Abstract
Background
There is a paucity of information about Brazilian COVID‐19 in‐hospital mortality probability of death combining risk factors.
Objective
We aimed to correlate COVID‐19 Brazilian in‐hospital patients' mortality to demographic aspects, biomarkers, tomographic, echocardiographic findings, and clinical events.
Methods
A prospective study, single tertiary center in Brazil, consecutive patients hospitalized with COVID‐19. We analyzed the data from 111 patients from March to August 2020, performed a complete transthoracic echocardiogram, chest thoracic tomographic (CT) studies, collected biomarkers and correlated to in‐hospital mortality.
Results
Mean age of the patients: 67 ± 17 years old, 65 (58.5%) men, 29 (26%) presented with systemic arterial hypertension, 18 (16%) with diabetes, 11 (9.9%) with chronic obstructive pulmonary disease. There was need for intubation and mechanical ventilation of 48 (43%) patients, death occurred in 21/111 (18.9%) patients. Multiple logistic regression models correlated variables with mortality: age (OR: 1.07; 95% CI 1.02–1.12; p: 0.012; age >74 YO AUC ROC curve: 0.725), intubation need (OR: 23.35; 95% CI 4.39–124.36; p < 0.001), D dimer (OR: 1.39; 95% CI 1.02–1.89; p: 0.036; value >1928.5 ug/L AUC ROC curve: 0.731), C‐reactive protein (OR: 1.18; 95% CI 1.05–1.32; p < 0.005; value >29.35 mg/dl AUC ROC curve: 0.836). A risk score was created to predict intrahospital probability of death, by the equation: 3.6 (age >75 YO) + 66 (intubation need) + 28 (C‐reactive protein >29) + 2.2 (D dimer >1900).
Conclusions
A novel and original risk score were developed to predict the probability of death in Covid 19 in‐hospital patients concerning combined risk factors.
Keywords: COVID‐19, in‐hospital patients, mortality
In‐hospital COVID 19 mortality.
1. INTRODUCTION
In December 2019, an infectious outbreak was observed in the center of China which would achieve global epidemic dimensions with exponential pandemic spread in the first few months of 2020. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Viral pneumonia cases were noted, along with the identification of a novel viral agent SARS‐CoV 2 (COVID‐19), previously called “2019 novel coronavirus” (2019‐nCoV). 1 , 2 Its mortality rate was reported to be very variable concerning investigations throughout different continents (from 2.3% to 21%). 10 , 13 , 14 , 15 It observed is an increase in cytokines in patients affected by the virus, 16 , 17 , 18 as well as in the natriuretic peptide pro BNP NT‐proBNP, levels of ultra‐sensitive troponin (cTnI), and D‐dimer. 19 , 20
Imaging approaches can lead to diagnostic information of COVID‐19 complications and the extent of the disease and could also provide prognostic information. Echocardiography may be employed to diagnose myocardial dysfunction in order to demonstrate cardiac involvement of COVID 19. Few complete echocardiographic studies have been undertaken so far, but the most relevant findings correlate right ventricle dilatation with a troponin increase 21 and lower free wall longitudinal strain to a worse prognosis. 22 Thoracic computed tomography can aid in the possible diagnosis of COVID‐19 infection and it can be used to detect the pulmonary infectious process and to estimate the extent of pulmonary involvement. 23 , 24
There is a paucity of information concerning the Brazilian COVID‐19 in‐hospital mortality rate and its relationship to biomarkers, clinical data, and echocardiographic and tomographic findings. To our best knowledge, this is the first study to address the clinical, laboratory, and imaging data concerning Brazilian COVID‐19 in‐hospital mortality probability taking into consideration combined risk factors. Thus, we performed an investigation to study COVID‐19 in‐hospital mortality concerning demographic data, clinical events during the in‐hospital period, biomarkers, and echocardiographic and tomographic findings.
