ABSTRACT
Nontuberculous mycobacteria (NTM) are opportunistic pathogens that cause chronic pulmonary disease (PD). NTM infections are thought to be acquired from the environment; however, the basal environmental factors that drive and sustain NTM prevalence are not well understood. The highest prevalence of NTM PD cases in the United States is reported from Hawai’i, which is unique in its climate and soil composition, providing an opportunity to investigate the environmental drivers of NTM prevalence. We used microbiological sampling and spatial logistic regression complemented with fine-scale soil mineralogy to model the probability of NTM presence across the natural landscape of Hawai’i. Over 7 years, we collected and microbiologically cultured 771 samples from 422 geographic sites in natural areas across the Hawaiian Islands for the presence of NTM. NTM were detected in 210 of these samples (27%), with Mycobacterium abscessus being the most frequently isolated species. The probability of NTM presence was highest in expansive soils (those that swell with water) with a high water balance (>1-m difference between rainfall and evapotranspiration) and rich in Fe-oxides/hydroxides. We observed a positive association between NTM presence and iron in wet soils, supporting past studies, but no such association in dry soils. High soil-water balance may facilitate underground movement of NTM into the aquifer system, potentially compounded by expansive capabilities allowing crack formation under drought conditions, representing further possible avenues for aquifer infiltration. These results suggest both precipitation and soil properties are mechanisms by which surface NTM may reach the human water supply.
IMPORTANCE Nontuberculous mycobacteria (NTM) are ubiquitous in the environment, being found commonly in soils and natural bodies of freshwater. However, little is known about the environmental niches of NTM and how they relate to NTM prevalence in homes and other human-dominated areas. To characterize NTM environmental associations, we collected and cultured 771 samples from 422 geographic sites in natural areas across Hawai’i, the U.S. state with the highest prevalence of NTM pulmonary disease. We show that the environmental niches of NTM are most associated with highly expansive, moist soils containing high levels of iron oxides/hydroxides. Understanding the factors associated with NTM presence in the natural environment will be crucial for identifying potential mechanisms and risk factors associated with NTM infiltration into water supplies, which are ultimately piped into homes where most exposure risk is thought to occur.
KEYWORDS: nontuberculous mycobacteria, Hawai’i, environmental niches, spatial modeling
INTRODUCTION
Nontuberculous mycobacteria (NTM) are a large group of environmentally acquired opportunistic pathogens that cause insidious pulmonary disease (NTM PD), among other conditions (1). Symptoms of NTM PD, including chronic cough, shortness of breath, and fatigue, are indicative of many other diseases, making diagnosis of NTM PD difficult (2). The global prevalence and mortality associated with NTM PD is climbing steadily, with some countries reporting prevalence rates of up to 9.8 cases/100,000 people (3). Prevalence rates in the United States are also rising, making NTM PD an emerging disease of concern both globally and within the United States (4).
It is posited that pulmonary NTM infections are acquired from environmental exposures (1), although certain species (e.g., Mycobacterium abscessus) may have a human-human transmission component in immunocompromised populations (5). Respiratory-relevant NTM have been isolated from a variety of natural habitats, including natural water sources (6, 7) and soils (8, 9); however, most exposures are thought to take place in human-dominated environments. Exposure is thought to be especially common in homes, where NTM are readily recovered from freshwater biofilms (e.g., showerheads, tubs; 6, 10, 11), places of work (e.g., mines, agriculture fields; 12), or elsewhere where water typically originating from municipal sources containing NTM is aerosolized and inhaled or ingested (11, 13). In water supply systems, NTM are suited for proliferation due to their innate resistance to chlorine (14). Recent work has shown the potential for NTM to originate from natural sources (e.g., marshes, riparian areas, losing streams) and enter municipal water supplies through natural aquifers (7), but a comprehensive understanding of the natural niches of NTM, possible routes of inoculation, and mechanisms of transport from natural environments to built environments, including homes, is still lacking.
To better understand the environmental niches of NTM, we focused on the state of Hawai’i, which has the highest prevalence of NTM PD in the United States, with up to four times higher prevalence than the rest of the country (15). The Hawaiian Islands are unique in their climate, topography, and soil composition. Most notable is the high spatial heterogeneity in rainfall (16), evapotranspiration (17), and soil type (18). From our prior work in Hawai’i, the prevalence of environmental NTM is related to the mineralogy of soils (9), rainfall, humidity, evapotranspiration, elevation, and population density (19), but it is unclear how the complex interplay of these factors in the natural environment intersect to collectively support or inhibit NTM presence. For example, NTM are associated with minerals in the soil (e.g., magnetite, hematite, ilmenite, maghemite; 9), but relative mineral content changes with water balance (i.e., precipitation minus evapotranspiration [millimeters]) and depends on soil permeability (20). Thus, given the high spatial variation in both soil type and moisture across the Hawaiian Islands, solely considering one factor in the absence of others may not result in strong predictions of NTM presence across the landscape.
Characterizing the natural environmental niches of NTM while considering multiple interacting environmental factors are important steps to addressing gaps in our current knowledge of NTM environmental requirements. A better understanding of these relationships can help shed light on risk factors associated with NTM infiltration of water supplies and homes. In the current study, we identified NTM from natural areas in Hawai’i and applied a spatial modeling framework to estimate and predict the probability of NTM presence. We considered “natural” areas to be those receiving little human traffic or intervention, but samples were not solely from remote areas, being located along the entire gradient of human population density.
RESULTS
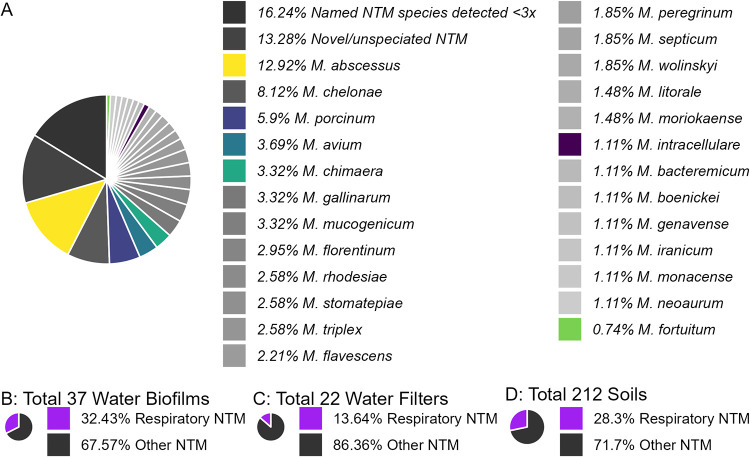
Of the 771 natural samples collected, 210 (27.2%) were culture positive for NTM with 55 different NTM species identified (Table 1). Potentially novel NTM species represented 13.3% of all samples by demonstrating rpoB sequence similarity to the genus Mycobacterium but were different enough from reference NTM species (see Fig. 2). M. abscessus represented 12.9% of the NTM species identified, followed by M. chelonae (8.1%), M. porcinum (5.9%), M. avium (3.7%), and M. chimaera (3.3%) (see Fig. 2). Among the 210 NTM-positive samples, 73 (34.7%) were positive for respiratory-relevant species.
TABLE 1.
