Background.
Living donor liver transplantation is the main source of organs in the Middle East. Therefore, well balanced criteria are needed to avoid unnecessary exclusion of potential donors, while prioritizing donor safety. We face a high incidence of sickle cell trait (SCT; and disease). Therefore, there is vast experience in general and cardiac surgeries in SCT carriers at our center. After studying their management in detail, we considered accepting SCT carriers as living liver donors, on an exceptional basis. This the first single-center case series of living donor liver transplantation with SCT.
Methods.
Between January 2012 and September 2021, 20 donors with SCT were reviewed for age, gender, relation to the recipient, hemoglobin, hemoglobin S (HbS), surgical approach, intensive care unit stay, donor and recipients’ complications, and graft and recipient survival.
Results.
Average age of donors was 28.4 y. Sixteen donated the left lateral segment, 4 the left lobe. Recipients were related children or adults. HbS ranged from 21.2% to 39.9%, being ≥30% in 14 donors. HbS was reduced by phlebotomy or exchange transfusion. We performed 7 open, one laparoscopic, and 12 robotic donor surgeries. Operating room time, blood loss, and intensive care unit stay were comparable to non-SCT donors. There was no SCT-related complication. All donors are alive and free of thromboembolic events. Graft and recipient survival is 100% until follow-up.
Conclusion.
Our experience should encourage other countries with high incidence of SCT to report their experience with this donor population.
INTRODUCTION
The limiting factor in liver transplantation is the lack of suitable organs, not only due to the low deceased organ quality faced by the Western world but also due to the very low donation rate in most of the Middle Eastern and Asian countries. This limitation leaves living donor liver transplantation as the primary option for the majority of patients with life-threatening liver disease, that is, in our center, approximately 90%. Although Middle Eastern and Asian countries can count themselves as very privileged to have large caring families with strong bonds, providing an astonishing rate of potential living organ donors, donor safety remains the main concern in any living donor program.
We are constantly working on decreasing donor morbidity and improving donors’ quality of life by reducing surgical trauma through minimally invasive techniques such as laparoscopic and robotic surgery. At the same time, we regularly review possible contraindications for living liver donation. For example, there are no guidelines for accepting donors with isolated abnormalities such as sickle cell trait (SCT), a hemoglobinopathy which is not considered as a pathology.
As opposed to sickle cell disease, SCT is a heterozygous hemoglobinopathy of a mutation of the β1-globin gene on chromosome 11 (glutamine 6 valine). In SCT, the consequent sickle hemoglobin (Hb; hemoglobin S [HbS]) concentration in each erythrocyte typically only ranges within 35% to 40%. SCT is protective against lethal childhood malaria, and, therefore, its prevalence is higher in endemic malaria areas, being the highest in Sub-Saharan Africa (30%–40%), the Mediterranean countries, and India. In Saudi Arabia, it is found in 4.2% of the general population and up to 20% of those living in the eastern and southern provinces.1-3 In the United States, it is more common in African-Americans with an incidence of 8% as compared to 0.05% in Caucasians.4
SCT is considered a benign condition, and most countries don’t screen for the disease because it is very rare. In countries with high incidence, blood and deceased organ donation is accepted from those carriers.5 As opposed to homozygous sickle cells disease, SCT does not cause sickling crises; however, there are certain conditions favoring sickling, including dehydration, hypothermia/hyperthermia, and severe hypoxia.6 These events have been described in some case reports of uncommon but potentially lethal complications from sickling-induced vaso-occlusive events mainly involving the renal papillae and medulla, spleen, eyes, and skeletal muscle (exertional rhabdomyolysis) with a risk of cascading into lethal events during times of physiologic stress, such as a major surgery or excessive exercise.7-9 The patient collectives in this literature are mainly North American college athletes or Navy recruits. There is evidence that these events are more common within the North American population than in the Middle East, because these ethnicities are very diverse and overall thromboembolic events are significantly lower in the Middle East.10
Any major operation may lead to hypovolemia, hypoxia, acidosis, and metabolic disturbance with the potential risk of inducing the abovementioned sickling-related complications. At our center, protocols were established for major cardiac surgery and minor abdominal surgery in patients with SCT.11 Adherence to these protocols resulted in very safe procedures for these patients without any sickling events.11 Living donation, however, is purely altruistic, so any harm or potential risk to the donor should be avoided. Since we did face a number of evaluated donors to incidentally be SCT carriers, we reviewed the protocols for abovementioned surgeries, discussed the potential risks in interdisciplinary donor evaluation meetings and started to allow fully informed SCT, and specially consented liver donors to undergo initially only left lateral segment donation, followed by full left lobe donation to directly related children and small adults with end-stage liver disease in situations where there were no other suitable donors.
