ABSTRACT
Streptococcus suis has been increasingly recognized as a porcine zoonotic pathogen that threatens the health of both pigs and humans. Metal homeostasis plays a critical role in the antioxidative capability of bacteria, thus facilitating the escape of pathogenic species from the innate immunity systems of hosts. Here, we revealed that manganese increased the ability of S. suis to resist oxidative stress. RNA sequencing was used to identify potential candidate genes involved in the maintenance of intracellular manganese homeostasis. Four genes, termed troABCD, were identified by NCBI BLASTp analysis. The troA, troB, troC, and troD deletion mutant strains exhibited decreased intracellular manganese content and tolerance to H2O2 compared to the wild-type strain. Thus, troABCD were determined to be involved in manganese uptake and played an important role in H2O2 tolerance in S. suis. Furthermore, the inactivation of perR increased the survival of H2O2-pulsed S. suis 2.18-fold and elevated the intracellular manganese content. H2O2-pulsed S. suis and perR deletion mutants upregulated troABCD. This finding suggested that H2O2 released the suppression of troABCD by perR. In addition, an electrophoretic mobility shift assay (EMSA) showed that PerR at 500 ng binds to the troABCD promoter, indicating that troABCD were directly regulated by PerR. In conclusion, this study revealed that manganese increases tolerance to H2O2 by upregulating the expression of troABCD. Moreover, PerR-regulated Mn import in S. suis and increased the tolerance of S. suis to oxidative stress by regulating troABCD.
IMPORTANCE During infection, it is extremely important for bacteria to defend against oxidative stress. While manganese plays an important role in this process, its role is unclear in S. suis. Here, we demonstrated that manganese increased S. suis tolerance to oxidative stress. Four manganese ABC transporter genes, troABCD, were identified. Oxidative stress increased the content of manganese in the cell. Furthermore, PerR increased the tolerance to oxidative stress of S. suis by regulating troABCD. Manganese played an important role in bacterial defense against oxidative stress. These findings provide novel insight into the mechanism by which S. suis resists oxidative stress and approaches to inhibit bacterial infection by limiting manganese intake.
KEYWORDS: Streptococcus suis, manganese, troABCD, oxidative stress, PerR
INTRODUCTION
Bacteria encounter oxidative stress when exposed to reactive oxygen species (ROS) and have consequently developed various mechanisms of protection (1, 2). ROS, including superoxide ion (O2−), hydroxyl radical (HO·), and hydrogen peroxide (H2O2), can cause severe damage to lipids, proteins, and DNA (3). Multiple factors, including superoxide dismutase (SOD), NADH oxidase, catalase, and DNA-binding protein, from starved cells play an important role in tolerance to oxidative stress (4, 5). Although H2O2 is not particularly toxic to microorganisms, it can react with cellular Fe2+ via Fenton chemistry to form highly toxic HO· (6).
The most well-known effect of manganese is related to its involvement in oxidative stress defense mechanisms (7). Manganese is an essential cofactor of manganese-containing SOD (8) and also acts as an inorganic enzyme to dismutate O2− by complexation with small molecules of bacteria (9). The host manganese chelator calprotectin and the deletion of manganese importers have been shown to diminish SOD activity in Staphylococcus aureus (10, 11). Manganese inhibits ferroxidation and prevents damage to Dps-1-bound DNA derived from iron leakage from the core, followed by the generation of ·OH through Fenton chemistry (12). Additionally, manganese functions as an antioxidant via a protein-independent mechanism (9).
A Fur family regulator, PerR, has been identified in various bacteria and is associated with significant variability in bacterial sensitivity to oxidation (13). PerR is associated with the suppression of oxidative stress detoxification genes, including sodA, dpr, pmtA, and ahpCF (14–17). There are various compositions of regulons governed by PerR among bacterial species and even between strains within a species. The genes regulated by PerR can be divided into two major groups: (i) genes involved in metal homeostasis and (ii) genes directly involved in ROS detoxification (13). PerR has been reported to be involved in the oxidative stress response in Streptococcus pyogenes (16), Streptococcus oligofermentans (18), and Streptococcus mutans (19). Moreover, Streptococcus suis is an increasingly recognized porcine zoonotic pathogen responsible for huge economic losses to the pig industry (20). Of the 29 known serotypes (21), S. suis serotype 2 is considered to be the most pathogenic. Two large-scale outbreaks of S. suis occurred in China in 1998 and 2005 (22, 23). S. suis can cause a variety of severe infections, including arthritis, meningitis, endocarditis, septicemia, and even death, in both swine and humans (21). It is extremely important for pathogenic bacteria to adapt to the host environment and develop defenses against oxidative stress (24). The regulation of responses to oxidative stress is extremely important for bacterial survival in vivo when bacteria encounter oxidative stress during infection. PerR has been demonstrated to regulate manganese uptake and defend against oxidative stress in Streptococcus oligofermentans (18). However, in S. suis, PerR has been reported only to regulate the oxidative stress response through the regulation of dpr (25). In addition, the relationship between PerR and manganese uptake is poorly understood. Furthermore, the role of manganese in S. suis tolerance to oxidative stress and how manganese uptake responds to oxidative stress remain unclear. In this study, we revealed the importance of manganese in S. suis H2O2 tolerance. In addition, PerR derepressed the manganese transporter troABCD genes in the presence of H2O2.
