Abstract
The synthesis of inorganic rod shape nanostructures is important in chromatography, dentistry, and medical applications such as bone implants, and drug and gene delivery systems. Herein, calcium carbonate (CaCO3) nanowires were synthesized using a plant extract and the ensuing nanoparticles were characterized by XRD, FESEM, and HR-TEM. Then, the leishmanicidal effects of biogenic calcium carbonate nanowires were investigated against Leishmania major including the toxicity of varying concentrations of nanoparticles, and the percentage of viable and apoptotic cells based on flow cytometry analysis. Based on the results, the IC50 of these polymorphs were calculated to be 800 μg mL−1. An ecofriendly, inexpensive, and novel biogenic method for the production of a new advanced inorganic nanostructure, CaCO3 nanowires, is described without using hazardous chemicals; calcium carbonate nanowires maybe used as a smart drug carrier.
The synthesis of inorganic rod shape nanostructures is important in chromatography, dentistry, and medical applications such as bone implants, and drug and gene delivery systems.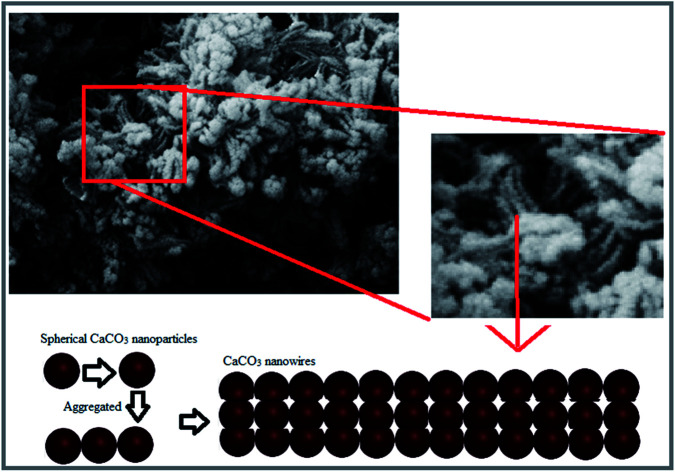
1. Introduction
In recent years, scientists have been scrutinizing the anti-parasitic properties of nanostructures and special effort has been made to treat common human and animal diseases.1,2 In view of the universal spread of leishmaniasis, and its drug resistance, novel therapeutic nano-medicine approaches are of particular significance.3,4 So far, various nanoparticles such as ZnO,5 TiO2,6 and Au7 have been studied for their leishmanicidal properties.
CaCO3 is abundant in nature,8 and is available in various forms namely, calcite, aragonite, and vaterite.9 This mineral is formed during the nature's biomineralization process.10 In recent decades, calcium carbonate nanocomposite has garnered much attention due to their high cell biocompatibility.11 Although vaterite is the least abundant among polymorphs of calcium carbonate, it is a biologically valuable element in organisms.12 Vaterite is weak in terms of chemical stability13 and rapidly gets converted to the calcite form. Different factors like pH, presence of the organic precursor, type of solvent, and concentration of precursors are effective in transforming the vaterite to calcite form.14 The calcium carbonate nanoparticles has been widely used due to their widespread applications in engineering,15 dentistry,16,17 drug delivery,18 biotechnology,19 bone regeneration20 and environmental protection.21 The use of calcite nanoparticles has increased significantly because of their pervasive availability, low cost and ease of synthesis,22 biocompatibility, and the potential for absorption and for bone tissue repairs in the biomedical field. Yu et al.23 have reported excellent potential for calcium carbonate nanostructure scaffolds in bone regeneration. Fujihara et al.24 reported that calcium carbonate nanostructure improved the proliferation of osteoblast cells.