As survey studies of American and British kidney transplant centers reflect, hardly any center in the West includes SCT screening in their donor evaluation, leaving the actual number of SCT donors unknown.12,13
Even though especially the kidney is potentially harmed by sickling events,14-17 living kidney donors with SCT have been accepted in various centers within the last years.13,18,19 For living liver donation, this is the first observational series.
MATERIALS AND METHODS
Between January 2012 and September 2021, 20 donors with SCT underwent living liver donation at our center. All our donors are screened by Hb electrophoresis. We only considered SCT donors; if not, other donors were available. Patient charts were reviewed retrospectively. The study was conducted in accordance with the ethical principles in the Declaration of Helsinki (2000), the International Conference on Harmonisation Harmonized Tripartite Good Clinical Practice Guidelines, the policies and guidelines of the institution, and the laws of the country. Patient outcomes were analyzed through the use of the institutional database.
Donors were evaluated for age, gender, relation to the recipient, body mass index, preoperative Hb, mean corpuscular volume, blood group, complete blood counts, liver function tests, metabolic testing, infectious disease testing including viral serologies, autoimmune screening, urinalysis, chest radiograph, and computed tomography (CT) of the abdomen. Donors underwent thrombophilia testing and Hb electrophoresis to yield concentrations of HbS, HbA1, HbA2, and HbF. All postoperative courses were analyzed for postoperative Hb and hematocrit, surrogates of perioperative tissue perfusion in the form of arterial pH and lactate, type of donor surgery, pre- and postoperative creatinine level, intensive care unit stay, hospital stay, postoperative pain score, early and late complication, and donor survival. Recipients’ files were only investigated for thrombotic events and organ and patient survival.
All donors received, at the least, hyperhydration as prophylaxis against a sickling event; this protocol consisted of 0.9% normal saline given at a rate of 2× the calculated maintenance rate during the preoperative night. More complex interventions, namely phlebotomy or exchange blood transfusion, were reserved for those donors with HbS levels >30%. If any preoperative reduction of HbS was necessary, we measured postprocedural HbS in almost every donor. Mechanical thromboprophylaxis in the form of sequential compression devices was initiated just before anesthesia, and postoperative anticoagulation (heparin 5000 IU SQ BID) was routinely started on the first postoperative morning. Complication severity was based on the Clavien-Dindo perioperative severity scoring system. Before accepting donors with SCT, all families were screened for alternative donors, but in most cases, other siblings were carriers as well.
RESULTS
During this period, 1465 liver transplantations were performed in our center, of whom 1212 were living donor liver transplants (82.7%). Of these transplants, 20 donors were identified as carriers of SCT. The average age of the 14 male and 6 female donors was 28.4 (18–40) y. Sixteen donated their left lateral segment (LL, segments II and III), and 4 donated their left lobe (segments I–IV). All donors donated their part of the liver to a direct relative. Body weight of the donors ranged from 52 to 95 kg with a mean of 70.3 kg. Donor height ranged from 152 to 185 cm with an average height of 168.2 cm. Donor body mass index ranged from 18.5 to 25 kg/m² with an average of 23.9 g/m² (Figure 1).
FIGURE 1.
Donor characteristics. Details of all 20 donors with regard to sex, age, height, body weight, body mass index (BMI), lobe donated, and relationship to the recipient. f, female; LL, left lateral segment; m, male.
