Abstract
Despite technological advances in diagnostic abilities and improved treatment methods, the burden of cancers remains high, leading to significant morbidity and mortality. One primary reason is that cancer cell secretory factors modulate the tumor microenvironment, supporting tumor growth and circumvents anticancer activities of conventional therapies. Macrophage inhibitory cytokine-1 (MIC-1) is a pleiotropic cytokine elevated in various cancers. MIC-1 regulates various cancer hallmarks, including sustained proliferation, tumor-promoting inflammation, avoiding immune destruction, inducing invasion, metastasis, angiogenesis, and resisting cell death. Despite these facts, the molecular regulation and downstream signaling of MIC-1 in cancer remain elusive, partly because its receptor (GFRAL) was unknown until recently. Binding of MIC-1 to GFRAL recruits the coreceptor tyrosine kinase RET to execute its downstream signaling. So far, studies have shown that GFRAL expression is restricted to the brain stem and is responsible for MIC-1/GFRAL/RET-mediated metabolic disorders. Nevertheless, abundant levels of MIC-1 expression have been reported in all cancer types and have been proposed as a surrogate biomarker. Given the ubiquitous expression of MIC-1 in cancers, it is crucial to understand both upstream regulation and downstream MIC-1/GFRAL/RET signaling in cancer hallmark traits.
Keywords: Cancer hallmarks, MIC-1/GFRAL/RET signaling, cancer, surrogate biomarker, immunosurveillance
1. INTRODUCTION
Cancer is a leading cause of death worldwide, with a prevalence of 19.3 million in 2020, and 50% of patients succumbed to the disease in 2020 [1]. Cancer’s burden remains high despite technological advances that improved diagnostic abilities, treatment methods, and understanding of molecular mechanisms. As a result, cancer cases are expected to increase to 28.4 million cases worldwide by 2040 [1]. The loss of homeostasis between multifunctional and multilineage cell types within the tumor microenvironment is one of the primary causes of most cancers. Significantly, such multifunctional cells-derived intrinsic and extrinsic factors impact tissue homeostasis and cancer progression. As a result, developing new treatments necessitates a thorough understanding of how the communication of cellular factors within the environment affects tissue homeostasis.
Macrophage inhibitory cytokine-1 (MIC-1) is best known as a TGF-β like cytokine and is associated with cellular stress response and macrophage activation. MIC-1 is also known by growth differentiation factor 15 (GDF15), nonsteroidal anti-inflammatory drug (NSAID)-activated gene-1 (NAG1), placental bone morphogenetic protein (PLAB), placental TGF-β (PTGFB), and prostate derived factor (PDF) based on its expression on a particular tissue type or functional association. Since its identification, evidence suggests that MIC-1 is expressed at very minimal levels under physiological conditions except placenta and prostate, but its expression is induced during injury and pathological conditions [2, 3]. MIC-1 exerts multifunctional actions and has received much attention due to its involvement in embryonic, osteogenic, and hematopoietic development, proliferation and migration of epithelial cells, cellular differentiation, stress, inflammation, and immunomodulation [2, 3].
Considerable evidence demonstrates that MIC-1 is abundantly expressed in almost all cancer types and that its alteration promotes tumorigenic properties in in vitro and in vivo settings. The tumorigenic properties of MIC-1 are supported by its pleiotropic functions, such as sustained proliferative signaling, evading growth suppressors, avoiding immune destruction, tumor-promoting inflammation, activating invasion and metastasis, inducing angiogenesis, and resisting cell death. Patients with elevated serum MIC-1 are generally associated with aggressive disease, metastasis, poor response to therapy, and disease prognosis [4–14]. Additionally, efforts have shed light on the immune-modulatory function of MIC-1 and thus prevent immune surveillance properties in the tumor microenvironment [15–18]. Despite all these facts, much of the downstream molecular pathways remain elusive partly due to unknown receptor information until recently. Since the identification of glial cell-derived neurotrophic factor (GDNF) family receptor alpha like (GFRAL) in 2017 [19–22], plenty of evidence suggests that MIC-1/GFRAL/ret proto-oncogene (RET) signaling as a therapeutic target for metabolic disorders and cachexia. However, the receptor expression and MIC-1/GFRAL downstream signaling remain an enigma in cancers. Furthermore, coordinated signaling lies in both the upstream and downstream signaling components. Given the ubiquitous expression of MIC-1 in cancers, it is also vital to understand upstream regulation and downstream MIC-1/GFRAL/RET signaling. This review presents a current understanding of MIC-1 signaling in the regulation of various hallmark cancer traits [23] and associated signaling in cancer progression. For a better understanding, this article includes the regulation of MIC-1, and its biomarker role. Finally, we also include our perspective on how the current advancement in the immunological and pharmacological approaches would help target MIC-1/GFRAL/RET signaling in deadly diseases such as cancer.
2. MIC-1 as a surrogate biomarker in cancer
Interestingly, the level of expression of MIC-1 is variable between tissues and individuals. Under physiological conditions in adults, MIC-1 is expressed very high in the placenta, followed by the prostate, liver, kidney, pancreas, and lungs [24]. In fetal tissue, the expression of MIC-1 is much greater in the lungs, followed by the liver and kidneys. [24]. Whereas MIC-1 is induced in almost all disease conditions studied [2, 11, 25]. In line with literature evidence, baseline protein expression of MIC-1 from the Human Protein Atlas shows that the placenta expresses a significant amount of MIC-1 under physiological conditions [26]. In addition, the urinary bladder and prostate constitute the second-highest expressing tissues. Furthermore, the stomach, colon, rectum, pancreas, kidney, and appendix also express detectable levels of MIC-1. Similarly, antibody staining data from the Human Protein Atlas showed that most cancers express MIC-1 [26]. Where prostate cancer expresses the highest amount of MIC-1, followed by colorectal, head and neck, carcinoid, urothelial, stomach, pancreatic, melanoma, testis, renal, endometrial, glioma, breast and ovarian cancers [26].
Irrespective of the expression of MIC-1 in normal/benign tissues, the serum level of MIC-1 is significantly increased in cancer conditions. In fact, MIC-1 is one of the most prominently overexpressed soluble factors in cancers [10]. Furthermore, subsequent studies showed that MIC-1 protein levels in tumor samples are significantly higher than adjacent normal or precancerous lesions and showed their association with tumor characteristics and clinical outcomes [11, 13]. For instance, serum MIC-1 levels accurately categorize pancreatic adenocarcinoma from pancreatitis [9]. MIC-1 has also been shown to predict the prevalence of advanced tumors in nonsymptomatic patients with a genetic predisposition to pancreatic cancer [27]. Similarly, MIC-1 expression differentiates preinvasive (high-grade prostatic intraepithelial neoplasia and prostate cancer from benign prostate epithelium [28]. In combination with prostate-specific antigen (PSA), the serum level of MIC-1 differentiated prostate cancer from healthy controls and benign prostatic hyperplasia [12, 29]. Colorectal carcinoma patients had significantly higher serum MIC-1 levels than polyps subjects [30]. In breast cancer, elevated serum MIC-1 levels have been shown in early stage, and further suggest that MIC-1, independently and in combination with CA15–3, a known blood biomarker of BC, predicts breast cancer with greater sensitivity [31]. Serum MIC-1 of esophageal squamous cell carcinoma was significantly higher than that of the control group and was positively associated with tumor invasion and lymph node metastasis [32]. It was suggested that MIC-1 could serve as a potential biomarker for esophageal squamous cell carcinoma [32].
MIC-1 is not only a surrogate marker for tumor progression; MIC-1 levels in serum and tissue also predict the clinical outcome of various cancers. Evidence from research groups suggests that serum MIC-1 is significantly higher in patients with aggressive tumors of the prostate. Of particular interest, MIC-1 has a better predictive ability of the chemotherapy response in prostate cancer. Men with lower levels of MIC-1 before treatment had a better prognosis after docetaxel therapy than men with higher serum MIC-1 status [4]. Serum levels of MIC-1 showed a clear discriminatory predictive capability for the outcome and mortality of prostate cancer [33]. Furthermore, there was an important link between supraphysiological MIC-1 in the serum of patients and PCa metastasis [33]. In prostate cancer, MIC-1 levels vary between 1300 ng/L and ~30,000 ng/L [34]. Furthermore, serum MIC-1 levels in prostate cancer patients with cachexia were 4-fold higher than those without cachexia [34]. Supportively, the expression pattern of immune gene signatures containing MIC-1 has been linked to shorter recurrence-free survival (RFS) in prostate cancer [35].
Serum concentrations of MIC-1 correlate with the development of adenomatous polyps and are proposed as a predictive marker for the progression of colorectal cancer and tumor recurrence [36, 37]. Furthermore, MIC-1 levels in the serum of colorectal cancer patients were correlated with the severity of liver involvement, and patients with higher MIC-1 levels had significantly worse outcomes [38]. A meta-analysis study on colorectal cancer revealed that increased expression of MIC-1 in the serum of the patient is associated with a poor prognosis and lower survival [39]. Furthermore, MIC-1 distinguishes pancreatitis from pancreatic adenocarcinoma more accurately than CA19–9 [40]. MIC-1 levels were significantly higher in chemo-resistant patients than in chemo-sensitive epithelial ovarian cancer patients and independently predicted the progression-free survival (PFS) [41]. In multiple myeloma, patients with high plasma levels of MIC-1 exhibit reduced event-free and overall survival chances compared to patients with low plasma levels of MIC-1 [42]. Similarly, increased MIC-1 was associated with the presence of metastases in ocular melanoma [43]. In non-small cell lung cancer, elevated serum MIC-1 levels are an independent risk factor for shorter overall survival [44]. Studies have also highlighted that serum MIC-1 levels serve as a useful independent predictor of therapy response and tumor recurrence in various cancers [6, 45, 46]. Besides the studies described above, several clinical studies have focused on the association between MIC-1 levels and disease risk, which is given in Table 1.