Leishmaniasis is a serious public health concern and the disease comprise 3 typical groups namely visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL); CL form has the most prevalent rate while Zoonotic CL (ZCL) is caused by Leishmania (L.) major. Due to the recent spread of the disease the development of new effective therapies has become a priority for the World Health Organization (WHO).25–27 Nanotechnology-based strategies using nanoscopic tools is one of the most effective treatment for protozoan infections.28,29 In the meantime, antibacterial, antivirals, and anti-parasitic properties of nanomaterials have been extensively investigated due to their favorable physicochemical properties.30–38 So far, the anti-leishmanial activity of various nanomaterials such as silver, zinc oxide, liposomes, titanium oxide has generated positive results but the calcium carbonate nanostructures have not been studied although calcium nanostructure have been considered in various fields due to their non-toxicity, suitability for intravenous and oral administration, high biodegradability, and good absorption in the human body.39
In view of our ongoing studies exploiting the plant-mediated strategies for the assembly of nanoparticles, the synthesis of calcium carbonate nanowires and their anti-leishmanicidal effects were undertaken; calcium nanowires were synthesized using a plant extract and then, their anti-leishmanicidal effects were examined on Leishmania major in in vitro.
2. Experimental
2.1. Materials
All chemicals and reagents were prepared from Merck Chemicals Co. Deionized water (DI) was used at all stages of the experimental work. The plant material used in the synthesis was purchased from a local supermarket.
2.2. Green synthesis of calcium carbonates polymorphs
At the outset, the young branches of Nasturtium officinale were washed three times with deionized water and were dried at room temperature for 7 days. The plant surface moisture was removed at room temperature then milled to a fine powder by electric Stainless Steel Home Grinding Milling Machine. To N. officinale young branches powder (10 g), deionized water (10 mL) was added and shaken on orbital shaker for 24 hours, then centrifuged at 8000 rpm for 15 min and filtered with Whatman filter paper no. 42. A stock solution (200 mL of 0.1 M CaCl2·2H2O) was added drop wise to 100 cm3 plant extract at 80 °C. The pH of the mixture was raised to 11 by addition of sodium hydroxide solution (1 M); precipitate ensued after increasing the pH. The obtained mixture was stored at room temperature for two hours, then cooled at room temperature, and incubated at 70 °C for 1 day. The resulting nanoparticles were centrifuged and washed with ethanol and DI, respectively, then dried at 120 °C and calcined in the furnace at 600 °C for 5 h.
2.3. Physicochemical characterization of nanoparticles
The structure and size of resulting nanoparticles were analyzed by field-emission SEM (FESEM) (Sigma VP, ZEISS) and a high-resolution TEM (HRTEM) (Tecnai, 20FEI). The XRD was recorded on X'PertPro Advance with CuKα radiation 1.54 Å at 40 kV and 30 mA. Also, EDAX analysis was performed to determine the purity of the nanoparticles.
2.4. Leishmanicidal activity
In this study, the L. major culture was treated with varying concentrations of biogenic CaCO3 nanowire and amphotericin-B; amphotericin-B has been used to treat fungal infections associated with AIDS, central nervous system (CNS) infections, lung infections, unicellular infections such as VL, etc.
Amphotericin and nanoparticles were prepared in DMSO. An adequate amount of promastigotes was prepared by culturing the L. major in RPMI 1640 (Gibco) with 20% FCS (Gibco).20 Promastigote proliferation was measured by MTT [3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. About 3 × 106 promastigotes of L. major per well were cultured in RPMI 1640 (Gibco) and 20% FCS (Gibco) and permitted to multiply for 72 h in the medium (control group), in solvent (other control groups) or the presence of CaCO3 NPS in 3 separate 96-well microtiter plates and in concentration of 100, 200, 400 and 800 μg mL−1. Then, 20 μL of tetrazolium (Roche, Germany) (5 mg mL−1) was poured to each well and incubated in 18 °C for 4 h to be centrifuged in 1000g for 10 min. The supernatant was removed and 100 μL of DMSO was poured to each well and resuspended. Finally, the ELISA reader was used to read OD at 450 nm.
