KM, et al. Patient-derived xenograft models of colorectal cancer in pre-clinical research: a systematic review. Oncotarget. 2016;7(40):66212. doi: 10.18632/oncotarget.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledford H. US cancer institute overhauls cell lines: veteran cells to be replaced by human tumours grown in mice. Nature. 2016;530(7591):391–392. doi: 10.1038/nature.2016.19364. [DOI] [PubMed] [Google Scholar]
- 11.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241(3):313–315. doi: 10.1002/path.4824. [DOI] [PubMed] [Google Scholar]
- 13.Lu P, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13(1):4. doi: 10.1186/s13045-019-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto T. Patient-derived tumor xenograft models: toward the establishment of precision cancer medicine. J Pers Med. 2020;10(3):64. doi: 10.3390/jpm10030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tentler JJ, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019;8(8):889. doi: 10.3390/cells8080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murayama T, Gotoh N. Patient-derived xenograft models of breast cancer and their application. Cells. 2019;8(6):621. doi: 10.3390/cells8060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrolecki LE, et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2016;35(4):547–573. doi: 10.1007/s10555-016-9653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Subrenal capsule grafting technology in human cancer modeling and translational cancer research. Differentiation. 2016;91(4–5):15–19. doi: 10.1016/j.diff.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Behbod F, et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009;11(5):R66. doi: 10.1186/bcr2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin K, et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12(7):473–480. doi: 10.1007/s12094-010-0540-6. [DOI] [PubMed] [Google Scholar]
- 23.Gock M, et al. Tumor take rate optimization for colorectal carcinoma patient-derived xenograft models. BioMed Res Int. 2016 doi: 10.1155/2016/1715053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao L-C, et al. Target identification and validation in drug discovery. Springer; 2019. Creation of pdx-bearing humanized mice to study immuno-oncology; pp. 241–252. [DOI] [PubMed] [Google Scholar]
- 25.John T, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011;17(1):134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 26.Merk J, et al. Patient-derived xenografts of non-small-cell lung cancer: a pre-clinical model to evaluate adjuvant chemotherapy? Eur J Cardiothorac Surg. 2009;36(3):454–459. doi: 10.1016/j.ejcts.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Simpson-Abelson MR, et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rγnull mice. J Immunol. 2008;180(10):7009–7018. doi: 10.4049/jimmunol.180.10.7009. [DOI] [PubMed] [Google Scholar]
- 28.Fichtner I, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14(20):6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 29.Razmara AM, et al. Tumor shedding and metastatic progression after tumor excision in patient-derived orthotopic xenograft models of triple-negative breast cancer. Clin Exp Metas. 2020;37(3):413–424. doi: 10.1007/s10585-020-10033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huo K-G, D’Arcangelo E, Tsao M-S. Patient-derived cell line, xenograft and organoid models in lung cancer therapy. Transl Lung Cancer Res. 2020;9(5):2214. doi: 10.21037/tlcr-20-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong KSM, Her Z, Chen Q. Humanized mice as unique tools for human-specific studies. Arch Immunol Ther Exp. 2018;66(4):245–266. doi: 10.1007/s00005-018-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu W, Zheng J. Suppression and regulation of immune responses. New York: Springer; 2016. Application of humanized mice in immunological research; pp. 157–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De La Rochere P, et al. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39(9):748–763. doi: 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Gao H, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 35.Oh D-Y, Bang Y-J. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 36.Corso S, et al. Rituximab treatment prevents lymphoma onset in gastric cancer patient-derived xenografts. Neoplasia. 2018;20(5):443–455. doi: 10.1016/j.neo.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo M, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne AT, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 39.Evrard YA, et al. The National Cancer Institute’s patient-derived models repository (PDMR). AACR; 2017.
- 40.Evrard YA, et al. The National Cancer Institute’s patient-derived models repository (PDMR). In: Cancer research. Philadelphia: AMER Assoc Cancer Research; 2018.