All liver function tests were within normal range preoperatively. No hematuria was noticed in preoperative urine analysis.
All 20 donors were identified as carriers for SCT, based on the characteristic Hb electrophoresis findings. HbS ranged between 21.2% and 40.8 % with a mean of 33.1%. In 6 donors, HbS was ≤30%, ranging from 21.2% to 27.3% with a mean of 24.5%. Fourteen donors had an HbS ≥30%, ranging from 32.0% to 40.8% with a mean of 36.6% (Table 1). Different procedures were undertaken in this donor group to reduce the elevated HbS. Two donors had no special procedure, 1 donor solely had phlebotomy of 450 cc blood and substitution with normal saline, 10 donors had exchange transfusions, of whom 4 had only 1 or 2 units, and 2 donors had 3 units (Table 2). Postprocedural HbS ranged from 20.6% to 29.9% with a mean of 27.5% (Table 2). The mean red blood indices were all in the normal range: mean Hb was 13.6 mg/dL (10.0–16.0), and mean corpuscular volume was 77.9 (59.9–90.9).
TABLE 1.
HbS percentage among all donors
| Patient sex | Hb, g/dL | HbS, % |
|---|---|---|
| M | 14.7 | 35.3 |
| F | 11.9 | 32.8 |
| M | 12.5 | 21.2 |
| M | 16.0 | 36.3 |
| M | 14.4 | 23.9 |
| M | 15.7 | 25.0 |
| F | 11.3 | 27.3 |
| M | 14.8 | 39.6 |
| M | 15.8 | 37.1 |
| M | 13.2 | 26.4 |
| F | 11.0 | 37.6 |
| M | 15.2 | 32.0 |
| M | 13.3 | 24.5 |
| M | 15.0 | 39.1 |
| M | 13.8 | 40.8 |
| M | 12.9 | 37.6 |
| M | 14.5 | 38.2 |
| F | 12.2 | 33.1 |
| F | 12.1 | 34.9 |
| F | 12.7 | 38.5 |
Preoperative hemoglobin concentration and percentage of HbS in all 20 donors. Fourteen donors had HbS concentration >30%.
F, female; Hb, hemoglobin; HbS, hemoglobin S; M, male.
TABLE 2.
Management of HbS >30%
| Procedure | Pre-HbS, % | Post-HbS, % |
|---|---|---|
| Ø | 35.3 | NR |
| 1 unit | 32.8 | 27.5 |
| 32.0 | 29.2 | |
| 38.3 | NR | |
| 34.9 | 29.9 | |
| 2 units | 36.6 | 30.6 |
| 37.1 | 30.7 | |
| 37.7 | 27.4 | |
| 39.0 | 29.1 | |
| 33.3 | NR | |
| 37.6 | NR | |
| 3 units | 40.8 | 28.7 |
| 38.5 | 25.1 | |
| Phlebotomy | 39.6 | NR |
| Mean | 36.64 | 27.5 |
Procedure performed in donors with HbS >30% to decrease HbS levels.
HbS, hemoglobin S; NR, not recorded.