RESULTS
Manganese increases the ability of S. suis to tolerate oxidative stress.
Manganese has been illustrated to increase the tolerance of Streptococcus oligofermentans (18), Staphylococcus aureus (26), and Deinococcus radiodurans (27) to oxidative stress. To explore the role of cellular manganese in oxidative stress tolerance, mid-log-phase cultures of S. suis were collected and spotted onto tryptic soy agar (TSA) plates supplemented with H2O2 or MnCl2 at different concentrations. As shown in Fig. 1, no colonies formed from 10 mM H2O2, and with the addition of 0.25 to 0.5 mM Mn2+, colonies formed in the presence of 10 mM H2O2. These results revealed that manganese played an important role in S. suis resistance to H2O2.
FIG 1.
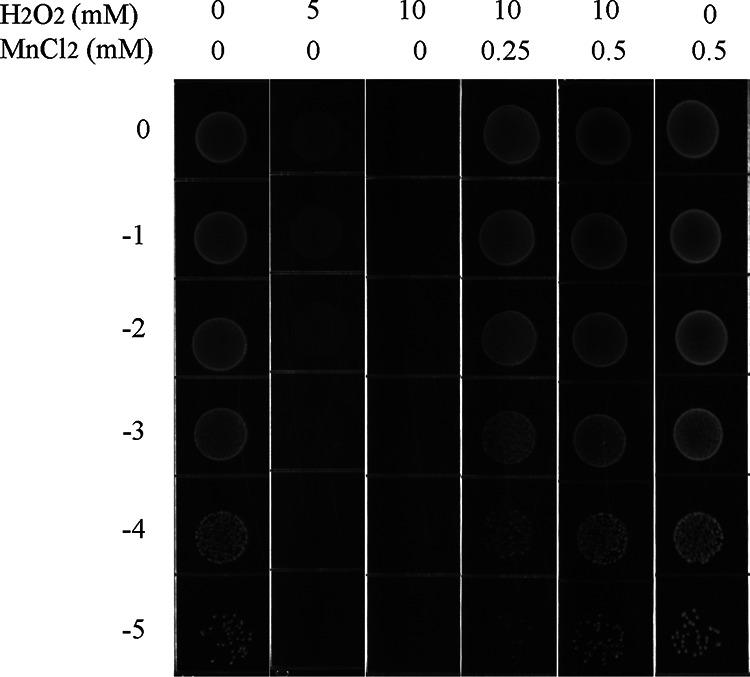
Spot dilution assays of the wild-type strain with or without H2O2 or manganese.
Transcriptional response of S. suis to excess exogenous manganese.
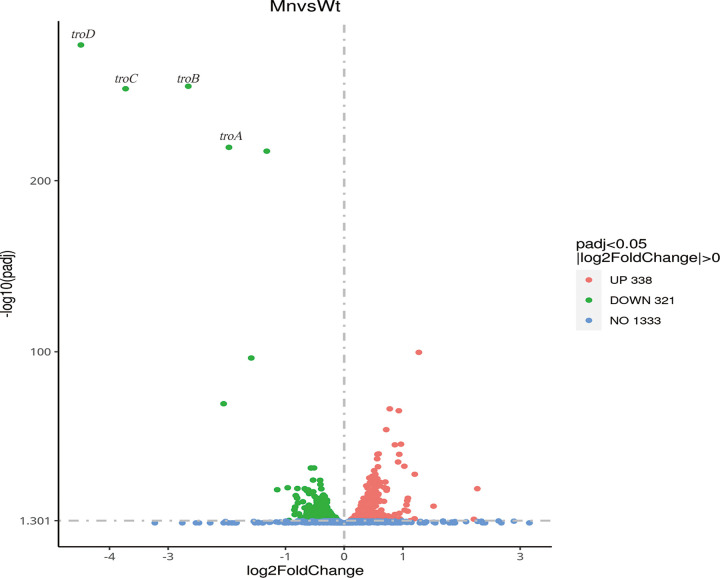
To identify potential candidate genes involved in the maintenance of intracellular manganese homeostasis, RNA sequencing (RNA-seq) was used to investigate the transcriptional response of S. suis exposed to 1 mM MnCl2 compared to untreated cells (Fig. 2).