- 41.Thakur MD, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494(7436):251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maru Y, Hippo Y. Current status of patient-derived ovarian cancer models. Cells. 2019;8(5):505. doi: 10.3390/cells8050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schatton T, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nardella C, et al. The APL paradigm and the “co-clinical trial” project. AACR; 2011. [DOI] [PMC free article] [PubMed]
- 45.Clohessy JG, Pandolfi PP. Mouse hospital and co-clinical trial project—from bench to bedside. Nat Rev Clin Oncol. 2015;12(8):491. doi: 10.1038/nrclinonc.2015.62. [DOI] [PubMed] [Google Scholar]
- 46.Nanni P, et al. Bone sarcoma patient-derived xenografts are faithful and stable preclinical models for molecular and therapeutic investigations. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-48634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauli C, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 2014;344(1):1–12. doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopetz S, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33(34):4032. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67(10):1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanmamed MF, et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2−/− IL2Rγnull immunodeficient mice. Can Res. 2015;75(17):3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 52.Stecklum M, et al. Abstract B126: correlation of tumor growth inhibition by check point inhibitors with PD-L1 expression in preclinical patient derived xenograft (PDX) models. AACR; 2016.
- 53.Sobarzo A, et al. A36 patient-specific humanized PDX model for overcoming tumor resistance to immune checkpoint inhibitors in NSCLC patients. J Thorac Oncol. 2020;15(2):S24. doi: 10.1016/j.jtho.2019.12.065. [DOI] [Google Scholar]
- 54.Helleday T. Using personalized immune-humanized xenograft mouse models to predict immune checkpoint responses in malignant melanoma: potential and hurdles. Ann Oncol. 2020;31(2):167–168. doi: 10.1016/j.annonc.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Can Res. 2010;70(22):9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mhaidly R, Verhoeyen E. Humanized mice are precious tools for preclinical evaluation of CAR T and CAR NK cell therapies. Cancers. 2020;12(7):1915. doi: 10.3390/cancers12071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, et al. Efficient growth suppression in pancreatic cancer PDX model by fully human anti-mesothelin CAR-T cells. Protein Cell. 2017;8(12):926–931. doi: 10.1007/s13238-017-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Garcia A, et al. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01109/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ny L, et al. Supporting clinical decision making in advanced melanoma by preclinical testing in personalized immune-humanized xenograft mouse models. Ann Oncol. 2020;31(2):266–273. doi: 10.1016/j.annonc.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Walsh NC, et al. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puchalapalli M, et al. NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (nude) mice. PLoS ONE. 2016;11(9):e0163521. doi: 10.1371/journal.pone.0163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int J Cancer. 2017;140(3):662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 63.Ilie M, et al. Setting up a wide panel of patient-derived tumor xenografts of non–small cell lung cancer by improving the preanalytical steps. Cancer Med. 2015;4(2):201–211. doi: 10.1002/cam4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 65.Cutz J-C, et al. Establishment in severe combined immunodeficiency mice of subrenal capsule xenografts and transplantable tumor lines from a variety of primary human lung cancers: potential models for studying tumor progression-related changes. Clin Cancer Res. 2006;12(13):4043–4054. doi: 10.1158/1078-0432.CCR-06-0252. [DOI] [PubMed] [Google Scholar]
- 66.Nik-Zainal S, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holen I, et al. In vivo models in breast cancer research: progress, challenges and future directions. Dis Model Mech. 2017;10(4):359–371. doi: 10.1242/dmm.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Plater L, et al. Establishment and characterisation of a new breast cancer xenograft obtained from a woman carrying a germline BRCA2 mutation. Br J Cancer. 2010;103(8):1192–1200. doi: 10.1038/sj.bjc.6605900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeRose YS, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eirew P, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518(7539):422. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cassidy JW, Caldas C, Bruna A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Can Res. 2015;75(15):2963–2968. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee W-S, et al. Genomic profiling of patient-derived colon cancer xenograft models. Medicine. 2014;93(28):e298. doi: 10.1097/MD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, et al. Establishing metastatic patient-derived xenograft model for colorectal cancer. Jpn J Clin Oncol. 2020;50(10):1108–1116. doi: 10.1093/jjco/hyaa089. [DOI] [PubMed] [Google Scholar]
