Abstract
Background
Multiple sclerosis (MS) patients receive immunomodulatory treatments which can influence their ability to maintain vaccine specific serological response overtime. MS patients treated with cladribine tablets developed a positive serology response following two doses of mRNA COVID-19 vaccine. However, there is only limited data regarding the effect of cladribine tablets on long-term humoral response after the second and the third booster.
Methods
Serology response to SARS-CoV-2 was tested in healthy controls (HCs) and MS patients treated with cladribine tablets 6 and 9–12 months after the second dose, and 1 and 3–6 months following the third booster-dose of the BTN162b2 mRNA vaccine.
Results
Thirty-five out of 36 MS patients treated with cladribine tablets and 100% (46/46) of HCs had a positive serology response up to 10 months after the second vaccine dose. In addition, all cladribine tablets -treated MS patients (22/22) and HCs (24/24) had a positive robust serology response following the third vaccine with a positive humoral response sustain up to 6 months. One month after the third vaccine dose IgG levels were significantly lower in patients treated with cladribine tablets compared to HCs (15,598+11,313 vs 26,394+11,335, p<0.01). Six-month post second vaccine and 3–6 months post third vaccine there was no difference in IgG levels between the groups (1088.0 ± 1072.0 vs 1153.0 ± 997.1, p = 0.79; 5234+4097 vs 11,198+14,679, p = 0.4).
Conclusion and relevance
MS patients treated with cladribine tablets have sustained positive vaccine specific serology response following the second and third SARS-CoV-2 vaccine dose.
Keywords: Multiple sclerosis, Cladribine tablets, COVID-19 vaccination, Long term serology response
Abbreviations: COVID-19, Coronavirus disease; MS, Multiple Sclerosis; DMTs, disease-modifying treatments; RBD, Receptor Binding Domain; EDSS, Expanded Disability Status Scale
1. Introduction
The resurgence of corona virus disease (COVID-19) worldwide despite the fact that high percentages of the population have been vaccinated with two doses of mRNA vaccine, along with increase data of waning vaccine immunity of the mRNA vaccines over time, has led to the recommendation of a third vaccine dose (Mizrahi et al., 2021; Goldberg et al., 2021). The third dose of mRNA vaccines has been shown to elicit a strong humoral response (Patalon et al., 2022; Gilboa et al., 2021; Eliakim-Raz et al., 2021).
COVID-19 vaccines have been reported to be safe for multiple sclerosis (MS) patients, with no evidence of increased risk of relapse (Achiron et al., February 2021; Dreyer-Alster et al., 2022; König et al., 2022). Patients with MS are treated with various disease-modifying treatments (DMTs) that affect different levels of the immune system and may affect the humoral immune response to COVID-19 vaccines (Kalincik et al., 2021).
Cladribine tablets (Mavenclad), a purine analogue selectively target lymphocytes and causes marked reduction of B cells and a modest reduction in T and NK cells (Beutler, 1992; Baker et al., 2017; Stuve et al., 2019). We and others have shown that MS patients treated with cladribine tablets develop a humoral response to two doses of the mRNA SARS-CoV-2 vaccines (Capone et al., 2021; Brill et al., 2021; Tortorella et al., 2022). However, only a few studies have examined the effect of cladribine tablets on a long-term humoral response after the second vaccination and data on the response following the third booster vaccine dose is limited (Achiron et al., 2021).
In the current work, we present a study assessing the serology response of MS patients treated with cladribine tablets, 6 and 9–12 months after the second vaccine dose as well as following the third vaccine dose.
2. Methods
2.1. Ethical considerations
The Hadassah and Rambam Medical Organization Ethics Committees approved this study. All patients provided written informed consent (975-20-HMO, 0188-21-RMB).
2.2. Patients
Patients with MS treated with cladribine tablets who received two or three doses of COVID-19 mRNA vaccine (BTN162b2 Pfizer/BioNTech) were included. None of the patients were infected with COVID-19 until 30.11.2021 (Before the omicron variant outbreak in Israel). Patients who were pregnant, breastfeeding, or diagnosed with another autoimmune disease were excluded from the study.
The study cohort include 44 MS patients treated with cladribine tablets (Female:Male = 33:11, average age 39.45±12.88 years, EDSS 2.88±1.98, average disease duration 8.24±7.44 years, and lymphocytes count 1.35±0.86) and forty-six HCs. Thirty-six patients with MS treated with cladribine tablets and 46 HCs provided blood samples 6 months following the second vaccine dose, 5 cladribine tablets treated MS patients gave blood 9–12 months following the second vaccine. Blood samples were analyzed 1 month following the third vaccine in 17 cladribine treated MS patients and 24 HCs, and from 5 cladribine tablets treated patients and 8 HCs, 3–6 months following the third vaccine dose.