Table 1:
Correlation between MIC-1 circulatory levels and clinical outcome in various clinical conditions.
| Cancer type | Patient cohort/Study | MIC-1 levels | Plasma/serum | Outcomes | Ref. |
|---|---|---|---|---|---|
| Prostate cancer | 1,442 patients | 1,466 pg/mL | Serum | Pretreatment serum MIC-1 levels had a stronger association with the disease outcome. Decreased post-treatment MIC-1 serum levels (associated with reduced prostate cancer mortality | [9] |
| 38 advanced prostate cancer patients with and without cachexia | 12,416 ± 10,235 pg/ml versus 3,265 ± 6,370 pg/ml in non-cachexic patients. | Serum | Higher serum levels of MIC-1 correlate with weight loss in patients with advanced prostate cancer | [45] | |
| Colorectal cancer | 618 patients from 2 independent cohorts | 1,060 pg/mL | Plasma | High levels of MIC-1 are associated with higher mortality from colorectal cancer-specific events. | [55] |
| 97 patients with metastatic | 7,000 pg/mL | Serum | Higher levels of MIC-1 in colorectal cancer compared to healthy controls and serum correlate with significantly worse outcomes. | [56] | |
| 473 patients | 1058.6±703.2 pg/mL to (1848.6±950.9 pg/mL (tumor recurrence). | Serum | MIC-1 increases in 100% of metastatic liver samples and independently predicts overall survival. | [13] | |
| 525 patients from 2 independent cohorts | 1,150 pg/mL Vs. | Serum | An abnormal serum MIC-1 level was associated with a 2.8-fold probability of distant metastasis | [46] | |
| Endometrial Cancer | 466 patients | 1,077 ng/L | Plasma | High levels of MIC-1 are associated with poor survival and a predictor of lymph node metastasis. | [57] |
| 235 patients | 1857 ng/L | Plasma | Higher levels of MIC-1 are associated with aggressive disease and lymph node metastasis | [58] | |
| Esophageal cancer | 286 patients | 990.5 pg/mL | Serum | Serum MIC-1 decreased after surgical removal and increased at relapse. MIC-1 above the threshold correlates with shorter relapse-free survival and tumor-specific survival. Patients with higher MIC-1 are associated with poor relapse-free and tumor-specific survival. | [42] |
| Esophageal adenocarcinoma | 138 patients | 1,140 pg/mL | Plasma | Plasma MIC-1 above threshold correlates with shorter OS in esophageal adenocarcinoma. | [65] |
| Gastric cancer | 217 patients | 1,372 pg/mL | Serum | Patients with high MIC-1 levels had shorter OS. | [59] |
| Glioblastoma | 61 patients | 229 pg/ml | CSF | Patients with glioblastoma with increased CSF MIC-1 had a shorter survival | [60] |
| Head and neck cancer | 64 patients | 875 pg/ml | Serum | Higher serum levels of MIC-1 are associated with a lower median survival of oral squamous cell carcinoma patients. | [14] |
| 60 patients | 346.9 ng/l | serum | Patients with <346.9 ng/l MIC-1 serum levels had a non-significant but improved 3-year disease-free survival rate. | [61] | |
| Melanoma | 761 patients with stage III/IV melanoma | 1,500 pg/mL | Serum | Elevated MIC-1 correlates with shorter OS Serum MIC-1 levels of ≥ 1,500 pg/ml were strongly associated with a reduction in OS. | [62] |
| 56 patients with non-resectable or metastatic melanoma. | ∼500 pg/mL | Serum | Serum MIC-1 levels were persistently higher in non survivors under ipilimumab treatment | [15] | |
| Hepatocellular carcinoma | 223 patients | 2.463 ng/mL | Serum | Higher serum levels were observed in patients with larger tumors. | [63] |
| Multiple myeloma | 131 patients | 0.90 ± 1.10 ng/mL | Plasma | Higher plasma levels of MIC-1 had a lower probability of event-free and overall survival after diagnosis. | [52] |
| 138 patients | 1.08 ng/mL | Serum | Low serum MIC-1 levels were associated with better OS | [64] | |
| Non-small cell lung cancer | 152 stage I and II patients | 1,465 pg/mL | Serum | Higher serum levels of MIC-1 correlate with reduced OS. | [54] |
| Ovarian cancer | 145 patients | 748 pg/mL | Serum | High expression of MIC-1 is associated with poor PFS and OS. | [12] |
| 122 patients | 960 pg/mL | Serum | Higher MIC-1 is related to first-line chemo-resistance, and higher levels are associated with shorter PFS. | [51] | |
| 312 ovarian cancer patients | 1,242 pg/mL | Plasma | Higher plasma MIC-1 levels correlate with shorter OS | [66] | |
| Renal cell cancer | 94 patients | 1,200 pg/mL | Serum | Higher serum levels of MIC-1 were associated with metastases and tumor cancer. | [67] |
| Uveal melanoma | 188 patients | 1.47 ng/mL | Serum | Higher serum levels of MIC-1 were associated with clinical metastases and tumor relapse. | [53] |
Footnotes: In some instances, values were represented as mean ± s.d.
Abbreviations: CSF - cerebrospinal Fluid ELISA - enzyme-linked immunoassay; MIC-1 - Macrophage inhibitory cytokine 1; OS – overall survival; PFS - Progression-free survival.
3. Transcriptional regulation of MIC-1
MIC-1 was cloned and characterized, and its presence was shown in multiple cell types and species [2, 3, 16, 26]. The prevalent notion is that MIC-1 is a secretory protein highly expressed in the placenta, epithelial cells, and macrophages. MIC-1 expression and its activity are regulated at transcriptional levels by multiple signal transduction pathways, and several transcription factors (TFs) bind and participate in the transcriptional activity of MIC-1 (Figure 1). Based on restricted expression in the placenta and prostate, MIC-1 was primarily studied for its regulation by endogenous growth factors. Androgens induce MIC-1 in prostate secretory epithelial cells but not in other male accessory glands [4]. Kannan et al. observed that MIC-1 expression is mediated by p53 in the lung cancer cell line [47] and was later confirmed in LNCaP prostate cancer cells [48]. Consistent with this observation, two consensus binding sites for p53 have been observed in the promoter region of MIC-1, which may be transcriptionally activated upon binding of p53 [49, 50]. Similarly, the expression of MIC-1 was upregulated by a transcription factor related to p53 and TAp63a [51]. The P53 mediated regulation of MIC-1 in cell growth inhibition could be associated with prosurvival protein activating transcription factor 3 (ATF3), which is known to be negatively regulated by P53 [52]. Furthermore, various drugs and growth factors, such as NSAIDs, peroxisome proliferator-activated receptor-γ ligands, dietary compounds such as genistein, retinoids, and resveratrol, as well as chemotherapeutic drugs, including etoposide, docetaxel, and doxorubicin, to increase MIC-1 expression levels independently or dependent on p53 in cancer cells [50, 52–55]. In most conditions where MIC-1 is expressed, stress-responsive TFs, such as nuclear factor NF-κB, activator protein-1 (AP-1), a tumor suppressor protein early growth response 1 (EGR1), and hypoxia-inducible factor-1a (HIF-1a), regulate MIC-1 in both wild-type and p53-independent conditions (Figure 1) [56–59].
Figure 1:
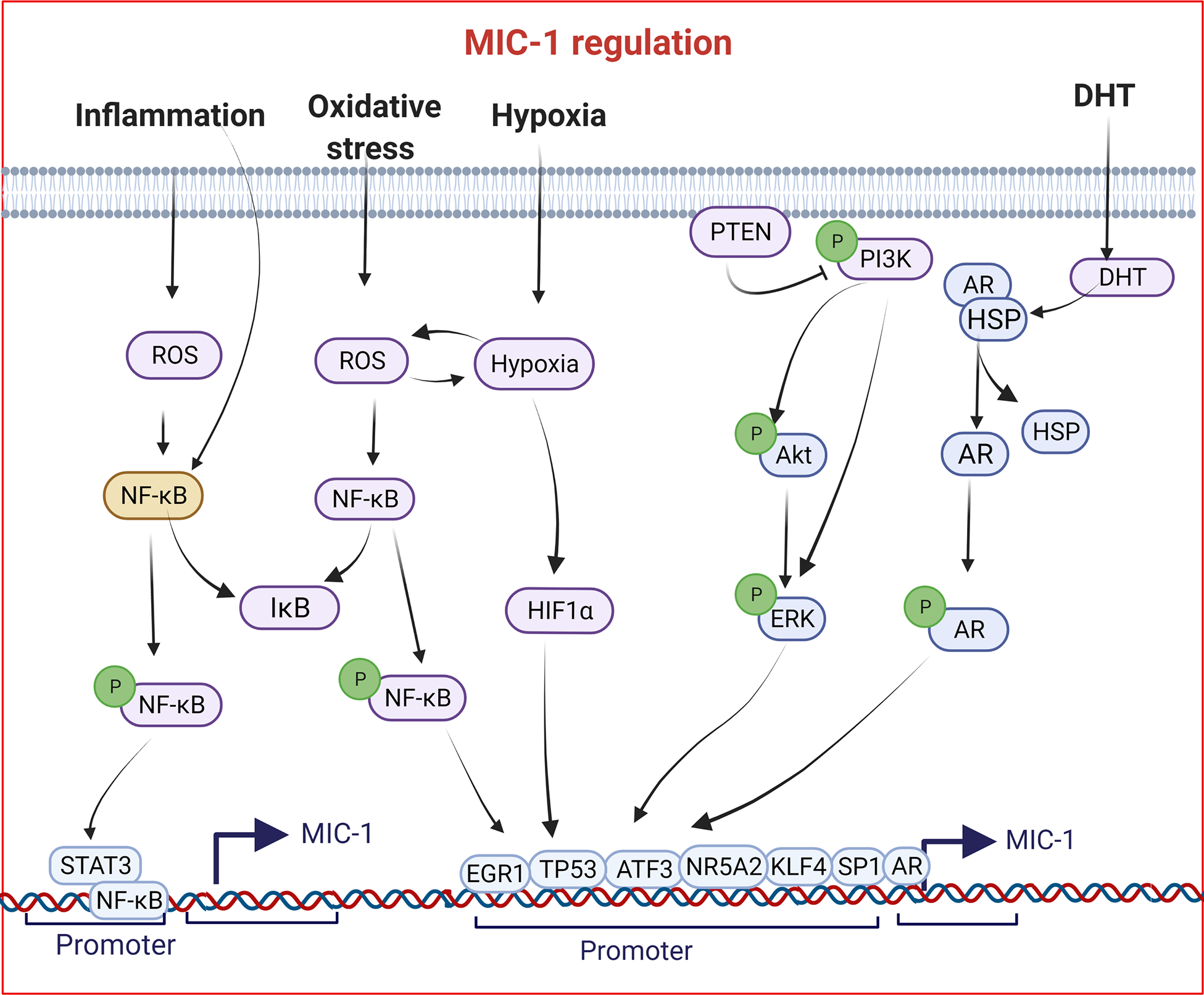
Genomic regulation of Macrophage inhibitory cytokine-1 (MIC-1) in cancer cells. Evidence from the literature suggests that various factors induce MIC-1 in a context-dependent manner.
Under inflammatory conditions, MIC-1 expression was induced by various cytokines and growth factors [60]. The expression of MIC-1 in anorexia is induced independently of P53 and HIF-1 [55]. Since then, based on the association between MIC-1, stress, and inflammation, several downstream signaling molecules, including MAPK, PI3K/AKT, and PTEN, shown to regulate MIC-1 expression in a context-dependent manner [49, 61–64]. The CCAAT/enhancer-binding protein (CEBPB) binds to the promoter region and regulates MIC-1 expression in ovarian cancer cell lines [65]. MIC-1 transcript levels are also observed under various stages of cancers and are context-dependent. MIC-1 has been regulated by NF-κB, p53, and the androgen receptor (AR) under pathological conditions [16, 49, 50, 66]. In therapy non-responsive non-small cell lung cancer cells, KLF4 regulates the transcription of MIC-1 [67]. Jones et al. showed that secretory factors (MIC-1 and PDGFα) and two TFs (EGR1 and FASN) were elevated in prostate tumors and normal adjacent benign tissues [68]. Later, the same group investigated whether EGR1 is a key transcription factor that regulates MIC-1, FASN, and PDGFα in normal tissues adjacent to prostate cancer [69]. They found that all four markers were elevated in cancerous tissues, and basal and lower expression levels were reported in disease-free tissues. Furthermore, in the same study, they demonstrated that EGR1 suppresses MIC-1 expression demonstrated by in vitro studies of ectopic EGR1 overexpression in an immortalized normal human prostate epithelium-derived RWPE-1 cell line [69]. However, further research is needed to identify EGR1’s involvement in MIC-1 regulation in malignant cells and mouse models. In a recent study, BRD4-mediated overexpression of NR5A2 promoted pancreatic cancer cell growth in vitro and in vivo, where MIC-1 is transcriptionally regulated by NR5A2 [70].
