Abstract
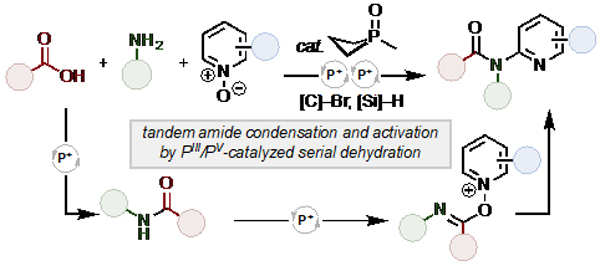
An organophosphorus (PIII/PV redox) catalyzed method for the three-component condensation of amines, carboxylic acids, and pyridine N-oxides to generate 2-amidopyridines via serial dehydration is reported. Whereas amide synthesis and functionalization usually occur under divergent reaction conditions, here a phosphetane catalyst—together with a mild bromenium oxidant and terminal hydrosilane reductant—is shown to drive both steps chemoselectively in an auto-tandem catalytic cascade. The ability to both prepare and functionalize amides under the action of a single organocatalytic reactive intermediate enables new possibilities for the efficient and modular preparation of medicinal targets.
Graphical Abstract
Amides are common targets in biological and medicinal chemistry,1,2 but challenging substrates for chemical derivatization.3 Resultantly, a diverse synthetic toolbox of mild reagents supports amide coupling,4 but amide activation5 generally employs strongly electrophilic reagents—chiefly Tf2O6 as exemplified in the work of Charette,7 Movassaghi,8 Huang,9 and Maulide10,11—to accomplish functionalization of the typically inert amide moiety.12–13,14 Notwithstanding the power of Tf2O-mediated amide activation, the highly electrophilic nature of this reagent necessitates sequencing of the amide preparation and functionalization operations, and generally precludes the use of substrates containing Lewis basic functionalities, including amines.15 This lack of cross-compatibility between amide coupling and activation enforces a practice whereby these two related reactions are taken as disparate synthetic tasks—performed in sequence (Figure 1A, top).
Figure 1.
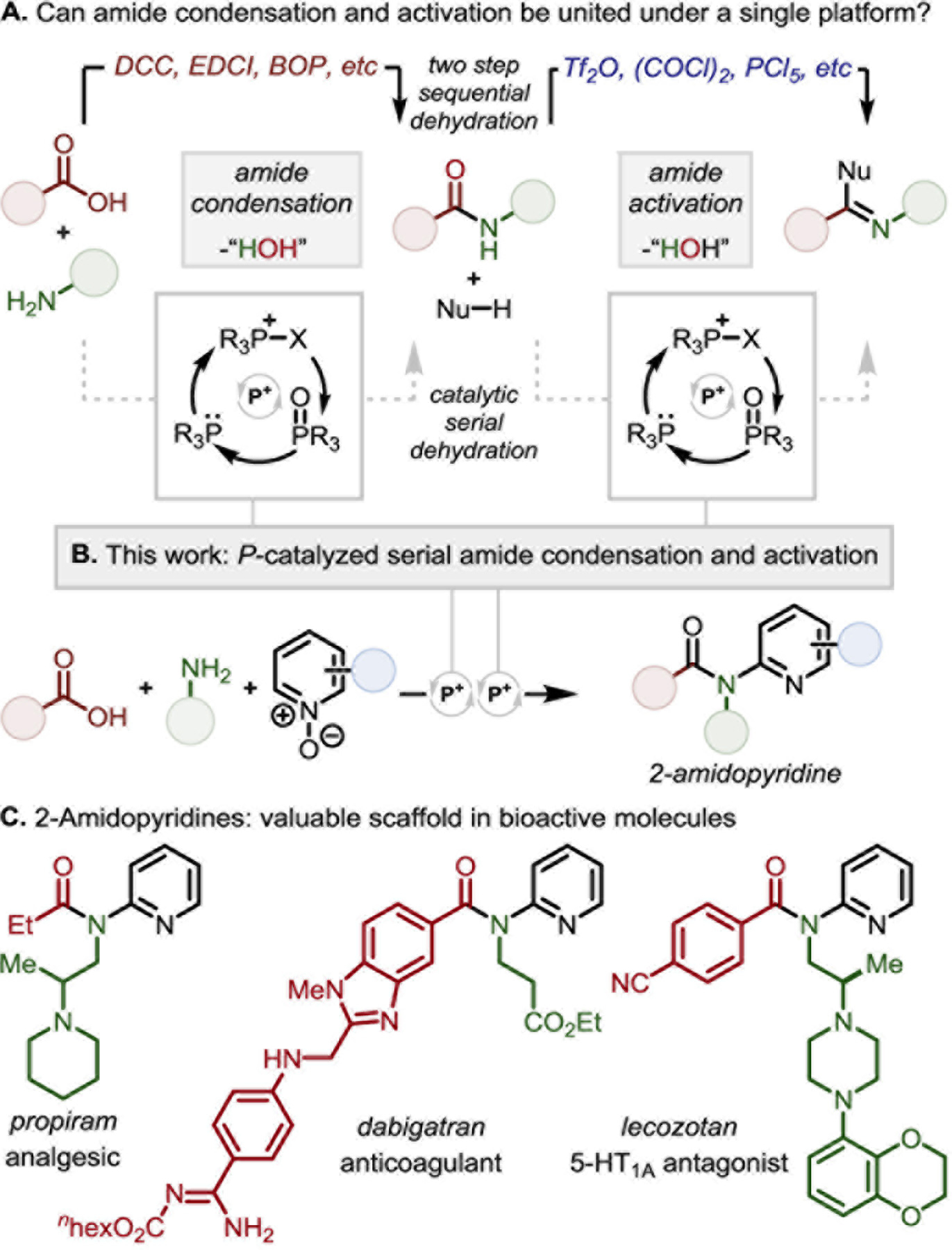
(A) Amide condensation and activation utilize different reagents due to reactivity differences – can they be chemoselectively carried out in tandem via a unified phosphacatalytic platform? (B) Organophosphorus-catalyzed condensation of amines, carboxylic acids, and pyridine N-oxides to 2-amidopyridines via serial dehydration. (C) Pharmaceutical agents containing 2-amidopyridine core.
Recognizing that amide synthesis and electrophilic amide activation are both condensation processes, we considered whether a mild dehydrating electrophile—generated iteratively under catalytic conditions—would permit tandem dehydrative amidation/activation events in which the amide serves as a reactive intermediate. In line with Mukaiyama’s “oxidation-reduction condensation” concept for irreversible dehydration,16 recent results from our lab have established that iterative generation of a mild halophosphonium species within the PIII/PV redox cycle17 permits recursive dehydration (Figure 1A, bottom).18 Under such a manifold, amides can be generated through condensation19 and utilized in situ as valuable synthetic intermediates for further activation20 and functionalization.21
Here, we report a PIII/PV=O catalyzed22 multicomponent cascade amide condensation and activation for intermolecular coupling, enabled by serial dehydration (Figure 1B). This approach provides rapid access to the valuable 2-amidopyridine pharmacophore,23 typified by the analgesic propiram, WHO essential medicine dabigatran, and anti-Alzheimer’s candidate lecozotan (Figure 1C). By expressing a net redox neutral reactivity in the PIII/PV=O redox couple to drive serial condensation, a chemoselective assemblage of simple starting materials is achieved in a single reaction,24 establishing a perspective on amides as veritable synthetic intermediates in catalytic tandem cascades.