2.3. Study design and serology
Enrolled patients provided a blood sample 6 and/or 9–12 months after the second COVID-19 mRNA vaccine, and 1 and 3–6 months after the third vaccine. Serology response to SARS-CoV-2 Spike Receptor Binding Domain (RBD) was measured using the Architect SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics). A positive response was defined by an IgG titer of ≥50 arbitrary units (AU/ml).
2.4. Statistical analysis
The Kolmogorov–Smirnov test was used to assess the distribution of all parameters considered in this study. A student t-test was performed to measure differences in serology response between the HCs and MS patients treated with cladribine tablets. Correlations between demographic and clinical variables and serology response were assessed by Pearson's correlation. Differences were considered significant when P <0.05.
3. Results
3.1. Long-term serology response after the second vaccine dose
In order to find the long-term vaccine specific serology response in MS patients treated with cladribine tablets we analyzed blood samples 6 and 9–12 months following the second vaccine dose. This study follows our recent report on serology responses 2–3 weeks following second vaccine in patients treated with cladribine tablets (Brill et al., 2021). Thirty-five out of 36 MS patients treated with cladribine tablets and 100% (46/46) of HCs had a positive serology response 6 months after the second vaccine dose. No difference was found between SARS-CoV-2-IgG titer of HCs and cladribine tablets-treated MS patients 6 months following the second vaccine (1153.0 ± 997.1 vs 1088.0 ± 1072.0, p = 0.79). All additional 5 samples from cladribine tablets treated patients obtained 9–12 months following the second vaccine had positive serology response (612.40±316.82) (Fig. 1 ). In both HCs and MS patients treated with cladribine tablets, IgG titers significantly decreased 6 months following the second vaccine dose (compared to 1 month following second dose), however between the groups, the decline was not significantly different (Δ 13,552±8573 vs Δ 12,029±12,260, p = 0.62, supplement figure 1).
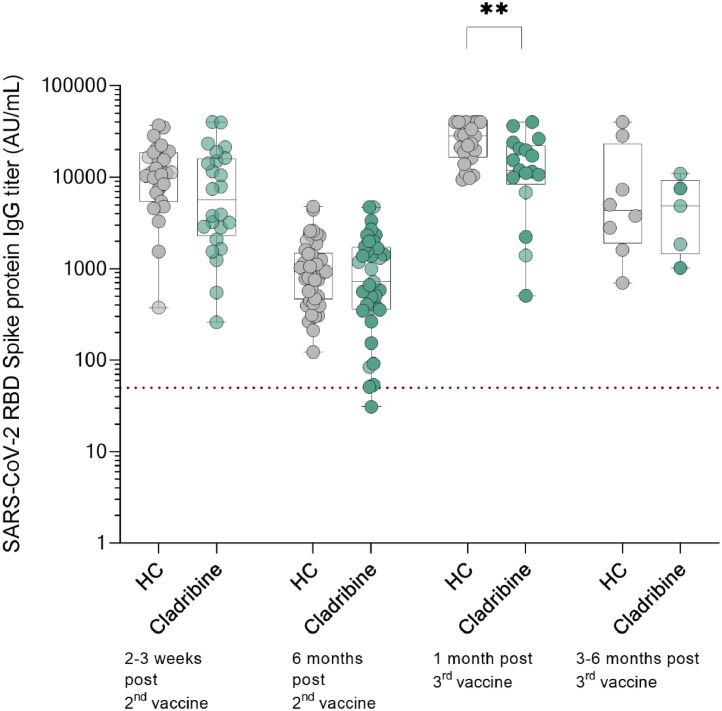
Fig. 1.
Longitudinal serology response to SARS-CoV-2 mRNA vaccine of cladribine treated MS patients and HCs.Fig. 1 Serology response of HCs and cladribine tablets-treated MS patients following the 2nd and 3rd mRNA vaccine dose on logarithmic amplitude scale. 2–3 weeks (n = 31, 13,003±8893AU/ml vs n = 21, 11,135±12,029, p = 0.52), and 6 months (n = 46, 1153.0 ± 997.1 vs n = 36, 1088.0 ± 1072.0, p = 0.79) following the 2nd vaccine, and 1 month (n = 24, 26,394±11,335 vs n = 17, 15,598±11,313, p = 0.40) and 3–6 months (n = 8, 11,198±14,679 vs n = 5, 5234±4097, p = 0.40) following the 3rd vaccine. Data presented as mean±SD. Dotted line indicates positive threshold (≥50 AU/ml).