2. MATERIAL AND METHODS
2.1. Patients
This study included all symptomatic patients admitted to a tertiary Hospital, in Brazil, with a positive diagnosis of COVID‐19 (positive reverse‐transcriptase polymerase chain reaction assay) from March to August 2020. We have prospectively studied 111 from 154 consecutive adult patients (>18 years old) since 43/154 (27.8%) patients were not included due to insufficient data or poor quality of information. Data collection considered demographic data, comorbidities and clinical information, laboratory findings (biomarkers), full transthoracic echocardiographic and thoracic computed tomographic findings, and in‐hospital clinical events. This investigation was approved by the Research Ethics Committee of the Hospital (No 3.960.096). Informed consent for the echocardiographic examination was signed by the patient or by a person legally responsible.
2.2. Echocardiography
Echocardiography was performed following clinical needs. The echocardiographic examination was performed following current international recommendations for COVID 19 echocardiographic performance. 25 , 26 All echocardiographic examinations were performed in a standard way with the same equipment (Epiq 7, Philips Medical Systems, Bothell, WA, USA) by cardiologists with full echocardiographic information. All echocardiographic data were acquired from bedside studies performed in COVID‐19 intensive care units or internal ward units, with posterior offline analysis in the Echolab section at a workstation employing the software Q lab 13.0 (Philips Medical Systems, Bothell, WA, USA). All patients were submitted to a two‐dimensional echocardiogram with color Doppler, spectral Doppler and tissue Doppler, as well as longitudinal strain measurements derived from speckle tracking, according to the American Society of Echocardiography guideline. 25 , 26 Left ventricular volumes and ejection fraction were calculated using the Simpson biplane method with an automatic calculation algorithm. The left atrial volume was also analyzed using the Simpson biplane method. The analysis of the diastolic function was performed employing pulsed Doppler as well as Tissue Doppler (from the apical four‐chamber view, with the Doppler sample in the basal region of the ventricular septum [medial mitral annulus], lateral mitral annulus). The E/e “ratio considered the average between the septal and lateral e” velocities. Pulmonary artery systolic pressure was estimated by the tricuspid reflux. The left ventricular global longitudinal strain was obtained from the apical planes four, three, and two chambers. Images of the apical plane four chambers were also obtained and optimized for a better visualization of the right ventricle for the measurement of the RV strain. The right ventricular myocardial performance was analyzed by quantification of the tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), tissue Doppler (S wave), and longitudinal strain parameters.
2.3. Thoracic computed tomography
Thoracic computed tomography was performed upon the request of the attending physician responsible for the patient, based on the patient's clinical needs. Thoracic involvement of the parenchyma was considered as follows: >25%, 25%–50%, and >50%.
2.4. Statistical analysis
The qualitative characteristics evaluated by absolute and relative frequencies; quantitative characteristics of the patients were described by summary measures (mean, SD, median, minimum, and maximum). 27 , 28 Deaths were described according to the qualitative characteristics evaluated using absolute and relative frequencies, and the association was verified with the use of chi‐square tests or exact tests (Fisher's exact test or likelihood ratio test). The quantitative characteristics were described according to the mortality of the patients using summary measures and were compared using Student's t‐tests or Mann–Whitney tests according to the probability distribution of the variables. 27 , 28 Multiple logistic regression models were adjusted, maintaining the variables age, need for intubation, diabetes, and heart disease and a laboratory or cardiological characteristic was inserted separately in each model due to the small number of cases of death in the sample, all characteristics being maintained in the final model “full model”. 27 , 28
To perform the analyses, IBM‐SPSS for Windows version 20.0 software was used and Microsoft Excel 2003 software was used to tabulate the data. The tests were performed with a 5% significance level.
2.5. Patient and public involvement statement
Echocardiography, tomographic examinations, and laboratory investigation were performed according to the request of the attending physician responsible for the patient, in response to the clinical need during in‐hospital stay. Patients and legal responsibles were aware of the report of the findings of the clinical investigation in medical journals.
3. RESULTS
The demographic information, comorbidities, clinical in‐hospital course, laboratory data, and the echocardiographic and thoracic computed tomography findings are presented in Tables 1, 2, 3, and 4. A total of 111 patients with COVID‐19 were included (median age 70, range 19–101 years; 58.5% male). The most common comorbidities were hypertension (38, 34.2%) and diabetes (26, 23.3%), and obesity was found in 28 (32.2%) patients. Biomarkers (D‐dimer, C‐reactive protein, Troponin‐I, Interleukin‐6, BNP) presented with elevated median values. Pro‐calcitonin was also markedly elevated.