- 74.Puig I, et al. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res. 2013;19(24):6787–6801. doi: 10.1158/1078-0432.CCR-12-1740. [DOI] [PubMed] [Google Scholar]
- 75.Chou J, et al. Phenotypic and transcriptional fidelity of patient-derived colon cancer xenografts in immune-deficient mice. PLoS ONE. 2013;8(11):e79874. doi: 10.1371/journal.pone.0079874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Julien S, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314–5328. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 77.Day C-P, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163(1):39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho YB, et al. Colorectal cancer patient-derived xenografted tumors maintain characteristic features of the original tumors. J Surg Res. 2014;187(2):502–509. doi: 10.1016/j.jss.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Kim MK, et al. Characterization of an oxaliplatin sensitivity predictor in a preclinical murine model of colorectal cancer. Mol Cancer Ther. 2012;11(7):1500–1509. doi: 10.1158/1535-7163.MCT-11-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertotti A, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 81.Dangles-Marie V, et al. Establishment of human colon cancer cell lines from fresh tumors versus xenografts: comparison of success rate and cell line features. Can Res. 2007;67(1):398–407. doi: 10.1158/0008-5472.CAN-06-0594. [DOI] [PubMed] [Google Scholar]
- 82.Mullins CS, et al. Integrated biobanking and tumor model establishment of human colorectal carcinoma provides excellent tools for preclinical research. Cancers. 2019;11(10):1520. doi: 10.3390/cancers11101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Isella C, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47(4):312. doi: 10.1038/ng.3224. [DOI] [PubMed] [Google Scholar]
- 84.Tian X, et al. 3,3'-Diindolylmethane inhibits patient-derived xenograft colon tumor growth by targeting COX1/2 and ERK1/2. Cancer Lett. 2019;448:20–30. doi: 10.1016/j.canlet.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 85.Weiswald L, et al. A short-term colorectal cancer sphere culture as a relevant tool for human cancer biology investigation. Br J Cancer. 2013;108(8):1720. doi: 10.1038/bjc.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18(19):5160–5162. doi: 10.1158/1078-0432.CCR-12-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen HM, et al. LuCaP prostate cancer patient-derived xenografts reflect the molecular heterogeneity of advanced disease and serve as models for evaluating cancer therapeutics. Prostate. 2017;77(6):654–671. doi: 10.1002/pros.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navone NM, et al. Movember GAP1 PDX project: an international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models. Prostate. 2018;78(16):1262–1282. doi: 10.1002/pros.23701. [DOI] [PubMed] [Google Scholar]
- 89.Lam H-M, Nguyen HM, Corey E. Prostate cancer. New York: Springer; 2018. Generation of prostate cancer patient-derived xenografts to investigate mechanisms of novel treatments and treatment resistance; pp. 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staack A, et al. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate. 2003;54(3):212–219. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate. 2005;64(2):149–159. doi: 10.1002/pros.20225. [DOI] [PubMed] [Google Scholar]
- 92.Vidal SJ, et al. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell. 2015;27(2):223–239. doi: 10.1016/j.ccell.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grisanzio C, et al. Orthotopic xenografts of RCC retain histological, immunophenotypic and genetic features of tumours in patients. J Pathol. 2011;225(2):212–221. doi: 10.1002/path.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Risbridger GP, Lawrence MG. Patient-derived xenograft models of human cancer. Cham: Springer; 2017. Towards best practice in establishing patient-derived xenografts; pp. 11–28. [Google Scholar]
- 95.Steinestel J, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2019;10(41):4213. doi: 10.18632/oncotarget.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laitinen S, et al. Chromosomal aberrations in prostate cancer xenografts detected by comparative genomic hybridization. Genes Chromosom Cancer. 2002;35(1):66–73. doi: 10.1002/gcc.10097. [DOI] [PubMed] [Google Scholar]