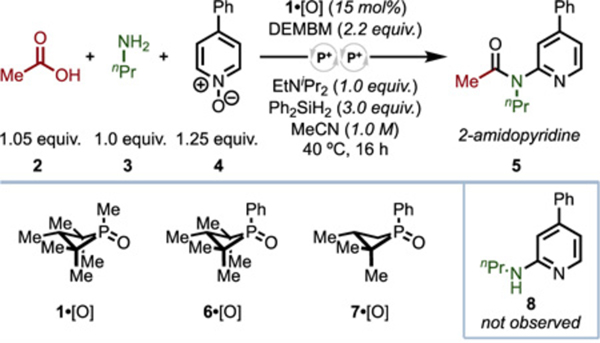
Inspired by amide functionalization methods25 from Abramovitch26 and Movassaghi,27 we envisioned a chemoselective tandem dehydrative three-component coupling28 of amines, carboxylic acids, and pyridine N-oxides,29 assembling 2-amidopyridines in a modular fashion. To probe this hypothesis, 1,2,2,3,4,4-hexamethyl phosphetane P-oxide 1·[O]30 was evaluated in the coupling of acetic acid (2), propylamine (3), and 4-phenylpyridine N-oxide (4) to 2-amidopyridine 5. In practice, 93% yield of 5 (86% isolated yield on 0.5 mmol scale) was obtained with 15 mol% 1·[O], along with 2.2 equiv. diethyl(methyl)bromomalonate (DEMBM)31 as oxidant, 3.0 equiv. diphenylsilane as reductant, and 1.0 equiv. of Hünig’s base at 40 °C in 1.0 M acetonitrile (Table 1, entry 1). The stoichiometry of oxidant and reductant required are in line with iterative PIII/PV redox cycling to formally strip two equivalents of H2O from the substrate molecules, whereas omitting any of 1·[O], DEMBM, or Ph2SiH2 resulted in no product formation, indicating that PIII/PV redox cycling is essential (see SI for expanded table).32 Critically, this reaction allows for evaluation of the chemoselectivity of the organophosphorus redox catalyst for the intended amide condensation/activation manifold, as phosphonium electrophiles, such as peptide-coupling reagent PyBroP,33 are known to activate pyridine N-oxides for reaction with nucleophiles.34 Notably, 2-aminopyridine 8 is not observed, even at lower conversion to product 5 (vide infra). While precise tuning of Hünig’s base loading to effectively quench the acid generated under this redox condensation manifold was necessary for maximal efficiency (entries 2 and 3), lutidine could be used in place of Hünig’s base with minimal effect (entry 4). With respect to catalyst, the P- phenyl phosphetane 6·[O] could be used at the expense of some efficiency, while 7·[O]35 proved to be ineffective, presumably due to lability at the α-unsubstituted cyclic methylene centers (entries 5 and 6).
Table 1.
Optimized conditions and variations.
| ||
|---|---|---|
| Entry | deviation from standard | Yield of 5 (%)a |
| 1 | none | 93 (86)b |
| 2 | 2.0 equiv. EtNiPr2 | 71 |
| 3 | 0 equiv. EtNiPr2 | 78 |
| 4 | 2,6-lutidine in place of EtNiPr2 | 88 |
| 5 | 6·[O] in place of 1·[O] | 85 |
| 6 | 7·[O] in place of 1·[O] | 0 |
Yield determined by 1H NMR against internal standard on 0.125 mmol scale reaction.
Isolated yield on 0.5 mmol scale reaction. DEMBM = diethyl(methyl)bromomalonate, 1,2-DCE = 1,2-dichloroethane.
In order to probe more specifically the sequence of bond-forming events, the coupling of 2 and 4 with 4-fluoroaniline (9) to 10 was monitored by 19F NMR spectroscopy (Figure 2A).36 Over the course of the first six hours, depletion of 9 and formation of the corresponding amide intermediate 11 was observed, with no formation of 2-aminopyridine 12. Subsequently, once 9 was completely consumed, formation of product 10 was observed as the acetanilide 11 was dehydrated. This reaction profile is consistent with sequential dehydrative activation via an amide intermediate as proposed.37 Independently, 11 and 4 could be dehydratively coupled to 10 under identical conditions with adjustment of reagent stoichiometry (see SI). In contrast, in the isolated reaction of 4 with 9, no coupling to 2-aminopyridine 12 was observed by 19F NMR or LC-MS (see SI). Thus, catalytically-generated bromophosphonium 1·Br+ is not effective for activation of the N-oxide, in contrast to N-oxide activation observed with PyBroP. This observed reactivity profile is in good agreement with stoichiometric reaction of [1·Br]Br with the reaction components.38 In concert, these data support a reaction pathway of amide condensation followed by amide activation to generate imidoyloxypyridinium 13 and rearrangement to yield 2-amidopyridine39 product 10 (Figure 2B), enabled by the catalytic generation of bromophosphonium ion 1·Br+ as a general, mild, and selective dehydrating species (see SI for full catalytic cycle).40
Figure 2.
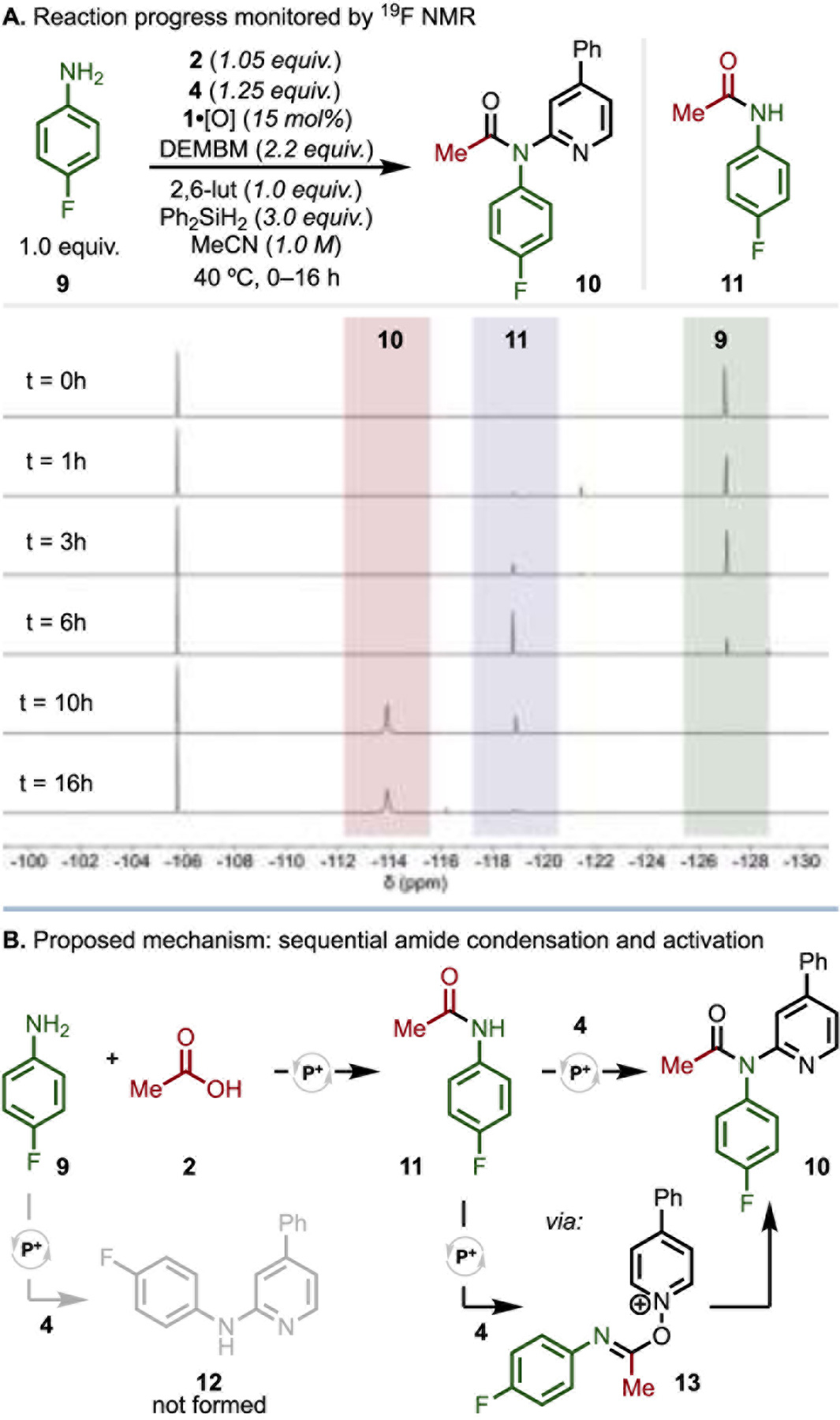
(A) Time-stacked 19F NMR spectra (in CDCl3) of the coupling of 9 with 2 and 4 at the indicated time points, showing aniline 9 (δ −127.3 ppm), acetanilide 11 (δ −118.8 ppm), and 2-amidopyridine 10 (δ −113.9 ppm), with 4,4’-difluorobenzophenone internal standard (δ −105.8 ppm). (B) Proposed reaction mechanism proceeding through amide condensation and activation, not pyridine N-oxide activation.