4. Role of MIC-1 signaling in cancer hallmark traits
Cancer is a progressive disease in which neoplastic cells undergo uncontrolled division and invade other critical organs and tissues. Tumor progression is accompanied by various concerted changes within the tumor microenvironment. Various tumor-promoting oncogenes, tumor suppressors, microenvironment modulatory factors such as secretory proteins play critical roles in the onset and progression of cancer. The pleiotropic cytokine MIC-1 plays an important role in tumors and activates oncogenic pathways. The primary sources of MIC-1 in the tumor microenvironment are tumor epithelial and myeloid cells like macrophages [2, 11, 16, 18]. MIC-1 supports tumorigenesis by regulating nearly half of the cancer hallmarks, such as by directly affecting cancer cell proliferation, evading apoptosis, promoting angiogenesis, invasiveness, migratory and metastasis, and immune evasion (Figure 2). Below, the role of MIC-1 and its downstream signaling in the regulation of cancer hallmark traits was discussed in detail.
Figure 2:
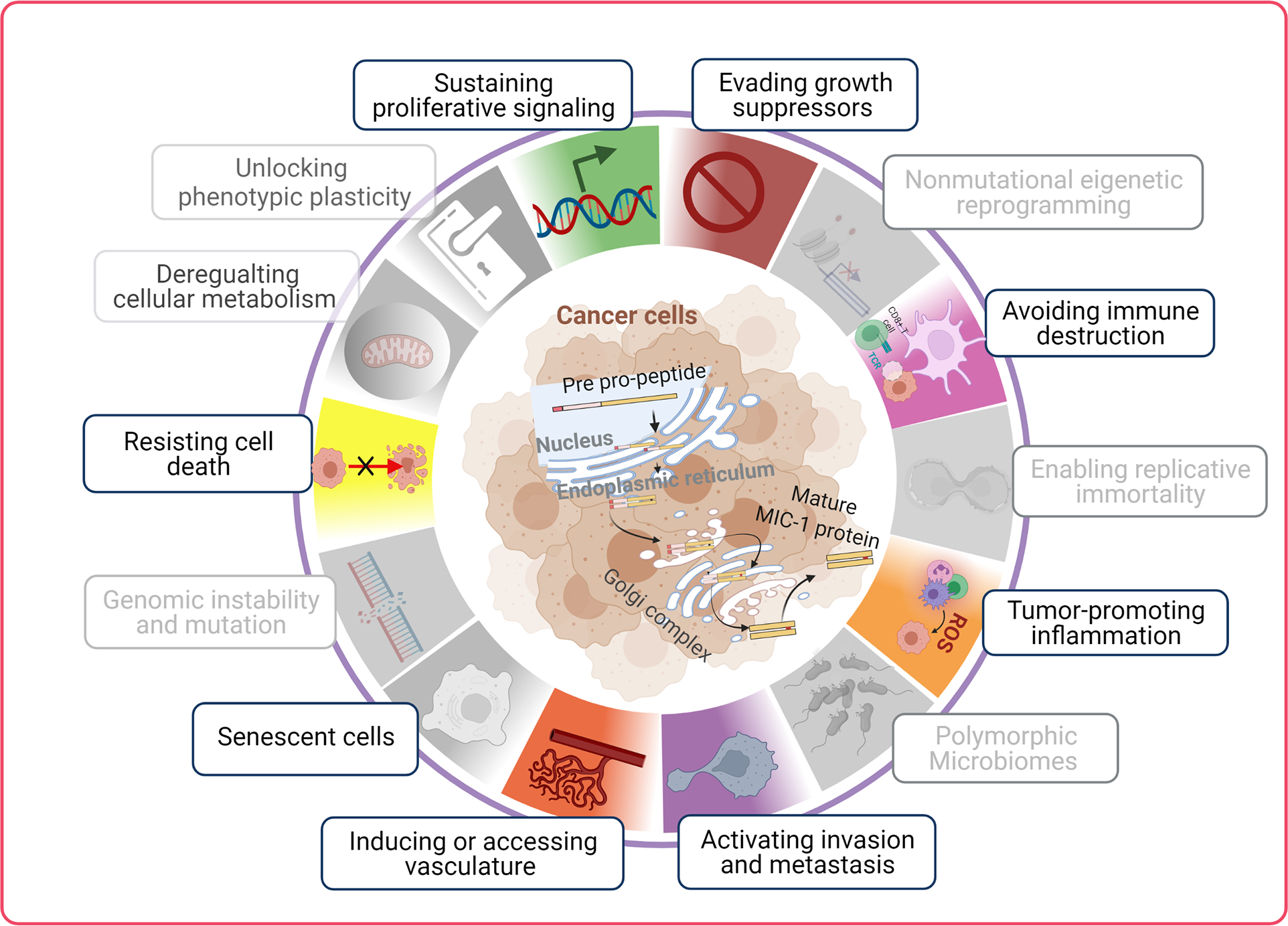
Macrophage inhibitory cytokine-1 (MIC-1) mediated cellular effects. MIC-1 association with cancer hallmarks traits through various biological features. The categories were masked (given in grey) where it lacks scientific evidence. The illustration were recreated based on a recent review by Hanahan [23].
4.1. MIC-1 signaling and cellular proliferation
Elevated MIC-1 expression has been reported in various cancers and induces cell proliferation in multiple cancer cells through canonical and noncanonical signaling pathways [71, 72]. Despite such evidence, the specific role of MIC-1 in tumor development remains in debate. MIC-1 has been well studied with respect to tumorigenic properties in prostate cancer. In androgen-sensitive LNCaP C-33 cells, MIC-1 overexpression leads to increased cell proliferation, clonogenicity, and tumor formation in vivo. Mechanistically, MIC-1 overexpression induced TGF-β alternative pathway involving pERK and p90RSK, but not SMAD activation [73]. Clearly, a highly metastatic variant, the LN3 subline of LNCaP prostate cancer cells, shows reduced cell proliferation after MIC-1 knockdown. However, MIC-1 overexpression in AR-negative DU145 prostate cancer cells reduced tumor growth in vivo and showed a reduction in cell adhesion by decreasing RhoE and catenin δ1 expression [74]. The antitumor effect of MIC-1 is also shown in the TRAMP prostate cancer metastatic model [75]. Since the T-antigen inhibits P53 expression, the observed inhibition of tumor growth in TRAMP mice after MIC-1 overexpression indirectly suggests that MIC-1 inhibits tumor formation, possibly through P53-mediated regulation of cell growth [52]. In SK-BR-3 and SNU-216 cancer cells, recombinant MIC-1 protein is shown to induce AKT and ERK phosphorylation through ErbB2 and ErbB3 activation [71]. Similarly, recombinant MIC-1 has been shown to induce the proliferation of esophageal squamous cells by activating ERK and AKT [76]. While siRNA-mediated knockdown of ErbB2 alone prevented MIC-1-induced activation of AKT and ERK. Similarly, in oral squamous cell carcinoma, MIC-1 overexpression sensitizes oral squamous cell carcinoma cells to chemotherapies through a caspase-9 dependent pathway in vitro and in vivo [72]. Mechanistically, MIC-1 induced oral squamous cell carcinoma cell proliferation through ErbB2 mediated AKT and ERK activation. While blocking ErbB-2 with a small molecule inhibitor, CI-1033 prevented oral squamous cell carcinoma cell proliferation, colony formation, and xenograft growth [72]. Also, MIC-1 knockdown attenuates head and neck cancer cell proliferation, migration, and invasion through the SMAD2/3, PI3K/AKT, and MEK/ERK-mediated epithelial to mesenchymal transition (EMT) mechanisms [56]. Interestingly, EGR1 is found to be downstream of MIC-1 signaling activating MIC-1 at the transcriptional levels through a positive feedback loop [56]. Whereas in thyroid cancer cells, MIC-1 induces tumor progression through the activation of STAT3 [77]. In hepatocellular carcinoma, MIC-1 KO cells reduced liver tumor size caused by the liver stellate cells induced tumorigenesis model [78]. In pancreatic cancer cells, MIC-1 knockdown attenuates ASPC-1 pancreatic cancer cell proliferation in vitro and xenograft tumor growth through the GFRAL receptor [79]. However, the study did not consider the involvement of the coreceptor RET. Overall, MIC-1 is shown to play a dualistic role in cancer progression (Figure 3).