FIG 2.
Exposure to 1 mM MnCl2 alters the S. suis transcriptome. Shown are results from RNA sequencing analysis measuring fold changes in transcript abundances for S. suis wild-type cells treated with 1 mM MnCl2 compared to untreated controls.
Four transcripts (SSUSC84_1887, SSUSC84_1888, SSUSC84_1889, and SSUSC84_1891), representing the most downregulated genes encoding metal ABC transporters, exhibited 3.9- to 22.45-fold decreases in transcript abundance in cells exposed to 1 mM Mn. SSUSC84_1891 has been reported previously in S. suis as troA (28). SSUSC84_1889 exhibited approximately 41.77%, 40.17%, 41.35%, 39.66%, and 41.83% amino acid identities to the manganese transporters PsaA from Streptococcus pneumoniae, MtsA from Streptococcus pyogenes, MntA from Streptococcus oligofermentans, MntA from Staphylococcus aureus, and EfaA from Enterococcus faecalis, respectively (see Fig. S1 in the supplemental material). Therefore, SSUSC84_1887, SSUSC84_1888, SSUSC84_1889, and SSUSC84_1891 were designated troABCD and inferred to be involved in manganese transport.
TroABCD promotes S. suis growth in Mn-restricted environments and functions as a manganese transporter.
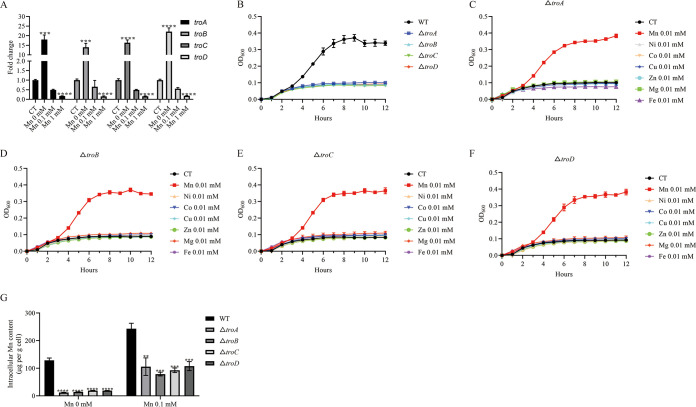
To explore the role of TroABCD in Mn environments, we examined the transcription levels of troA, troB, troC, and troD in chemically defined medium (CDM) (29) with or without Mn. As shown in Fig. 3A, the transcription levels of troA, troB, troC, and troD were significantly higher (14- to 22.12-fold) in CDM without Mn than in CDM containing Mn. Furthermore, the transcription levels decreased with higher concentrations of Mn (Fig. 3A). Next, we generated ΔtroA, ΔtroB, ΔtroC, and ΔtroD mutant strains using a markerless system (see Fig. S2 in the supplemental material). Upon genetic confirmation that all gene deletions occurred as planned, the growth of wild-type (WT) and mutant strains was monitored in Mn-depleted CDM. None of the mutant strains could grow in Mn-deficient medium, whereas the WT strain grew normally (Fig. 3B). The mutant strains could grow when supplemented with Mn but not other metal ions (Ni, Cu, Co, Zn, Mg, and Fe) (Fig. 3C to F). Collectively, these results demonstrated that the transcription levels of troABCD increased in Mn-restricted environments and that TroABCD contributed to the growth of S. suis in Mn-restricted environments.
FIG 3.
TroABCD promotes the growth of S. suis in Mn-restricted environments. (A) Transcription levels of troA, troB, troC, and troD of S. suis in CDM supplemented with or without Mn. (B) Growth curves of the WT, ΔtroA, ΔtroB, ΔtroC, and ΔtroD strains in CDM without Mn. (C to F) Growth curves of the ΔtroA, ΔtroB, ΔtroC, and ΔtroD strains in CDM with or without metal ions. (G) Intracellular Mn contents of the WT, ΔtroA, ΔtroB, ΔtroC, and ΔtroD strains treated with or without 0.1 mM Mn. The data are expressed as means ± standard deviations from three independent experiments. Asterisks indicate P values of <0.05 by Student’s t tests on the samples. CT, control; OD600, optical density at 600 nm.
To verify the primary function of the ABC transporter system in the process of manganese transport, we used a flame atomic absorption spectrophotometer to quantify the cellular Mn content in each of the different Mn transport mutants. As shown in Fig. 3G, mutations in the troABCD components caused a significant reduction in the intracellular Mn pool, with or without supplementary Mn. These results demonstrated that TroABCD was the main Mn importer in S. suis. The Mn uptake system promoted S. suis growth in Mn-restricted environments.
TroABCD contributes to S. suis resistance to H2O2.