With an understanding of the reaction parameters and mechanism, scope of the reaction upon variation of the carboxylic acid coupling partner was evaluated (Figure 3). Straight-chain alkyl carboxylic acids (14–18, 61–78% yield) bearing various functional groups, including aryl fluoride (15), alkyl bromide (16), ether (17), and sulfonamide (18)could be transformed under the standard reaction conditions. Carboxylic acids bearing branching α-carbon centers (19 and 20, 77 and 83% yield, respectively) demonstrated equivalent efficiency. Further, benzoic acids bearing a wide variety of substitutions were successfully utilized in this reaction (21–24, 60–71% yield). A benzoic acid bearing a pendant alkyl ethyl ester provided the desired coupled product 24 in 63% yield with no evidence of ester reactivity, highlighting the mild nature of this catalytic dehydrative platform. A variety of heterocycle-containing carboxylic acids could be incorporated into this reaction, as benzotriazole41 (25, 60% yield), unprotected N-H indole42 (26, 71% yield), thiophene (27, 72% yield), N-Me benzimidazole43 (28, 63% yield), and benzothiazole (29, 62% yield) moieties evidenced no deleterious side effects from the inherent reactivity of the heterocycle. A variety of undesired side-reaction pathways are available to these heterocyclic substrates through reaction with strong electrophiles, highlighting the advantageous nature of catalytic generation of a mild, selectively-activating phosphonium ion for tandem dehydration sequences on functionality-rich substrates.
Figure 3.
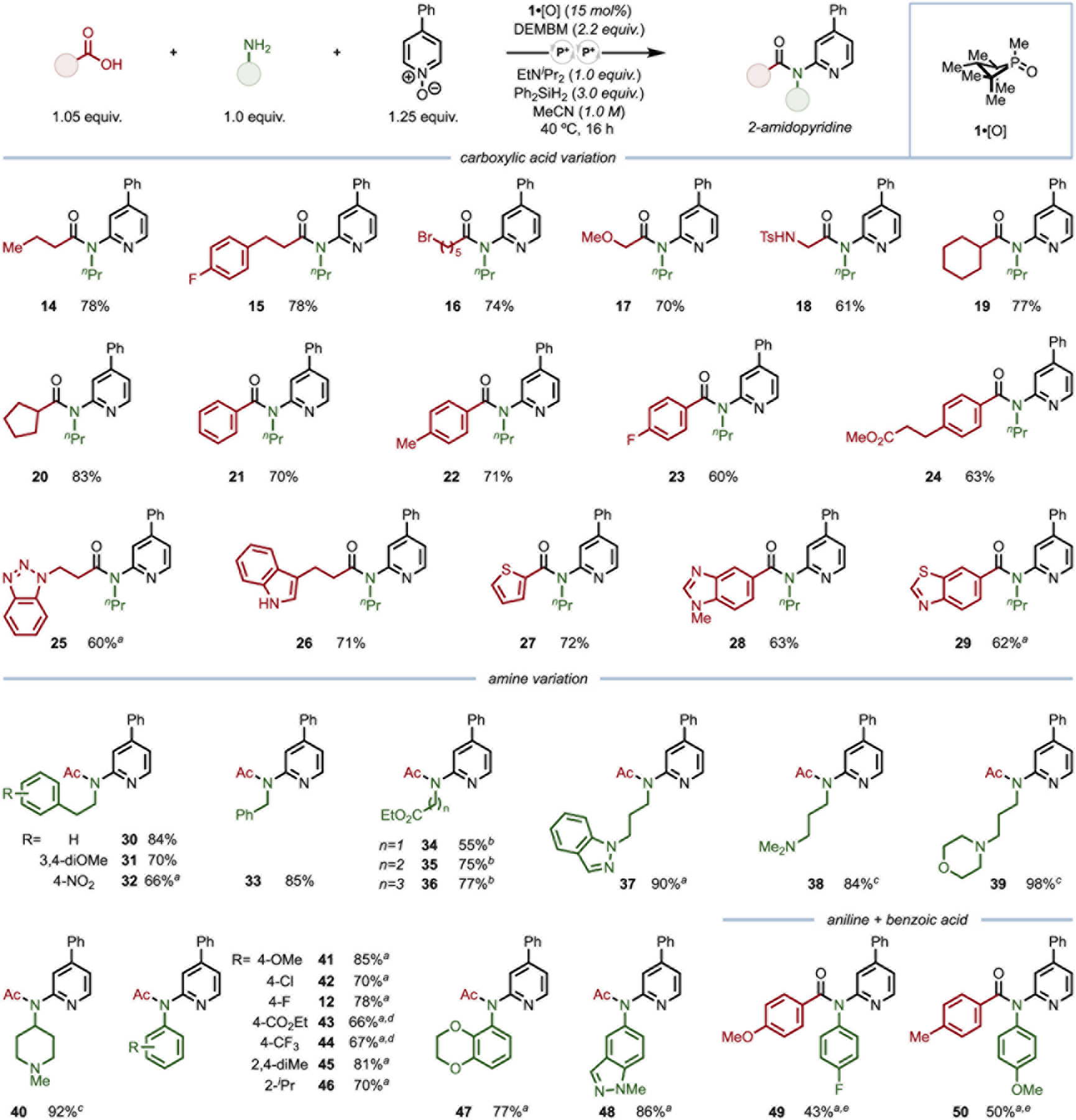
Synthetic scope of three-component serial dehydrative coupling of amines, carboxylic acids, and pyridine N-oxides. All yields isolated on 0.5 mmol scale. a 2,6-Lutidine in place of EtNiPr2. b Amine hydrochloride salt, 2,6-lutidine (2.0 equiv.) in place of EtNiPr2. c No EtNiPr2. d 60 °C. e 80 °C.