- 97.Hausser H-J, Brenner RE. Phenotypic instability of Saos-2 cells in long-term culture. Biochem Biophys Res Commun. 2005;333(1):216–222. doi: 10.1016/j.bbrc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 98.Murphy B, et al. Evaluation of alternative in vivo drug screening methodology: a single mouse analysis. Can Res. 2016;76(19):5798–5809. doi: 10.1158/0008-5472.CAN-16-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keysar SB, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776–790. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sivanand S, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med. 2012;4(137):137ra75. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin K, et al. Establishment of a PDTT xenograft model of gastric carcinoma and its application in personalized therapeutic regimen selection. Hepatogastroenterology. 2011;58(110–111):1814–1822. doi: 10.5754/hge11136. [DOI] [PubMed] [Google Scholar]
- 102.Read M, et al. Intramuscular transplantation improves engraftment rates for esophageal patient-derived tumor xenografts. Ann Surg Oncol. 2016;23(1):305–311. doi: 10.1245/s10434-015-4425-3. [DOI] [PubMed] [Google Scholar]
- 103.Fujii E, et al. Establishment and characterization of in vivo human tumor models in the NOD/SCID/γcnull mouse. Pathol Int. 2008;58(9):559–567. doi: 10.1111/j.1440-1827.2008.02271.x. [DOI] [PubMed] [Google Scholar]
- 104.Zhang T, et al. Patient-derived gastric carcinoma xenograft mouse models faithfully represent human tumor molecular diversity. PLoS ONE. 2015;10(7):e0134493. doi: 10.1371/journal.pone.0134493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang L, et al. The extent of inflammatory infiltration in primary cancer tissues is associated with lymphomagenesis in immunodeficient mice. Sci Rep. 2015;5:9447. doi: 10.1038/srep09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vockerodt M, et al. The Epstein–Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235(2):312–322. doi: 10.1002/path.4459. [DOI] [PubMed] [Google Scholar]
- 107.Petrillo LA, et al. Xenografts faithfully recapitulate breast cancer-specific gene expression patterns of parent primary breast tumors. Breast Cancer Res Treat. 2012;135(3):913–922. doi: 10.1007/s10549-012-2226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sprouffske K, et al. Genetic heterogeneity and clonal evolution during metastasis in breast cancer patient-derived tumor xenograft models. Comput Struct Biotechnol J. 2020;18:323–331. doi: 10.1016/j.csbj.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boehm JS, Golub TR. An ecosystem of cancer cell line factories to support a cancer dependency map. Nat Rev Genet. 2015;16(7):373. doi: 10.1038/nrg3967. [DOI] [PubMed] [Google Scholar]
- 110.Wang M, et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32(3):1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho S-Y. Patient-derived xenografts as compatible models for precision oncology. Lab Anim Res. 2020;36(1):1–11. doi: 10.1186/s42826-020-00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Covassin L, et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2r γnull (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013;174(3):372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalavska K, et al. Lymphoma transformation of tumor infiltrating lymphocytes observed in testicular patient-derived xenograft models. Oncol Rep. 2018;40(6):3593–3602. doi: 10.3892/or.2018.6769. [DOI] [PubMed] [Google Scholar]
- 114.Choi YY, et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci Rep. 2016;6(1):1–12. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wetterauer C, et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate. 2015;75(6):585–592. doi: 10.1002/pros.22939. [DOI] [PubMed] [Google Scholar]
- 116.Choi YY, et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci Rep. 2016;6:22172. doi: 10.1038/srep22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCreery MQ, Balmain A. Chemical carcinogenesis models of cancer: back to the future. Annu Rev Cancer Biol. 2017 doi: 10.1146/annurev-cancerbio-050216-122002. [DOI] [Google Scholar]