Types of natural samples collected across Hawai’i and microbiologically cultured for NTM
| Location | No. of biofilm samples | No. of filter samples | No. of soil samples |
|---|---|---|---|
| Soil | 492 | ||
| Stream/river | 35 | ||
| Well | 6 | 4 | |
| Stream/aquatic vegetation | 62 | ||
| Lava rocks | 7 | ||
| Stream/aquatic rock | 165 | ||
| Total | 240 | 39 | 492 |
FIG 2.
Total of 210 samples collected from natural environments of Hawai’i were culture positive for NTM. We detected 80 species, including 26 novel or unnamed species and five species of respiratory relevance, of which M. abscessus and M. porcinum were most common. (A) All samples are shown, with all named species detected at least 2 times shown. The composition of respiratory-relevant species compared to other NTM species is shown based on sample type: water biofilms (B), stream water filters (C), and soil with respiratory-relevant species being more common in water biofilms (D).
We profiled 240 water biofilm swabs, of which 37 (15.4%) were culture positive for NTM. The predominant species identified from water biofilms were M. chelonae (29.4%), M. avium (11.7%), and M. porcinum (8.8%; Table 1). Of the 39 freshwater filters cultured, 22 (43.5%) were positive for NTM. The most frequently isolated NTM species were M. stomatepiae (31.8%), M. florentinum (9%), and M. mucogenicum (9%) (Table 1). Of the 492 soil samples collected, 212 (32.9%) were NTM culture positive. M. abscessus (15%), M. chelonae (5.6%), and M. porcinum (5.6%) comprised the most commonly isolated NTM species from soil (Table 1). Respiratory-relevant species were detected in 32.4%, 13.6%, and 28.3% of NTM-positive biofilm swabs, stream water filters, and soil samples, respectively (see Fig. 2). Recovery of M. intracellulare and M. fortuitum was infrequent among the samples tested.
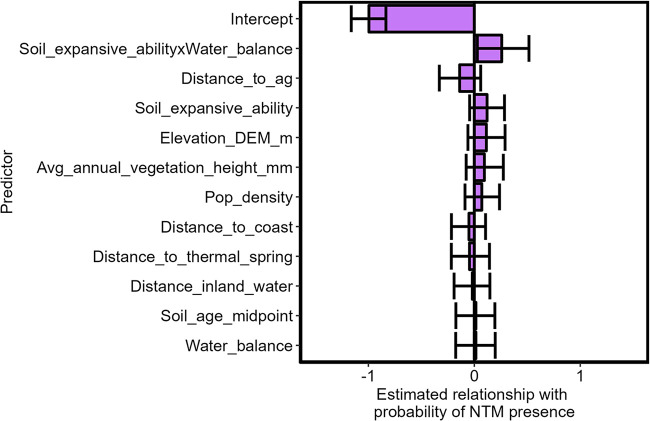
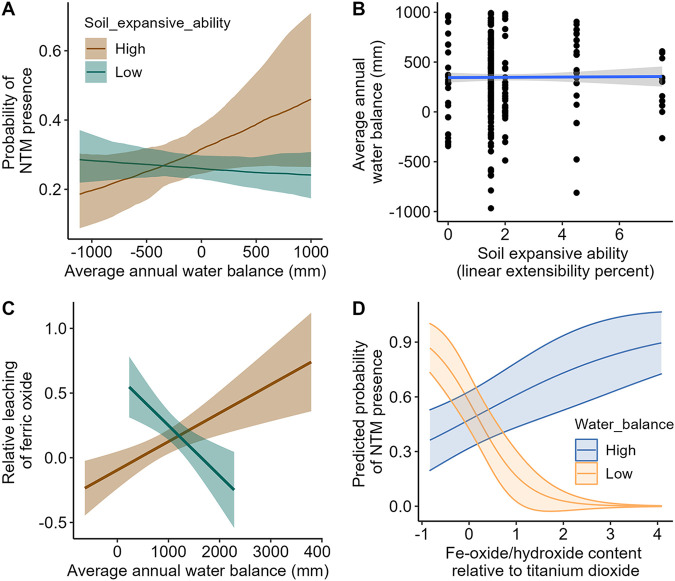
Although we noted differences in diversity and relative sample prevalence of NTM species across the different sample types, the distribution of samples along gradients of each model predictor was similar when we considered all species together and when respiratory-relevant species were considered alone (two-sample Kolmogorov-Smirnov P > 0.05; Fig. 1). This supports our approach of using all species and suggests our assumption that all species of NTM in this study have similar environmental requirements is valid for this data set. Our model fit assessed by 5-fold cross-validation accuracy (predicted response versus observed response) showed a score of 0.6 and area under the receiver operating characteristic (ROC) curve of 0.58 (Fig. 2A). While not representing strong statistical support, these scores indicate more support for our model over an alternative nonspatial model run in a maximum likelihood framework (cross-validation accuracy score of 0.58). Most environmental variables tested were not strong predictors of the probability of NTM presence across the state, except for the interaction between soil expansive ability and water balance, which had a slope coefficient significantly different from zero (Fig. 3, Table 2). This relationship indicates that the NTM presence related to water balance is different in high- and low-expansive-ability soils, with all levels of expansive ability being present at all levels of water balance considered (up to 1 m/year) (Fig. 4). Specifically, the probability of NTM presence was positively associated with water balance in highly expansive soils but negatively associated with water balance in low-expansion soils (Fig. 4). NTM were most likely to be present in highly expansive soils with a high water balance (i.e., precipitation greater than evapotranspiration; moist soils) (Fig. 4).
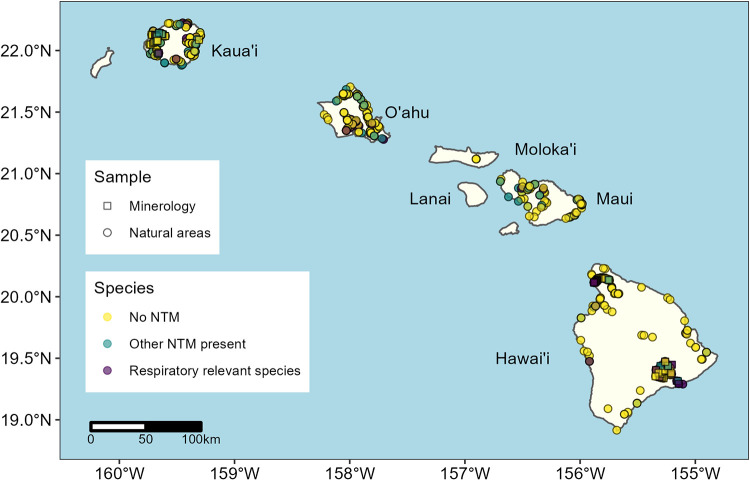
FIG 1.
Locations of 771 natural samples collected from 422 geographic sites (circles) on the Hawaiian Islands that were analyzed for the presence of NTM. Samples were taken from soils, rivers, streams, shorelines, rocks, and vegetation (Table 1). Also pictured are soil sample locations from three regions in which detailed soil mineralogy was associated with NTM presence or absence (n = 121, minerology, squares). Samples positive for respiratory-relevant NTM species (Table 1) are shown in purple, and samples positive for other NTM species are shown in green.
FIG 3.