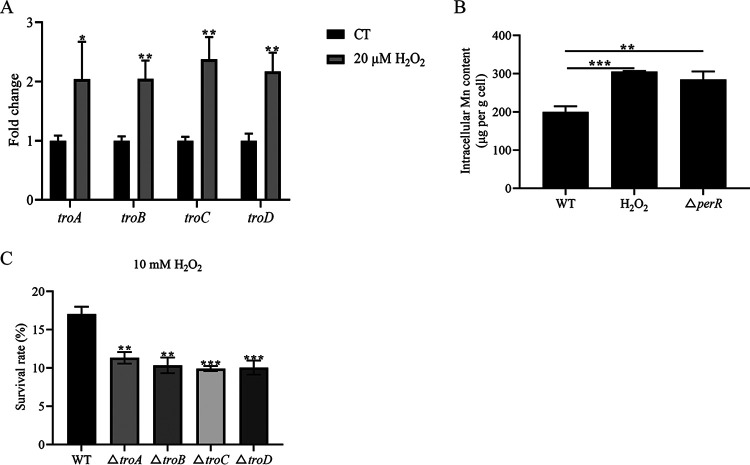
The transcription of mntABC has been reported to be induced by H2O2 and to play an important role in H2O2 resistance (30). To investigate the role of TroABCD in S. suis resistance to H2O2, the WT strain was cultured with 0 or 20 μM H2O2 to mid-log phase. The RNA expression of troABCD was measured, and as shown in Fig. 4A, the troABCD expression level was higher in 20 μM H2O2 than the control one. The intracellular Mn content of S. suis treated with H2O2 was increased compared to the control (Fig. 4B).
FIG 4.
TroABCD contributed to the resistance to H2O2 of S. suis. (A) Transcription levels of troABCD of S. suis treated with or without H2O2. (B) Intracellular Mn content. (C) Survival rates of WT and deletion mutant strains treated with 10 mM H2O2. The data are expressed as means ± standard deviations from three independent experiments. Asterisks indicate P values of <0.05 by Student’s t tests on the samples.
Furthermore, troABCD deletion mutant strains were used to evaluate H2O2 tolerance. As shown in Fig. 4C, the WT and deletion mutant strains were cultured with or without H2O2, and the survival rates of the deletion mutant strains were low compared to that of the WT strain. These data suggested that the manganese transporter TroABCD played an important role in the H2O2 tolerance of S. suis.
The perR deletion increased tolerance to H2O2 and the cellular manganese content.
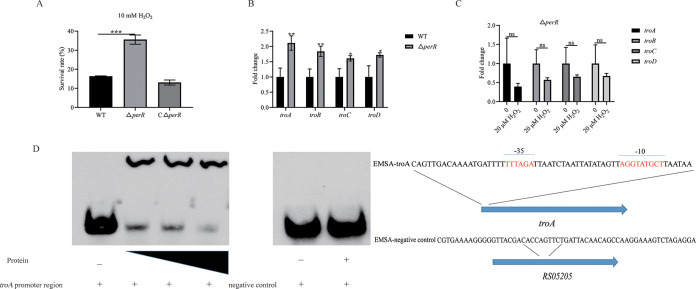
PerR was indicated to be a peroxide-responsive repressor in S. suis (25). To identify the function of PerR in S. suis, a perR deletion mutant was constructed and verified by PCR (Fig. S1B) and sequencing. The WT strain, the perR deletion mutant (ΔperR), and the complementation strain (CΔperR) were cultured with or without H2O2. As shown in Fig. 5A, the survival of the ΔperR mutant was 2.18-fold higher than that of the WT strain. PerR has been reported to regulate metal ion homeostasis in Bacillus subtilis (31) and to downregulate cellular manganese in S. oligofermentans (18). To explore whether PerR played a similar role in S. suis, cells in the mid-log phase were collected to measure the levels of cellular manganese using a flame atomic absorption spectrophotometer. As shown in Fig. 4B, the manganese content was higher in the ΔperR mutant than in the WT and CΔperR strains. These results revealed that PerR downregulates cellular manganese in S. suis.
FIG 5.
PerR played an important role in resistance to H2O2 and regulating troABCD. (A) Survival rates of WT and deletion mutant strains treated with 10 mM H2O2. (B) Transcription levels of troABCD of S. suis or the ΔperR mutant. (C) Transcription levels of troABCD in the perR mutant treated with or without H2O2. (D) An EMSA was performed to evaluate the direct binding of PerR to the promoter region of troABCD. The left panel shows PerR (0 ng, 500 ng, 600 ng, and 700 ng) and a biotin-labeled DNA probe. The right panel shows PerR (700 ng) and a nonspecific DNA probe. The locations of the two probes in the genome are also shown. The data are expressed as means ± standard deviations from three independent experiments. Asterisks indicate P values of <0.05 by Student’s t tests on the samples. ns, not significant.