Variation of the amine coupling partner to demonstrate scope and generality is also shown in Figure 3. Various primary alkyl amines containing reactive functionalities were incorporated into 2-amidopyridines with good to excellent efficiency (30–40, 55–98% yield). β-Phenethylamines are readily functionalized (30–32, 66–84% yield); notably, 3,4-dimethoxyphenethylamine yielded the desired product 31 in 70% yield, with minimal (<5%) cyclodehydration onto the pendant electron-rich arene ring observed. Similarly, owing to the mild reaction temperature and inclusion of the bromenium oxidant, 4-nitrophenethylamine could be incorporated into this tandem dehydrative platform with no reaction at the nitroarene moiety (32, 66% yield).44 Benzylamine could also be functionalized as desired (33, 85% yield), despite potential degradation pathways available to a putative N-benzyl nitrilium cation.45 Further, amino acid esters of various chain lengths could be incorporated into the transformation directly from their commercial hydrochloride salts (34–36, 55–77% yield). Indazole-containing product 37 was delivered in 90% yield, demonstrating the ability of this system to incorporate pharmaceutically relevant heterocyclic motifs.46 Similarly, primary amines with tethered basic tertiary amines47 provided the desired products (38–40, 84–98% yield) without the need for exogenous amine base, evidencing the mild, chemoselective conditions are highly tolerant of Brønsted and Lewis basic functionality.48 α-Secondary amine 4-amino-1-methylpiperidine could deliver 40 in 92% yield, despite the possibility of retro-Ritter reaction occurring from a putative nitrilium cation.49
A wide variety of anilines of varying electronic natures were competent in the tandem dehydrative catalytic transformation, with lutidine serving as the ideal base (41–48, 66–86% yield). Tolerated functionality include ether (41, 85% yield), chloro (42, 70% yield), fluoro (12, 78% yield), ester (43, 66% yield), and trifluoromethyl (44, 67% yield) groups, as well as sterically-encumbering ortho-substitution (45 and 46, 81 and 70% yield, respectively). This lack of apparent deleterious steric effects highlights the utility of complementary C–N bond-forming platforms. Bis-heteroaryl amide products containing benzodioxane50 (47, 77% yield) and indazole46 (48, 86% yield) cores demonstrate the efficacy of this catalytic protocol for incorporating heterocyclic motifs common in pharmaceutical chemistry into complex molecular scaffolds. Further, when varying both amine and carboxylic acid components, it was observed that less electron-rich N-aryl benzamide intermediates do not undergo appreciable activation under the standard conditions. However, this can be mitigated upon heating the reaction slightly to 80 °C delivering the amide-activated, three-component coupled product (49 and 50, 43 and 50% yield, respectively).
Variation of the pyridine N-oxide coupling partner was also evaluated (Figure 4A).51 Replacement of the 4-Ph substituent with 4-Me and 4-H had minimal effect on efficiency, as products 51 and 52 were both formed in excellent yield (82 and 84%, respectively). From a 10 mmol scale reaction, 52 was able to be isolated in equal yield, affording 1.50 g of product, demonstrating the scalability of this transformation. 3-Methylpyridine N-oxide was functionalized to 53 in 71% yield, with 1.2:1 rr, indicating minimal steric or electronic influence on the C–N regioselectivity.52 Given the broad generality of the transformation with respect to the amine and acid coupling partners, the functionalization53 of bioactive pyridine scaffolds54 via their N-oxides was undertaken to assess the utility of this new catalytic transformation in complex molecule derivatization (Figure 4B). Commonly-used pesticide pyriproxyfen, containing multiple ether linkages, was derivatized to yield 54 in 66% yield. Further, basal cell carcinoma drug vismodegib was functionalized to product 55 in 63% yield. Notably, the N-aryl benzamide moiety, a competent substrate for activation under the catalytic system, was untouched in the reaction carried out at 40 °C. In addition, the oxidized sulfone was not observed to undergo any reductive reaction. In combination with earlier results, these examples demonstrate the utility of this organophosphorus-catalyzed transformation in the derivatization of complex molecules bearing sensitive functionality frequently present in biologically active compounds.
Figure 4.
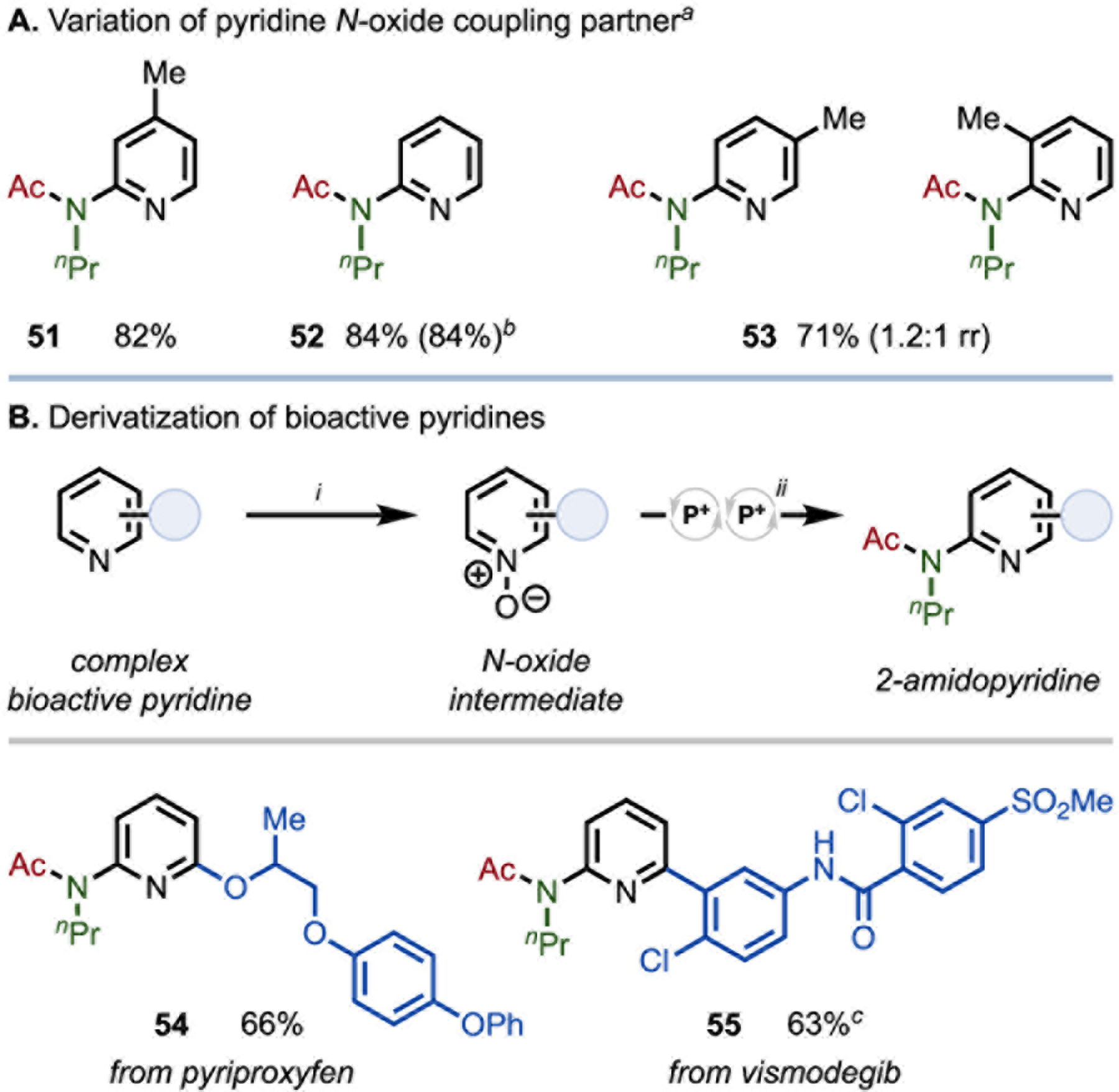
(A) Variation of pyridine N-oxide in tandem dehydration coupling. (B) Derivatization of pharmaceuticals by phosphacatalytic serial dehydrative amide condensation and amide activation. See SI for full synthetic details. All yields isolated. i mCPBA (1.05 equiv., 77%), CH2Cl2, 18 h. ii 2 (1.05 equiv.), 3 (1.0 equiv.), pyridine N-oxide (1.25 equiv.), 1·[O] (15 mol%), DEMBM (2.2 equiv.), EtNiPr2 (1.0 equiv.), Ph2SiH2 (3.0 equiv.) MeCN (1.0 M), 40 °C, 16 h. aConditions as in Figure 3. bYield in parentheses on 10.0 mmol scale; 1.50 g 52 isolated. c20 h. mCPBA = meta-chloroperoxybenzoic acid.