TABLE 1.
Baseline characteristics of COVID‐19 in‐hospital patients
| Demographic information | N (%) |
|---|---|
| Total No. | 111 |
| Age, (mean, SD, median, range), year | 67, 17, 70 (19–101) |
| Sex | |
| Male | 65(58.5) |
| Female | 46 (41.5) |
| Comorbidities | |
| Diabetes | 26 (23.4) |
| Cardiovascular disease | |
| Hypertension | 38 (34.2) |
| Coronary artery disease | 3 (2.7) |
| Dyslipidemia | 13 (11.7) |
| Congestive heart failure | 3 (2.7) |
| Chronic respiratory disease | |
| Chronic obstructive pulmonary disease | 11 (9.9) |
| Smoking | 5 (4.5) |
| Ex‐smoker | 6 (5.4) |
| Kidney disease | 7 (6.3) |
| Neoplasms | 8 (7.2) |
| BMI | |
| Normal | 33 (29.7) |
| Overweight | 46 (41.4) |
| Obesity (>30) | 32 (28.8) |
Abbreviations: BMI, body mass index (expressed as weight in kilograms divided by height in meters square); COVID‐19, coronavirus disease 2019.
TABLE 2.
Laboratory characteristics of COVID‐19 in‐hospital patients
| Parameter | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Creatinine, mg/dl | 2.91 | 12.33 | 1.025 | 0.26 | 115 |
| Lactate dehydrogenase, U/L | 362.32 | 268.59 | 311 | 4.7 | 2500 |
| Glucose, mg/dl | 132.42 | 51.20 | 115 | 41 | 379 |
| Magnesium, mg/dl | 4.29 | 19.13 | 1.8 | 1.3 | 175 |
| Potassium, mmol/L | 4.97 | 4.39 | 4.5 | 3 | 48 |
| C‐reactive protein, (NV <0.3) mg/dl | 50.28 | 78.67 | 16.2 | 0.3 | 444.3 |
| Sodium, mmol/L | 142.62 | 17.07 | 141 | 128 | 310.8 |
| Aspartate aminotransferase, U/L | 144.60 | 771.84 | 31 | 14 | 7000 |
| Alanine transaminase, U/L | 129.59 | 692.27 | 36 | 9 | 7000 |
| Blood urea nitrogen, mg/dl | 68.60 | 65.31 | 46 | 12 | 357 |
| Erytrocythes, g/dl | 3.87 | 0.83 | 3.81 | 1.72 | 5.34 |
| Hemoglobin, g/dl | 11.44 | 2.49 | 10.9 | 6 | 16.4 |
| White blood cells, μl | 2901.31 | 7513.77 | 9.94 | 3 | 54 480 |
| Lymphocytes, μl | 1643.21 | 4397.32 | 838.,5 | 1 | 44 674 |
| Platelets,103/μl | 250.45 | 110.57 | 237 | 29 | 601 |
| D‐dimer, (NV < 500) ug/L | 2403.99 | 2291.71 | 1410 | 1,31 | 7650 |
| Fibrinogen, (NV: 200–400) mg/dl | 533.70 | 853.89 | 418 | 138 | 7650 |
| Troponin‐I, (NV < 10) ug/L | 97.19 | 168.60 | 31 | 5 | 968 |
| Interleukin‐6, (NV: 5–15) pg/ml | 359.1 | 864.12 | 84.4 | 1.4 | 5000 |
| BNP, (NV < 100) pg/ml | 233.92 | 302.76 | 120 | 5 | 1578 |
| Ferritin, (NV men: 20–500, women: 20–200) ng/ml | 848.46 | 685.08 | 649.5 | 43.6 | 3187 |
| Pro‐calcitonin, (NV < 0.5) ng/ml | 6.59 | 41.87 | 0.19 | 0.02 | 311 |
| Lactate, (NV < 10) mg/dl | 16.80 | 8.73 | 15 | 11 | 32 |
| Glycated hemoglobin, mmol/L | 5.93 | 0.78 | 5.8 | 4.6 | 7.5 |
Abbreviations: COVID‐19, coronavirus disease 2019; NV, normal value.