Beta coefficient estimates with associated 95% credible interval (error bars) for predictors in a spatial logistic regression model for the probability of NTM presence across the Hawaiian Islands. All variables were included in the model, along with spatial random effects. All credible intervals overlap zero except for the interaction between soil expansive ability and average annual water balance (average annual precipitation [mm] – average annual evapotranspiration [mm]). Estimates are based on 771 samples collected from 422 geographic sites.
TABLE 2.
Variables used in logistic regression with spatial random effects, with variable description and sources given
| Variable | Description | Source |
|---|---|---|
| Soil_expansive_ability | Linear extensibility percent (LEP): change in soil vol upon wetting and drying | Hawai'i Soil Atlas, College of Tropical Agriculture and Human Resources, University of Hawai'i at Manoa |
| Distance_thermal_spring | Distance (m) to locations of thermal springs identified by Geothermex, Inc., in 2000 | Hawai'i Department of Business, Economic Development and Tourism |
| Distance_to_ag | Distance (m) to edge of crop polygons identified from 2020 agricultural land use update for state of Hawai'i | The University of Hawai'i at Hilo Spatial Data Analysis and Visualization (SDAV) Laboratory in conjunction with the Hawai'i State Department of Agriculture, 2021 |
| Distance_to_coast | Distance (m) to coast | National Hydrography Dataset (72) |
| Distance_inland_water | Distance (m) to bodies of freshwater (rivers, streams, lakes, ponds) | National Hydrography Dataset (72) |
| Pop_density | Population density estimate (people per square km) extrapolated to 2015 based on the 2010 census at 30 arc-second resolution aggregrated at 2.5 arc-minutes | Gridded Population of the World, version 4 (GPWv4) (73) |
| Vegetation_ht | Vegetation ht (m) at 30-m resolution | Climate of Hawai'i, Geography Deptartment, University of Manoa (17) |
| Water_balance | Mathematical difference of mean annual rainfall (mm) and evapotranspiration, 30-m resolution | Climate of Hawai'i, Geography Department, University of Manoa (17) |
| Elevation_DEM_m | Elevation (m) at 10-m resolution | USGS digital elevation model (74) |
| Soil_age_midpoint | The midpoint of the soil age range | USGS geologic map of Hawai’i (37) |
| Soil_expansive_ability × Water_balance | A multiplicative interaction term between Soil_expansive ability and Water_balance |
FIG 4.
Model-predicted relationships between average annual water balance (average annual precipitation [mm] – average annual evapotranspiration [mm]) and soil expansive capability (linear extensibility percent) on the probability of NTM presence. (A) Relationships across the Hawaiian Islands based on predictions generated from a spatial model accounting for spatial correlation and sampling bias. (B) Predictions were generated for levels of water balance over which there was no confounding relationship between soil expansive capability and water balance. (C) Highly expansive soils with a high water balance have less leaching of Fe-oxide/hydroxide (Fe2O3) than TiO2, an oxide that is not readily leached from soils. (D) Probability of NTM presence was positively related to the relative prevalence of Fe-oxide/hydroxide in wet soils but negatively related in dry soils.
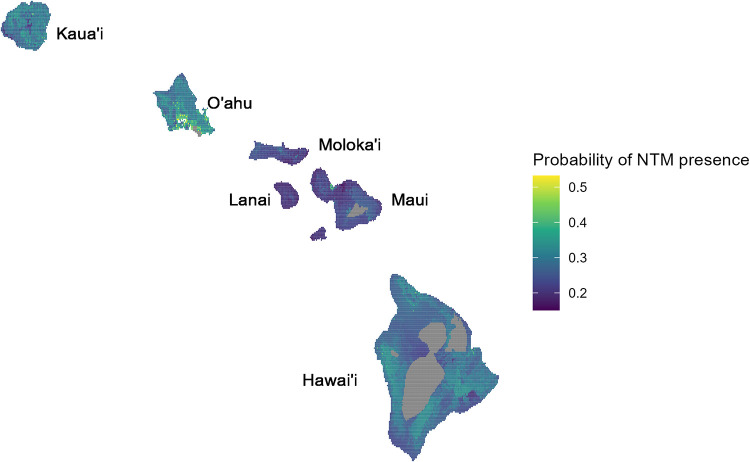
Highly expansive soils with a high water balance had both the highest probability of NTM presence and the highest relative prevalence of Fe-oxide/hydroxide (Fig. 4). Our mineralogy subset indicated that the probability of NTM presence was positively associated with the relative amount of Fe-oxide/hydroxide in the soil but only in wet soils (i.e., those with a high water balance; 1,839 mm, 90th quantile). In soils with a low water balance (−635 mm, 0 quantile), the probability of NTM presence was instead negatively associated with the relative prevalence of Fe-oxide/hydroxide in the soil (Fig. 4). Our model predictions indicate that the probability of NTM presence, but also uncertainty in those probabilities, in natural areas of Hawai’i is expected to be highest on O’ahu, especially around the city of Honolulu and on Kaua’i around Kahului (Fig. 3 and 5).
FIG 5.
Map of the probability of NTM presence across the main Hawaiian Islands. Missing is the island of Ni’ihau for which we were missing coverage of predictor variables. Predictions are generated from a spatial model using remotely sensed predictors and accounting for spatial bias in sampling effort. Gray areas are those with higher elevation, population density, and/or soil expansive ability than measured by our sample data and thus have no associated predictions.
DISCUSSION
By profiling environmental samples from a variety of natural areas of Hawai’i using gold-standard microbiological approaches and molecular sequencing, we identified M. abscessus as the most frequently recovered NTM species from this collection, likely attributed to the large representation of outdoor soil. This varies from other reports suggesting rare recovery of M. abscessus from the environment (21). NTM species of respiratory relevance most often found in homes and clinical samples in Hawai’i are M. chimaera, M. intracellulare, and M. fortuitum (6, 19), all of which had relative low rates of isolation in the present study, especially M. intracellulare and M. fortuitum. This is curious, as M. fortuitum infections are thought to originate from contaminated water supplies and M. intracellulare from contaminated soils, which we sampled (19, 22–24). This underscores the need for a better understanding of the reservoirs and environmental niches of NTM species that cause respiratory infections in Hawai’i, particularly M. fortuitum and M. intracellulare.
We found support for the contention that all NTM species have similar environmental requirements, with respiratory-relevant species being found predominantly where other NTM species were found in our samples. This is consistent with recent work that found all NTM species, as a proxy for respiratory-relevant species in soils and water associated with the built environment, was a strong predictor of human disease (25). However, we note that further study on niche variation among NTM species is warranted, with particular consideration paid to variation in nutritional needs (e.g., when NTM grow as part of a biofilm or planktonically) and species-specific metabolic pathways (e.g., anaerobic versus aerobic) that may confer advantages in certain environments.