In conclusion, we have demonstrated that organophosphorus-catalyzed serial dehydration serves as an effective platform for both amide coupling and activation, enabling a convergent synthesis of 2-amidopyridines by three-component coupling of amines, carboxylic acids, and pyridine N-oxides. The ability to both access and functionalize an amide in situ establishes organophosphorus redox catalysis as a distinct, valuable modality within the expanding amide activation toolbox. The evident chemoselectivity and functional interplay of the catalyst, reagents and substrates in the title transformation portends further development of cascade condensation reactions driven by this catalytic manifold.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by NIH NIGMS (GM114547). J.M.L. thanks the Camille and Henry Dreyfus Foundation for a postdoctoral fellowship in Environmental Chemistry.
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
General methods and synthetic procedures; mechanistic studies; 1H, 13C, and 19F NMR spectra.
The authors declare no competing financial interest.
REFERENCES
- 1.The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science; Greenberg A, Breneman CM, Liebman JF, Eds.; Wiley: New York, 2003. [Google Scholar]
- 2. Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458. Schneider N; Lowe DM; Sayle RA; Tarsellli MA; Landrum GA Big Data from Pharmaceutical Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter. J. Med. Chem 2016, 59, 4385–4402. Tomberg A; Boström J Can easy chemistry produce complex, diverse, and novel molecules? Drug Discov. Today 2020, 25, 2174–2181.
- 3. Kaiser D; Bauer A; Lemmerer M; Maulide N Amide activation: an emerging tool for chemoselective synthesis. Chem. Soc. Rev 2018, 47, 7899–7925. Huang P-Q Direct Transformations of Amides: Tactics and Recent Progress. Acta Chim. Sinica 2018, 76, 357–365.
- 4. El-Faham A; Albericio F Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev 2011, 111, 6557–6602. Pattabiraman VR; Bode JW Rethinking amide bond synthesis. Nature 2011, 480, 471–479.
- 5.Kaiser D; Maulide N Making the Least Reactive Electrophile the First in Class: Domino Electrophilic Activation of Amides. J. Org. Chem 2016, 81, 4421–4428. [DOI] [PubMed] [Google Scholar]
- 6.Baraznenok IL; Nenajdenko VG; Balenkova ES Chemical Transformations Induced by Triflic Anhydride. Tetrahedron 2000, 56, 3077–3119. [Google Scholar]
- 7. Charette AB; Chua P A New Method for the Conversion of Secondary and Tertiary Amides to Bridged Orthoesters. Tetrahedron Lett 1997, 38, 8499–8502. Charette AB; Grenon M Spectroscopic studies of the electrophilic activation of amides with triflic anhydride and pyridine. Can. J. Chem 2001, 79, 1694–1703. Barbe G; Charette AB Highly Chemoselective Metal-Free Reduction of Tertiary Amides. J. Am. Chem. Soc 2008, 130, 18–19. Pelletier G; Bechara WS; Charette AB Controlled and Chemoselective Reduction of Secondary Amides. J. Am. Chem. Soc 2010, 132, 12817–12819. Bechara WS; Pelletier G; Charette AB Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nature Chem 2012, 4, 228–234.
- 8. Movassaghi M; Hill MD Single-Step Synthesis of Pyrimidine Derivatives. J. Am. Chem. Soc 2006, 128, 14254–14255. Movassaghi M; Hill MD; Ahmad OK Direct Synthesis of Pyridine Derivatives. J. Am. Chem. Soc 2007, 129, 10096–10097. Movassaghi M; Hill MD A Versatile Cyclodehydration Reaction for the Synthesis of Isoquinoline and β-Carboline Derivatives. Org. Lett 2008, 10, 3485–3488. Leypold M; D’Angelo KA; Movassaghi M Chemoselective α-Sulfidation of Amides Using Sulfoxide Reagents. Org. Lett 2020, 22, 8802–8807.
- 9. Xiao K-J; Luo J-M; Ye K-Y; Wang Y; Huang P-Q Direct, One-pot Sequential Reductive Alkylation of Lactams/Amides with Grignard and Organolithium Reagents through Lactam/Amide Activation. Angew. Chem., Int. Ed 2010, 49, 3037–3040. Xiang S-H; Xu J; Yuan H-Q; Huang P-Q Amide Activation by Tf2O: Reduction of Amides to Amines by NaBH4 under Mild Conditions. Synlett 2010, 1829–1832. Xiao K-J; Wang A-E; Huang P-Q Direct Transformation of Secondary Amides into Secondary Amines: Triflic Anhydride Activated Reductive Alkylation. Angew. Chem., Int. Ed 2012, 51, 8314–8317. Huang P-Q; Huang Y-H; Xiao K-J; Wang Y; Xia X-E A General Method for the One-Pot Reductive Functionalization of Secondary Amides. J. Org. Chem 2015, 80, 2861–2868.
- 10. Madelaine C; Valerio C; Maulide N Unexpected Electrophilic Rearrangements of Amides: A Stereoselective Entry to Challenging Substituted Lactones. Angew. Chem., Int. Ed 2010, 49, 1583–1586. Peng B; Geerdink D; Maulide N Electrophilic Rearrangements of Chiral Amides: A Traceless Asymmetric α-Allylation. J. Am. Chem. Soc 2013, 135, 14968–14971. Peng B; Geerdink D; Farès C; Maulide N Chemoselective Intermolecular α-Arylation of Amides. Angew. Chem., Int. Ed 2014, 53, 5462–5466. Li J; Oost R; Maryasin B; González L; Maulide N A redox-neutral synthesis of ketones by coupling of alkenes and amides. Nature Commun 2019, 10, 2327.
- 11.For amide activation and reaction via a formal enolonium intermediate, see: de la Torre A; Kaiser D; Maulide N Flexible and Chemoselective Oxidation of Amides to α-Keto Amides and α-Hydroxy Amides. J. Am. Chem. Soc 2017, 139, 6578–6581.Kaiser D; Teskey CJ; Adler P; Maulide N Chemoselective Intermolecular Cross-Enolate-Type Coupling of Amides. J. Am. Chem. Soc 2017, 139, 16040–16043.Gonçalves CR; Lemmerer M; Teskey CJ; Adler P; Kaiser D; Maryasin B; González L; Maulide N Unified Approach to the Chemoselective α-Functionalization of Amides with Heteroatom Nucleophiles. J. Am. Chem. Soc 2019, 141, 18437–18443.Adler P; Teskey CJ; Kaiser D; Holy M; Sitte HH; Maulide N α-Fluorination of carbonyls with nucleophilic fluorine. Nature Chem 2019, 11, 329–334.
- 12.For alternative SmI2-mediated approaches to amide activation via single electron reduction, see: Ogawa A; Takami N; Sekiguchi M; Ryu I; Kambe N; Sonoda N The First Deoxygenative Coupling of Amides by an Unprecedented Samarium/Samarium Diiodide System. J. Am. Chem. Soc 1992, 114, 8729–8730.Szostak M; Spain M; Eberhart AJ; Procter DJ Highly Chemoselective Reduction of Amides (Primary, Secondary, Tertiary) to Alcohols using SmI2/Amine/H2O under Mild Conditions. J. Am. Chem. Soc 2014, 136, 2268–2271.Huang H-M; Procter DJ Dearomatizing Radical Cyclizations and Cyclization Cascades Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc 2017, 139, 1661–1667.Jiao J; Wang X Merging Electron Transfer with 1,2-Metalate Rearrangement: Deoxygenative Arylation of Aromatic Amides with Arylboronic Esters. Angew. Chem., Int. Ed 2021, 60, 17088–17093.