TABLE 3.
Clinical evolution in COVID‐19 in‐hospital patients
| Patients–Total No: 111 | N (%) |
|---|---|
| Clinical in‐hospital evolution | |
| Discharge | 87 (78.4) |
| Still in‐hospital | 3 (2.7) |
| Death | 21 (18.9) |
| Intubation and mechanical ventilation need | 48 (43.2) |
| Clinical events (No: 54) | |
| Myocardial infarction | 10 (9.3) |
| Pulmonary thromboembolism | 7 (6.3) |
| Deep vein thrombosis | 5 (4.5) |
| Renal failure | 12 (10.8) |
| Hemodyalisis | 9 (9.8) |
| ECMO | 3 (2.7) |
TABLE 4.
Echocardiographic and tomographic thoracic data in COVID‐19 in hospital patients
| Diagnostic imaging technique | |||||
|---|---|---|---|---|---|
| Transthoracic echocardiography | Mean | SD | Median | Minimum | Maximum |
| LVEF (%) | 58.20 | 8.18 | 59.65 | 16.4 | 70.6 |
| LV longitudinal global strain (%) | −21.01 | 3.79 | −21.1 | −27.3 | −13 |
| RV longitudinal global strain (%) | −15.69 | 3.90 | −16.15 | −21.3 | −6.8 |
| RV free wall longitudinal strain (%) | −25.38 | 5.48 | −23.95 | −36.5 | −14.5 |
| Left atrium (mm) | 37.88 | 6.03 | 38 | 24 | 54 |
| Septum (mm) | 13.33 | 9.40 | 11 | 7 | 61 |
| Left ventricle posterior wall (mm) | 10.55 | 3.93 | 10 | 7 | 46 |
| Left ventricle diastolic diameter (mm) | 43.13 | 11.10 | 45 | 8 | 60 |
| Left ventricle systolic diameter (mm) | 32,03 | 7.14 | 30.5 | 22 | 55 |
| RV diameter (mm) | 27.51 | 7.30 | 26 | 18 | 74 |
| Aortic root diameter (mm) | 33.45 | 4.32 | 33.5 | 23 | 42 |
| PASP (mmHg) | 39.40 | 9.56 | 42 | 23 | 55 |
| TAPSE (mm) | 19.18 | 6.29 | 19 | 1.9 | 38 |
| Tricuspid inflow velocity (m/s) | 2.56 | 0.51 | 2.55 | 1.7 | 4.2 |
| FAC (%) | 41.26 | 10.23 | 42 | 8.1 | 60 |
| RV diastolic 4 chamber area (cm2) | 16.21 | 5.34 | 15.95 | 6.8 | 37 |
| RV systolic 4 chamber area (cm2) | 9.71 | 7.88 | 8.2 | 2.1 | 73 |
| E/A wave ratio | 1.08 | 0.49 | 1 | 0.4 | 2,9 |
| Mitral valve deceleration time (ms) | 233.09 | 76.15 | 208 | 99 | 494 |
| e' average wave | 8.57 | 2.68 | 8.5 | 3.4 | 14 |
| E/e’ average ratio | 9.64 | 3.77 | 8.7 | 4.2 | 25.4 |
| Chest computed thoracic tomography No: 54 | |||||
|---|---|---|---|---|---|
| Thoracic involvement of parenchyma | N (%) | ||||
| >25% | 3 (3.3) | ||||
| 25%–50% | 46 (86.7) | ||||
| >50% | 5 (10) | ||||
Abbreviations: FAC: fractional area change; LV, left ventricle; LVEF, left ventricle ejection fraction; PASP, pulmonary artery systolic pressure; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
The mortality rate was 18.9% (21 patients). There were 54 clinical events in 111 patients (48.6%). The most common complication was respiratory failure requiring intubation and mechanical ventilation in 48 (43.2%) patients, with an intubation period between 7 and 20 days in 29/48 (60.6%) patients, and a pronation need in 4/48 (8.3%) patients.