3. Discussion and results
3.1. XRD analysis
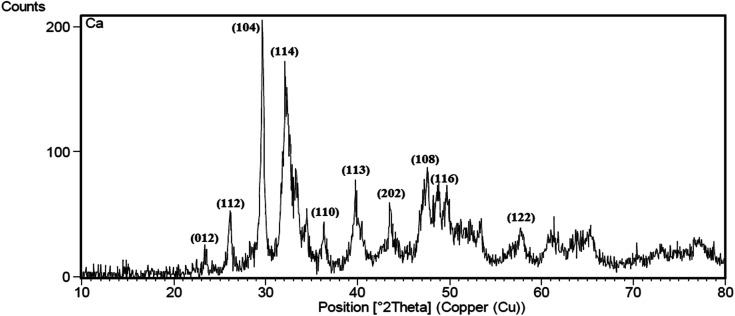
XRD pattern for CaCO3 nanowire obtained by the green method at pH = 11 after calcination at 600 °C is depicted in Fig. 1. According to XRD data, there are two crystalline phases of CaCO3 (calcite and vaterite) in the final product. No peak of the aragonite crystalline phase was observed in the graph.40 The peaks observed in 2θ = 23, 29.9, 36, 39.5, 47, 48.5, 58, 61, 64, 65.5 and 76.50 related to the presence of calcite phase in the nanowires.40 Also, the peaks observed in 2θ = 26, 32, 33, 34, 43.5, 49, and 53.50 are the main features of the vaterite phase in the nanowires41 based on the standard JCPDS (no. 85-1108). These findings are similar to previously published reports on CaCO3 nanoparticles that synthesized by using chemical methods.40–43
Fig. 1. X-ray diffraction spectrum of CaCO3 nanowire synthesis using plant extract.
3.2. Field-emission scanning electron microscope and energy dispersive spectrometer (EDS)
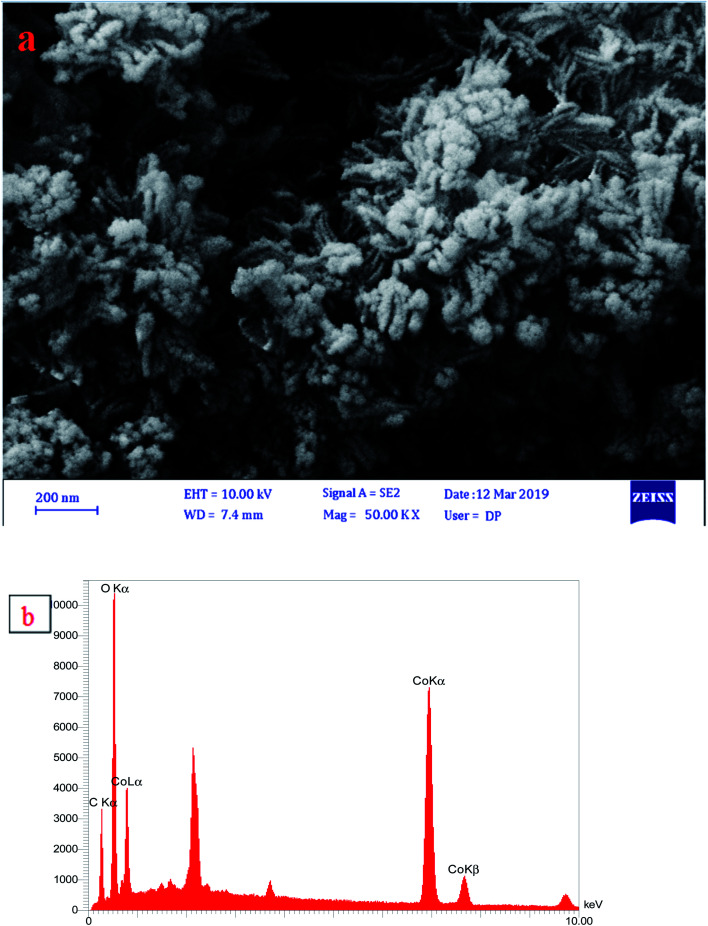
The morphology of CaCO3 nanostructures was investigated by SEM analysis (Fig. 2). The uniform addition of calcium chloride solution to the plant extract in the air and CO2 environment for two hours with a continuous stirring resulted in the formation of calcium carbonate nanoparticles. The addition of the NaOH solution to calcium carbonate particles produced polycrystals of vaterite. The incubation of the mixture resulted in the accumulation of particles and the formation of star-like structures, presumably due to the increase in salt content in a small volume of space. A 50% increase in sodium hydroxide salts reduced the stability of calcium carbonate particles and reduced the number of calcium ions relative to carbonate, resulting in an increase in the number of elliptical particles and the formation of irregular star-like structures.44 The components of calcium carbonate powders have been calcined at 600 °C are shown in Fig. 2b. The EDX analysis (Fig. 2b) of CaCO3 nanoparticles showed that nanoparticles comprise 59.8 (wt%) calcium, 33.4 oxygen, and 6.8 carbon.