NTM in Hawai’i have shown a positive association with water, rainfall, and wet environments (19), iron in the soil and drinking water (9, 26, 27), low elevations, and high population densities (19). We used predictors in our current model to determine if these factors remain important at a larger scale, finding that most were not strong predictors of the probability of NTM presence in non-human-dominated environments. We note that although population density was not a strong predictor in our model, our highest model-predicted probabilities of NTM presence were in the most urban areas we sampled (Honolulu and Kahului). However, these relationships were highly uncertain, most likely due to a relatively low sampling effort compared to less human-influenced areas. Future data analysis that focuses on samples collected in and around homes in Hawai’i will likely shed more light on the importance of human population density to NTM prevalence. The overall lack of strong predictors in our model and model accuracy values only marginally above 50% suggest important sources of variation in NTM remain unaccounted for in our model. Potential sources of this unaccounted-for variation that we were unable to consider in our model due to a paucity of data include the ecology of multiple hosts (i.e., humans and wildlife species), fine-scale habitat and water characteristics (i.e., those unavailable from remote-sensing), and competitive interactions with other soil microbes. The role of multiple hosts is particularly interesting and can be affected by environmental factors such as soil properties and moisture, potentially seeding natural environments according to their own ecological niches, demographics, and susceptibility (e.g., see reference 28). A better understanding of the roles of different host species on NTM environmental prevalence will be important moving forward.
Despite these limitations, we did find a strong association between the probability of NTM presence and the interaction between soil properties and moisture in natural environments, indicating that these factors are important to NTM prevalence in Hawai’i. Whereas we could not use iron content of the soil per se as a large-scale predictor in our model, since such information is not available on the statewide scale, by using a subset of soil samples for which we analyzed detailed mineralogy, we showed that soil expansive ability is related to relative iron content in our samples, particularly the relative amount of Fe-oxide/hydroxide. Iron acquisition in the environment, and potentially within the host, is an important component of bacterial survival (29). Furthermore, iron is an important cofactor found in a number of critical bacterial enzyme active sites (e.g., see reference 30), including catalase peroxidase enzymes, which have been leveraged for the treatment of tuberculosis (31). Our work suggests that iron is critical to the environmental niches of NTM, but its importance may depend on soil moisture.
We found a positive relationship between the probability of NTM presence and Fe-oxide/hydroxide prevalence in the soil that only occurred in soils with a high water balance (i.e., water balance > 0; precipitation exceeds evapotranspiration and results in more water percolating through the soil; 32). Iron is generally not leached from soils under oxidizing conditions because the solubility of Fe-oxides/hydroxides is low compared to that of other cations that are readily leached (i.e., Mg, Na, Ca, K; 33, 34). Thus, greater movement of water through the soil increases the relative prevalence of Fe-oxide/hydroxide as other elements are lost (35). This phenomenon is demonstrated in the positive relationship found between water balance and Fe-oxide/hydroxide relative prevalence in our study. This also explains the strong negative relationship found between Fe-oxide/hydroxide and the probability of NTM presence in soils with low water balance, since Fe-oxide/hydroxide content of those soils is low relative to other minerals (i.e., less leaching of non-Fe minerals and ions). Our quantification of total Fe-oxide/hydroxide content included a number of Fe3+-bearing secondary minerals, including hematite, maghemite, and goethite, all of which are weathering products of primary minerals in basalt. NTM use iron in its reduced form, Fe2+, not its oxidized form, Fe3+, as it exists in most soil Fe-oxides/hydroxides, using binding agents (i.e., siderophores), short-term storage molecules (i.e., mycobactin), and reductases to facilitate conversion of Fe3+ to useable Fe2+ (36). It is possible that certain forms and mineral compounds of Fe-oxide/hydroxides in the soil are easier for NTM to use than others (e.g., hematite, an alpha polymorph of Fe203; 9), suggesting an avenue of further research on the metabolism of naturally occurring forms of iron by NTM. We note that the soils in Hawai'i tend to be more highly enriched in iron than other soils in the United States, being primarily tholeiitic to transitional alkalic lavas (37) known to enrich overlying soils with Fe3+ during the weathering process (38). This suggests an avenue of further study on absolute iron content of soils relative to NTM presence. Although the probability of NTM presence was lower in soils with low water balance, it was not zero, indicating that NTM prevalence in these soils is dependent on other materials, possibly noniron minerals (e.g., molybdenum; 39) and/or organic matter.
We hypothesized that soils with high water-holding capabilities are ideal environments for NTM by satisfying niche requirements for both ample moisture and soil minerals. Our finding that NTM were most associated with expansive, moist soils, which have both high moisture and iron content relative to other soils, supports that hypothesis. Highly expansive soils hold large amounts of moisture for potentially long periods of time (40), making ideal environments for soil microbes (41), but can also be prone to anaerobic conditions (42). Since NTM are considered aerobic organisms (43), it is likely that moist soils that remain predominantly aerobic are favored; however, we note that NTM in the human body are capable of shifting metabolic pathways to grow under anaerobic conditions (44). Furthermore, recent studies of NTM PD have shown a positive association with hydric soils, which are high-moisture soils, providing further support for niche dependence on soil moisture. However, hydric soils are defined as those that are periodically rendered anaerobic (45), suggesting NTM are capable of withstanding anaerobic environmental conditions to some extent, but more research on this tolerance is warranted. Highly expansive soils in our study were also associated with high Fe-oxide/hydroxide prevalence, which may further promote bacterial growth (46, 47). The positive relationship between relative Fe-oxide/hydroxide prevalence, water balance, soil expansive ability, and the probability of NTM presence indicate that soil expansive ability is a useful large-scale proxy for soil Fe-oxide/hydroxide content when modeling the environmental niches of NTM and predicting areas of high NTM prevalence at large scales. Our results highlight the complex interplay between NTM and moisture availability and soil properties, such as water-holding ability and iron content.
Highly expansive soils are unique in that they shrink and crack, causing deep fissures in the ground during times of low moisture availability (48). These fissures are ideal pathways for the movement of soil microbes to lower regions of the soil and into the water table (49). Where water balances are high, groundwater recharge occurs, regardless of soil expansive ability, inoculating aquifers and thereby allowing movement of particles below the ground (50). This could allow soil bacteria like NTM an alternate route to enter underground aquifer systems. Thus, if NTM are more common in highly expansive soils with high water balances, we might expect a higher risk of NTM entry into the water table both within and outside drought conditions. Indeed, prior work has shown the potential for NTM to flow from natural environments into ground water supplies and into human dwellings, with infiltration through the soil being dependent on soil composition and rainfall rates (7). Our work highlights the importance of several interactive climate and soil factors on the environmental prevalence of NTM in natural areas of Hawai’i and suggests that additional targeted work regarding the potential for NTM transport in the human water supply via expansive soils is warranted.
MATERIALS AND METHODS
Study site.
Hawai’i is an archipelago in the Pacific Ocean made up of eight main islands. The climate of Hawai’i is notable for consistent mild temperatures year-round (range, 18 to 32°C), moderate humidity (mean, 76.4%; standard deviation, 10.7%), northeasterly trade winds (51), and high spatial heterogeneity in rainfall (16). For example, mean annual rainfall varies from <0.25 m/year on the leeward side of Hawai’i to >10 m/year at the summit of Kaua’i (52). The elevation of Hawai’i varies from sea level at its lowest to 4,207 m at the peak of the highest mountain, Mauna Kea. The variable elevation and strong trade winds contribute to the high spatial variation in rainfall (53). Soils of the Hawaiian Islands are highly variable in mineral content and age, typically being composed of basalt lavas, which can range widely in weathering products depending on their location (54). Highly expansive 2:1 clay soils are rare in Hawai’i (37, 55); however, Hawaiian soils nevertheless are capable of expansion and contraction (37).