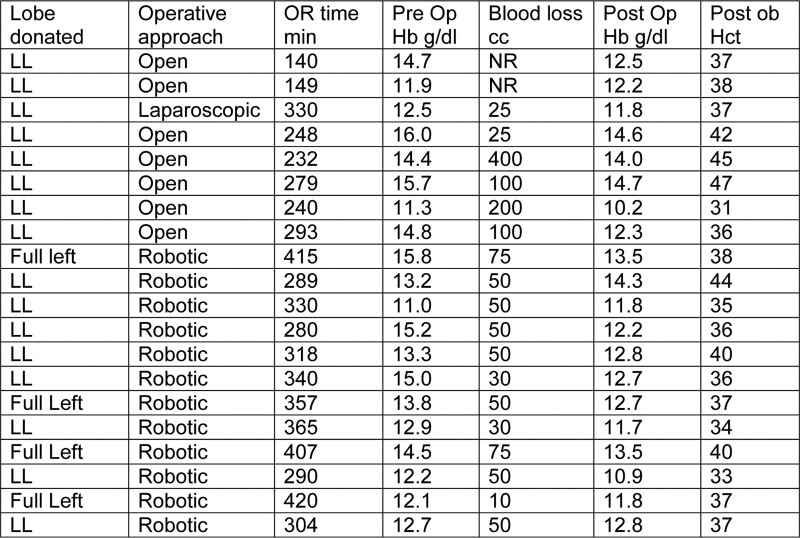
Our surgical approach advanced over the study period. Therefore, in 7 donors, the operation was performed in an open technique, 1 donor had a laparoscopic approach, and the remaining 12 donors were subject to robotic donor operation. We did not consider minimal invasive surgery as contraindication for donors with SCT, since this approach is a way to minimize trauma and, therefore, the risk of sickling events. The liver quality was excellent in all donors except for a single donor with 10% macrosteatosis. Mean postoperative Hb was 12.7 (10.2–14.6) g/dL. Mean postoperative hematocrit values were (0.382) ranges between 0.316 and 0.474. Average blood loss was 83.3 (25–400) cc (Figure 2). Mean overall operating room (OR) time was 300.9 min, ranging from 140 to 415 min. The laparoscopic case had an OR time of 330 min. Open donor surgeries (n = 7) had a mean OR time of 196 min, ranging from 140 to 293 min. Robotic donor operations (n = 14) had a mean OR time of 343 min, ranging from 280 to 415 min (Figure 3).
FIGURE 2.
Operative characteristics. Details of the donor surgery with regard to donated graft, operative approach, operating room (OR) time, pre- and postoperative hemoglobin (Hb), postoperative hematocrit (Hct), blood loss in cc, left lateral segment (LL), not recorded (NR), and operation room (OR) time.
FIGURE 3.
Operating room (OR) time. Mean operative time of open (n = 7), laparoscopic (n = 1), and robotic (n = 12) donor surgery.
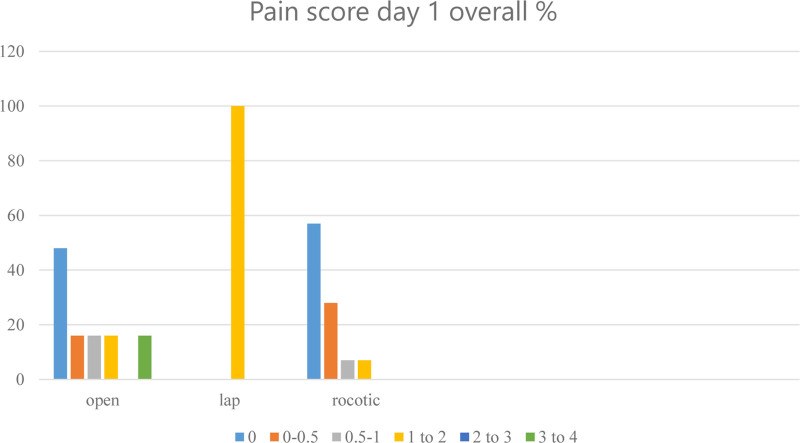
Postoperative lactic acid levels at day 1 (intensive care unit) ranged from 0.7 to 2.5 mg/dL with a mean of 1.41 mg/dL. The laparoscopic donors’ lactate level was 1.3 mg/dL. Average lactate level of open donors was 1.3 mg/dL (0.8–2.5 g/dL). Average lactate level of robotic donors was 1.41 mg/dL (0.7–2.3 mg/dL; Figure 4). International normalized ratio was measured between 0.9 and 1.1 preoperatively and between 1.0 and 1.4 on day 1. Postoperative pain score was between 1 and 2 in the open donors and below 1 in the robotic donors’ evaluation (Figure 5). Donors stayed in intensive care unit for 1 d, except one open donor who stayed for 2 d, having bilateral pleural effusions resolved by chest physiotherapy.
FIGURE 4.
Lactate day 1. Mean lactate day levels on day 1 after donor surgery, comparing open, laparoscopic, and robotic donor surgery. ICU, intensive care unit.