PerR inactivation upregulates troABCD, and PerR binds to the troABCD promoter.
Because the level of cellular manganese was increased in the ΔperR mutant, troABCD expression was evaluated. As shown in Fig. 5B, the expression of troABCD in the ΔperR mutant was upregulated compared to the WT strain. Furthermore, in the perR mutant, the transcription levels of troABCD were not increased following treatment with H2O2 (Fig. 5C). To explore whether PerR-regulated troABCD indirectly or directly, we assessed PerR binding to the promoter region of troA using an electrophoretic mobility shift assay (EMSA). As shown in Fig. 5D, PerR bound directly to the troABCD promoter region but was not able to bind to a nonspecific DNA fragment (Fig. 5D).
These results demonstrated that PerR represses the expression of troABCD and that H2O2 released the repression. Moreover, PerR increased S. suis tolerance to H2O2 by increasing the expression of troABCD.
DISCUSSION
Most streptococci are commensals, pathogens, or opportunistic pathogens of animals and humans (32). During the process of infection, bacteria may encounter situations of both metal starvation and oxidative stress (33, 34). For pathogenic bacteria, adaptation to the host environment is crucial for their survival and the establishment of a successful infection (24). During host-pathogen interactions, neutrophils use an “oxidative burst” to kill pathogenic bacteria, which is extremely important for bacteria to resist oxidative stress (35, 36). Insufficient intracellular manganese can have wide-ranging consequences, affecting the Mn-dependent enzymes involved in transcription, metabolism, and defense against oxidative stress (26, 37, 38). Mn is the most well-known metal ion involved in antioxidant stress, which increases the antioxidant capacity of bacteria through a variety of mechanisms (7). In this study, we report that S. suis could resist H2O2 by regulating the import of Mn through PerR.
Transition metals are essential trace metal elements for bacteria. Lactic acid bacteria (e.g., streptococci) are notoriously Mn-centric organisms, exhibiting a much higher nutritional demand for Mn than other bacterial groups (39). This particular metabolic phenomenon might explain why the striking loss of virulence observed here is in contrast to the moderate virulence attenuation of Mn transport mutants in Gram-negative and other Gram-positive pathogens (10, 40). In this work, S. suis increased the uptake of manganese in the presence of H2O2 and the ability to tolerate H2O2 by supplementing manganese (Fig. 1 and Fig. 4B).
To explore the genes involved in S. suis manganese regulation, RNA-seq was used to analyze the levels of RNA expression. Four genes displayed a large degree of downregulation when the WT strain was treated with manganese. Using amino acid sequence analysis, the genes had high homology with mntABC of other streptococci. In addition, the manganese ABC transporter is the primary transporter for bacterial uptake of manganese, which has been reported in Staphylococcus aureus (41) and Bacillus anthracis (42). It has been identified to affect the oxidative stress resistance of Streptococcus gordonii (18, 43). However, the role of TroABCD has seldom been reported in S. suis. To investigate the role of troABCD in resistance to H2O2, we constructed deletions of troA, troB, troC, and troD. The deletion mutant strains decreased intracellular manganese and attenuated H2O2-challenged survival. Furthermore, the troABCD expression levels were increased in the WT strain in response to treatment with H2O2 (Fig. 4A). These findings indicated that troABCD-mediated manganese uptake was pivotal to antioxidative stress tolerance in S. suis.
PerR has been reported to regulate the oxidative stress response by increasing dpr expression in S. suis (25). Because Dpr has iron storage properties, cytosolic iron can be efficiently scavenged when dpr is overexpressed in S. suis (44). To determine whether PerR was involved in manganese uptake, the mRNA expression of troABCD was detected in ΔperR. The expression of troABCD was increased in the perR deletion mutant strain compared with that of the WT strain (Fig. 5B). Furthermore, the intracellular manganese content of the ΔperR mutant was higher than that of the WT strain. In the presence of endogenous or exogenous H2O2, PerR derepressed the expression of the manganese transporter genes troABCD. This resulted in increased Mn contents and H2O2 tolerance.
Oxidative stress tolerance is critical for the ability of bacteria to invade a host. There are multiple mechanisms used by bacteria to resist oxidative stress. In this study, S. suis was found to increase the intracellular manganese content in the presence of H2O2, and supplementation with manganese can increase the tolerance to H2O2 of S. suis. Moreover, manganese played an important role in the tolerance to H2O2 of S. suis. PerR, a Fur family regulator, has been identified in various bacteria and is involved in oxidative stress tolerance. In this study, PerR was found to regulate the sensitivity of bacteria to H2O2 by repressing troABCD expression. These findings are in accordance with those of a previous report (18). Therefore, manganese plays an important role in oxidative stress defense, and PerR increases the tolerance of S. suis to oxidative stress by regulating troABCD.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and growth conditions.