Figure 3:
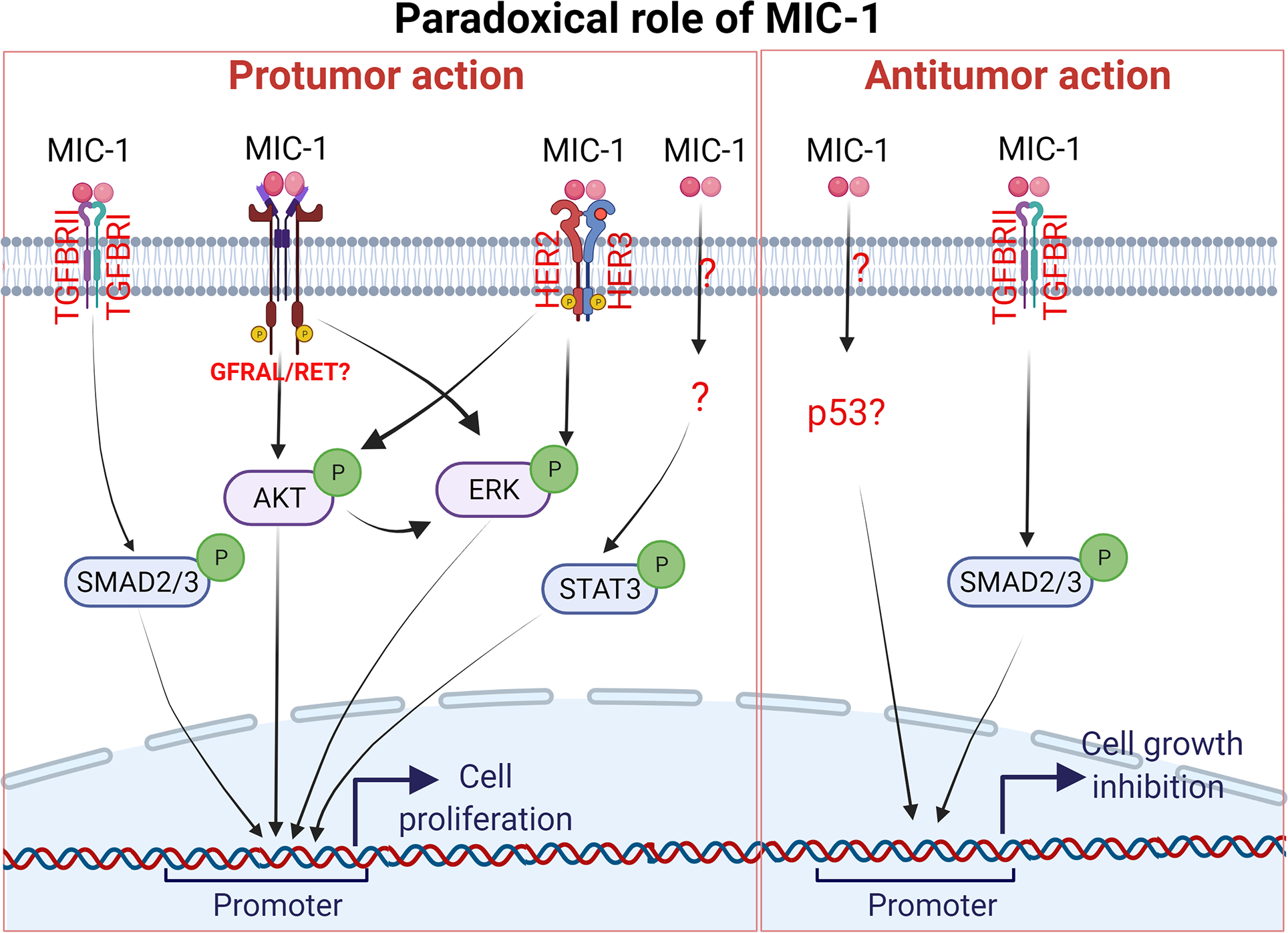
A schematic representation of the Macrophage inhibitory cytokine-1 (MIC-1) signaling pathway in cell proliferation. MIC-1 has been shown to have dualistic regulation of MIC-1 signaling. The protumor function of MIC-1 is shown mainly through the activation of ERK and AKT in cancer cells and the antitumor function is mainly through p53-dependent and independent mechanisms..
4.2. MIC-1 and evasion of growth suppression
One of the cancer hallmarks is the cell’s inherent ability to evade growth suppressor-mediated regulation. TP53, PTEN, RB, and p16 are the major cell growth suppressors which frequently lost during tumor formation. When the external stimulus hits, the tumor-suppressive signaling network activates to induce cell cycle arrest, senescence, and apoptosis. Literature has shown a strong association between MIC-1 levels and expression of growth-suppressive genes such as TP53 and PTEN downstream signaling such as PI3K and AKT. Ectopic overexpression of p53 and chemotherapies induces P53-mediated MIC-1 inducing apoptosis or cell growth arrest [52, 74, 80]. Furthermore, PTEN or PIK3CA mutations have also been shown to activate PI3K signaling and, subsequently, p53-mediated MIC-1 expression and growth suppression in bladder cancer cells [49]. Conversely, PTEN downstream PI3K/AKT and MAPK/ERK signaling pathways are also associated with the tumor-promoting effects of MIC-1 [81, 82].
4.3. MIC-1 and tumor-promoting inflammation
Local infection and tissue injury (such as diet, ROS, or mechanical assault) generate local inflammation, where cellular proliferation will increase at the expense of inflammatory mediators (immune cells) to resolve and restore homeostasis. While sustained inflammation, along with mutagenic or loss of tumor suppressors, could lead to continued proliferation and tumor progression. The cancer-associated inflammation phenotype involves infiltrating immune cells such as tumor-associated macrophages and the production of cytokines [83]. Since the initial discovery of macrophage inhibitory activity, MIC-1 expression has been identified in various tissues and cell types. In fact, the first evidence came from a study in which MIC-1 was induced in activated macrophages, a vital component of the immune response [60]. The association between MIC-1 and inflammation is supported by higher serum MIC-1 levels and inflammatory markers in tumor tissues [11, 84]. Recent evidence suggests that MIC-1 is a central mediator of inflammation-induced tissue tolerance and inflammation-mediated tumor progression [11, 85, 86]. Furthermore, MIC-1 is significantly higher in the adult prostate, inflammatory conditions, viral infections, and inflammation-associated pathological conditions [66, 85, 87].
The primary players of inflammation, such as NF-κB, shown to induce MIC-1 in the early stage of the tumor; however, there is an inverse association between NF-κB and MIC-1 expression at advanced stages. Prostate cancer is well studied for the association between inflammation and MIC-1. Prostate cancer has abundant tumor-associated macrophages (TAMs), acting as tumor suppressor type M1 in early tumor development and pro-tumorigenic type M2 in the advanced stage of prostate cancer [88]. Recently, an elegant study characterized the prostate inflammatory environment and its association with MIC-1 expression levels [84]. Expression levels of M2-type macrophages and MIC-1 increased in prostate tumors compared to benign prostate biopsy. Co-expression analyses of M1/M2 macrophages and MIC-1 in paired prostate tissue revealed a strong negative association between inflammation grade and MIC-1 expression. However, there was a positive correlation between M2-type macrophages and MIC-1 expression levels in tumors and tumor-adjacent benign compared to benign tissue and correlated with biochemical relapse [84].
The immunomodulatory functions of MIC-1 are biphasic; most of the evidence supports the hypothesis that MIC-1 could act as a tumor suppressor in early development through the activation of M1 macrophages mediated by NF-κB [16]. Whereas tumor-derived MIC-1 polarizes TAMs into M2 tumor-promoting macrophages at a later stage [18, 84]. The immune regulatory functions are supported by the fact that MIC-1 production is mainly contributed by tumor epithelial cells in the tumor microenvironment. Supportively, studies using genetically engineered animal tumor models suggest a strong association with immune modulation during tumor development. Furthermore, researchers have also revealed that MIC-1 affects embryonic development, cell proliferation, and other metabolic processes, in part by altering immune cell functions [2, 3, 11].
4.4. MIC-1 and avoiding immune destruction
Immunosurveillance is a key regulator in the tumor environment, where immune cells (such as macrophages, cytotoxic T cells, and natural killer cells) recognize and destroy malignant epithelial cells. Emerging evidence suggests that altered expression of MIC-1 plays critical roles in the early and progressive phases of tumors [16, 17]. An earlier study indicated that MIC-1 knockdown SMA-560 glioma cells were more susceptible to NK-cell mediated lysis in vitro, and increased T cell infiltration in vivo compared to MIC-1 competent glioma cells. The increased infiltration of T cells improved the immune response and was associated with increased survival of mice impanated with MIC-1 depleted glioma cells [17]. Similarly, MIC-1 depleted Kras/P53 mutant syngeneic mouse cell lines showed increased tumor-infiltrating macrophages and reduced tumor growth in an orthotopic pancreatic ductal adenocarcinoma mouse model [16]. Mechanistically, inflammation-induced NF-κB mediates MIC-1 secretion in tumor cells, which suppresses TGF-β–activated kinase (TAK1) signaling, thus preventing macrophages’ tumor-infiltrating ability [16]. Supportively, MIC-1 was observed to be one of the main secretory molecules in tumor-derived conditioned media while inhibiting dendritic cell function [89]. In vitro functional assays showed that MIC-1 suppresses dendritic cell maturation, ultimately inhibiting major histocompatibility complex (MHC) and other costimulatory molecules. Further, MIC-1 alters dendric cell-mediated IL-12 and TGF- β secretion [89]. In hepatocellular carcinoma model, MIC-1 depletion significantly decreased CD4+ T cell frequency in xenograft tumors and in mouse spleen [18]. These experimental studies were supported by the correlative observation of higher MIC-1 levels of immune cell infiltration in the prostate cancer patient sample [84].
4.5. MIC-1 effects on invasion and metastasis
Cancer cells acquire a mesenchymal phenotype during metastatic tumor growth, invade nearby tissues, and migrate to distant organs. Once reached the metastatic site, the tumor cells revert to mesenchymal to epithelial phenotype (MET) and proliferate into metastatic tumors. Transitions between EMT and MET are well-accepted phenotypes, and MIC-1 plays an essential step in tumor aggressiveness. Various clinical studies showed that higher serum MIC-1 levels could be an independent predictor of metastatic diseases of the prostate, breast, and colorectal carcinomas [11, 34, 76]. The available evidence suggests that MIC-1 promotes the invasive capacity in various cancer cells [90]. Using MIC-1 gain and loss of functional analyses, we have shown that MIC-1 regulates migration, invasion, and metastasis [91, 92]. MIC-1 overexpression in LNCaP C-33 androgen-dependent cells promotes cell proliferation and depletion of MIC-1 action using genetic knockdown and MIC-1 neutralizing antibody reduced anchorage-independent growth [73]. Furthermore, MIC-1 overexpression in MIC-1 low expressing PC-3 cells promotes motility and invasiveness. Moreover, MIC-1 knockdown in PC-3M metastatic variant prostate cancer cells reversed the invasive ability of MIC-1 [92]. Mechanistically, MIC-1 alters actin rearrangement and activates the downstream FAK–RhoA signaling pathway to promote invasiveness [92] (Figure 4). At distant sites, MIC-1 induces CCL2 production in osteoblasts, which in turn helps recruit osteal macrophages and increase bone resorption. The aberrant osteoblast and osteoclasts formation fuel prostate cancer cell growth and alters bone integrity [91] (Figure 4). In colorectal cancer cells, MIC-1 expression induced TGF-β-mediated SMAD2 activation and induced EMT, invasiveness, and metastasis [39]. Autocrine production of MIC-1 induced oral squamous cell carcinoma cell proliferation through ErbB2 mediated AKT and ERK activation. While blocking ErbB2 with a small molecule inhibitor, CI-1033 prevented oral squamous cell carcinoma cell proliferation, colony formation, and xenograft growth [72]. Conversely, MIC-1 knockdown attenuates head and neck and oral squamous cell carcinoma cell proliferation, migration, and invasion through PI3K/AKT and MEK/ERK, SMAD2/3 dependent, and independent mechanisms, respectively [56, 90]. In hepatocellular carcinoma cells, MIC-1 KO reduced liver tumor size caused by the liver stellate cells induced streptozotocin/high-fat diet induced liver tumorigenesis model [78]. In pancreatic cancer cells, MIC-1 knockdown attenuated the tumorigenic properties of AsPC-1 pancreatic cancer cells in vitro and in the xenograft model through the GFRAL [79]. Interestingly, MIC-1 overexpression did not alter tumor incidence, but induced higher metastatic events in athymic nude mice [92]. Supportively, MIC-1 transgenic mice delayed tumor progression in TRAMP mice while increased metastasis at a later stage [75]. In few instances, the MIC-1 is also associated with reduced tumor cell invasion and metastasis. MIC-1 overexpression in AR-negative DU145 prostate cancer cells reduced tumor growth in vivo, undergone caspase-dependent apoptosis, and cell adhesion by decreasing RhoE and catenin δ1 expression [74]. Lentiviral-mediated MIC-1 overexpression in lung cancer cells reduced tumor growth and metastasis, possibly through the TGF-β/SMAD pathway [93]. The discrepancies may result from different expression levels of MIC-1, cell lines, experimental models, context, and microenvironment effects.