Demographic, clinical evolution, laboratory, echocardiographic, and tomographic data analysis related to mortality among the COVID‐19 in‐hospital patients are presented in Table 5 (just the parameters with statistical significance plus the TAPSE with p = 0.064; all other studied parameters did not reach the statistical significance level). The multiple logistic regression model for predicting COVID‐19 in‐hospital mortality is presented in Table 6. In‐hospital mortality was related to age, need for intubation and mechanical ventilation, and the CRP and D dimer levels. The area under the receiver operating characteristic curves for predicting COVID‐19 patients in‐hospital mortality concerning age, D‐dimer and C‐reactive protein are shown in Table 7 and Graph 1.
TABLE 5.
Demographic, clinical evolution, laboratory, echocardiographic and tomographic data analysis related to mortality in COVID‐19 in‐hospital patients (n: 111 patients)
| Parameter | p |
|---|---|
| Demographic | |
| Age | <0.001 |
| Clinical evolution | |
| Intubation and mechanical ventilation need | <0.001 |
| Laboratory | |
| Creatinine | 0.007* |
| Lactate dehydrogenase | <0.001* |
| C‐reactive protein | <0.001* |
| Intreleukin 6 | |
| D‐dimer | 0.004* |
| BNP | 0.035* |
| Echocardiographic findings | |
| LVEF | 0.008 |
| Tricuspid inflow velocity | 0.004 |
| TAPSE | 0.064 |
Note: T‐Student test; * Mann–Whitney test.
Abbreviations: LVEF, left ventricle ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
TABLE 6.
Multiple logistic regression model for predicting COVID‐19 patients in‐hospital mortality
| Parameter | OR | CI (95%) | p | |
|---|---|---|---|---|
| Inferior | Superior | |||
| Age (years) | 1.07 | 1.02 | 1.12 | 0.012 |
| Intubation need | 23.35 | 4.39 | 124.36 | <0.001 |
| Diabetes | 1.60 | 0.47 | 5.45 | 0.454 |
| Cardiopathy | 2.00 | 0.50 | 7.99 | 0.328 |
| LVEF (%) | 0.96 | 0.90 | 1.02 | 0.160 |
| Creatinine | 1.00 | 0.93 | 1.07 | 0.926 |
| TAPSE | 4.05 | 0.51 | 31.98 | 0.185 |
| CRP | 1.18 | 1.05 | 1.32 | 0.005 |
| D‐dimer | 1.39 | 1.02 | 1.89 | 0.036 |
Abbreviations: CRP, C‐reactive protein; LVEF: left ventricle ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
TABLE 7.
The area under the receiver operating characteristic curve for predicting COVID‐19 patients in‐hospital mortality
| Parameter | Value | Specificity | Sensitivity | AUC | CI (95%) | |
|---|---|---|---|---|---|---|
| Inferior | Superior | |||||
| Age (years) | 74 | 63.3 | 61.9 | 0.725 | 0.617 | 0.832 |
| CRP | 29.35 | 67.5 | 73.7 | 0.836 | 0.741 | 0.931 |
| D‐dimer | 1928.5 | 68.4 | 67.5 | 0.731 | 0.618 | 0.845 |
Abbreviation: CRP, C‐reactive protein.
GRAPH 1.