- 13.For alternative transition metal-catalyzed approaches to amide activation via reductive hydrosilylation, see: Katahara S; Kobayashi S; Fujita K; Matsumoto T; Sato T; Chida N An Iridium-Catalyzed Reductive Approach to Nitrones from N-Hydroxyamides. J. Am. Chem. Soc 2016, 138. 5246–5249.Fuentes de Arriba ÁL; Lenci E; Sonawane M; Formery O; Dixon DJ Iridium-Catalyzed Reductive Strecker Reaction for Late-Stage Amide and Lactam Cyanation. Angew. Chem., Int. Ed 2017, 56, 3655–3659.Takahashi Y; Yoshii R; Sato T; Chida N Iridium-Catalyzed Reductive Nucleophilic Addition to Secondary Amides. Org. Lett 2018, 20, 5705–5708.Ou W; Han F; Hu X-N; Chen H; Huang P-Q Iridium-Catalyzed Reductive Alkylations of Secondary Amides. Angew. Chem., Int. Ed 2018, 57, 11354–11358.Chen D-H; Sun W-T; Zhu C-J; Lu G-S; Wu D-P; Wang A-E; Huang P-Q Enantioselective Reductive Cyanation and Phosphonylation of Secondary Amides by Iridium and Chiral Thiourea Sequential Catalysis. Angew. Chem., Int. Ed 2021, 60, 8827–8831.
- 14.For alternative transition metal-catalyzed approaches to amide activation via C-N oxidative addition, see: Hie L; Fine Nathel NF; Shah TK; Baker EL; Hong X; Yang Y-F; Liu P; Houk KN; Garg NK Conversion of amides to esters by the nickel-catalysed activation of amide C–N bonds. Nature 2015, 524, 79–83.Li G; Ma S; Szostak M Amide Bond Activation: The Power of Resonance. Trends Chem 2020, 2, 914–928.Liu C; Szostak M Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J 2017, 7157–7173.
- 15.Valerio V; Petkova D; Madelaine C; Maulide N Direct Room-Temperature Lactonisation of Alcohols and Ethers onto Amides: An “Amide Strategy” for Synthesis. Chem. Eur. J 2013, 19, 2606–2610. [DOI] [PubMed] [Google Scholar]
- 16. Mukaiyama T Oxidation-Reduction Condensation a New Method for Peptide Synthesis. Synth. Commun 1972, 2, 243–265. Mukaiyama T Oxidation-Reduction Condensation. Angew. Chem., Int. Ed 1976, 15, 94–103.
- 17.For redox-neutral (all PV) organophosphorus-catalyzed dehydration catalysis, see: Denton RM; An J; Adeniran B Phosphine oxide-catalysed chlorination reactions of alcohols under Appel conditions. Chem. Commun 2010, 46, 3025–3027.Denton RM; An J; Adeniran B; Blake AJ; Lewis W; Poulton AM Catalytic Phosphorus(V)-Mediated Nucleophilic Substitution Reactions: Development of a Catalytic Appel Reaction. J. Org. Chem 2011, 76, 6749–6767.Beddoe RH; Andrews KG; Magné V; Cuthbertson JD; Saska J; Shannon-Little AL; Shanahan SE; Sneddon HF; Denton RM Redox-neutral organocatalytic Mitsunobu reactions. Science 2019, 365, 910–914.
- 18.Lecomte M; Lipshultz JM; Kim-Lee S-H; Li G; Radosevich A Driving Recursive Dehydration by PIII/PV Catalysis: Annulation of Amines and Carboxylic Acids by Sequential C–N and C–C Bond Formation T. J. Am. Chem. Soc 2019, 141, 12507–12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JB Preparation of Acyl Halides under Very Mild Conditions. J. Am. Chem. Soc 1966, 88, 3440–3441. Barstow LE; Hruby VJ A Simple Method for the Synthesis of Amides. J. Org. Chem 1971, 36, 1305–1306. Luo Q-L; Lv L; Li Y; Tan J-P; Nan W; Hui Q An Efficient Protocol for the Amidation of Carboxylic Acids Promoted by Trimethyl Phosphite and Iodine. Eur. J. Org. Chem 2011, 6916–6922.
- 20.Phosphonium ion-mediated amide coupling and activation to imidoyl halides, which have been trapped in situ by nucleophiles, has been described under Appel conditions, see: Appel R; Ziehn K-D; Warning K Über die gemeinsame Einwirkung von Phosphinen und Tetrachlorkohlenstoff auf Ammoniak(Derivate), 10. Über eine neue Synthese von Chlorformamidinen. Chem. Ber 1973, 106, 2093–2097.Appel R; Warning K; Ziehn K-D Über die gemeinsame Einwirkung von Phosphinen und Tetrachlorkohlenstoff auf Ammoniak (Derivate), 12. Über zwei neue Verfahren zur Darstellung von Imidhalogeniden. Chem. Ber 1973, 106, 3450–3454.Appel R; Warning K; Ziehn K-D Über die Darstellung neuer Chlorformamidine. Chem. Ber 1974, 107, 698–705.Phakhodee W; Wangngae S; Pattarawarapan M Approach to the Synthesis of 2,3-Disubstituted-3H-quinazolin-4-ones Mediated by Ph3P–I2. J. Org. Chem 2017, 82, 8058–8066.
- 21.Reductive N-alkylation of amines with carboxylic acids, transiting through amide intermediates, has been previously described, see: Cabrero-Antonino JR; Adam A; Beller M Catalytic Reductive N-Alkylations Using CO2and Carboxylic Acid Derivatives: Recent Progress and Developments. Angew. Chem., Int. Ed 2019, 58, 12820–12838.Fu M-C; Shang R; Cheng W-M; Fu Y Boron-Catalyzed N-Alkylation of Amines Using Carboxylic Acids. Angew. Chem., Int. Ed 2015, 54, 9042–9046.Stoll EL; Tongue T; Andrews KG; Valette D; Hirst DJ; Denton RM A practical catalytic reductive amination of carboyxlic acids. Chem. Sci 2020, 11, 9494–9500.
- 22.Lipshultz JM; Li G; Radosevich AT Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15. J. Am. Chem. Soc 2021, 143, 1699–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiltmann R; Hoffmeister F; Niemers S; Schlichting U; Wollweber H 2-Acylaminopyridine derivatives with morphine agonistic and morphine antagonistic effects. Arzneim. Forsch 1974, 24, 584–600. Zhang D; Ai J; Liang Z; Li C; Peng X; Ji Y; Jiang H; Geng M; Luo C; Liu H Discovery of novel 2-aminopyridine-3-carboxamides as c-Met kinase inhibitors. Bioorg. Med. Chem 2012, 20, 5169–5180. Sivaprakasam P; Han X; Civiello RL; Jacutin-Porte S; Kish K; Pokross M; Lewis HA; Ahmed N; Szapiel N; Newitt JA; Baldwin ET; Xiao H; Krause CM; Park H; Nophsker M; Lippy JS; Burton CR; Langley DR; Macor JE et al. Discovery of New Acylaminopyridines as GSK-3 Inhibitors by a Structure Guided in-Depth Exploration of Chemical Space around a Pyrrolopyridinone Core. Bioorg. Med. Chem. Lett 2015, 25, 1856–1863. McClure KF; Piotrowski DW; Petersen D; Wei L; Xiao J; Londregan AT; Kamlet AS; Dechert-Schmitt AM; Raymer B; Ruggeri RB; Canterbury D; Limberakis C; Liras S; DaSilva-Jardine P; Dullea RG; Loria PM; Reidich B; Salatto CT; Eng H et al. Liver-Targeted Small-Molecule Inhibitors of Proprotein Convertase Subtilisin/Kexin Type 9 Synthesis. Angew. Chem., Int. Ed 2017, 56, 16218–16222. Londregan AT; Wei L; Xiao J; Lintner NG; Petersen D; Dullea RG; McClure KF; Bolt MW; Warmus JS; Coffey SB; Limberakis C; Genovino J; Thuma BA; Hesp KD; Aspnes GE; Reidich B; Salatto CT; Chabot JR; Cate JHD et al. Small Molecule Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors: Hit to Lead Optimization of Systemic Agents. J. Med. Chem 2018, 61, 5704–5718.