Figure 4:
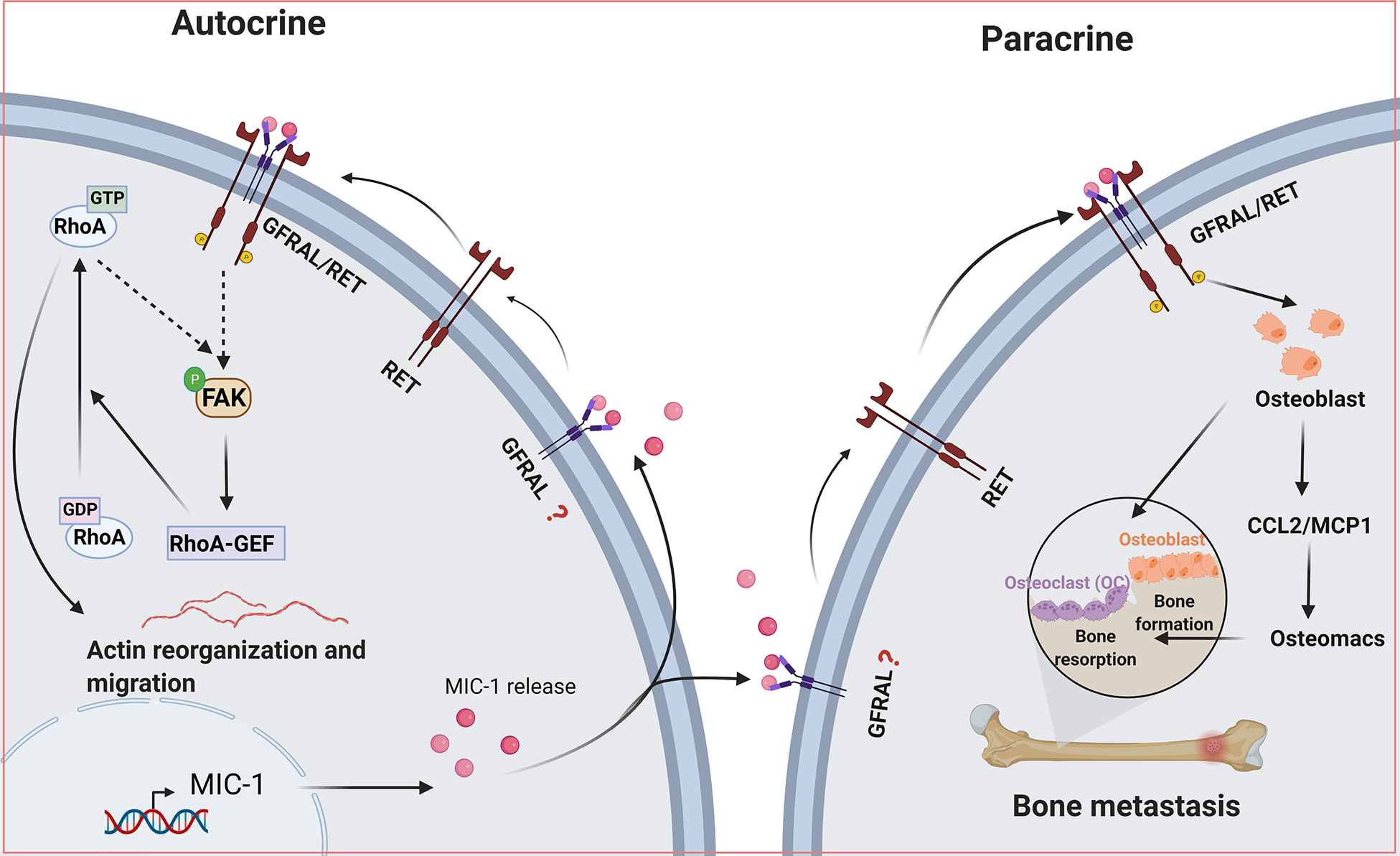
Autocrine and paracrine effects of Macrophage inhibitory cytokine-1 (MIC-1) in cancer cell invasion and metastasis. Tumor cell-derived MIC-1 could induce autocrine action at the primary tumor site and paracrine action in tumor microenvironment and in metastasis.
4.6. MIC-1 and cell death resistance mechanism
Antiapoptotic mechanisms are one of the primary reasons cancer cells escape chemotherapeutic agent-induced apoptosis. Various DNA damage-inducing therapies induce MIC-1 expression and secretion in cancer cells. Early evidence showed that the association between MIC-1 and apoptosis is its induction by various chemotherapeutic agents, natural product derivatives, and cytokines [3]. DNA damage-induced MIC-1 expression in cancer cells through the cell cycle regulator p53 [47]. In accordance with the antitumor effect, MIC-1 overexpression inhibits prostate tumor growth in vitro and in vivo [94, 95]. MIC-1 overexpression in DU145 and A549 prostate and lung cancer cells, respectively, induced apoptosis [96]. A recent study revealed that defined serum components induced endoplasmic reticulum (ER) stress mediated MIC-1 upregulation and apoptosis in multiple cancer cells [97]. Alternatively, the suppression of MIC-1 using siRNA improved the efficacy of docetaxel in prostate cancer cells [98]. Furthermore, rhMIC-1 treatment and MIC-1 overexpression in PC-3 prostate cancer cells contribute to chemoresistance to docetaxel [98]. MIC-1 persistent pancreatic cancer syngeneic cells had a lower probability of undergoing apoptosis than MIC-1 knockdown cells, which is further reflected in mice injected with MIC-1 knockdown cells that survived longer [16]. These results suggest that MIC-1 plays a paradoxical role in cell growth and tumor progression depending on the context and cell type.
4.7. MIC-1 and angiogenesis
Angiogenesis plays an important role in the development of tumors by supplying nutrients and oxygen and removing metabolic by-products from actively proliferating cells. Angiogenesis is also an integral factor in tumor proliferation and protection against immune destruction. Higher MIC-1 levels have been associated with malignancy and facilitate tumor angiogenesis and VEGF expression [99]. Not only in tumor progression, but MIC-1 expression has also been shown to be provoked by various chemotherapeutic agents [100]. The formation of neovascular vessels in tumor cells is activated by hypoxia-mediated expression of VEGF, which helps tumor progression. Tumor-derived epithelial cells can act on endothelial cells to induce angiogenesis. Under hypoxic conditions, tumor-derived MIC-1 was shown to promote angiogenesis by inhibiting p53 activity, which then increased and stabilized HIF-1α expression in endothelial cells [101]. In addition, MIC-1-mediated activation of phosphoinositide 3-kinase (PI3K)/AKT could induce NO, and thus induce endothelial cell proliferation. Furthermore, hypoxia-mediated activation of the transcription factor STAT3 could also regulate angiogenesis [101].
Several studies have shown that MIC-1 plays a crucial role in the regulation of endothelial function after vascular injury by promoting neovascularization. However, MIC-1 has been shown to interact with CCN2 and inhibit αVβ3 integrin clustering, and FAK activation in HUVEC cells and ultimately inhibits tube formation [102]. Additionally, the action of MIC-1 on endothelial cells under physiological conditions is very different where the induction of NO-signaling is through constitutive activation of eNOS [103]. The endothelium could reduce injuries and accelerate repair through various inherent mechanisms. In cancers, there are excessive blood vessels, and new vessel formation depends on hypoxia-mediated VEGF secretion and endothelial cell proliferation. It has also been shown that tumor cells secreting MIC-1 could increase angiogenesis by activating the PI3K/AKT signaling pathway and enhancing the E2F-dependent expression of G(1)-cyclins in endothelial cells [104]. Anti-hMIC-1 monoclonal antibody-treated esophageal squamous cell carcinoma xenograft mice showed reduced neovascularization, suggesting that MIC-1 could induce angiogenesis in tumor progression. These results suggest the significance of MIC-1 in angiogenesis (Figure 5); however, further studies are necessary to determine the context-dependent downstream signaling in cancer.
Figure 5:
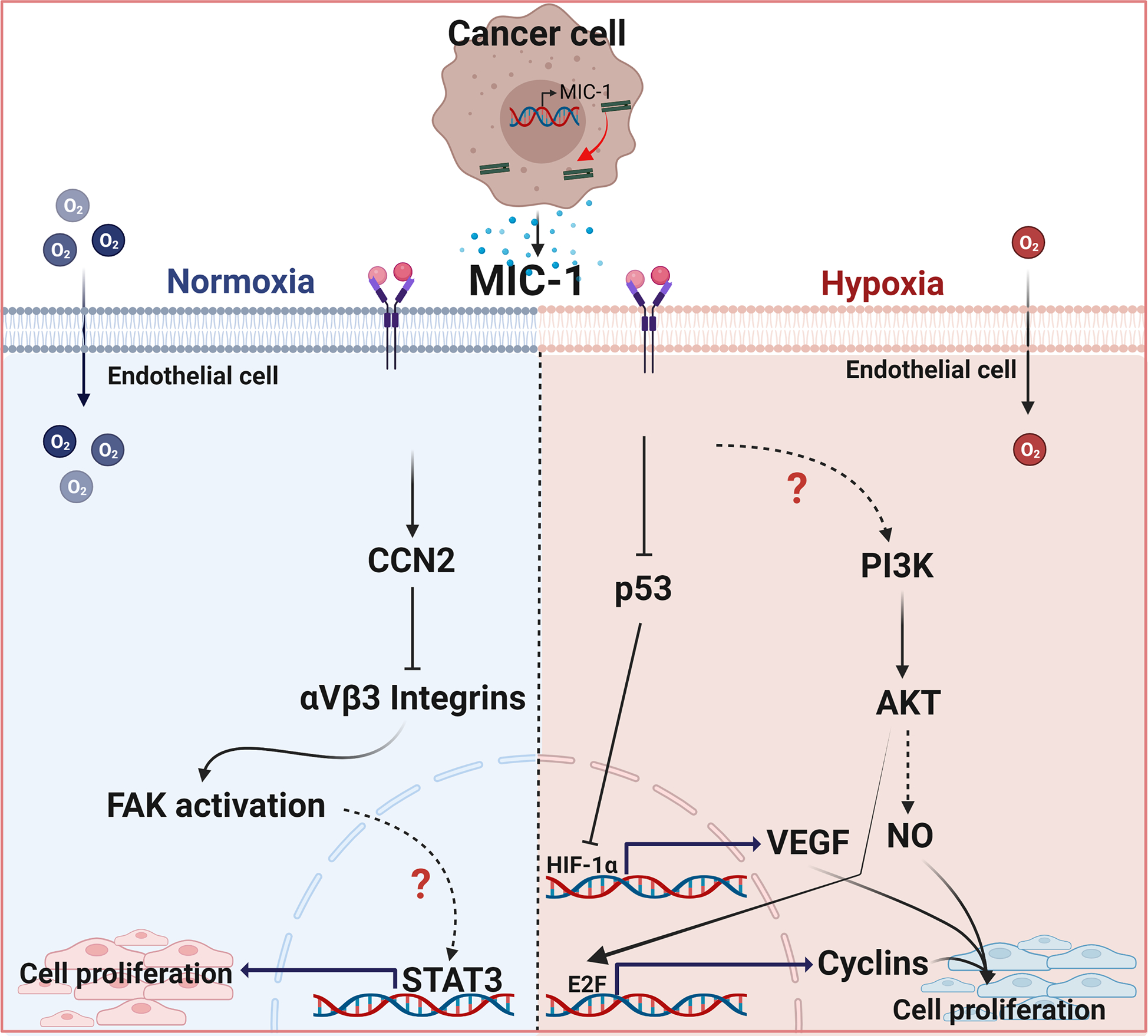
Macrophage inhibitory cytokine-1 (MIC-1) mediated action on endothelial cells. Under normoxic conditions, MIC-1, possibly through GFRAL, induces CCN2 and inhibits αVβ3 integrin clusteringand prevent endothelial cell proliferation. In hypoxic conditions, MIC-1 could inhibit P53 which then stabilizes the HIF-1α and induces VEGF expression.