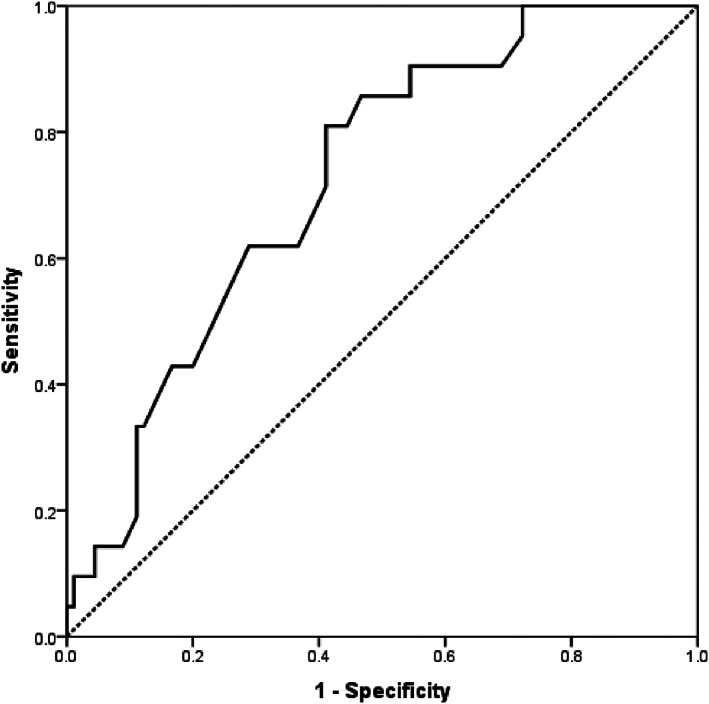
The area under the receiver operating characteristic curve of age for predicting in‐hospital mortality
A risk score was created to predict intrahospital probability of death, by the equation: 3.6 (age >75 YO) + 66 (intubation need) + 28 (C‐reactive protein >29) + 2.2 (D dimer >1900).
4. DISCUSSION
To our best knowledge, this is the first investigation to study data including a complete transthoracic echocardiogram, computed thoracic tomographic (CT) studies, biomarkers, laboratory data, and clinical information in hospitalized patients with COVID‐19 in a tertiary hospital in Brazil that aimed to predict the probability of in‐hospital death combining risk factors. These data seem to be relevant, taking into consideration the number of Brazilian patients affected by COVID‐19 (third highest in the world as of early November 2021), with more than 608 thousand deaths and most important the need for a better understanding of the COVID‐19 pandemic mortality regarding demographic and clinical aspects, biomarkers, tomographic and echocardiographic features.
The mortality rate was high (18.9%), close to the mortality rate observed in the New York City area (21%), 15 different from previous Chinese studies in the Wuhan area, 10 , 13 (most probably due to the characteristics of the studied population with a mean age 67 ± 17 years old, and to the characteristics of the hospital, a tertiary center that receives severe COVID‐19 patients from secondary hospitals and from different parts of the country). The severity of the disease was confirmed by the rate of respiratory failure leading to intubation and the need for mechanical ventilation (43.2% of the patients).
The most frequent comorbidities in the studied population were hypertension and diabetes. In other investigations, it was observed there was a less favorable follow‐up in patients presenting with hypertension, diabetes, previous cardiopathies, chronic pulmonary diseases, and immunosuppressive diseases. 3 , 6 , 7 , 10 , 11 In our population, a less favorable follow‐up and increased in‐hospital mortality was seen for older patients (age > 74 years, OR: 1.07; 95% CI 1.02–1.12, p: 0.012; AUC ROC curve: 0.725). We could not demonstrate increased in‐hospital mortality under multiple logistic regression models for patients with hypertension, diabetes, or obesity. We wonder if this could be related to the number of patients studied (111).
In our study, we observed increased levels of C‐reactive protein, D‐dimer, BNP, troponin‐I, ferritin, and interleukin 6 (Table 2). The relevance of the increased biomarkers to in‐hospital mortality was demonstrated by the relationship to mortality of D‐dimer (OR: 1.39; 95% CI 1.02–1.89; p: 0.036; value >1928.5 ug/L, AUC ROC curve: 0.731) and C‐reactive protein (OR: 1.18; 95% CI 1.05–1.32, p <0.005; value >29.35 mg/dL, AUC ROC curve: 0.836). No doubt an excessive inflammatory state led to a less favorable outcome in those patients. It was also observed there were high levels of pro‐calcitonin, a precursor to the thyroid hormone calcitonin, which could also represent the severity of the infectious state, associated systemic bacterial infection, and sepsis.