NTM sampling and isolation.
From 2012 to 2019, we collected and cultured 771 environmental samples from 422 natural geographic sites (i.e., those receiving little human traffic and influence) across O’ahu, Kaua’i, Hawai’i Island, Molokai, and Maui for the presence of NTM (Fig. 1). We sampled semiannually between November-December (representing winter months) and May-June (representing the summer months), using all samples in this analysis. We used a combination of opportunistic and transect sampling to collect natural samples from soils, rivers, streams, shorelines, rocks, and vegetation from each island. Opportunistic sampling involved sampling areas that could be accessed readily, as many natural areas in Hawaii are difficult to access due to land restrictions and/or topography. Transect sampling was conducted along a 15-km transect traversing the rainfall gradient of Kohala, the northwestern peninsula of Hawai’i (37 to 283 cm/year). We collected 46 soil samples along this transect with adjacent samples spaced at least 250 m apart.
We used standard microbiological approaches, as described in reference 7 and detailed below, for the isolation of environmental NTM and confirmation of presence within a sample. We picked and inoculated grown colonies into Middlebrook 7H9 broth to create bacterial stocks and then centrifuged 1 mL of turbid bacterial stock at 13,000 × g for 1 min to create bacterial pellets. We stored bacterial pellets at −80°C until they were used for DNA isolation, following the DNA extraction and species identification procedures by rpoB gene sequencing detailed below.
Water biofilm samples.
We collected water biofilm samples using sterile synthetic dual-tipped swabs (Puritan HydraFlock sterile flocked collection devices 25-3306 2HBT; Guilford, ME) on a variety of natural surfaces previously shown to harbor NTM (Table 1) (1). We immersed and vortexed swabs in 2 mL of autoclaved ultrapure water and transferred 450 μL of each sample to sterile tubes with 50 μL of 1% cetylpyridinium chloride (CPC). After vortexing a second time, we incubated samples at room temperature for 30 min before vortexing a third time and spreading 100 μL in duplicate onto Middlebrook 7H10 agar plates supplemented with oleic acid/glycerol enrichment. We incubated one plate at 30°C and the other at 37°C for 21 days and then examined them for colony growth.
Water filter samples.
We collected water samples by hand-filtering 0.5 to 1 liter of water through 0.2-μm syringe filters. We then extracted filter membranes from filter cartridges using snip shears sterilized with 70% ethanol. After cutting each filter membrane into four pieces using sterile forceps and razor blades, we transferred them into 5-mL screwcap tubes containing 2 mL of autoclaved ultrapure water. After vortexing on high for 30 s, we transferred 450 μL of each sample to sterile tubes with 50 μL of 1% CPC, vortexed them again, and incubated them at room temperature for 30 min. We vortexed samples a third time before spreading 100 μL in triplicate on 7H10 agar plates supplemented with oleic acid/glycerol enrichment. We incubated plates at 22°C, 30°C, and 37°C for 21 days and then examined them for colony growth.
Soil samples.
We collected soil samples by clearing away surface debris to expose top soil and then scooped soil into sterile 50-mL conical vials. We immersed 1 g of soil into 10 mL of enzymatic solution containing 1 mg/mL pronase, 1 mg/mL lipase, 1 mg/mL cellulase, 1% Tween 80, and 1% NaCl. After briefly vortexing on high, we incubated samples on a rocking platform at 37°C for 1 h and then allowed them to settle upright for 1 h. Once soil particles had settled, we transferred 450 μL to sterile tubes with 50 μL of 1% CPC, vortexed them again, and incubated them at room temperature for 30 min. Following one more vortex, we spread 100 μL in duplicate on 7H10 agar plates supplemented with oleic acid/glycerol enrichment and amphotericin B. We incubated one plate at 30°C and the other at 37°C for 21 days and then examined them for colony growth.
DNA extraction and identification.
We thawed bacterial pellets from frozen and followed established methods for DNA extraction (56). Specifically, we immersed bacterial pellets in 100 μL of lysis buffer and 25 μL of 100 mg/mL lysozyme, vortexed them briefly, and then incubated them for 1 h at 37°C. We added 25 μL of 2.5 mg/mL proteinase K and 50 μL of 20% SDS and then incubated it for 10 min at 65°C. We transferred the entire volume of each sample to a deep-well 96-well plate containing 100 μL of zirconia beads and added 400 μL of chromatin immunoprecipitation binding buffer from the ZR-96 Genomic DNA Clean and Concentrator-5 kit (no. D4067; Zymo Research) to each well. Using a Qiagen TissueLyzer II, we bead beat the deep-well plate for 3 min at 30 Hz and then centrifuged at maximum speed for 2 min to pellet debris. We transferred the sample supernatants to the column provided with the kit, and the remainder of the DNA extraction followed the manufacturer’s instructions.
To determine the identity of the cultured environmental NTM, we performed Sanger sequencing of an ∼700-bp region of the RNA polymerase region five beta subunit (rpoB) gene. We set up and submitted PCRs for Sanger sequencing (Quintara Biosciences, San Francisco, CA), which included 1 to 10 ng of DNA template, 4 μL mixture of 5 μM forward and reverse primers, and nuclease-free water. We trimmed the resulting sequences for quality and compared them to sequences in the National Center for Biotechnology Information (NCBI) GenBank using the BLAST algorithm. Putatively novel NTM were defined as isolates that highly matched (greater than 90% sequence similarity and query coverage to reference sequence) uncharacterized mycobacteria in the NCBI BLAST GenBank database.
Mineralogy.
A subset of the 771 samples included 121 soil samples from three different areas on Kaua’i and Hawai’i Island (Fig. 1), which were analyzed further for detailed mineral content. We used a Rigaku MiniFlex 600 X-ray diffractometer (XRD) employing copper radiation and a scintillation detector with a graphite monochromator as a practical tool for rapid screening and characterization of complex soil mixtures. Mineral and cation abundances were quantified by standard Rietveld methods embedded in the Rigaku PDXL2 software. The poorly crystalline nature of many minerals in the soil makes it difficult to resolve magnetite (primary), maghemite (secondary), and chromite (primary) from one another, as they share similar crystal structures. For example, their most intense peak (311) has a d-spacing from 2.5135 to 2.5314 Å. In addition, soils may contain a mixture of primary and secondary Fe-Ti-Cr-oxides because primary minerals may persist a long time despite deep weathering (57, 58). Relative leaching and prevalence of Fe-oxides/hydroxides, including all forms of iron, was therefore determined by comparing abundances to TiO2 in parent basalt, an oxide that is not readily leached from soils. Leaching relative to TiO2, denoted τ, was calculated according to Brantley and White (59) and expressed as a percentage. Weathered material was treated as if it were air-dried soil by adjusting the weight-based abundances by adding back losses on ignition. Samples were cultured for NTM by following the methods outlined above. We used a simple logistic regression to determine the strength and direction of any relationship between soil Fe-oxide/hydroxide relative prevalence and the probability of NTM presence.
Spatial model.