Fig. 2. (a) SEM image of star-like shaped and (b) EDX profile of CaCO3 nanostructures.
3.3. HRTEM analysis
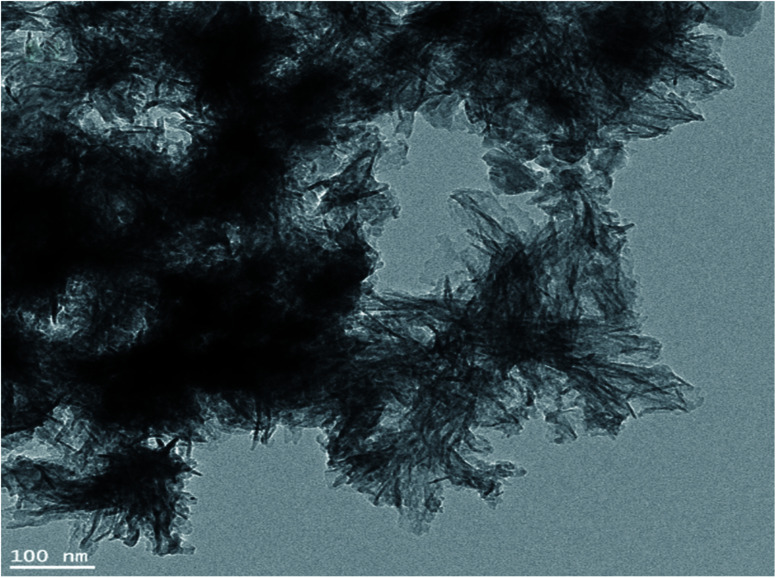
Fig. 3 depicts the image of calcium carbonate nanowires45 and their nanostring like particles (Fig. 3) at pH = 11, respectively. The minimum and maximum sizes of these single crystals are 3 to 76 nm, respectively. Nanowires shape (or needle-like) structures with a full center confirms the precursor conductivity of calcium for the calcite nanoparticles formation.46
Fig. 3. HRTEM micrographs of CaCO3 nanowires.
3.4. Leishmanicidal activity of nanowires calcium carbonate nanowires
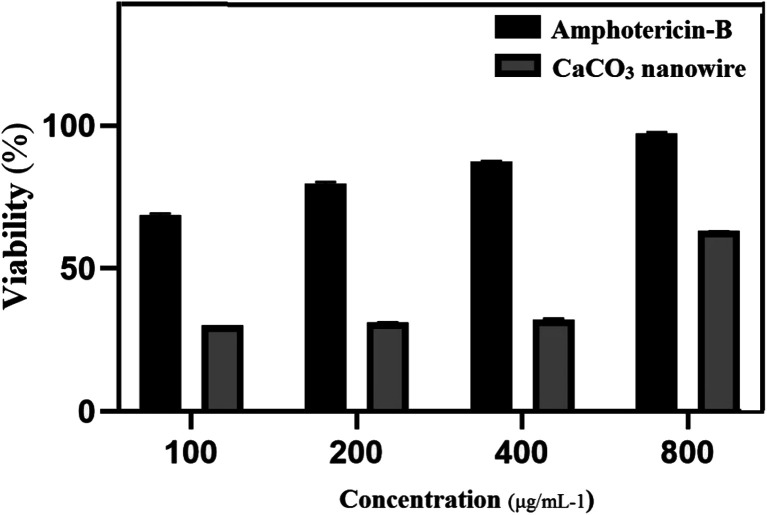
Leishmanicidal activity of varying concentrations of amphotericin-B was investigated in comparison with the CaCO3 nanowire on L. major cultures. Fig. 4 shows the toxicity of CaCO3 nanowire as compared to the control group after 72 h. The MTT assay was applied to measure the cytotoxic effect of CaCO3 on L. major promastigotes. Promastigotes growth inhibition of L. major were evaluated in the presence of 100, 200, 400, and 800 μg mL−1 concentrations of CaCO3 nanowire. Following the application of nanowires calcium carbonate, promastigote IC50 was measured as 800 μg mL−1; results identified the CaCO3 nanowire that have the low toxicity against L. major.