5. Conclusions and future perspectives
Cancer cell proliferation and tumor progression result from the combined action of many factors, including an inflammatory-rich environment, immune dysfunction, loss of tumor suppressors, activation of oncogenes, presence of cytokines and growth factors, and activated stroma. Over the past two decades, accumulated evidence shows that MIC-1 can play an important role in cancer pathophysiology. Research shown that MIC-1 is contributed by cancer cells and plays a pleiotropic role by acting on the same tumor cells or other cell types present in the tumor microenvironment. In fact, this cytokine acts and regulates many cell types for the concerted action of tumor progression. Furthermore, MIC-1 has been well considered as a surrogate biomarker in most cancer conditions, such as tumor progression, tumor relapse, and resistance to therapy. This review has discussed the transcriptional regulation of MIC-1 and how MIC-1 regulates cancer hallmarks and downstream signaling. In fact, MIC-1 exerts both pro- and antitumorigenic roles. This is likely due to differential signal transduction and immunomodulatory pathways executed by MIC-1 in cell-type and context-dependent manner [11, 84, 85]. Although earlier studies hypothesized that MIC-1 executes downstream signaling through classical TGF-β receptors [34, 105], most underlying mechanisms of MIC-1 signaling in cancers remain elusive. Some studies have even proposed noncanonical signaling through ErbB2 signaling through the feedback loop. Recently, GFRAL has been identified as a receptor for MIC-1, and since then, MIC-1 has received much attention for its anti-obesity action. However, downstream GFRAL signaling is not explicit and is becoming a significant challenge based on the report that GFRAL is restricted to the brain stem under physiological conditions. However, considering the abundant expression of MIC-1 in the placenta and prostate and the ubiquitous induction of MIC-1 in pathological conditions, studies on the expression profile of GFRAL are of utmost need. Interestingly, the GFRAL coreceptor RET is expressed in most aggressive tumors [106]. Therefore, the functional roles of MIC-1 in various cancer phenotypes may have discrete roles in early tumor development, progression, and metastasis. Therefore, future studies on downstream signaling in cancer are essential in developing novel treatment strategies against cancer progression.
Highlights:
MIC-1 regulates various cancer hallmarks, including metastasis and immune evasion
MIC-1 acts as a surrogate biomarker of cancers progression and therapy response
MIC-1 regulation is context and cell-type dependent
MIC-1 downstream signaling majorly occurs via ERK and AKT activation
MIC-1 plays a biphasic role in cancer, which is context and signaling dependent
Acknowledgments
The authors are, in part, supported by the National Institutes of Health (NIH) Grant U01 CA185148 (SKB) and Department of Defense Award W81XWH-18-1-0308 (SKB). The figures were created using Biorender.com.
Abbreviations
- AP-1
Activator protein-1
- AR
Androgen receptor
- ATF3
Activating transcription factor 3
- BRD4
Bromodomain-containing protein 4
- CEBPB
the CCAAT/enhancer binding protein
- EGR1
Early growth response 1
- eNOS
endothelial nitric oxide synthase
- ErbB2
Human epidermal growth factor receptor 2
- ErbB3
Human epidermal growth factor receptor 3
- FAK
Focal Adhesion Kinase
- FASN
Fatty Acid Synthase
- GFRAL
Glial-derived neurotrophic factor family receptor α-like
- HIF-1a
Hypoxia-inducible factor-1a
- HUVEC
Human umbilical vein endothelial cells
- KLF4
Kruppel Like Factor 4
- Kras
KRAS Proto-oncogene, GTPase
- MAPK
Mitogen-activated protein kinase1
- PI3K/Akt
Phosphoinositide-3-kinase/ AKT Serine/Threonine kinase
- PTEN
Phosphatase and tensin homolog
- MIC-1
Macrophage inhibitory cytokine-1
- NF-κB
Nuclear factor κ B subunit
- NR5A2
Nuclear receptor subfamily 5 group A member 2
- p53
Tumor protein P53
- PDGFα
Platelet derived growth factor subunit A
- RET
Rearranged during transfection
- RFS
Recurrence-free survival
- SMAD2
SMAD family member 2
- STAT3
Signal transducer and activator of transcription 3
- TAK1
TGF-β–activated kinase 1
- TAMs
Tumor-associated macrophages
- TGF-β
Transforming Growth Factor β 1
- VEGF
Vascular endothelial growth factor
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
SKB is a founding member of Sanguine Diagnostics and Therapeutics, Inc. Other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J Clin, 71 (2021) 209–249. [DOI] [PubMed] [Google Scholar]
- [2].Mimeault M, Batra SK, Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer, J Cell Physiol, 224 (2010) 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN, The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases, Cell Metab, 28 (2018) 353–368. [DOI] [PubMed] [Google Scholar]
- [4].Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, Sutherland RL, Henshall SM, Horvath LG, Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling, Cancer Res, 69 (2009) 7696–7703. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Y, Hua W, Niu LC, Li SM, Wang YM, Shang L, Zhang C, Li WN, Wang R, Chen BL, Xin XY, Zhang YQ, Wang J, Elevated growth differentiation factor 15 expression predicts poor prognosis in epithelial ovarian cancer patients, Tumour Biol, 37 (2016) 9423–9431. [DOI] [PubMed] [Google Scholar]
- [6].Wang X, Yang Z, Tian H, Li Y, Li M, Zhao W, Zhang C, Wang T, Liu J, Zhang A, Shen D, Zheng C, Qi J, Zhao D, Shi J, Jin L, Rao J, Zhang W, Circulating MIC-1/GDF15 is a complementary screening biomarker with CEA and correlates with liver metastasis and poor survival in colorectal cancer, Oncotarget, 8 (2017) 24892–24901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schiegnitz E, Kammerer PW, Koch FP, Kruger M, Berres M, Al-Nawas B, GDF 15 as an anti-apoptotic, diagnostic and prognostic marker in oral squamous cell carcinoma, Oral Oncol, 48 (2012) 608–614. [DOI] [PubMed] [Google Scholar]
- [8].Nyakas M, Aamdal E, Jacobsen KD, Guren TK, Aamdal S, Hagene KT, Brunsvig P, Yndestad A, Halvorsen B, Tasken KA, Aukrust P, Maelandsmo GM, Ueland T, Prognostic biomarkers for immunotherapy with ipilimumab in metastatic melanoma, Clin Exp Immunol, 197 (2019) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A, Brand R, Guha S, Jain M, Wittel U, Singh SK, Batra SK, Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer, PLoS One, 8 (2013) e55171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson HF Jr., Hampton GM, Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum, Proc Natl Acad Sci U S A, 100 (2003) 3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wischhusen J, Melero I, Fridman WH, Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint, Front Immunol, 11 (2020) 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li J, Veltri RW, Yuan Z, Christudass CS, Mandecki W, Macrophage inhibitory cytokine 1 biomarker serum immunoassay in combination with PSA is a more specific diagnostic tool for detection of prostate cancer, PLoS One, 10 (2015) e0122249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arfsten H, Cho A, Freitag C, Raderer M, Goliasch G, Bartko PE, Wurm R, Strunk G, Gisslinger H, Marosi C, Kornek G, Zielinski C, Hulsmann M, Pavo N, GDF-15 in solid vs non-solid treatment-naive malignancies, Eur J Clin Invest, 49 (2019) e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Gronberg H, Breit SN, Wiklund FE, Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer, Clin Cancer Res, 15 (2009) 6658–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao Y, Xu Y, Zhao S, Qian L, Song T, Zheng J, Zhang J, Chen B, Growth differentiation factor-15 promotes immune escape of ovarian cancer via targeting CD44 in dendritic cells, Exp Cell Res, 402 (2021) 112522. [DOI] [PubMed] [Google Scholar]
- [16].Ratnam NM, Peterson JM, Talbert EE, Ladner KJ, Rajasekera PV, Schmidt CR, Dillhoff ME, Swanson BJ, Haverick E, Kladney RD, Williams TM, Leone GW, Wang DJ, Guttridge DC, NF-kappaB regulates GDF-15 to suppress macrophage surveillance during early tumor development, J Clin Invest, 127 (2017) 3796–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN, Tabatabai G, Wick W, Weller M, Wischhusen J, GDF-15 contributes to proliferation and immune escape of malignant gliomas, Clin Cancer Res, 16 (2010) 3851–3859. [DOI] [PubMed] [Google Scholar]
- [18].Wang Z, He L, Li W, Xu C, Zhang J, Wang D, Dou K, Zhuang R, Jin B, Zhang W, Hao Q, Zhang K, Zhang W, Wang S, Gao Y, Gu J, Shang L, Tan Z, Su H, Zhang Y, Zhang C, Li M, GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma, J Immunother Cancer, 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X, The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL, Nat Med, 23 (2017) 1215–1219. [DOI] [PubMed] [Google Scholar]
- [20].Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, To C, Mondal K, Li B, Kekatpure A, Wang M, Laird T, Horner G, Chan J, McEntee M, Lopez M, Lakshminarasimhan D, White A, Wang SP, Yao J, Yie J, Matern H, Solloway M, Haldankar R, Parsons T, Tang J, Shen WD, Alice Chen Y, Tian H, Allan BB, Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15, Nature, 550 (2017) 255–259. [DOI] [PubMed] [Google Scholar]
- [21].Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM, GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates, Nat Med, 23 (2017) 1150–1157. [DOI] [PubMed] [Google Scholar]
- [22].Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Norgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jorgensen SB, GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand, Nat Med, 23 (2017) 1158–1166. [DOI] [PubMed] [Google Scholar]
- [23].Hanahan D, Hallmarks of Cancer: New Dimensions, Cancer Discov, 12 (2022) 31–46. [DOI] [PubMed] [Google Scholar]
- [24].Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN, MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation, J Leukoc Biol, 65 (1999) 2–5. [DOI] [PubMed] [Google Scholar]