FIGURE 5.
Pain score day 1. Pain score according to our postoperative pain chart, comparing open, laparoscopic, and robotic donor surgery.
The mean postoperative hospital stay was 4.8 (4–6) d, and at a mean follow-up of 6 y and 6 mo (from 8 d to 9 y and 5 mo), no donors developed a significant (Clavien grade ≥3) complication (Table 3). No sickling-related complications or changes in the red blood cell indices developed in the postoperative period. A single, Clavien grade 1 or 2 complication occurred in a donor undergoing laparoscopic surgery; this manifested in the form of a self-limited hematoma at the Pfannenstiel incision extraction site, 2 open donors had hypertrophic scarring in the later follow-up, 1 donor had bilateral self-limiting pleural effusions. All liver function tests normalized within a period comparable to those of donors without SCT. No hematuria was detected postoperatively. Clinical follow-up was completed up to date in all 20 donors.
TABLE 3.
Complications
| Clavien grade | Early, n | Specification | Late, n | Specification |
|---|---|---|---|---|
| Ø | 18 | 18 | ||
| Grade I | 0 | 2 | Hypertrophic scarring | |
| Grade II | 2 | Hematoma, pleural effusion | 0 | |
| Grade III | 0 | 0 | ||
| Grade IV | 0 | 0 |
Complications in the donors according to the Clavien-Dindo classification.
No SCT-related complications were observed in the recipient. However, the recipient population is very heterogenous because some were SCT as well. Therefore, in some recipients, risks could be donor and recipient related.
DISCUSSION
In living donor liver transplantation, the importance of evidence-based donor selection with regard to donor safety cannot be overstated and is the foundation of its success. SCT is a condition that increases potential perioperative risks to the donor. Because of the very low incidence in the West, there is no guideline with regard to screening of potential donors. If identified, however, these gene carriers have been mostly excluded from living donation, although they are routinely accepted as blood and bone marrow donors. After studying safe protocols for major operations up to living donor kidney transplantation12,18 in SCT carriers, we started to consider these candidates as potential donors when the following criteria were met: absence of any other potentially hypercoagulable state, no contraindications to anticoagulation, agreeable to the potential use of interventions such as phlebotomy ± blood transfusion. Initially we only accepted donations with the least operative trauma, that is, left lateral section.
Controversy is generated by the fact that, although SCT mainly causes renal manifestations,14-17 only one-third of the US centers screen for SCT within their kidney donors. Centers that do screen mostly consider the trait as an absolute contraindication.12,13 There is no clear recommendation on how to address or gain experience with SCT in donors, the only recommendation stated in an expert review of kidney donation in the American Journal of Kidney Disease is to encourage experienced centers to publish their outcomes to better clarify the safety profile in this group of donors.19 There are only very few reports about the outcome for living kidney donors12,18 and none for liver donors.
The favorable outcome in our slowly growing cohort is reassuring and may also reflect our strict donor selection, followed by optimal and informed care in the perioperative setting. Our interdisciplinary team of hepatologists, surgeons, anesthetists, and critical care clinicians closely monitored possible complications inherent to the SCT state in each phase of the donation process.
Quantification of HbS during the evaluation phase of any donor confirms the diagnosis and stratifies the risk of sickling events. Our standard liver donor evaluation will identify any SCT-related renal abnormalities by urine analysis and CT abdomen.14-17 Splenic infarcts, which are at risk of developing with an HbS >40%, would also have been ruled out accordingly.
All experience with perioperative exchange transfusions has evolved from managing patients homozygot sickle cell disease, with two inherited hemoglobin S genes. The procedure of removing HbS carrying cells reduces the risk of vaso-occlusive events and hemolysis, in addition to augmenting the oxygen-carrying capacity of the blood without increasing the viscosity.20
The widely accepted goal for a preoperative HbS ≤30% is based on little clinical evidence.1,20,21 The Preoperative Transfusion in Sickle Cell Disease Study Group did provide prospective, randomized data in their 1995 New England Journal of Medicine study. Vichinsky et al20 compared 2 arms, of each 300 patients, with one arm to reach an Hb of 10 g/dL by conservative management and an exchange transfusion arm to decrease HbS to <30%. The safety of both arms was similar with fewer transfusion-associated complications in the conservative arm.