Fig. 4. MTT assay of CaCO3 nanowire on L. major after 72 h.
4. Discussion
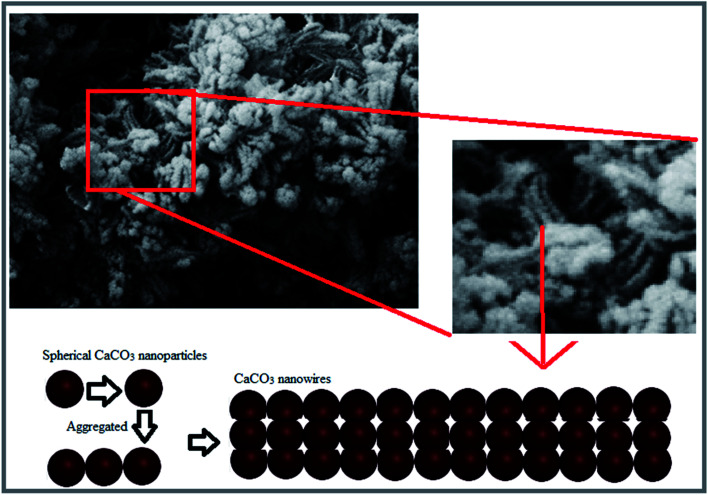
In this study, unprecedented synthesis of CaCO3 nanowires was accomplished using plant extract. The characterization results of the synthesized nanoparticles confirmed the morphology of nanowires and their star-shaped polycrystalline structures. Schematic of the formation process of the CaCO3 nanowire are shown in Fig. 5, nanostring like CaCO3 particles with homogeneous size distribution were initially formed and then aggregated and finally the growth of CaCO3 nanowires ensued.
Fig. 5. Schematic of formation process of the CaCO3 nanowire.
Due to the high toxicity of plant physicochemical on Leishmania parasite,47 the use of nanoparticles containing these compounds reduces the medical costs for the disease treatment by minimizing the adverse effects, improving the solubility, without generating cytotoxicity.
Also, the mechanisms for the conventional drug resistance are often associated with lower drug uptake, faster drug metabolism, increased efflux, drug target variations, and the over-expression of drug transporters. The high outbreak rate of CL and the presence of resistance to conventional drugs highlight a demand for promoting and exploring new, low toxic and minimal cost drugs. According to the results of Borrego-Sánchez et al., the solubility of praziquantel antiparasitic drugs with calcium carbonate increased in the acidic medium and cytotoxicity study revealed no cell death in HTC116 cells.48 Tessarolo et al., showed which benznidazole delivery with calcium carbonate was more toxic on Trypanosoma cruzi compared with benznidazole alone.49 One of the biggest challenges in treating tropical diseases, as a public health problem, has been its cost. Herein, the CaCO3 nanowires were synthesized using an inexpensive method. Additionally, calcium carbonate nanostructures may be used as a smart anticancer drug carrier.50,51
5. Conclusion
Calcium nanowires were synthesized using a plant extract. Their leishmanicidal effects were evaluated on Leishmania major in in vitro. These results confirm that the plant extract offer an easy, inexpensive and an effective method to synthesize calcium carbonate nanowires without using any harmful chemicals detrimental to humans and or environment.