The bacterial strains, plasmids, and primers used in this study are listed in Tables S1 and S2 in the supplemental material. The S. suis strain was cultured at 37°C in brain heart infusion (BHI) broth (Oxoid), in tryptic soy broth (TSB; BD), or on tryptic soy agar (TSA; BD) containing 10% (vol/vol) newborn bovine serum. Escherichia coli DH5α competent cells were grown in Luria-Bertani (LB) broth or on LB agar at 37°C.
Oxidative stress sensitivity assays.
The strains were cultured with or without H2O2 for 3 h in BHI broth. The bacterial solution was serially diluted 10-fold up to an appropriate dilution for viability counts. The survival rate was calculated by the number of viable strains.
RNA-seq analysis.
The S. suis wild-type strain was cultured with or without supplementation of 1 mM MnCl2 until the mid-log phase. The cells were then centrifuged and subjected to RNA extraction. RNA sequencing was performed by Wuhan GeneCreate Biological Engineering Co., Ltd., using an Illumina Novaseq platform. The clean reads were obtained by removing reads containing an adapter, reads containing an N base, and low-quality reads from the raw data. The clean reads were mapped to the S. suis SC84 genome using Bowtie2-2.2.3. FPKM, the expected number of fragments per kilobase of transcript sequence per million base pairs sequenced, was used to quantify the levels of gene expression. Differential expression analysis of two groups was performed using the DESeq R package (1.18.0). Genes with an adjusted P value of <0.05 obtained by DESeq were considered to be differentially expressed.
Construction of deletion mutants and complementation strains.
The method for obtaining a mutant was performed as previously described (45). To obtain a deletion mutant, the up-F/up-R and down-F/down-R primers were used to separately amplify the upstream and downstream regions of the target gene by PCR. The PCR products were directly cloned to a pSET4s vector following digestion with the corresponding restriction enzymes. The recombinant plasmid pSET4s::perR was transformed into SC19. The mutant strain was selected on TSA based on its sensitivity to spectinomycin. The resultant mutant strain was confirmed by PCR and sequencing. The complementary strain was acquired by transforming pSET2, which contained the target gene, into the mutant strain.
RNA extraction and reverse transcription-quantitative real-time PCR.
The total RNA in the strains was extracted using a bacterial total RNA isolation kit (Sangon Biotech, China). HiScript II Q RT SuperMix for reverse transcription and quantitative real-time PCR (qRT-PCR) (+gDNA wiper) (Vazyme, China) was used to synthesize the cDNA. AceQ quantitative PCR (qPCR) SYBR green master mix (Vazyme, China) was used to measure the levels of mRNA. The 16S rRNA gene was used as an endogenous control. The 2−ΔCT method was used to quantify and compare the levels of gene transcription.
H2O2 survival assay.
Bacterial strains were cultured to mid-log phase. Cells (1 mL) were harvested by centrifugation and subsequently resuspended in phosphate-buffered saline (PBS). The bacterial solution was serially diluted 10-fold up to a 10−5 dilution, and 5 μL of each dilution was spotted onto TSA plates supplemented with 10% (vol/vol) newborn bovine serum and H2O2 (0, 5, or 10 mM) or MnCl2 (1, 0.25, or 0.5 mM). Plates were incubated at 37°C for 12 h.
Analysis of the intracellular manganese content.
Measurement of the intracellular manganese content was performed as previously described, with some modifications (46, 47). The harvested strains were washed three times with PBS containing 0.25 mM EDTA and subsequently washed three times with PBS. Cells were then desiccated at 60°C overnight. The dry cell weight was measured, and the cells were resuspended in 35% (vol/vol) HNO3 and boiled at 95°C for 1 h prior to the removal of debris by centrifugation. Samples were diluted to a final concentration of 3.5% (vol/vol) HNO3 and analyzed using a flame atomic absorption spectrophotometer.
EMSAs.
The troA-F and troA-R primers were used to obtain the DNA probe, which was end labeled with biotinylated ribonucleotides. PerR and the DNA probe were incubated in electrophoretic mobility shift assay (EMSA) binding buffer for 20 min, and the mixture was separated on a 4% (wt/vol) native polyacrylamide gel at 70 V and then transferred to a positively charged nylon membrane at 100 V for 20 min, followed by UV cross-linking and incubation with horseradish peroxidase (HRP). Finally, the probe was detected using a ChemiDoc Touch imaging system (Bio-Rad, USA).
Statistical analysis.
GraphPad Prism 7 software was used to analyze the data. Student’s t test for analysis of variance was used to analyze the results. For all tests, a P value of <0.05 was considered the threshold for significance.