Modeling of respiratory-relevant NTM species (i.e., those implicated in human cases of NTM PD: Mycobacterium chimaera, Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium abscessus, Mycobacterium porcinum, and Mycobacterium fortuitum; 1) is key to understanding the environmental niches of NTM and their relevance to human health. However, positivity rates for respiratory-relevant species of NTM in areas outside human homes is often low (19), presenting a challenge for spatial modeling. Since previous research has suggested similar environmental niches among different species of NTM (8), we used data on all NTM species (respiratory relevant and not respiratory relevant) in our model, making the assumption that respiratory-relevant species have environmental requirements similar to those of non-respiratory-relevant species. To verify this assumption, we compared the distribution of positive data for all NTM species and positive data for respiratory-relevant species along a gradient of each covariate used in our model using a two-sample Kolmogorov-Smirnov test (60) in program R (v3.6.1; 61). We also present descriptive data on the sample prevalence of different NTM species, highlighting those of respiratory relevance.
We modeled factors affecting the probability of NTM presence on a larger scale than possible with our minerology samples by using all 771 samples from natural areas across the state. We fit a logistic regression model (i.e., a model based on a binary outcome: NTM detected/not detected) with spatial random effects within a Bayesian framework using Markov chain Monte Carlo (MCMC) techniques (62). We used a multivariate normal prior with mean = 0 and variance = 1000 for the slope coefficients. Spatial random effects helped account for marginal levels of spatial autocorrelation in regression residuals (Morans I measure of spatial autocorrelation: observed, −0.03, expected, −0.001, P = 0.048; 63). We assumed an exponential covariance function for our spatial random effects with parameters estimated for range , partial sill , and nugget (64). We assumed a uniform prior for the range given by
where d is the maximum distance (in kilometers) between samples. We assumed inverse gamma (IG) priors for the partial sill and nugget parameters with
Computations were performed in program R using the spGLM routine in the spBayes package (64). We chose 11 remotely sensed predictors that best represented our current knowledge of NTM in the Hawai’i environment, allowing hypothesis testing with coverage available for the entire state (Table 2). Predictors included those related to moisture (i.e., water balance, a derived variable of precipitation minus evapotranspiration), water sources (i.e., distance to the coast, freshwater sources, thermal springs, and agriculture), and soils (i.e., soil expansive ability and the midpoint of estimated soil age range). Soil expansive ability in our study was evaluated by the USDA Natural Resources and Conservation Service and gives the relative expected change in volume with changes in moisture content (i.e., extent of shrinking when soil dries out or swelling when wet; 65). Since they have been found to be important in past studies, we included predictors for population density and elevation (19). We included a proxy for UV radiation, to which NTM may be sensitive (e.g., see reference 66), using average vegetation height, which was correlated with availability of UV but less correlated with other included variables. We included one interaction term in our model, the interaction between soil expansive ability and water balance. After our model was run and beta coefficients estimated, we simplified visualization of the relationship between the interaction of two continuous predictors and the probability of NTM presence by selecting low and high levels of each predictor: high and low water balance, 1,839 mm (90th quantile) and −635 mm (0th quantile), respectively, and high and low soil expansive ability of 4.5 linear extensibility percent (90th quantile) and 1.5 linear extensibility percent (10th quantile), respectively. Quantiles were based on the lowest and highest values that had good sample coverage over the values of the interacting predictor.
We confirmed that all predictors were correlated less than 0.4 based on a Pearson correlation coefficient, considered low-moderate correlation (67), with variance inflation factors of <2.5 (68), supporting our ability to model all predictors together without risking multicollinearity. Thus, we included all predictors in a single global model to facilitate analysis. We confirmed the absence of overfitting and tested the model’s predictive ability by assessing 5-fold cross-validation accuracy, training on a new 1/5 of the data set each time without replacement, and AUC (area under the ROC curve; 69, 70). All predictors were centered and scaled prior to analysis. Our model converged after 30,000 iterations following 500 iterations of burn-in (i.e., discarding initial MCMC samples before chains reached posterior distribution from initial values; 62), which was confirmed with traceplots and Gelman-Rubin statistics of <1.1 (71). We used the spPredict function in the spBayes package to predict the probability of NTM presence across the entire state, given statewide predictor values on a 1-km grid, eliminating prediction for some areas where predictor values were beyond the range of our sample (i.e., elevation of >2,000 m, population density of >2,000 people/km2, and soil expansive ability of >10%). Most soil expansive ability values fell in the range of 0 to 8%, with only a small area of Hawai’i Island attaining a higher value (20%). We note removal of a single data point, the only one to sample in the >8% soil expansive ability area, to avoid extrapolation based on a single data point when estimating beta coefficient relationships.
Data availability.
rpoB sequence data from this study have been deposited in the NCBI database under accession numbers ON011722 to ON012029.
ACKNOWLEDGMENTS
This work was funded, in part, by the National Science Foundation grant number 1743587, Division of Environmental Biology, as part of the Ecology and Evolution of Infectious Diseases program. J.R.H. also acknowledges support from the Padosi Foundation.
Footnotes
Supplemental material is available online only.
Contributor Information
Arielle W. Parsons, Email: ahwaldst@ncsu.edu.
Jeremy D. Semrau, University of Michigan-Ann Arbor
REFERENCES
- 1.Honda JR, Virdi R, Chan ED. 2018. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 9:2029. 10.3389/fmicb.2018.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA. 2016. Non-tuberculous mycobacterial (NTM) lung infection public meeting: October 15, 2015. FDA, Silver Spring, MD. [Google Scholar]
- 3.Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34. 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed I, Tiberi S, Farooqi J, Jabeen K, Yeboah-Manu D, Migliori GB, Hasan R. 2020. Non-tuberculous mycobacterial infections—a neglected and emerging problem. Int J Infect Dis 92:S46–S50. 10.1016/j.ijid.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda JR, Hasan NA, Davidson RM, Williams MD, Epperson LE, Reynolds PR, Smith T, Iakhiaeva E, Bankowski MJ, Wallace RJ, Chan ED, Falkinham JO, Strong M. 2016. Environmental nontuberculous mycobacteria in the Hawaiian Islands. PLoS Negl Trop Dis 10:e0005068. 10.1371/journal.pntd.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson ST, Robinson S, Rey K, Brown L, Jones N, Dawrs SN, Virdi R, Norton GJ, Epperson LE, Hasan NA, Chan ED, Strong M, Honda JR. 2021. Exposure pathways of nontuberculous mycobacteria through soil, streams, and groundwater, Hawai'i, USA. GeoHealth 5:e2020GH000350. 10.1029/2020GH000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh CM, Gebert MJ, Delgado-Baquerizo M, Maestre FT, Fierer N. 2019. A global survey of mycobacterial diversity in soil. Appl Environ Microbiol 85:e01180-19. 10.1128/AEM.01180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glickman CM, Virdi R, Hasan NA, Epperson LE, Brown L, Dawrs SN, Crooks JL, Chan ED, Strong M, Nelson ST, Honda JR. 2020. Assessment of soil features on the growth of environmental nontuberculous mycobacterial isolates from Hawai'i. Appl Environ Microbiol 86:e00121-20. 10.1128/AEM.00121-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe J, Alop-Mabuti A, Burger P, Button J, Ellsberry M, Hitzeman J, Morgenstern D, Nunies K, Strother M, Darling-Munson J, Chan YL, Cassady R, Vasconcellos SMK, Iseman MD, Chan ED, Honda JR. 2016. Comparing the temporal colonization and microbial diversity of showerhead biofilms in Hawai'i and Colorado. FEMS Microbiol Lett 363:fnw005. 10.1093/femsle/fnw005. [DOI] [PubMed] [Google Scholar]
- 11.Falkinham IIJ. 2011. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 17:419–424. 10.3201/eid1703.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada S, Ito Y, Hirai T, Murase K, Tsuji T, Fujita K, Mio T, Maekawa K, Fujii T, Ono S, Nishimura T, Hayashi A, Komori T, Fujita N, Niimi A, Ichiyama S, Chin K, Mishima M. 2016. Impact of industrial structure and soil exposure on the regional variations in pulmonary nontuberculous mycobacterial disease prevalence. Int J Mycobacteriol 5:170–176. 10.1016/j.ijmyco.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Covert TC, Rodgers MR, Reyes AL, Stelma GN. 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol 65:2492–2496. 10.1128/AEM.65.6.2492-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor RH, Falkinham IIJ, Norton CD, LeChevallier MW. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl Environ Microbiol 66:1702–1705. 10.1128/AEM.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stidd CK, Leopold LB. 1951. The geographic distribution of average monthly rainfall, Hawaii. American Meteorological Society, Boston, MA, USA. [Google Scholar]
- 17.Giambelluca TW, Shuai X, Barnes ML, Alliss RJ, Longman RJ, Miura T, Chen Q, Frazier AG, Mudd RG, Cuo L, Businger AD. 2014. Evapotranspiration of Hawai‘i. Final report submitted to the U.S. Army Corps of Engineers—Honolulu District, and the Commission on Water Resource Management, State of Hawai‘i.