Authors' contributions
The authors read and approved the final manuscript.
Conflicts of interest
The authors confirm that there are no competing interests.
Supplementary Material
Acknowledgments
This work is partially supported by the Bam and Shahid Beheshti Medical Sciences University.
References
- Khashan K. S. Sulaiman G. M. Hussain S. A. Marzoog T. R. Jabir M. S. J. Inorg. Organomet. Polym. Mater. 2020:1–17. [Google Scholar]
- Safaei M. Foroughi M. M. Ebrahimpoor N. Jahani S. Omidi A. Khatami M. TrAC, Trends Anal. Chem. 2019;118:401–425. doi: 10.1016/j.trac.2019.06.007. [DOI] [Google Scholar]
- Mostafavi M. Sharifi I. Farajzadeh S. Khazaeli P. Sharifi H. Pourseyedi E. Kakooei S. Bamorovat M. Keyhani A. Parizi M. H. Khosravi A. Khamesipour A. Biomed. Pharmacother. 2019;116:108942. doi: 10.1016/j.biopha.2019.108942. [DOI] [PubMed] [Google Scholar]
- Noorani B. Tabandeh F. Yazdian F. Soheili Z.-S. Shakibaie M. Rahmani S. Int. J. Polym. Mater. Polym. Biomater. 2018;67:754–763. doi: 10.1080/00914037.2017.1362639. [DOI] [Google Scholar]
- Khatami M. Alijani H. Q. Heli H. Sharifi I. Ceram. Int. 2018;44:15596–15602. doi: 10.1016/j.ceramint.2018.05.224. [DOI] [Google Scholar]
- Sepúlveda A. A. L. Velásquez A. M. A. Linares I. A. P. de Almeida L. Fontana C. R. Garcia C. Graminha M. A. S. Photodiagn. Photodyn. Ther. 2020;30:101676. doi: 10.1016/j.pdpdt.2020.101676. [DOI] [PubMed] [Google Scholar]
- Lubis K. Chudapongse N. Doan H. V. Weeranantanapan O. Curr. Nanosci. 2020;16:214–225. doi: 10.2174/1573413715666181128142258. [DOI] [Google Scholar]
- Pavez J. Silva J. F. Melo F. Electrochim. Acta. 2005;50:3488–3494. doi: 10.1016/j.electacta.2004.12.025. [DOI] [Google Scholar]
- de Leeuw N. H. Parker S. C. J. Phys. Chem. B. 1998;102:2914–2922. doi: 10.1021/jp973210f. [DOI] [Google Scholar]
- Azizi Z. Pourseyedi S. Khatami M. Mohammadi H. J. Cluster Sci. 2016;27:1613–1628. doi: 10.1007/s10876-016-1024-9. [DOI] [Google Scholar]
- Ma M.-G. Dong Y.-Y. Fu L.-H. Li S.-M. Sun R.-C. Carbohydr. Polym. 2013;92(2):1669–1676. doi: 10.1016/j.carbpol.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Weiner S. Levi-Kalisman Y. Raz S. Addadi L. Connect. Tissue Res. 2003;44:214–218. doi: 10.1080/03008200390181681. [DOI] [PubMed] [Google Scholar]
- Turnbull A. G. Geochim. Cosmochim. Acta. 1973;37:1593–1601. doi: 10.1016/0016-7037(73)90093-8. [DOI] [Google Scholar]
- Rao M. S. Bull. Chem. Soc. Jpn. 1973;46:1414–1417. doi: 10.1246/bcsj.46.1414. [DOI] [Google Scholar]
- Biswas A. Nagaraja A. T. McShane M. J. ACS Appl. Mater. Interfaces. 2014;6:21193–21201. doi: 10.1021/am5061195. [DOI] [PubMed] [Google Scholar]