- [25].Corre J, Hebraud B, Bourin P, Concise review: growth differentiation factor 15 in pathology: a clinical role?, Stem Cells Transl Med, 2 (2013) 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science, 347 (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [27].O’Neill RS, Emmanuel S, Williams D, Stoita A, Macrophage inhibitory cytokine-1/growth differentiation factor-15 in premalignant and neoplastic tumours in a high-risk pancreatic cancer cohort, World J Gastroenterol, 26 (2020) 1660–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rasiah KK, Kench JG, Gardiner-Garden M, Biankin AV, Golovsky D, Brenner PC, Kooner R, O’Neill G F, Turner JJ, Delprado W, Lee CS, Brown DA, Breit SN, Grygiel JJ, Horvath LG, Stricker PD, Sutherland RL, Henshall SM, Aberrant neuropeptide Y and macrophage inhibitory cytokine-1 expression are early events in prostate cancer development and are associated with poor prognosis, Cancer Epidemiol Biomarkers Prev, 15 (2006) 711–716. [DOI] [PubMed] [Google Scholar]
- [29].Bansal N, Kumar D, Gupta A, Chandra D, Sankhwar SN, Mandhani A, Relevance of MIC-1 in the Era of PSA as a Serum Based Predictor of Prostate Cancer: A Critical Evaluation, Sci Rep, 7 (2017) 16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, Laiyemo AO, Wang Z, Cross AJ, Schatzkin A, Danta M, Srasuebkul P, Amin J, Law M, Breit SN, Lanza E, Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer?, Cancer Epidemiol Biomarkers Prev, 21 (2012) 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Modi A, Purohit P, Gadwal A, Roy D, Fernandes S, Vishnoi JR, Pareek P, Elhence P, Misra S, Sharma P, A Combined Analysis of Serum Growth Differentiation Factor-15 and Cancer Antigen 15–3 Enhances the Diagnostic Efficiency in Breast Cancer, EJIFCC, 32 (2021) 363–376. [PMC free article] [PubMed] [Google Scholar]
- [32].Wang XB, Jiang XR, Yu XY, Wang L, He S, Feng FY, Guo LP, Jiang W, Lu SH, Macrophage inhibitory factor 1 acts as a potential biomarker in patients with esophageal squamous cell carcinoma and is a target for antibody-based therapy, Cancer Sci, 105 (2014) 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Selander KS, Brown DA, Sequeiros GB, Hunter M, Desmond R, Parpala T, Risteli J, Breit SN, Jukkola-Vuorinen A, Serum macrophage inhibitory cytokine-1 concentrations correlate with the presence of prostate cancer bone metastases, Cancer Epidemiol Biomarkers Prev, 16 (2007) 532–537. [DOI] [PubMed] [Google Scholar]
- [34].Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN, Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1, Nat Med, 13 (2007) 1333–1340. [DOI] [PubMed] [Google Scholar]
- [35].Luan JC, Zhang QJ, Zhao K, Zhou X, Yao LY, Zhang TT, Zeng TY, Xia JD, Song NH, A Novel Set of Immune-associated Gene Signature predicts Biochemical Recurrence in Localized Prostate Cancer Patients after Radical Prostatectomy, J Cancer, 12 (2021) 3715–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN, MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma, Clin Cancer Res, 9 (2003) 2642–2650. [PubMed] [Google Scholar]
- [37].Danta M, Barber DA, Zhang HP, Lee-Ng M, Baumgart SWL, Tsai VWW, Husaini Y, Saxena M, Marquis CP, Errington W, Kerr S, Breit SN, Brown DA, Macrophage inhibitory cytokine-1/growth differentiation factor-15 as a predictor of colonic neoplasia, Aliment Pharmacol Ther, 46 (2017) 347–354. [DOI] [PubMed] [Google Scholar]
- [38].Vocka M, Langer D, Fryba V, Petrtyl J, Hanus T, Kalousova M, Zima T, Petruzelka L, Growth/differentiation factor 15 (GDF-15) as new potential serum marker in patients with metastatic colorectal cancer, Cancer Biomark, 21 (2018) 869–874. [DOI] [PubMed] [Google Scholar]
- [39].Li C, Wang J, Kong J, Tang J, Wu Y, Xu E, Zhang H, Lai M, GDF15 promotes EMT and metastasis in colorectal cancer, Oncotarget, 7 (2016) 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hogendorf P, Durczynski A, Skulimowski A, Kumor A, Poznanska G, Strzelczyk J, Growth differentiation factor (GDF-15) concentration combined with Ca125 levels in serum is superior to commonly used cancer biomarkers in differentiation of pancreatic mass, Cancer Biomark, 21 (2018) 505–511. [DOI] [PubMed] [Google Scholar]
- [41].Zhao D, Wang X, Zhang W, GDF15 predict platinum response during first-line chemotherapy and can act as a complementary diagnostic serum biomarker with CA125 in epithelial ovarian cancer, BMC Cancer, 18 (2018) 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Corre J, Labat E, Espagnolle N, Hebraud B, Avet-Loiseau H, Roussel M, Huynh A, Gadelorge M, Cordelier P, Klein B, Moreau P, Facon T, Fournie JJ, Attal M, Bourin P, Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma, Cancer Res, 72 (2012) 1395–1406. [DOI] [PubMed] [Google Scholar]
- [43].Suesskind D, Schatz A, Schnichels S, Coupland SE, Lake SL, Wissinger B, Bartz-Schmidt KU, Henke-Fahle S, GDF-15: a novel serum marker for metastases in uveal melanoma patients, Graefes Arch Clin Exp Ophthalmol, 250 (2012) 887–895. [DOI] [PubMed] [Google Scholar]
- [44].Liu YN, Wang XB, Wang T, Zhang C, Zhang KP, Zhi XY, Zhang W, Sun KL, Macrophage Inhibitory Cytokine-1 as a Novel Diagnostic and Prognostic Biomarker in Stage I and II Nonsmall Cell Lung Cancer, Chin Med J (Engl), 129 (2016) 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fisher OM, Levert-Mignon AJ, Lord SJ, Lee-Ng KK, Botelho NK, Falkenback D, Thomas ML, Bobryshev YV, Whiteman DC, Brown DA, Breit SN, Lord RV, MIC-1/GDF15 in Barrett’s oesophagus and oesophageal adenocarcinoma, Br J Cancer, 112 (2015) 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Windrichova J, Fuchsova R, Kucera R, Topolcan O, Fiala O, Finek J, Slipkova D, MIC1/GDF15 as a Bone Metastatic Disease Biomarker, Anticancer Res, 37 (2017) 1501–1505. [DOI] [PubMed] [Google Scholar]
- [47].Kannan K, Amariglio N, Rechavi G, Givol D, Profile of gene expression regulated by induced p53: connection to the TGF-beta family, FEBS Lett, 470 (2000) 77–82. [DOI] [PubMed] [Google Scholar]
- [48].Kelly JA, Lucia MS, Lambert JR, p53 controls prostate-derived factor/macrophage inhibitory cytokine/NSAID-activated gene expression in response to cell density, DNA damage and hypoxia through diverse mechanisms, Cancer Lett, 277 (2009) 38–47. [DOI] [PubMed] [Google Scholar]
- [49].Kim JS, Lee C, Bonifant CL, Ressom H, Waldman T, Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA, Mol Cell Biol, 27 (2007) 662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC, Klamut HJ, Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression, J Biol Chem, 275 (2000) 20127–20135. [DOI] [PubMed] [Google Scholar]
- [51].Ichikawa T, Suenaga Y, Koda T, Ozaki T, Nakagawara A, TAp63-dependent induction of growth differentiation factor 15 (GDF15) plays a critical role in the regulation of keratinocyte differentiation, Oncogene, 27 (2008) 409–420. [DOI] [PubMed] [Google Scholar]
- [52].Tan M, Wang Y, Guan K, Sun Y, PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway, Proc Natl Acad Sci U S A, 97 (2000) 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yamaguchi K, Lee SH, Eling TE, Baek SJ, Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway, J Biol Chem, 279 (2004) 49617–49623. [DOI] [PubMed] [Google Scholar]
- [54].Modlich O, Prisack HB, Munnes M, Audretsch W, Bojar H, Immediate gene expression changes after the first course of neoadjuvant chemotherapy in patients with primary breast cancer disease, Clin Cancer Res, 10 (2004) 6418–6431. [DOI] [PubMed] [Google Scholar]
- [55].Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME, Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1, Oncogene, 21 (2002) 4212–4219. [DOI] [PubMed] [Google Scholar]
- [56].Jin Y, Jung SN, Lim MA, Oh C, Piao Y, Kim HJ, Liu L, Kang YE, Chang JW, Won HR, Song K, Koo BS, Transcriptional Regulation of GDF15 by EGR1 Promotes Head and Neck Cancer Progression through a Positive Feedback Loop, Int J Mol Sci, 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE, Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein, Mol Pharmacol, 67 (2005) 356–364. [DOI] [PubMed] [Google Scholar]
- [58].Shim M, Eling TE, Protein kinase C-dependent regulation of NAG-1/placental bone morphogenic protein/MIC-1 expression in LNCaP prostate carcinoma cells, J Biol Chem, 280 (2005) 18636–18642. [DOI] [PubMed] [Google Scholar]
- [59].Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ, Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth, Mol Cell Biol, 30 (2010) 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN, MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily, Proc Natl Acad Sci U S A, 94 (1997) 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang Y, Moszczynski LA, Liu Q, Jiang J, Zhao D, Quan D, Mele T, McAlister V, Jevnikar A, Baek SJ, Liu K, Zheng X, Over-expression of growth differentiation factor 15 (GDF15) preventing cold ischemia reperfusion (I/R) injury in heart transplantation through Foxo3a signaling, Oncotarget, 8 (2017) 36531–36544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, Goldspink DA, Miedzybrodzka EL, Konopka AR, Esponda RR, Huang JT, Tung YCL, Rodriguez-Cuenca S, Tomaz RA, Harding HP, Melvin A, Yeo GSH, Preiss D, Vidal-Puig A, Vallier L, Nair KS, Wareham NJ, Ron D, Gribble FM, Reimann F, Sattar N, Savage DB, Allan BB, O’Rahilly S, GDF15 mediates the effects of metformin on body weight and energy balance, Nature, 578 (2020) 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, Cimino I, Maurin AC, Roberts GP, Meek CL, Virtue S, Sparks LM, Parsons SA, Redman LM, Bray GA, Liou AP, Woods RM, Parry SA, Jeppesen PB, Kolnes AJ, Harding HP, Ron D, Vidal-Puig A, Reimann F, Gribble FM, Hulston CJ, Farooqi IS, Fafournoux P, Smith SR, Jensen J, Breen D, Wu Z, Zhang BB, Coll AP, Savage DB, O’Rahilly S, GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans, Cell Metab, 29 (2019) 707–718 e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li D, Zhang H, Zhong Y, Hepatic GDF15 is regulated by CHOP of the unfolded protein response and alleviates NAFLD progression in obese mice, Biochem Biophys Res Commun, 498 (2018) 388–394. [DOI] [PubMed] [Google Scholar]