ACKNOWLEDGMENTS
We thank T. Sekizaki (National Institute of Animal Health, Japan) for supplying plasmids pSET4s and pSET2.
We report no potential conflict of interest.
This work was supported by the Natural Science Foundation of China (NSFC) (grant no. 31672560 and 31802189), the Natural Science Foundation of Hubei Province (no. 2018CFA045), the Technical Innovation Project of Hubei Province (no. 2020ABA016), the National Key R&D Program of China (no. 2017YFD0500201), and the National Key Research and Development Program of China (2021YFD1800400).
Footnotes
Supplemental material is available online only.
Contributor Information
Yongxiang Tian, Email: tyxanbit@163.com.
Fangyan Yuan, Email: fangyanyuan12@163.com.
Weicheng Bei, Email: beiwc@mail.hzau.edu.cn.
Charles M. Dozois, INRS
REFERENCES
- 1.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lushchak VI. 2011. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol 153:175–190. 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Henle ES, Linn S. 1997. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem 272:19095–19098. 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 4.Moparthi VK, Moparthi SB, Howe C, Raleiras P, Wenger J, Stensjö K. 2019. Structural diffusion properties of two atypical Dps from the cyanobacterium Nostoc punctiforme disclose interactions with ferredoxins and DNA. Biochim Biophys Acta 1860:148063. 10.1016/j.bbabio.2019.148063. [DOI] [PubMed] [Google Scholar]
- 5.Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect Immun 69:5098–5106. 10.1128/IAI.69.8.5098-5106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadtman ER, Berlett BS. 1991. Fenton chemistry. Amino acid oxidation. J Biol Chem 266:17201–17211. 10.1016/S0021-9258(19)47359-6. [DOI] [PubMed] [Google Scholar]
- 7.Juttukonda LJ, Skaar EP. 2015. Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol 97:216–228. 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun 68:2819–2826. 10.1128/IAI.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culotta VC, Daly MJ. 2013. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid Redox Signal 19:933–944. 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, Rathi S, Chazin WJ, Caprioli RM, Skaar EP. 2013. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 81:3395–3405. 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen KH, Grove A. 2012. Metal binding at the Deinococcus radiodurans Dps-1 N-terminal metal site controls dodecameric assembly and DNA binding. Biochemistry 51:6679–6689. 10.1021/bi300703x. [DOI] [PubMed] [Google Scholar]
- 13.Pinochet-Barros A, Helmann JD. 2018. Redox sensing by Fe(2+) in bacterial Fur family metalloregulators. Antioxid Redox Signal 29:1858–1871. 10.1089/ars.2017.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenot A, Weston BF, Caparon MG. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol Microbiol 63:1185–1196. 10.1111/j.1365-2958.2006.05577.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Keramati L, Helmann JD. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc Natl Acad Sci USA 92:8190–8194. 10.1073/pnas.92.18.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifantini R, Toukoki C, Colaprico A, Gryllos I. 2011. Peroxide stimulon and role of PerR in group A Streptococcus. J Bacteriol 193:6539–6551. 10.1128/JB.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsburgh MJ, Clements MO, Crossley H, Ingham E, Foster SJ. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun 69:3744–3754. 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Tong H, Dong X. 2014. PerR-regulated manganese ion uptake contributes to oxidative stress defense in an oral streptococcus. Appl Environ Microbiol 80:2351–2359. 10.1128/AEM.00064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruxin TR, Schwartzman JA, Davidowitz CR, Peters Z, Holtz A, Haney RA, Spatafora GA. 2021. Regulatory involvement of the PerR and SloR metalloregulators in the Streptococcus mutans oxidative stress response. J Bacteriol 203:e00678-20. 10.1128/JB.00678-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. 2014. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 3:e45. 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okura M, Osaki M, Nomoto R, Arai S, Osawa R, Sekizaki T, Takamatsu D. 2016. Current taxonomical situation of Streptococcus suis. Pathogens 5:45. 10.3390/pathogens5030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, Yu H, Pan X, Zhou X, Wang H, Wu B, Wang H, Zhao H, Lin Y, Yue J, Wu Z, He X, Gao F, Khan AH, Wang J, Zhao G-P, Wang Y, Wang X, Chen Z, Gao GF. 2006. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 3:e377. 10.1371/journal.pmed.0030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, Yu H, Pan X, Zhou X, Wang H, Wu B, Wang H, Zhao H, Lin Y, Yue J, Wu Z, He X, Gao F, Khan AH, Wang J, Zhao G-P, Wang Y, Wang X, Chen Z, Gao GF. 2006. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 3:e151. 10.1371/journal.pmed.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopoulou N, Granneman S. 2022. The role of RNA-binding proteins in mediating adaptive responses in Gram-positive bacteria. FEBS J 289:1746–1764. 