According to these findings, some experts in hematology advocate for no modulation of the fractional HbS% or Hb levels in the SCT state.20 To be on the safer side, our center adopted a practice of selective phlebotomy ± exchange transfusion to start with, but over the whole study period, we did not follow a strict protocol, and, therefore, the treatment of donors with HbS ≥30% does differ with regard to the procedure allied and the number of exchanged units (Table 2).
Our SCT donors had an average Hb of 13.6 g/dL, and 65% (13/20) had a preprocedural HbS ≥30%. The recommendation that the study of Vichinsky et al (the SCT study group) was based on was made on a patient collective with an average Hb of ≈8 g/dL, which makes the patient collectives not directly comparable. However, blood viscosity increases from Hb levels of >10 to 11 g/dL in the HbS and hemoglobin S genes population compared to Hb >14 to 16 g/dL in normal individuals.22
Not having any clear recommendations for preoperative transfusion approaches in SCT, we tried to adhere to a more aggressive treatment in our living donors because intraoperative volume constriction may lead to a rise in Hb. We felt safe following the best practice paper (Hematology Reviews, 2009) advice to reduce HbS levels below 30%.23 Fourteen out of 20 donors had HbS levels above 30% and therefore underwent (slightly heterogeneous) sequential phlebotomy or blood transfusion 2 d preoperatively to achieve the aimed HbS levels. No sickling- or transfusion-related complications were seen.
Venous thromboembolism is a feared perioperative complication in any living liver donation. The potentially hypercoagulable state in SCT, in addition to inducing a proinflammatory thrombophilic situation during major liver resection, has been a major concern in accepting SCT carriers as donors.24-26 This concern is supported by US reports of hospitalized black male SCT carriers having a 2× to 4× higher risk for venous thromboembolism.27
We therefore implemented an aggressive thromboembolic prophylaxis with SC heparin or low-molecular-weight heparin plus pneumatic intermittent compression devices in all donors. Needless to say, that additional procoagulant factors (protein C/S, deficiency, oral contraceptive use) are exclusion criteria included in the screening protocol.
Although SCT is caused by the same monogenetic abnormality, its phenotypic expression is heterogenous among ethnic groups.28 It is well known that thromboembolic events in the general population are fewer in the Middle Eastern countries compared to the Western world, having the lowest incidence in Asia. The Afro-American population probably is one of the ethnic groups having the highest risk of all.10 There might be other genetic factors involved in the stratification of SCT-related complications, that still need to be assessed. Hence, our results might not be equally reproducible in other ethnic settings, and every single donor’s candidacy must be evaluated by an experienced team to stratify the individual risk of this potential donor.
Exercise collapse associated with SCT leading even to exercise-related deaths in US college athletes, in Black US Army recruits, or Navy trainees has been reported in small case series.4,7,8 The pathophysiology of exercise collapse associated with SCT is not fully understood but might evolve from highly strenuous activity, with or without hyperthermia, leading to a combination of metabolic acidosis, hyperosmolality, red cell dehydration, and hypoxia, inducing irreversible sickling and microvascular occlusion. Hypoxia and subsequent rhabdomyolysis can lead to hyperkalemia and acidosis causing fatal cardiac arrhythmias and renal insufficiency.4-6
Even though surgery might introduce red blood sickling, some surgeries are lifesaving and mandatory. Open-heart surgery, which causes hypoxia and hypothermia and modern liver resection causing hypovolemia, can be performed safely, and exercise collapse associated with SCT has not been reported.29-31 Because of the likelihood of sickling during procedures with low Pao2 levels,11,22,32 the protocol for open-heart surgery at our center is more aggressive, and up to now, SCT and SCD patients are equally subjected to it. No increased incidence of sickling was reported in a retrospective study of 47 patients after aiming for exchange transfusions to achieve HbS <10% and hematocrit >30.11,30 A non-exchange and no active cooling protocol was published by the University of Toronto group, emphasizing the importance of temperature hemostasis to avoid hypothermia-induced tissue hypoxemia.33
In our series, after initial promising results with left lateral segment donation, we carefully expanded the donated liver volume to the full left lobe, experiencing equal results. Despite not being able to provide evidence, we do believe that as our technique has evolved, reducing surgical trauma by robotic donor surgery, the exposure of SCT donor to sickling-inducing events is equally reduced. We could also notice a reduction in postoperative pain after robotic donor surgery. After this initial experience, we believe that minimizing surgical trauma and postoperative pain are two major factors for safety in SCT donors. We would not consider using a cell-saving device in SCT carriers, if needed, because this might induce sickling in the device. Since none of our donors required blood transfusion during the donor surgery, we are not using any cell-saving devices in any donor operation.