No correlation was found between SARS-CoV-2 IgG levels 6 months following the second vaccine and lymphocytes count (r = 0.03, p = 0.86), EDSS (r=−0.11, p = 0.51), age (r=−0.03, p = 0.88), disease duration (r=−0.15, p = 0.4), and the time between last cladribine dose and vaccination (r = 0.15, p = 0.49). In addition, no difference was found between patients who received one or two courses of cladribine tablets prior to vaccination (835 ± 825.5 vs 1275 ± 1285, p = 0.4).
3.2. Serology response following the third vaccine dose
Following the third vaccine dose all participants, 22 MS patients treated with cladribine tablets and 24 HCs, had positive serology response. IgG levels of cladribine tablets-treated MS patients were significantly lower compared to HCs in samples obtained 1 month after the third vaccine dose (15,598±11,313 vs 26,394±11,335, p<0.01), but not 3 to 6 months following the third vaccine (11,198±14,679 vs 5234±4097, p = 0.4). All study participants had significantly increased IgG titers 1 month after the third vaccine compared to the IgG levels 6 months after the second vaccine (Δ 26,095.28±10,973.84 vs Δ 11,903.88±11,741, p = 0.001 Supplementary figure 2).
All HCs were vaccinated with the third dose 5–6 months after the second dose. Out of 17 MS patients treated with cladribine tablets, 6 were vaccinated 5–6 month following the second dose and 11 were vaccinated 7–12 months following the second dose. Average IgG titer in patients that were vaccinated more than 7 months after the second dose were lower, but the difference was not statistically significant (19,079±10,461 vs 13,699±11,782, p = 0.37, supplementary figure 3).
No correlation was found between IgG titers 1 months following the third vaccine dose and lymphocytes count (r=−0.27,p = 0.35), EDSS (r = 0.22,p = 0.40) age (r = 0.09, p = 0.74), disease duration (r = 0.13, p = 0.63), duration between last cladribine tablets dose and third vaccine (r=−0.21, p = 0.44), time between the second vaccine and third vaccine (r=−0.16, p = 0.61) and between patients who received two courses of cladribine tablets before the third vaccine to patients with only one course of cladribine tablets (12,804±11,443 vs 15,986±11,658, p = 0.56).
4. Discussion
A national third-dose vaccination campaign was initiated in Israel on August 1, 2021. To date, a third vaccine is recommended in many countries. Several groups have demonstrated a reduction in the odds of SARS-CoV-2 infection and hospitalization within a few weeks of receiving the third vaccine compared with receiving the 2 primary doses (Patalon et al., 2022; Barda et al., 2021; Thompson et al., 2022). According to a number of studies, serology response of healthy individuals following the third vaccine is greater compared to the second vaccine booster (Gilboa et al., 2021; Eliakim-Raz et al., 2021).
In the current study, we describe longitudinal serology response in MS patients treated with cladribine tablets at 6 and 9–12 months after the second dose as well as at 1 and 3–6 months after the third dose of the BNT162b2 mRNA vaccine. Our results show that 97% of MS patients treated with cladribine tablets have a positive serology response following 6 months and up to 12 months after the second dose. In addition, in all participants, the third vaccine results in a robust increased serological response; however, the average response in MS patients treated with cladribine tablets is lower than that of HCs. We did not find a correlation between IgG levels and age, EDSS, lymphocytes count, disease duration, and duration between last cladribine dose and vaccination. Also, no difference was found between patients who received one or two courses of cladribine tablets before the third vaccine dose.
To date, several reports show that the majority of MS patients treated with cladribine tablets have positive serology response after receiving two doses of the mRNA vaccine. Nevertheless, it is still unclear whether IgG levels are comparable to HCs (Capone et al., 2021; Brill et al., 2021; Tortorella et al., 2022; Grothe et al., 2021; Maniscalco et al., 2022).
The protection of the mRNA vaccines against COVID-19 fades after several months (Mizrahi et al., 2021; Goldberg et al., 2021). Goldberg et al., showed that the risk for infection was significantly higher for participants that were vaccinated 6 months before the study, compared to those who were vaccinated later (Goldberg et al., 2021). This parallels the known decrease in IgG levels 6 months following a second vaccination and infection (Zhong et al., 2021; Shrotri et al., 2021; Seow et al., 2020). In addition, 5–6 months following the second vaccine there is a significant overall decrease at memory B-cells and neutralizing antibodies, however T cell responses are preserved (Tarke et al., 2022). Of note is that it is still unclear what is cutoff IgG level that would provide protection against infection or from severe COVID-19 disease course (Islamoglu et al., 2021). In addition, the cross-sectional natures of the vaccine response studies to date do not reach consensus about the best timing of vaccines and effects of boosters or third doses to maximize humoral and cellular responses (Rieckmann et al., 2021).