- Melo M. A. S. Cheng L. Zhang K. Weir M. D. Rodrigues L. K. Xu H. H. Dent. Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong L. K. Foroughi M. M. Mirhosseini A. F. Safaei M. Jahani S. Mostafavi M. Ebrahimpoor N. Sharifi M. Varma R. S. Khatami M. RSC Adv. 2020;10:15430–15460. doi: 10.1039/D0RA00762E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki Dizaj S. Barzegar-Jalali M. Zarrintan M. H. Adibkia K. Lotfipour F. Expert Opin. Drug Delivery. 2015;12:1649–1660. doi: 10.1517/17425247.2015.1049530. [DOI] [PubMed] [Google Scholar]
- He X. Liu T. Chen Y. Cheng D. Li X. Xiao Y. Feng Y. Cancer Gene Ther. 2008;15:193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- Khoshraftar A. Noorani B. Yazdian F. Rashedi H. Vaez Ghaemi R. Alihemmati Z. Shahmoradi S. Int. J. Polym. Mater. Polym. Biomater. 2018;67:987–995. doi: 10.1080/00914037.2017.1417283. [DOI] [Google Scholar]
- Cai G.-B. Zhao G.-X. Wang X.-K. Yu S.-H. J. Phys. Chem. C. 2010;114:12948–12954. doi: 10.1021/jp103464p. [DOI] [Google Scholar]
- Islam K. Zuki A. Ali M. Hussein B. Zobir M. Noordin M. Loqman M. Wahid H. Hakim M. Hamid A. J. Nanomater. 2012;2012:1–5. doi: 10.1155/2012/534010. [DOI] [Google Scholar]
- Yu H.-D. Zhang Z.-Y. Win K. Y. Chan J. Teoh S. H. Han M.-Y. Chem. Commun. 2010;46:6578–6580. doi: 10.1039/C0CC01348J. [DOI] [PubMed] [Google Scholar]
- Fujihara K. Kotaki M. Ramakrishna S. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Bamorovat M. Sharifi I. Aflatoonian M. R. Sadeghi B. Shafiian A. Oliaee R. T. Keyhani A. Afshar A. A. Khosravi A. Mostafavi M. Parizi M. H. Khatami M. Arefinia N. Int. Immunopharmacol. 2019;69:321–327. doi: 10.1016/j.intimp.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Bamorovat M. Sharifi I. Aflatoonian M. R. Sharifi H. Karamoozian A. Sharifi F. Khosravi A. Hassanzadeh S. PLoS One. 2018;13:e0192236. doi: 10.1371/journal.pone.0192236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamorovat M. Sharifi I. Dabiri S. Mohammadi M. A. Fasihi Harandi M. Mohebali M. Aflatoonian M. R. Keyhani A. Iran. J. Public Health. 2015;44:1359–1366. [PMC free article] [PubMed] [Google Scholar]
- Shokri A. Fakhar M. Akhtari J. Gill P. J. Mazandaran Univ. Med. Sci. 2016;25:412–429. [Google Scholar]
- Saleem K. Khursheed Z. Hano C. Anjum I. Anjum S. Nanomaterials. 2019;9:1749. doi: 10.3390/nano9121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattarahmady N. Rahi A. Heli H. Sci. Rep. 2017;7:11238. doi: 10.1038/s41598-017-11680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein H. Masoud N. Recent Pat. Nanotechnol. 2017;11:1–12. [Google Scholar]
- Khatami M. Sharifi I. Nobre M. A. L. Zafarnia N. Aflatoonian M. R. Green Chem. Lett. Rev. 2018;11:125–134. doi: 10.1080/17518253.2018.1444797. [DOI] [Google Scholar]
- Iranmanesh T. Foroughi M. M. Jahani S. Shahidi Zandi M. Hassani Nadiki H. Talanta. 2020;207:120318. doi: 10.1016/j.talanta.2019.120318. [DOI] [PubMed] [Google Scholar]