During the clinical in‐hospital follow‐up, myocardial infarction occurred in 10 (9%) patients, pulmonary thromboembolism in 7 (6.3%) patients, renal failure in 12 (10.8%) patients, and the need for hemodialysis in 9 (9.8%) patients. Respiratory failure with intubation and a mechanical ventilation need was associated with increased mortality (intubation need (OR: 23.35; 95% CI 4.39–124.36; p < 0.001) under multiple logistic regression model analysis. We consider that although the OR is high for the need for intubation and mechanical ventilation need and mortality, it also has a very wide CI, which could lead to a certain concern about its accuracy and greater uncertainty in relation to the estimate. A study considering myocardial injury was observed that 1 in 5 patients presented myocardial involvement what could predict 30 day mortality. 29
The findings of the echocardiographic and thoracic tomographic studies were not associated with COVID‐19 in‐hospital mortality. Tomographic studies were performed in 54/111 (48.6) patients. Thoracic parenchyma involvement occurred between 25% and 50% in 26/30 (86.7%) patients who were submitted to computed thoracic tomography investigation, and mainly of them maintained the tomographic findings during the first weeks after hospital discharge.
A risk score was created to predict intrahospital probability of death, by the equation: 3.6 (age >75 YO) + 66 (intubation need) + 28 (C‐reactive protein >29) + 2.2 (D dimer >1900). This information seems to be of clinical value concerning the relative importance of each risk factor for in‐hospital probability of death. In this population it is clear that pulmonary acommitement due to the inflammatory response represented the key reason related to the probability of in‐hospital death.
5. LIMITATIONS
This study comprises data from a single tertiary hospital center enrolling a limited number of patients in a specific period of the Covid 19 Pandemic. Further studies involving higher number of patients and multicenter information will certainly enhance our understanding of COVID 19 and factors related to its mortality.
6. CONCLUSION
In conclusion, in hospitalized COVID‐19 patients, death occurred in 21/111 (18.9%). A novel and original risk score was created to predict in‐hospital probability of death, by the equation: 3.6 (age >75 YO) + 66 (intubation need) + 28 (C‐reactive protein >29) + 2.2 (D dimer >1900).
Vieira MLC, Afonso TR, Oliveira AJ, et al. A risk score for predicting death in COVID‐19 in‐hospital infection: A Brazilian single‐center study. J Clin Ultrasound. 2022;50(5):604‐610. doi: 10.1002/jcu.23195
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fisher D, Heymann D. Q&A: the novel coronavirus outbreak causing COVID‐19. BMC Med. 2020;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. https://www.worldometers.info/coronavirus/#countries.
- 13. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 14. Shaobo S, Um Q, Yuli C, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson S, Hirsch JS, Narasimhan M, et al. The Northwell COVID‐19 research consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kritas SK, Ronconi G, Caraffa A, et al. Mast cells contribute to coronavirus‐induced inflammation: new anti‐inflammatory strategy. J Biol Regul Homeost Agents. 2020;34(1):9‐14. [DOI] [PubMed] [Google Scholar]
- 17. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327‐331. [DOI] [PubMed] [Google Scholar]
- 19. Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360(15):1526‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2013;34(33):2636‐2648. [DOI] [PubMed] [Google Scholar]
- 21. Szekely Y, Lichter Y, Taieb P, et al. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID‐19)–a systematic echocardiographic study. Circulation. 2020;142:342‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Zhu S, Xie Y, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. JACC: Cardiovas Imaging 2020;13(11):2287‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology. 2020;296(2):E46‐E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng MY, Ey L, Yang J, et al. Imaging profile of the COVID‐19 infection: radiologic findings and literature review. Radiol Cardiothoracic Imaging. 2020;2(1):e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirkpatrick JN, Mitchell C, Taub C, et al. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020;75(24):3078‐3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233‐270. [DOI] [PubMed] [Google Scholar]
- 27. Kirkwood BR, Sterene JA. Essential Medical Statistics. 2nd ed. Blackwell Science; 2006:502. [Google Scholar]
- 28. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2a ed. Wiley; 2000:320. [Google Scholar]
- 29. Bardaji A, Carrasquer A, Sanchéz‐Giménez R, et al. Prognostic implications of myocardial injury in patients with and without COVID‐19 infection treated in an University hospital. Rev Esp Cardiol. 2021;74(1):24‐32. doi: 10.1016/j.rec.2020.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