Our ongoing strategy and recommendations aim at a preoperative HbS level of <30%, without being able to prove, at this stage, that it is necessary and by which protocol this should be achieved. To gain more evidence, further studies might be needed, preferably in a multicenter study from countries with high incidence in SCT.
CONCLUSION
Exclusion criteria for living donors should ideally be evidence based. Although this is the case for most common coagulopathies, there is no evidence that SCT carriers cannot safely undergo living liver donation. Encountering the high incidence of SCT in the Middle East, we felt encouraged to stratify their risk as donors. Studying a variety of protocols and studies on patients with hemoglobinopathies with favorable outcomes in the fields of general and cardiac surgery, we selectively adapted our criteria and accepted initially only left lateral segment liver donors. Our initial findings reassured us to expand the donation volume to the whole left lobe for the time being. We must, however, constantly readdress our main goal, while modifying our practice, to aim at the lowest risk to liver donors. Before we move on to right lobe donation, we would encourage other centers to report their results on any organ donation from SCT carriers.
Footnotes
M.Sc. participated in the writing of the paper, data analysis, and donor and recipient surgery. A.Z. participated in the donor protocol and management, writing of the paper, and donor and recipient surgery. M.St. participated in data analysis, writing of the paper, and donor and recipient surgery. Su.A. participated in analysis and collection of data. M.Sh. participated in donor and recipient management and protocol for pediatric recipients. K.B. participated in protocol for adult donors and recipients and writing of the paper. Sa.A. participated in adult recipient and donor management. D.C.B. participated in donor management, donor and recipient surgery, and writing of the paper.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Alhamdan NA, Almazrou YY, Alswaidi FM, et al. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med. 2007;9:372–377. [DOI] [PubMed] [Google Scholar]
- 2.Elsayid M, Al-Shehri MJ, Alkulaibi YA, et al. Frequency distribution of sickle cell anemia, sickle cell trait and sickle/beta-thalassemia among anemic patients in Saudi Arabia. J Nat Sci Biol Med. 2015;6:S85–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jastaniah W. Epidemiology of sickle cell disease in Saudi Arabia. Ann Saudi Med. 2011;31:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harmon KG, Drezner JA, Klossner D, et al. Sickle cell trait associated with a RR of death of 37 times in National Collegiate Athletic Association football athletes: a database with 2 million athlete-years as the denominator. Br J Sports Med. 2012;46:325–330. [DOI] [PubMed] [Google Scholar]
- 5.Tantawy AAG. The scope of clinical morbidity in sickle cell trait. Egypt J Med Hum Genet. 2014;15:319–326. [Google Scholar]
- 6.Ashorobi D, Ramsey A, Yarrarapu SNS, et al. Sickle Cell Trait. StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 7.Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28:939–944. [DOI] [PubMed] [Google Scholar]
- 8.Kark JA, Burr PQ, Wenger CB, et al. Exertional heat illness in Marine Corps recruit training. Aviat Space Environ Med. 1996;67:354–360. [PubMed] [Google Scholar]
- 9.Kark JA, Posey DM, Schumacher HR, et al. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317:781–787. [DOI] [PubMed] [Google Scholar]
- 10.Crous-Bou M, Harrington LB, Kabrhel C. Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost. 2016;42:808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousafzai SM, Ugurlucan M, Al Radhwan OA, et al. Open heart surgery in patients with sickle cell hemoglobinopathy. Circulation. 2010;121:14–19. [DOI] [PubMed] [Google Scholar]
