Abstract
Background
The COVID‐19 pandemic has greatly increased the incidence and clinical importance of critical illness myopathy (CIM), because it is one of the most common complications of modern intensive care medicine. Current diagnostic criteria only allow diagnosis of CIM at an advanced stage, so that patients are at risk of being overlooked, especially in early stages. To determine the frequency of CIM and to assess a recently proposed tool for early diagnosis, we have followed a cohort of COVID‐19 patients with acute respiratory distress syndrome and compared the time course of muscle excitability measurements with the definite diagnosis of CIM.
Methods
Adult COVID‐19 patients admitted to the Intensive Care Unit of the University Hospital Bern, Switzerland requiring mechanical ventilation were recruited and examined on Days 1, 2, 5, and 10 post‐intubation. Clinical examination, muscle excitability measurements, medication record, and laboratory analyses were performed on all study visits, and additionally nerve conduction studies, electromyography and muscle biopsy on Day 10. Muscle excitability data were compared with a cohort of 31 age‐matched healthy subjects. Diagnosis of definite CIM was made according to the current guidelines and was based on patient history, results of clinical and electrophysiological examinations as well as muscle biopsy.
Results
Complete data were available in 31 out of 44 recruited patients (mean [SD] age, 62.4 [9.8] years). Of these, 17 (55%) developed CIM. Muscle excitability measurements on Day 10 discriminated between patients who developed CIM and those who did not, with a diagnostic precision of 90% (AUC 0.908; 95% CI 0.799–1.000; sensitivity 1.000; specificity 0.714). On Days 1 and 2, muscle excitability parameters also discriminated between the two groups with 73% (AUC 0.734; 95% CI 0.550–0.919; sensitivity 0.562; specificity 0.857) and 82% (AUC 0.820; CI 0.652–0.903; sensitivity 0.750; specificity 0.923) diagnostic precision, respectively. All critically ill COVID‐19 patients showed signs of muscle membrane depolarization compared with healthy subjects, but in patients who developed CIM muscle membrane depolarization on Days 1, 2 and 10 was more pronounced than in patients who did not develop CIM.
Conclusions
This study reports a 55% prevalence of definite CIM in critically ill COVID‐19 patients. Furthermore, the results confirm that muscle excitability measurements may serve as an alternative method for CIM diagnosis and support its use as a tool for early diagnosis and monitoring the development of CIM.
Keywords: SARS‐CoV‐2, ICU‐acquired weakness, Sepsis, Neuromuscular blocking agents, Electromyography, Nerve conduction studies, Membrane potential, Propofol, Muscle velocity recovery cycle
Introduction
Critical illness myopathy (CIM) is an acute and acquired primary myopathy and is one of the most frequent neuromuscular complications following intensive care treatment. 1 , 2 Clinical signs of CIM include difficulty in weaning from the ventilator, flaccid paresis or plegia, and atrophy of muscles. Common consequences are delayed or only partial recovery, prolonged mechanical ventilation, intensive care unit (ICU) and hospital stay as well as a higher need for health care and rehabilitation resources. The outcome is heterogeneous, but in general, CIM correlates with functional limitations, increased morbidity and mortality, and decreased employment and quality of life. 3 , 4 , 5 , 6 , 7
CIM development is assumed to be multifactorial in nature. Several risk factors have been proposed, including the patients' premorbid health‐status, duration of intensive care treatment and mechanical ventilation, treatment with neuromuscular blocking agents (NMBA), and the degree of severity of the acute disease. 1 , 6 , 8 , 9 , 10 Recently, it has also been discussed if prolonged sedation with propofol, one of the most widely used drugs for sedation, may contribute to the development of CIM. 11 The clinical relevance of CIM has become more evident in the COVID‐19 pandemic, with an increasing number of clinical reports describing frequent and severe CIM in critical illness survivors. 12 , 13 , 14 , 15 , 16
The current diagnostic criteria for CIM are based on a multimodal approach and require (i) a positive history for critical illness with multiorgan dysfunction; (ii) clinical finding of limb weakness or difficulty in weaning the patient from the ventilator; (iii) electrophysiological studies, and (iv) a muscle biopsy showing primary myopathy with myosin loss. 1 The current criteria therefore only allow diagnosis of definite CIM at an advanced stage, so that patients, especially in the early stages, are at risk of being overlooked. Furthermore, the lack of a tool for early diagnosis of evolving CIM impedes pathophysiological research as well as the conducting and monitoring of therapeutic trials.
Z'Graggen and Bostock have previously developed a method to assess muscle excitability in vivo by recording muscle velocity recovery cycles (MVRCs). 17 , 18 This technique is based on standard neurophysiological techniques and measures how the velocity of a muscle action potential changes, depending on the time interval after a preceding action potential. The changes in conduction velocity of a muscle action potential in the wake of a previous stimulus provide an indirect indication of the afterpotential following the muscle action potential. The afterpotential itself and consequently the recovery cycle are strongly dependent on membrane potential. In patients with probable CIM, MVRC recordings revealed changes related to either muscle fibre membrane depolarization and/or a heightened sensitivity to potassium due to increased sodium channel inactivation. 19 , 20 In a porcine model of sepsis, similar alterations were found within 6 h of experimental sepsis onset, indicating that muscle membrane changes may represent an early sign of evolving CIM. 21
We aimed to prospectively determine the frequency of CIM in a cohort of COVID‐19 patients with acute respiratory distress syndrome (ARDS) using the current diagnostic criteria and to compare the time course of changes in muscle excitability measurements with the confirmatory definite diagnosis of CIM in order to develop a tool for early diagnosis of CIM.