- [65].Guo LL, Wang SF, Downregulated Long Noncoding RNA GAS5 Fails to Function as Decoy of CEBPB, Resulting in Increased GDF15 Expression and Rapid Ovarian Cancer Cell Proliferation, Cancer Biother Radiopharm, 34 (2019) 537–546. [DOI] [PubMed] [Google Scholar]
- [66].Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD, Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family, J Biol Chem, 273 (1998) 13760–13767. [DOI] [PubMed] [Google Scholar]
- [67].Zhao C, Li Y, Qiu W, He F, Zhang W, Zhao D, Zhang Z, Zhang E, Ma P, Liu Y, Ma L, Yang F, Wang Y, Shu Y, C5a induces A549 cell proliferation of non-small cell lung cancer via GDF15 gene activation mediated by GCN5-dependent KLF5 acetylation, Oncogene, 37 (2018) 4821–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jones AC, Trujillo KA, Phillips GK, Fleet TM, Murton JK, Severns V, Shah SK, Davis MS, Smith AY, Griffith JK, Fischer EG, Bisoffi M, Early growth response 1 and fatty acid synthase expression is altered in tumor adjacent prostate tissue and indicates field cancerization, Prostate, 72 (2012) 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jones AC, Antillon KS, Jenkins SM, Janos SN, Overton HN, Shoshan DS, Fischer EG, Trujillo KA, Bisoffi M, Prostate field cancerization: deregulated expression of macrophage inhibitory cytokine 1 (MIC-1) and platelet derived growth factor A (PDGF-A) in tumor adjacent tissue, PLoS One, 10 (2015) e0119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Guo F, Zhou Y, Guo H, Ren D, Jin X, Wu H, NR5A2 transcriptional activation by BRD4 promotes pancreatic cancer progression by upregulating GDF15, Cell Death Discov, 7 (2021) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim KK, Lee JJ, Yang Y, You KH, Lee JH, Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells, Carcinogenesis, 29 (2008) 704–712. [DOI] [PubMed] [Google Scholar]
- [72].Zhao TC, Zhou ZH, Ju WT, Liang SY, Tang X, Zhu DW, Zhang ZY, Zhong LP, Mechanism of sensitivity to cisplatin, docetaxel, and 5-fluorouracil chemoagents and potential erbB2 alternatives in oral cancer with growth differentiation factor 15 overexpression, Cancer Sci, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, Batra SK, Lin MF, Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells, Prostate, 67 (2007) 557–571. [DOI] [PubMed] [Google Scholar]
- [74].Liu T, Bauskin AR, Zaunders J, Brown DA, Pankhurst S, Russell PJ, Breit SN, Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells, Cancer Res, 63 (2003) 5034–5040. [PubMed] [Google Scholar]
- [75].Husaini Y, Qiu MR, Lockwood GP, Luo XW, Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA, Breit SN, Macrophage inhibitory cytokine-1 (MIC-1/GDF15) slows cancer development but increases metastases in TRAMP prostate cancer prone mice, PLoS One, 7 (2012) e43833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Urakawa N, Utsunomiya S, Nishio M, Shigeoka M, Takase N, Arai N, Kakeji Y, Koma Y, Yokozaki H, GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways, Lab Invest, 95 (2015) 491–503. [DOI] [PubMed] [Google Scholar]
- [77].Kang YE, Kim JM, Lim MA, Lee SE, Yi S, Kim JT, Oh C, Liu L, Jin Y, Jung SN, Won HR, Chang JW, Lee JH, Kim HJ, Koh HY, Jun S, Cho SW, Shong M, Koo BS, Growth Differentiation Factor 15 is a Cancer Cell-Induced Mitokine That Primes Thyroid Cancer Cells for Invasiveness, Thyroid, 31 (2021) 772–786. [DOI] [PubMed] [Google Scholar]
- [78].Myojin Y, Hikita H, Sugiyama M, Sasaki Y, Fukumoto K, Sakane S, Makino Y, Takemura N, Yamada R, Shigekawa M, Kodama T, Sakamori R, Kobayashi S, Tatsumi T, Suemizu H, Eguchi H, Kokudo N, Mizokami M, Takehara T, Hepatic Stellate Cells in Hepatocellular Carcinoma Promote Tumor Growth Via Growth Differentiation Factor 15 Production, Gastroenterology, 160 (2021) 1741–1754 e1716. [DOI] [PubMed] [Google Scholar]
- [79].Zhao Z, Zhang J, Yin L, Yang J, Zheng Y, Zhang M, Ni B, Wang H, Upregulated GDF-15 expression facilitates pancreatic ductal adenocarcinoma progression through orphan receptor GFRAL, Aging (Albany NY), 12 (2020) 22564–22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tsui KH, Hsu SY, Chung LC, Lin YH, Feng TH, Lee TY, Chang PL, Juang HH, Growth differentiation factor-15: a p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells, Sci Rep, 5 (2015) 12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Griner SE, Joshi JP, Nahta R, Growth differentiation factor 15 stimulates rapamycin-sensitive ovarian cancer cell growth and invasion, Biochem Pharmacol, 85 (2013) 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Joshi JP, Brown NE, Griner SE, Nahta R, Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells, Biochem Pharmacol, 82 (2011) 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A, Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability, Carcinogenesis, 30 (2009) 1073–1081. [DOI] [PubMed] [Google Scholar]
- [84].Sadasivan SM, Chen Y, Gupta NS, Han X, Bobbitt KR, Chitale DA, Williamson SR, Rundle AG, Tang D, Rybicki BA, The interplay of growth differentiation factor 15 (GDF15) expression and M2 macrophages during prostate carcinogenesis, Carcinogenesis, 41 (2020) 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, Zhang C, Ring AM, Young LH, Medzhitov R, GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance, Cell, 178 (2019) 1231–1244 e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Karan D, Holzbeierlein J, Thrasher JB, Macrophage inhibitory cytokine-1: possible bridge molecule of inflammation and prostate cancer, Cancer Res, 69 (2009) 2–5. [DOI] [PubMed] [Google Scholar]
- [87].Verhamme FM, Seys LJM, De Smet EG, Provoost S, Janssens W, Elewaut D, Joos GF, Brusselle GG, Bracke KR, Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure, Mucosal Immunol, 10 (2017) 1400–1411. [DOI] [PubMed] [Google Scholar]
- [88].Hayashi T, Fujita K, Matsushita M, Nonomura N, Main Inflammatory Cells and Potentials of Anti-Inflammatory Agents in Prostate Cancer, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou Z, Li W, Song Y, Wang L, Zhang K, Yang J, Zhang W, Su H, Zhang Y, Growth differentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specific immune response, PLoS One, 8 (2013) e78618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang CZ, Ma J, Zhu DW, Liu Y, Montgomery B, Wang LZ, Li J, Zhang ZY, Zhang CP, Zhong LP, GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma, Ann Oncol, 25 (2014) 1215–1222. [DOI] [PubMed] [Google Scholar]
- [91].Siddiqui JA, Seshacharyulu P, Muniyan S, Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM, Jain M, Datta K, Nasser MW, Batra SK, GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation, Bone Res, 10 (2022) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK, Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway, Oncogene, 29 (2010) 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Duan L, Pang HL, Chen WJ, Shen WW, Cao PP, Wang SM, Liu LL, Zhang HL, The role of GDF15 in bone metastasis of lung adenocarcinoma cells, Oncol Rep, 41 (2019) 2379–2388. [DOI] [PubMed] [Google Scholar]
- [94].Wang X, Chrysovergis K, Bienstock RJ, Shim M, Eling TE, The H6D variant of NAG-1/GDF15 inhibits prostate xenograft growth in vivo, Prostate, 72 (2012) 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cheng JC, Chang HM, Leung PC, Wild-type p53 attenuates cancer cell motility by inducing growth differentiation factor-15 expression, Endocrinology, 152 (2011) 2987–2995. [DOI] [PubMed] [Google Scholar]
- [96].Tarfiei GA, Shadboorestan A, Montazeri H, Rahmanian N, Tavosi G, Ghahremani MH, GDF15 induced apoptosis and cytotoxicity in A549 cells depends on TGFBR2 expression, Cell Biochem Funct, 37 (2019) 320–330. [DOI] [PubMed] [Google Scholar]
- [97].Scheffer D, Kulcsar G, Nagyeri G, Kiss-Merki M, Rekasi Z, Maloy M, Czompoly T, Active mixture of serum-circulating small molecules selectively inhibits proliferation and triggers apoptosis in cancer cells via induction of ER stress, Cell Signal, 65 (2020) 109426. [DOI] [PubMed] [Google Scholar]
- [98].Mimeault M, Johansson SL, Batra SK, Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1, Br J Cancer, 108 (2013) 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dong G, Zheng QD, Ma M, Wu SF, Zhang R, Yao RR, Dong YY, Ma H, Gao DM, Ye SL, Cui JF, Ren ZG, Chen RX, Angiogenesis enhanced by treatment damage to hepatocellular carcinoma through the release of GDF15, Cancer Med, 7 (2018) 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT, Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation, Mol Cancer Ther, 2 (2003) 1023–1029. [PubMed] [Google Scholar]
- [101].Song H, Yin D, Liu Z, GDF-15 promotes angiogenesis through modulating p53/HIF-1alpha signaling pathway in hypoxic human umbilical vein endothelial cells, Mol Biol Rep, 39 (2012) 4017–4022. [DOI] [PubMed] [Google Scholar]
- [102].Whitson RJ, Lucia MS, Lambert JR, Growth differentiation factor-15 (GDF-15) suppresses in vitro angiogenesis through a novel interaction with connective tissue growth factor (CCN2), J Cell Biochem, 114 (2013) 1424–1433. [DOI] [PubMed] [Google Scholar]
- [103].Mazagova M, Buikema H, Landheer SW, Vavrinec P, Buiten A, Henning RH, Deelman LE, Growth differentiation factor 15 impairs aortic contractile and relaxing function through altered caveolar signaling of the endothelium, Am J Physiol Heart Circ Physiol, 304 (2013) H709–718. [DOI] [PubMed] [Google Scholar]
- [104].Jin YJ, Lee JH, Kim YM, Oh GT, Lee H, Macrophage inhibitory cytokine-1 stimulates proliferation of human umbilical vein endothelial cells by up-regulating cyclins D1 and E through the PI3K/Akt-, ERK-, and JNK-dependent AP-1 and E2F activation signaling pathways, Cell Signal, 24 (2012) 1485–1495. [DOI] [PubMed] [Google Scholar]
- [105].Artz A, Butz S, Vestweber D, GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-betaRII heterodimer, Blood, 128 (2016) 529–541. [DOI] [PubMed] [Google Scholar]
- [106].Takahashi M, Kawai K, Asai N, Roles of the RET Proto-oncogene in Cancer and Development, JMA J, 3 (2020) 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]