10.1111/febs.15810. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Ding Y, Li T, Wan Y, Li W, Chen H, Zhou R. 2012. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol 12:85. 10.1186/1471-2180-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. 2011. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10:158–164. 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai S, Xie Z, Wang B, Yu N, Zhao J, Zhou Y, Hua Y, Tian B. 2021. Dynamic polyphosphate metabolism coordinating with manganese ions defends against oxidative stress in the extreme bacterium Deinococcus radiodurans. Appl Environ Microbiol 87:e02785-20. 10.1128/AEM.02785-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichgers Schreur PJ, Rebel JMJ, Smits MA, van Putten JPM, Smith HE. 2011. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol 193:5073–5080. 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun 27:444–448. 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Wang X, Yang F, Hu Q, Tong H, Dong X. 2017. Molecular insights into hydrogen peroxide-sensing mechanism of the metalloregulator MntR in controlling bacterial resistance to oxidative stresses. J Biol Chem 292:5519–5531. 10.1074/jbc.M116.764126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faulkner MJ, Helmann JD. 2011. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal 15:175–189. 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sader HS, Streit JM, Fritsche TR, Jones RN. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002–2004). Clin Microbiol Infect 12:844–852. 10.1111/j.1469-0691.2006.01550.x. [DOI] [PubMed] [Google Scholar]
- 33.Cotter PD, Hill C. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453. 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaspar J, Kim JN, Ahn S-J, Burne RA. 2016. An essential role for (p)ppGpp in the integration of stress tolerance, peptide signaling, and competence development in Streptococcus mutans. Front Microbiol 7:1162. 10.3389/fmicb.2016.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. 2018. Update on neutrophil function in severe inflammation. Front Immunol 9:2171. 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosales C. 2018. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol 9:113. 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handke LD, Gribenko AV, Timofeyeva Y, Scully IL, Anderson AS. 2018. MntC-dependent manganese transport is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 3:e00336-18. 10.1128/mSphere.00336-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausmann S, Guimarães VA, Garcin D, Baumann N, Linder P, Redder P. 2017. Both exo- and endo-nucleolytic activities of RNase J1 from Staphylococcus aureus are manganese dependent and active on triphosphorylated 5′-ends. RNA Biol 14:1431–1443. 10.1080/15476286.2017.1300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisher JP, Giedroc DP. 2013. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol 3:91. 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papp-Wallace KM, Maguire ME. 2006. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60:187–209. 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 41.Al‐Tameemi H, Beavers WN, Norambuena J, Skaar EP, Boyd JM. 2021. Staphylococcus aureus lacking a functional MntABC manganese import system has increased resistance to copper. Mol Microbiol 115:554–573. 10.1111/mmi.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gat O, Mendelson I, Chitlaru T, Ariel N, Altboum Z, Levy H, Weiss S, Grosfeld H, Cohen S, Shafferman A. 2005. The solute-binding component of a putative Mn(II) ABC transporter (MntA) is a novel Bacillus anthracis virulence determinant. Mol Microbiol 58:533–551. 10.1111/j.1365-2958.2005.04848.x. [DOI] [PubMed] [Google Scholar]
- 43.Jakubovics NS, Smith AW, Jenkinson HF. 2002. Oxidative stress tolerance is manganese (Mn2+) regulated in Streptococcus gordonii. Microbiology (Reading) 148:3255–3263. 10.1099/00221287-148-10-3255. [DOI] [PubMed] [Google Scholar]
- 44.Pulliainen AT, Kauko A, Haataja S, Papageorgiou AC, Finne J. 2005. Dps/Dpr ferritin-like protein: insights into the mechanism of iron incorporation and evidence for a central role in cellular iron homeostasis in Streptococcus suis. Mol Microbiol 57:1086–1100. 10.1111/j.1365-2958.2005.04756.x. [DOI] [PubMed] [Google Scholar]
- 45.Takamatsu D, Osaki M, Sekizaki T. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140–148. 10.1006/plas.2001.1532. [DOI] [PubMed] [Google Scholar]
- 46.Alquethamy SF, Khorvash M, Pederick VG, Whittall JJ, Paton JC, Paulsen IT, Hassan KA, McDevitt CA, Eijkelkamp BA. 2019. The role of the CopA copper efflux system in Acinetobacter baumannii virulence. Int J Mol Sci 20:575. 10.3390/ijms20030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colomer-Winter C, Flores-Mireles AL, Baker SP, Frank KL, Lynch AJL, Hultgren SJ, Kitten T, Lemos JA. 2018. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog 14:e1007102. 10.1371/journal.ppat.1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Tables S1 to S3. Download aem.00086-22-s0001.pdf, PDF file, 0.7 MB (778.6KB, pdf)