- 12.Nath J, McDaid J, Bentall A, et al. Sickle cell and renal transplant: a national survey and literature review. Exp Clin Transplant. 2012;10:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Reese PP, Hoo AC, Magee CC. Screening for sickle trait among potential live kidney donors: policies and practices in US transplant centers. Transpl Int. 2008;21:328–331. [DOI] [PubMed] [Google Scholar]
- 14.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol. 2000;63:205–211. [DOI] [PubMed] [Google Scholar]
- 15.Herard A, Colin J, Youinou Y, et al. Massive gross hematuria in a sickle cell trait patient with renal papillary necrosis. Conservative approach using a balloon ureteral catheter to tamponade the papilla bleeding. Eur Urol. 1998;34:161–162. [DOI] [PubMed] [Google Scholar]
- 16.Sesso R, Almeida MA, Figueiredo MS, et al. Renal dysfunction in patients with sickle cell anemia or sickle cell trait. Braz J Med Biol Res. 1998;31:1257–1262. [DOI] [PubMed] [Google Scholar]
- 17.Zadeii G, Lohr JW. Renal papillary necrosis in a patient with sickle cell trait. J Am Soc Nephrol. 1997;8:1034–1039. [DOI] [PubMed] [Google Scholar]
- 18.Rehman Su, Al-Amoudi A, Kelta M, et al. Kidney transplant from sickle cell trait donor to sickle cell trait recipient. Exp Clin Transplant. 2007;5:698–700. [PubMed] [Google Scholar]
- 19.Davis CL. Evaluation of the living kidney donor: current perspectives. Am J Kidney Dis. 2004;43:508–530. [DOI] [PubMed] [Google Scholar]
- 20.Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–213. [DOI] [PubMed] [Google Scholar]
- 21.Hemming AE. Pro: exchange transfusion is required for sickle cell trait patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2004;18:663–665. [DOI] [PubMed] [Google Scholar]
- 22.Maduska AL, Guinee WS, Heaton JA, et al. Sickling dynamics of red blood cells and other physiologic studies during anesthesia. Anesth Analg. 1975;54:361–365. [DOI] [PubMed] [Google Scholar]
- 23.Wun THK. Best practices for transfusion for patients with sickle cell disease. Hematol Rev. 2009;1:106–110. [Google Scholar]
- 24.Ataga KI, Brittain JE, Desai P, et al. Association of coagulation activation with clinical complications in sickle cell disease. PLoS One. 2012;7:e29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezeaud A, Denninger MH, Dondero F, et al. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252–1256. [PubMed] [Google Scholar]
- 26.Hilmi IA, Planinsic RM. Live liver donors: are they at a higher risk for post-operative thrombotic complications? World J Transplant. 2012;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014;38:28–32. [DOI] [PubMed] [Google Scholar]
- 28.Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–912. [DOI] [PubMed] [Google Scholar]
- 29.Atlas SA. The sickle cell trait and surgical complications. A matched-pair patient analysis. JAMA. 1974;229:1078–1080. [PubMed] [Google Scholar]
- 30.Métras D, Coulibaly AO, Ouattara K, et al. Open-heart surgery in sickle-cell haemoglobinopathies: report of 15 cases. Thorax. 1982;37:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oduro KA, Searle JF. Anaesthesia in sickle-cell states: a plea for simplicity. Br Med J. 1972;4:596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messent M. Con: exchange transfusion is not required for sickle cell trait patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2004;18:666–667. [DOI] [PubMed] [Google Scholar]
- 33.Djaiani GN, Cheng DC, Carroll JA, et al. Fast-track cardiac anesthesia in patients with sickle cell abnormalities. Anesth Analg. 1999;89:598–603. [DOI] [PubMed] [Google Scholar]