- Khan Z. U. H. Khan A. Chen Y. M. Shah N. S. Khan A. U. Muhammad N. Tahir K. Shah H. U. Khan Z. U. Shakeel M. Nadeem M. Imran M. Wan P. J. Photochem. Photobiol., B. 2018;180:208–217. doi: 10.1016/j.jphotobiol.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Singh H. Du J. Singh P. Yi T. H. J. Nanostruct. Chem. 2018;8:359–368. doi: 10.1007/s40097-018-0281-6. [DOI] [Google Scholar]
- Nozohouri S. Salehi R. Ghanbarzadeh S. Adibkia K. Hamishehkar H. Mater. Sci. Eng. C. 2019;99:752–761. doi: 10.1016/j.msec.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Khatami M. Iravani S. Varma R. S. Mosazade F. Darroudi M. Borhani F. Bioprocess Biosyst. Eng. 2019;42:2007–2014. doi: 10.1007/s00449-019-02193-8. [DOI] [PubMed] [Google Scholar]
- Azhdari S. Sarabi R. E. Rezaeizade N. Mosazade F. Heidari M. Borhani F. Abdollahpour-Alitappeh M. Khatami M. RSC Adv. 2020;10:29737–29744. doi: 10.1039/D0RA04071A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darroudi M. Bagherpour M. Hosseini H. A. Ebrahimi M. Ceram. Int. 2016;42:3816–3819. doi: 10.1016/j.ceramint.2015.11.045. [DOI] [Google Scholar]
- Pérez-Villarejo L. Takabait F. Mahtout L. Carrasco-Hurtado B. Eliche-Quesada D. Sánchez-Soto P. J. Ceram. Int. 2018;44:5291–5296. doi: 10.1016/j.ceramint.2017.12.142. [DOI] [Google Scholar]
- Saraya M. E.-S. I. Rokbaa H. Am. J. Nanomater. 2016;4:44–51. [Google Scholar]
- Ghiasi M. Malekzadeh A. Cryst. Res. Technol. 2012;47:471–478. doi: 10.1002/crat.201100240. [DOI] [Google Scholar]
- Schüler T. Tremel W. Chem. Commun. 2011;47:5208–5210. doi: 10.1039/C0CC05717G. [DOI] [PubMed] [Google Scholar]
- Donatan S. Yashchenok A. Khan N. Parakhonskiy B. Cocquyt M. Pinchasik B.-E. Khalenkow D. Möhwald H. Konrad M. Skirtach A. ACS Appl. Mater. Interfaces. 2016;8:14284–14292. doi: 10.1021/acsami.6b03492. [DOI] [PubMed] [Google Scholar]
- Butt A. Ejaz S. Baron J. Ikram M. Ali S. Dig. J. Nanomater. Biostructures. 2015;10(3):799–809. [Google Scholar]
- Bari S. S. Mishra S. Carbohydr. Polym. 2017;169:426–432. doi: 10.1016/j.carbpol.2017.04.023. [DOI] [PubMed] [Google Scholar]
- Silva A. R. Scher R. Santos F. V. Ferreira S. R. Cavalcanti S. C. Correa C. B. Bueno L. L. Alves R. J. Souza D. P. Fujiwara R. T. Molecules. 2017;22:815. doi: 10.3390/molecules22050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego-Sánchez A. Sánchez-Espejo R. Albertini B. Passerini N. Cerezo P. Viseras C. Sainz-Díaz C. I. Pharmaceutics. 2019;11:533. doi: 10.3390/pharmaceutics11100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarolo L. D. Mello C. P. Lima D. B. Magalhães E. P. Bezerra E. M. Sales F. A. M. Neto I. L. B. de Fátima Oliveira M. dos Santos R. P. Albuquerque E. L. Parasitology. 2018;145:1191–1198. doi: 10.1017/S0031182018000197. [DOI] [PubMed] [Google Scholar]
- Sun D. Peng H. Wang S. Zhu D. Nanoscale Res. Lett. 2015;10:948. doi: 10.1186/s11671-015-0948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. He C. Tong Z. Liu X. Ren B. Zeng F. Int. J. Pharm. 2006;308:160–167. doi: 10.1016/j.ijpharm.2005.11.004. [DOI] [PubMed] [Google Scholar]