- 18.Deenik J, McClellan AT. 2007. Cooperative Extension Service. University of Hawaii, Manoa. [Google Scholar]
- 19.Virdi R, Lowe ME, Norton GJ, Dawrs SN, Hasan NA, Epperson LE, Glickman CM, Chan ED, Strong M, Crooks JL, Honda JR. 2021. Lower recovery of nontuberculous mycobacteria from outdoor Hawai’i environmental water biofilms compared to indoor samples. Microorganisms 9:224. 10.3390/microorganisms9020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munn DA, McLean EO, Ramirez A, Logan TJ. 1973. Effect of soil, cover, slope, and rainfall factors on soil and phosphorus movement under simulated rainfall conditions. Soil Sci Soc Am J 37:428–431. 10.2136/sssaj1973.03615995003700030033x. [DOI] [Google Scholar]
- 21.Thomson R, Tolson C, Sidjabat H, Huygens F, Hargreaves M. 2013. Mycobacterium abscessus isolated from municipal water-a potential source of human infection. BMC Infect Dis 13:241–247. 10.1186/1471-2334-13-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Ingen J, Boeree MJ, Dekhuijzen PNR, Van Soolingen D. 2009. Environmental sources of rapid growing nontuberculous mycobacteria causing disease in humans. Clin Microbiol Infect 15:888–893. 10.1111/j.1469-0691.2009.03013.x. [DOI] [PubMed] [Google Scholar]
- 23.Nishiuchi Y, Iwamoto T, Maruyama F. 2017. Infection sources of a common non-tuberculous mycobacterial pathogen, Mycobacterium avium complex. Front Med (Lausanne) 4:27. 10.3389/fmed.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Groote MA, Pace NR, Fulton K, Falkinham IIJ. 2006. Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol 72:7602–7606. 10.1128/AEM.00930-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzou CL, Dirac MA, Becker AL, Beck NK, Weigel KM, Meschke JS, Cangelosi GA. 2020. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil: a case–control study. Ann Am Thorac Soc 17:57–62. 10.1513/AnnalsATS.201812-915OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, Chan ED, Adjemian J, Dunn RR, Fierer N. 2018. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio 9:e01614-18. 10.1128/mBio.01614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haig SJ, Kotlarz N, Kalikin LM, Chen T, Guikema S, LiPuma JJ, Raskin L. 2020. Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics. Environ Sci Water Res Technol 6:3032–3043. 10.1039/D0EW00723D. [DOI] [Google Scholar]
- 28.Greig J, Rajić A, Young I, Mascarenhas M, Waddell L, LeJeune J. 2015. A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food chain. Zoonoses Public Health 62:269–284. 10.1111/zph.12147. [DOI] [PubMed] [Google Scholar]
- 29.Sandy M, Butler A. 2009. Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109:4580–4595. 10.1021/cr9002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong SC, Roversi P, Lillington J, Rodriguez F, Krehenbrink M, Zeldin OB, Garman EF, Lea SM, Berks BC. 2014. A complex iron-calcium cofactor catalyzing phosphotransfer chemistry. Science 345:1170–1173. 10.1126/science.1254237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sritharan M, Yeruva VC, Sivasailappan SC, Duggirala S. 2006. Iron enhances the susceptibility of pathogenic mycobacteria to isoniazid, an antitubercular drug. World J Microbiol Biotechnol 22:1357–1364. 10.1007/s11274-006-9183-8. [DOI] [Google Scholar]
- 32.Savabi M, Williams J. 1989. Water balance and percolation. USDA Agricultural Research, Manhattan, KS. [Google Scholar]
- 33.Chen P, Sun J, Ma L, Chen Y, Xia J. 2022. Effects of shell sand content on soil physical properties and salt ions under simulated rainfall leaching. Geoderma 406:115520. 10.1016/j.geoderma.2021.115520. [DOI] [Google Scholar]
- 34.Nelson ST, Tingey DG, Selck B. 2013. The denudation of ocean islands by ground and surface waters: the effects of climate, soil thickness, and water contact times on Oahu, Hawaii. Geochim Cosmochim Acta 103:276–294. 10.1016/j.gca.2012.09.046. [DOI] [Google Scholar]
- 35.Jaworska H, Dąbkowska-Naskręt H, Kobierski M. 2016. Iron oxides as weathering indicator and the origin of Luvisols from the Vistula glaciation region in Poland. J Soils Sediments 16:396–404. 10.1007/s11368-015-1201-8. [DOI] [Google Scholar]
- 36.Ratledge C. 2004. Iron, mycobacteria and tuberculosis. Tuberculosis (Edinb) 84:110–130. 10.1016/j.tube.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Sherrod DR, Sinton JM, Watkins SE, Brunt KM. 2007. Geologic map of the State of Hawaii. U.S. Geological Survey report. US Geological Survey, Reston, VA. [Google Scholar]
- 38.Best MG. 2013. Igneous and metamorphic petrology. John Wiley & Sons, New York, NY. [Google Scholar]
- 39.Lipner EM, French J, Bern CR, Walton-Day K, Knox D, Strong M, Prevots DR, Crooks JL. 2020. Nontuberculous mycobacterial disease and molybdenum in Colorado watersheds. Ijerph 17:3854. 10.3390/ijerph17113854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warkentin BP. 1962. Water retention and swelling pressure of clay soils. Can J Soil Sci 42:189–196. 10.4141/cjss62-024. [DOI] [Google Scholar]
- 41.Baldrian P, Merhautová V, Petránková M, Cajthaml T, Šnajdr J. 2010. Distribution of microbial biomass and activity of extracellular enzymes in a hardwood forest soil reflect soil moisture content. Appl Soil Ecol 46:177–182. 10.1016/j.apsoil.2010.08.013. [DOI] [Google Scholar]
- 42.Tiedje JM, Sexstone AJ, Parkin TB, Revsbech NP. 1984. Anaerobic processes in soil. Plant Soil 76:197–212. 10.1007/BF02205580. [DOI] [Google Scholar]