- 118.Liu Y, et al. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. 2015;5(5):708. doi: 10.3978/j.issn.2223-4292.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olson B, et al. Mouse models for cancer immunotherapy research. Cancer Discov. 2018;8(11):1358–1365. doi: 10.1158/2159-8290.CD-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharpless NE, DePinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5(9):741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 121.Georges LMC, et al. Cell line derived xenograft mouse models are a suitable in vivo model for studying tumor budding in colorectal cancer. Front Med. 2019;6:139. doi: 10.3389/fmed.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat. 2018;50(1):1. doi: 10.4143/crt.2017.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pearson AT, et al. Patient-derived xenograft (PDX) tumors increase growth rate with time. Oncotarget. 2016;7(7):7993. doi: 10.18632/oncotarget.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu WN, et al. Establishment and characterization of humanized mouse NPC-PDX model for testing immunotherapy. Cancers. 2020;12(4):1025. doi: 10.3390/cancers12041025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marangoni E, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Can Res. 2013;73(15):4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nunes M, et al. Evaluating patient-derived colorectal cancer xenografts as preclinical models by comparison with patient clinical data. Can Res. 2015;75(8):1560–1566. doi: 10.1158/0008-5472.CAN-14-1590. [DOI] [PubMed] [Google Scholar]
- 128.Ricci F, et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Can Res. 2014;74(23):6980–6990. doi: 10.1158/0008-5472.CAN-14-0274. [DOI] [PubMed] [Google Scholar]
- 129.Huynh H, Ong R, Zopf D. Antitumor activity of the multikinase inhibitor regorafenib in patient-derived xenograft models of gastric cancer. J Exp Clin Cancer Res. 2015;34(1):132. doi: 10.1186/s13046-015-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X-C, et al. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med. 2013;11(1):168. doi: 10.1186/1479-5876-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garrido-Laguna I, et al. Tumor engraftment in nude mice and enrichment in stroma-related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17(17):5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nehls M, et al. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372(6501):103. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 133.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood J Am Soc Hematol. 2015;125(17):2630–2640. doi: 10.1182/blood-2014-11-570218. [DOI] [PubMed] [Google Scholar]
- 134.Blunt T, et al. Defective DNA-dependent protein kinase activity is linked to V (D) J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80(5):813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 135.Nonoyama S, et al. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150(9):3817–3824. [PubMed] [Google Scholar]
- 136.Greiner DL, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with CB-17-scid/scid mice. Am J Pathol. 1995;146(4):888. [PMC free article] [PubMed] [Google Scholar]
- 137.Christianson SW, et al. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scidMice and in C57BL/6J-scid bgMice. Cell Immunol. 1996;171(2):186–199. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 138.Shultz LD, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–191. [PubMed] [Google Scholar]
- 139.Pflumio F, et al. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88(10):3731–3740. doi: 10.1182/blood.V88.10.3731.bloodjournal88103731. [DOI] [PubMed] [Google Scholar]
- 140.Richard E, et al. The mammary ducts create a favourable microenvironment for xenografting of luminal and molecular apocrine breast tumours. J Pathol. 2016;240(3):256–261. doi: 10.1002/path.4772. [DOI] [PubMed] [Google Scholar]
- 141.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 142.Jung YJ, et al. Development of a protein biomarker panel to detect non-small-cell lung cancer in Korea. Clin Lung Cancer. 2017;18(2):e99–e107. doi: 10.1016/j.cllc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 143.Morgan KM, et al. Patient-derived xenograft models of non-small cell lung cancer and their potential utility in personalized medicine. Front Oncol. 2017;7:2. doi: 10.3389/fonc.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang Y, et al. Establishment of lung cancer patient-derived xenograft models and primary cell lines for lung cancer study. J Transl Med. 2018;16(1):138. doi: 10.1186/s12967-018-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuwata T, et al. Establishment of novel gastric cancer patient-derived xenografts and cell lines: pathological comparison between primary tumor, patient-derived, and cell-line derived xenografts. Cells. 2019;8(6):585. doi: 10.3390/cells8060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Y, Lin D, Gout PW. Patient-derived xenograft models of human cancer. Cham: Springer; 2017. [Google Scholar]
- 147.Du Manoir S, et al. Breast tumor PDXs are genetically plastic and correspond to a subset of aggressive cancers prone to relapse. Mol Oncol. 2014;8(2):431–443. doi: 10.1016/j.molonc.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.