- 24. Weber L The Application of Multi-Component Reactions in Drug Discovery. Curr. Med. Chem 2002, 9, 2085–2093. Hulme C; Gore V “Multi-component Reactions : emerging chemistry in drug discovery” ‘from xylocain to crixivan’. Curr. Med. Chem 2003, 10, 51–80. Ruitjer E; Orru RVA Multicomponent reactions – opportunities for the pharmaceutical industry. Drug Disc. Today Technol 2013, 10, e15–e20.
- 25. Manley PJ; Bilodeau MT A Mild Method for the Formation and in Situ Reaction of Imidoyl Chlorides: Conversion of Pyridine-1-oxides to 2-Aminopyridine Amides. Org. Lett 2002, 4, 3127–3129. Couturier M; Caron L; Tumidajski S; Jones K; White TD Mild and Direct Conversion of Quinoline N-Oxides to 2-Amidoquinolines with Primary Amides. Org. Lett 2006, 8, 1929–1932.
- 26. Abramovitch RA; Singer GM A Direct Alkyl and Aryl Amination of Heteroaromatic Nitrogen Compounds. J. Am. Chem. Soc 1969, 91, 5672–5673. Abramovitch RA; Singer BM Direct Acylamination of Pyridine 1-Oxides. J. Org. Chem 1974, 39, 1795–1802. Abramovitch RA; Shinkai I Aromatic Substitution via New Rearrangements of Heteroaromatic N-Oxides. Acc. Chem. Res 1976, 9, 192–200.
- 27.Medley JW; Movassaghi M Direct Dehydrative N-Pyridinylation of Amides. J. Org. Chem 2009, 74, 1341–1344. [DOI] [PubMed] [Google Scholar]
- 28.Jones DH; Kay ST; McLellan JA; Kennedy AR; Tomkinson NCO Regioselective Three-Component Reaction of Pyridine N-Oxides, Acyl Chlorides, and Cyclic Ethers. Org. Lett 2017, 19, 3512–3515. [DOI] [PubMed] [Google Scholar]
- 29. Wang L; Zhang W Recent Developments in the Chemistry of Heteroaromatic N-Oxides. Synthesis 2015, 47, 289–305. Wang D; Désaubry L; Li G; Huang M; Zheng S Recent Advances in the Synthesis of C2-Functionalized Pyridines and Quinolines Using N-Oxide Chemistry. Adv. Synth. Catal 2020, 363, 2–39. Malykhin RS; Sukhorukov AY Nucleophilic Halogenaion of Heterocyclic N-Oxides: Recent Progress and a Practical Guide. Adv. Synth. Catal 2021, 363, 3170–3188.
- 30.Nykaza TV; Cooper JC; Radosevich AT anti-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide. Org. Synth 2019, 96, 418–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halomalonates and tetrahalomethanes have been used as silane-compatible halenium oxidants in catalytic Appel halogenation and amidation: van Kalkeren HA; Leenders SAN; Hommersom CRA; Rutjes FPJT; van Delft FL In Situ Phosphine Oxide Reduction: A Catalytic Appel Reaction. Chem. Eur. J 2011, 17, 11290–11295.van Kalkeren HA; van Delft FL; Rutjes FPJT Catalytic Appel reactions. Pure Appl. Chem 2012, 85, 817–828.Lenstra DC; Rutjes FPJT; Mecinović J Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines. Chem. Commun 2014, 50, 5763–5766.
- 32.See SI for control and optimization results using 9 in place of 3. Of particular note is the formation of some (<10%) amide intermediate in the absence of DEMBM or 1·[O], attributable to Ph2SiH2-mediated amide coupling, see: Sayes M; Charette AB Diphenylsilane as a coupling reagent for amide bond formation. Green Chem 2017, 19, 5060–5064.D’Amaral MC; Jamkhou N; Adler MJ Efficient and accessible silane-mediated direct amide coupling of carboxylic acids and amines. Green Chem 2021, 23, 288–295.
- 33. Londregan AT; Jennings S; Wei L General and Mild Preparation of 2-Aminopyridines. Org. Lett 2010, 12, 5254–5257. Londregan AT; Jennings S; Wei L Mild Addition of Nucleophiles to Pyridine-N-Oxides. Org. Lett 2011, 13, 1840–1843.
- 34.For azine N-oxide activation with non-phosphonium electrophiles for 2-amination and related reactions, see: Yin J; Xiang B; Huffman MA; Raab CE; Davies IW A General and Efficient 2-Amination of Pyridines and Quinolines. J. Org. Chem 2007, 72, 4554–4557.Keith JM One-Step Conversion of Azine N-Oxides to α−1,2,4-Triazolo-, 1,2,3-Triazolo, Imidazolo-, and Pyrazoloheteroarenes. J. Org. Chem 2010, 75, 2722–2725.Farrell RP; Silva Elipe MV; Bartberger MD; Tedrow JS; Vounatsos F An Efficient, Regioselective Amination of 3,5-Disubstituted Pyridine N-Oxides Using Saccharin as an Ammonium Surrogate. Org. Lett 2013, 15, 168–171.Chen M-T; You X; Bai L-G; Luo Q-L Metal-free phosphonation of heteroarene N-oxides with trialkyl phosphite at room temperature. Org. Biomol. Chem 2017, 15, 3165–3169.Lemmerer M; Teskey CJ; Kaiser D; Maulide N Regioselective synthesis of pyridines by redox alkylaton of pyridine N-oxides with malonates. Monatsh. Chem 2018, 149, 15–719.Xie L-Y; Peng S; Liu F; Yi J-Y; Wang M; Tang Z; Xu X; He W-M Metal-free Deoxygenative 2-Amidation of Quinoline N-oxides with Nitriles via a Radical Activation Pathway. Adv. Synth. Catal 2018, 360, 4259–4264.Chen X; Peng M; Huang H; Zheng Y; Tao X; He C; Xiao Y TsOH·H2O-mediated N-amidation of quinoline N-oxides: Facile and regioselective synthesis of N-(quinolin-2-yl)amides. Org. Biomol. Chem 2018, 16, 6202–6205.Bugaenko DI; Yurovskaya MA; Karchava AV Reaction of Pyridine-N-Oxides with Tertiary sp2-N-Nucleophiles: An Efficient Synthesis of Precursors for N-(Pyrid-2-yl)-Substituted N-Heterocyclic Carbenes. Adv. Synth. Catal 2020, 362, 5777–5782.
- 35.Reichl KD; Dunn NL; Fastuca NJ; Radosevich AT Biphilic Organophosphorus Catalysis: Regioselective Reductive Transposition of Allylic Bromides via PIII/PV Redox Cycling. J. Am. Chem. Soc 2015, 137, 5292–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. 2,6-Lutidine was found to be the optimal base for reaction of aniline substrates, generally providing yields 5–15% above reactions using Hünig’s base. See SI for optimization and controls employing 9.
- 37.At this time, a cooperative catalysis pathway for amidation wherein pyridine N-oxide further activates an acyloxyphosphonium cannot be ruled out, see: Shiina I; Ushiyama H; Yamada Y.-k.; Kawakita Y.-i.; Nakata K 4-(Dimethylamino)pyridine N-oxide (DMAPO): An Effective Nucleophilic Catalyst in the Peptide Coupling Reaction with 2-Methyl-6-nitrobenzoic Anhydride. Chem. Asian J 2008, 454–461.Ishihara K; Lu Y Boronic acid–DMAPO cooperative catalysis for dehydrative condensation between carboxylic acids and amines. Chem. Sci 2016, 7, 1276–1280.
- 38. While secondary amides undergo reaction upon exposure to 1·Br+ (see ref 18), no reaction is observed by 31P NMR upon mixing of 4 and [1·Br]Br.