Data on the long-term durability of the serology response of MS patients treated with cladribine tablets is scarce. We found that up to 12 months following the second and up to 6 months after the third vaccine dose, both HCs and MS patients treated with cladribine tablets have a positive serology response.
The humoral response of patients treated with cladribine tablets was significantly lower 1 month following the third vaccine dose but not 6 months post second vaccine dose and 3–6 months after the third vaccination. Timing of the vaccine is known to affect humoral response (Moghadas et al., 2021). While all HCs were vaccinated with the third dose 5–6 months after the second dose, in the group of MS patients treated with cladribine tablets there was a wider range between the two doses. Patients that were vaccinated with the third dose more than 7 month after the second, had lower IgG titers in average. Further, larger MS cohort studies may provide insight on the cause of the difference between HCs and cladribine tablets treated patients.
5. Conclusion
In conclusion, we found that MS patients treated with cladribine tablets have sustained positive vaccine specific serology response following the second and third vaccine dose of the BNT162b2 mRNA vaccine.
Conflict of Interest
A. Vaknin-Dembinsky has served on scientific advisory boards for F. Hoffmann-La Roche, Biogen, Sanofi- Aventis and the healthcare business of Merck KGaA, Darmstadt, Germany and has received grants from F. Hoffmann-La Roche, Biogen and the healthcare business of Merck KGaA, Darmstadt, Germany.
Funding
This work was partially supported by an investigator-initiated study grant by Merck Serono Ltd., Herzliya, Israel, an affiliate of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). (Grant number MS700568_0167)
CRediT authorship contribution statement
Livnat Brill: Conceptualization, Validation, Investigation, Writing – original draft. Ariel Rechtman: Conceptualization, Validation, Investigation, Writing – original draft. Alla Shifrin: Resources, Writing – review & editing. Ayal Rozenberg: Resources, Writing – review & editing. Svetlana Afanasiev: Resources, Writing – review & editing. Omri Zveik: Investigation, Writing – review & editing. Nitzan Haham: Investigation, Writing – review & editing. Neta Levin: Resources, Writing – review & editing. Adi Vaknin-Dembinsky: Conceptualization, Validation, Resources, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A. Vaknin-Dembinsky has served on scientific advisory boards for F. Hoffmann-La Roche, Biogen, Sanofi- Aventis and the healthcare business of Merck KGaA, Darmstadt, Germany and; has received grants from F. Hoffmann-La Roche, Biogen and the healthcare business of Merck KGaA, Darmstadt, Germany.
Footnotes
This work was partially supported by an investigator-initiated grant by Merck KGaA, Darmstadt, Germany
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103863.
Appendix. Supplementary materials
References
- Achiron A., et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron, A. et al. COVID-19 vaccination in patients with multiple sclerosis : what we have learnt by February 2021. 1–7 (2021) doi:10.1177/13524585211003476. [DOI] [PMC free article] [PubMed]
- Baker D., et al. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol. Neuroimmunol. NeuroInflamm. 2017;4:1–10. doi: 10.1212/NXI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda N., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Cladribine (2-chlorodeoxyadenosine) Lancet. 1992;340:952–956. doi: 10.1016/0140-6736(92)92826-2. [DOI] [PubMed] [Google Scholar]
- Brill L., et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2021;103343 doi: 10.1016/j.msard.2021.103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone F., et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics. 2021 doi: 10.1007/s13311-021-01165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer-Alster S., et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliakim-Raz N., et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326:2203. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa M., et al. Early immunogenicity and safety of the third dose of BNT162b2 messenger RNA coronavirus disease 2019 vaccine among adults older than 60 years: real-world experience. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab584. [DOI] [PubMed] [Google Scholar]
- Goldberg Y., et al. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe C., Steffen F., Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J. Cent. Nerv. Syst. Dis. 2021;13 doi: 10.1177/11795735211060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islamoglu M.S., et al. Relationship between antibody levels and SARS-Cov-2 reinfection. Ann. Clin. Lab. Sci. 2021;51:750–755. [PubMed] [Google Scholar]
- König M., et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022 doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalincik T., et al. Effect of disease-modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology. 2021;96:e783–e797. doi: 10.1212/WNL.0000000000011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., et al. Interferon Beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects. Mult. Scler. Relat. Disord. 2022;58 doi: 10.1016/j.msard.2021.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi B., et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021;12:6379. doi: 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas S.M., et al. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLOS Biol. 2021;19 doi: 10.1371/journal.pbio.3001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patalon T., et al. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern. Med. 2022;182:179. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P., et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211058298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow J., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotri M., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O., et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022 doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.G., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION network, 10 States, August 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C., et al. Humoral- and T-cell–specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D., et al. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA. 2021;326:2524. doi: 10.1001/jama.2021.19996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.