- 43.McNeil MM, Brown JM. 1994. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev 7:357–417. 10.1128/CMR.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojony R, Danelishvili L, Campeau A, Wozniak JM, Gonzalez DJ, Bermudez LE. 2020. Exposure of mycobacterium abscessus to environmental stress and clinically used antibiotics reveals common proteome response among pathogenic mycobacteria. Microorganisms 8:698. 10.3390/microorganisms8050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeFlorio-Barker S, Egorov A, Smith GS, Murphy MS, Stout JE, Ghio AJ, Hudgens EE, Messier KP, Maillard JH, Hilborn ED. 2021. Environmental risk factors associated with pulmonary isolation of nontuberculous mycobacteria, a population-based study in the southeastern United States. Sci Total Environ 763:144552. 10.1016/j.scitotenv.2020.144552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hersman LE, Forsythe JH, Ticknor LO, Maurice PA. 2001. Growth of Pseudomonas mendocina on Fe (III)(hydr) oxides. Appl Environ Microbiol 67:4448–4453. 10.1128/AEM.67.10.4448-4453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojha A, Hatfull GF. 2007. The role of iron in Mycobacterium smegmatis biofilm formation: the exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol Microbiol 66:468–483. 10.1111/j.1365-2958.2007.05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Painuli DK, Mohanty M, Sinha NK, Misra AK. 2017. Crack formation in a swell–shrink soil under various managements. Agric Res 6:66–72. 10.1007/s40003-016-0241-7. [DOI] [Google Scholar]
- 49.Stagnitti F. 1999. A model of the effects of nonuniform soil-water distribution on the subsurface migration of bacteria: Implications for land disposal of sewage. Math Computer Modelling 29:41–52. 10.1016/S0895-7177(99)00038-2. [DOI] [Google Scholar]
- 50.Yeh HF, Lee CH, Chen JF, Chen WP. 2007. Estimation of groundwater recharge using water balance model. Water Resour 34:153–162. 10.1134/S0097807807020054. [DOI] [Google Scholar]
- 51.Wyrtki K, Meyers G. 1976. The trade wind field over the Pacific Ocean. J Appl Meteor 15:698–704. . [DOI] [Google Scholar]
- 52.Frazier AG, Giambelluca TW, Diaz HF, Needham HL. 2016. Comparison of geostatistical approaches to spatially interpolate month-year rainfall for the Hawaiian Islands. Int J Climatol 36:1459–1470. 10.1002/joc.4437. [DOI] [Google Scholar]
- 53.Carbone RE, Tuttle JD, Cooper WA, Grubišić V, Lee WC. 1998. Trade wind rainfall near the windward coast of Hawaii. Mon Weather Rev 126:2847–2863. . [DOI] [Google Scholar]
- 54.Stearns HT. 1946. Geology of the Hawaiian islands. US Geological Survey, Reston, VA. [Google Scholar]
- 55.Thomas PJ, Baker JC, Zelazny LW. 2000. An expansive soil index for predicting shrink–swell potential. Soil Sci Soc Am J 64:268–274. 10.2136/sssaj2000.641268x. [DOI] [Google Scholar]
- 56.Epperson LE, Strong M. 2020. A scalable, efficient, and safe method to prepare high quality DNA from mycobacteria and other challenging cells. J Clin Tuberc Other Mycobacterial Dis 19:100150. 10.1016/j.jctube.2020.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kierczak J, Neel C, Bril H, Puziewicz J. 2007. Effect of mineralogy and pedoclimatic variations on Ni and Cr distribution in serpentine soils under temperate climate. Geoderma 142:165–177. 10.1016/j.geoderma.2007.08.009. [DOI] [Google Scholar]
- 58.Cornu S, Lucas Y, Lebon E, Ambrosi JP, Luizão F, Rouiller J, Bonnay M, Neal C. 1999. Evidence of titanium mobility in soil profiles, Manaus, central Amazonia. Geoderma 91:281–295. 10.1016/S0016-7061(99)00007-5. [DOI] [Google Scholar]
- 59.Brantley SL, White AF. 2009. Approaches to modeling weathered regolith. Rev Mineral Geochem 70:435–484. 10.2138/rmg.2009.70.10. [DOI] [Google Scholar]
- 60.Pratt JW, Gibbons JD. 1981. Kolmogorov-Smirnov two-sample tests, p 318–344. Concepts of nonparametric theory. Springer, New York, NY. [Google Scholar]
- 61.R Core Development Team. 2008. R: a language and environment for statistical computing, v3.4.0. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 62.Gelman A, Rubin DB. 1996. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res 5:339–355. 10.1177/096228029600500402. [DOI] [PubMed] [Google Scholar]
- 63.Moran PA. 1950. Notes on continuous stochastic phenomena. Biometrika 37:17–23. 10.1093/biomet/37.1-2.17. [DOI] [PubMed] [Google Scholar]
- 64.Finley AO, S B, Gelfand A. 2015. spBayes for large univariate and multivariate point-referenced spatio-temporal data models. J Stat Soft 63:1–28. 10.18637/jss.v063.i13. [DOI] [Google Scholar]
- 65.Soil Survey Staff. 2021. Official soil series descriptions. U.S. Department of Agriculture, Silver Spring, MD. [Google Scholar]
- 66.Norton GJ, Williams M, Falkinham IIJ, Honda JR. 2020. Physical measures to reduce exposure to tap water–associated nontuberculous mycobacteria. Front Public Health 8:190. 10.3389/fpubh.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schober P, Boer C, Schwarte LA. 2018. Correlation coefficients: appropriate use and interpretation. Anesth Analg 126:1763–1768. 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 68.Johnston R, Jones K, Manley D. 2018. Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual Quant 52:1957–1976. 10.1007/s11135-017-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stone M. 1974. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc Series B 36:111–133. 10.1111/j.2517-6161.1974.tb00994.x. [DOI] [Google Scholar]
- 70.Jiménez-Valverde A. 2012. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob Ecol Biogeogr 21:498–507. 10.1111/j.1466-8238.2011.00683.x. [DOI] [Google Scholar]
- 71.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Statist Sci 7:457–472. 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 72.USGS. 2019. National hydrography dataset (ver. USGS national hydrography dataset best resolution (NHD) for hydrologic unit (HU) 4–2001). U.S. Geological Survey, Reston, VA. [Google Scholar]
- 73.Center for International Earth Science Information Network. 2016. Gridded population of the world, version 4 (GPWv4): administrative unit center points with population estimates. Columbia University, Palisades, NY. [Google Scholar]
- 74.USGS. 2019. USGS 3D elevation program digital elevation model. U.S. Geological Survey, Reston, VA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables SA1 and SA2 and Fig. SA1 to SA3. Download aem.00018-22-s0001.pdf, PDF file, 0.4 MB (371.2KB, pdf)
Data Availability Statement
rpoB sequence data from this study have been deposited in the NCBI database under accession numbers ON011722 to ON012029.