- 39.For site-specific C–H to C–N functionalization of pyridines, see: Allen LJ; Cabrera PJ; Lee M; Sanford MS N-Acyloxyphthalimides as Nitrogen Radical Precursors in the Visible Light Photocatalyzed Room Temperature C–H Amination of Arenes and Heteroarenes. J. Am. Chem. Soc 2014, 136, 5607–5610.Patel C; Mohnike M; Hilton MC; McNally A A Strategy to Aminate Pyridines, Diazines, and Pharmaceuticals via Heterocyclic Phosphonium Salts. Org. Lett 2018, 20, 2607–2610.Fier PS; Kim S; Cohen RD A Multifunctional Reagent Designed for the Site-Selective Amination of Pyridines. J. Am. Chem. Soc 2020, 142, 8614–8618.Greenwood JW; Boyle BT; McNally A Pyridylphosphonium salts as alternatives to cyanopyridines in radical–radical coupling reactions. Chem. Sci 2021, 12, 10538–10543.
- 40. In accordance with Movassaghi’s proposed mechanism (see ref 27) and our earlier work (see ref 18), we believe the amide activation step generates a nitrilium ion, which traps pyridine N-oxide 4 to generate the N-imidoyloxy pyridinium 13. Subsequent rearrangement provides product 10.
- 41. Kale RR; Prasad V; Mohapatra PP; Tiwari VK Recent developments in benzotriazole methodology for construction of pharmacologically important heterocyclic skeletons. Monatsh. Chem 2010, 141, 1159–1182. Katritzky AR; Rachwal S Synthesis of Heterocycles Mediated by Benzotriazole. 1. Monocyclic Systems. Chem. Rev 2010, 110, 1564–1610. Katritzky AR; Rachwal S Synthesis of Heterocycles Mediated by Benzotriazole. 2. Bicyclic Systems. Chem. Rev 2011, 111, 7063–7120.
- 42.Gee YS; Rivinoja DJ; Wales SM; Gardiner MG; Ryan JH; Hyland CJT Pd-Catalyzed Dearomative [3 + 2] Cycloaddition of 3-Nitroindoles with 2-Vinylcyclopropane-1,1-dicarboxylates. J. Org. Chem 2017, 82, 13517–13529. [DOI] [PubMed] [Google Scholar]; (b) Manneveau M; Tanii S; Gens F; Legros J; Chataigner I Dearomatization of 3-cyanoindoles by (3 + 2) cycloaddition: from batch to flow chemistry. Org. Biomol. Chem 2020, 18, 3481–3486. [DOI] [PubMed] [Google Scholar]
- 43. Baidya M; Brotzel F; Mayr H Nucleophilicities and Lewis basicities of imidazoles, benzimidazoles, and benzotriazoles. Org. Biomol. Chem 2010, 8, 1929–1935. Drexler MT; Foley DA; Ward HW II; Clarke HJ IR and NMR Reaction Monitoring Techniques for Nucleophilic Addition Reactions: In Situ Monitoring of the Addition of Benzimidazole to a Pyridinium Salt. Org. Proc. Res. Dev 2015, 19, 1119–1127.
- 44.1·[O] is an effective precatalyst for the reductive deoxygenative functionalization of nitro groups, see: Nykaza TV; Harrison TS; Ghosh A; Putnik RA; Radosevich AT A Biphilic Phosphetane Catalyzes N-N Bond-Forming Cadogan Heterocyclization via PIII/PV═O Redox Cycling. J. Am. Chem. Soc 2017, 139, 6839–6842.Nykaza TV; Ramirez A; Harrison TS; Luzung MR; Radosevich AT Biphilic Organophosphorus-Catalyzed Intramolecular Csp2–H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 2018, 140, 3103–3113.Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; Radosevich AT Intermolecular Reductive C-N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV═O Catalysis. J. Am. Chem. Soc 2018, 140, 15200–15205.Nykaza TV; Li G; Yang J; Luzung MR; Radosevich AT PIII/PV═O Catalyzed Cascade Synthesis of N‐Functionalized Azaheterocycles. Angew. Chem., Int. Ed 2020, 59, 4505–4510.Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV═O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc 2020, 142, 6786–6799.Li G; Qin Z; Radosevich AT P(III)/P(V)-Catalyzed Methylamination of Arylboronic Acids and Esters: Reductive C–N Coupling with Nitromethane as a Methylamine Surrogate. J. Am. Chem. Soc 2020, 142, 16205–16210.
- 45.In contrast, 1-phenethylamine results in poor yield of desired product, with substantial deleterious retro-Ritter reaction, likely owing to the high stability of the 2°-benzylic phenethyl carbocation. Reaction with 4-fluorobenzoic acid yielded <20% desired product and 30% 4-fluorobenzonitrile as determined by 19F NMR of the crude reaction mixture. Similarly, reaction of tert-butylamine with 4-fluorobenzoic acid yielded no detectable desired product and 13% 4-fluorobenzonitrile as determined by 19F NMR of the crude reaction mixture. For characterization of retro-Ritter pathways of nitrilium ions, see: Fodor Nagubandi, D. Correlation of the von Braun, Ritter, Bischler-Napieralski, Beckmann and Schmidt reactions via nitrilium salt intermediates. Tetrahedron 1980, 36, 1279–1300.Nagubandi S; Fodor G The Mechanism of the Bischler-Napieralski Reaction. J. Heterocycl. Chem 1980, 17, 1457–1463.Geng H; Huang P-Q Versatile and chemoselective transformation of aliphatic and aromatic secondary amides to nitriles. Tetrahedron 2015, 71, 3795–3801.
- 46. Gaikwad DD; Chapolikar AD; Devkate CG; Warad KD; Tayade AP; Pawar RP; Domb AJ Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem 2015, 90, 707–731. Zhang S-G; Liang C-G; Zhang W-H Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives. Molecules 2018, 23, 2783.
- 47. An excess of primary amine (including in a substrate with two primary amines) results in minimal conversion of the amide intermediate to the desired 2-amidopyridine.
- 48.Dyer RMB; Hahn PL; Hilinski MK Selective Heteroaryl N-Oxidation of Amine-Containing Molecules. Org. Lett 2018, 20, 2011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. See SI for crossover experiment conducted in CD3CN, which resulted in identical yield as in CH3CN, with no incorporation of the CD3 group onto the acetamide of 40 as measured by LCMS, indicating no measurable retro-Ritter/Ritter reaction.
- 50.Bolchi C; Bavo F; Appiani R; Roda G; Pallavicini M 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem 2020, 200, 112419. [DOI] [PubMed] [Google Scholar]
- 51. At present, the use of quinoline N-oxide and isoquinoline N-oxide is unsuccessful in this reaction.
- 52. Abramovitch RA; Rogers RB Direct acylamination of 3-substituted pyridine-1-oxides. Directive effect of the substituent. J. Org. Chem 1974, 39, 1802–1807. Manley PJ; Bilodeau MT A Mild Method for the Formation and in Situ Reaction of Imidoyl Chlorides: Conversion of Pyridine-1-oxides to 2-Aminopyridine Amides. Org. Lett 2002, 4, 3127–3129.
- 53. Anderson RG; Jett BM; McNally A A Unified Approach to Couple Aromatic Heteronucleophiles to Azines and Pharmaceuticals. Angew. Chem., Int. Ed 2018, 57, 12514–12518. Zhang X; McNally A Cobalt-Catalyzed Alkylation of Drug-Like Molecules and Pharmaceuticals Using Heterocyclic Phosphonium Salts. ACS Catal 2019, 9, 4862–4866. Kim I; Kang G; Lee K; Park B; Kang D; Jung H; Me Y-T; Baik M-H; Hong S Site-Selective Functionalization of Pyridinium Derivatives via Visible-Light-Driven Photocatalysis with Quinolinone. J. Am. Chem. Soc 2019, 141, 9239–9248.
- 54.Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