Material and methods
Study design and participants
This is a prospective monocentre cohort study investigating COVID‐19 patients admitted to the ICU of the University Hospital Bern, Switzerland, who were in need of mechanical ventilation due to the development of ARDS (Registration‐URL: ClinicalTrials.gov; unique identifier: NCT04397172). Patients were examined for the first time within 24 h after intubation (Day 1), followed‐up on Days 2 and 5, and completed on Day 10. Study examinations included clinical examination, muscle excitability measurements, medication record, and laboratory analyses on all study visits, and additionally nerve conduction studies, EMG, and muscle biopsy on Day 10.
Between April and December 2020, adult critically ill patients aged between 18 and 80 years admitted to the ICU due to COVID‐19 related ARDS requiring mechanical ventilation were recruited and assessed. Patients were screened for eligibility within 12 h of admission and enrolled within 24 h after intubation. Exclusion criteria were pre‐existing intubation for more than 24 h, pregnancy and breastfeeding, and pre‐existing polyneuropathy, Guillain‐Barré syndrome, spinal cord lesion, myasthenia gravis, or myopathy. All procedures were approved by the local ethics committee (Kantonale Ethikkomission, Bern, Switzerland: project‐ID 2020‐00730) and conformed to the Declaration of Helsinki and its amendments. Patients fulfilling the eligibility criteria were included if written informed consent by an independent physician acting as a surrogate was present. Next‐of‐kin was informed as soon as possible, and once the health condition of patients allowed, patient written informed consent was obtained.
Outcome measures
The primary outcome variable was frequency of definite CIM diagnosis 1 assessed at Day 10. In brief, definite CIM was diagnosed if patients (i) had a positive history of critical illness with multi‐organ dysfunction, (ii) showed limb weakness or difficulty in weaning from the ventilator after exclusion of non‐neuromuscular causes, (iii) showed signs of myopathy in nerve conduction studies and needle EMG 22 after exclusion of a neuromuscular transmission deficit, and (iv) had preferential myosin loss in muscle biopsy according to the protocol described by Marrero and colleagues. 23 Secondary outcome variables included the difference of the following parameters between patients with and without CIM: components of muscle excitability measurements (see below), classification of severity of disease according to the Sequential Organ Failure Assessment (SOFA) score, and clinical examination using the Functional Disability Score (FDS), all assessed at each study visit. Additionally, the following laboratory parameters were recorded as exploratory variables at each study visit: potassium, sodium, chloride, glucose, creatinine, creatine kinase, C‐reactive protein and arterial blood gas analysis (pO2, pCO2, pH, bicarbonate, base excess, lactate). Particular attention was paid to glucose and potassium variability (mean, standard deviation, median, minimum and maximum values were calculated) during the first 10 days of ICU stay. Other outcomes of interest were classification of severity of disease according to Acute Physiology and Chronic Health Evaluation II (APACHE‐II) and Charlson co‐morbidity index at admission, total duration of mechanical ventilation and extracorporeal membrane oxygenation during ICU stay, death during ICU stay, treatment with glucocorticoids, cumulative dosage of administered medication corrected for body weight (sedatives, opioids, NMBA, vasoactive agents), cumulative dosage of insulin and nutrition in kcal (Isosource Protein®), percentage of days patients received sedation (propofol (Propofol®, Fresenius Kabi (Switzerland) AG) and midazolam (Dormicum®, CPS Cito Pharma Services)), NMBA (rocuronium bromide (Esmeron®, MSD Merck Sharp & Dohme AG) and atracurium besilate (Tracrium®, Aspen Pharma Switzerland GmbH)) and mechanical ventilation, and muscle strength sum score 24 at Day 10.
Procedures
Electrophysiological studies
Muscle excitability recordings (Days 1, 2, 5 and 10)
The technique of muscle excitability measurements is illustrated in Figure 1 . Multi‐fibre responses to direct muscle stimulation through an intramuscular needle electrode were recorded from the patient's tibialis anterior muscle. The measurements were performed using an established protocol. 17 , 18 Stimulation and recording were controlled by QtracS software (copyright Institute of Neurology, London, UK), using the recording protocol M3REC5. The protocol consisted of two successive parts. The first was the recording of a MVRC with 1, 2, and 5 conditioning stimuli. The following MVRC parameters were assessed: (i) Muscle relative refractory period (MRRP) in ms, defined as the shortest interpolated interstimulus interval (ISI) at which the latencies of the unconditioned and conditioned test response were identical, (ii) early supernormality (ESN), measured as the peak percentage reduction in latency at ISIs shorter than 15 ms, (iii) late supernormality (LSN), measured as the average percentage reduction in latency at ISIs between 50 and 150 ms, (iv) extra late supernormality (XLSN) due to two, and (v) 5XLSN due to five conditioning stimuli. The second part consisted of the frequency ramp protocol, where a 1 s train of stimuli were delivered every 2 s. The number of stimuli in the train was increased by 1 from 2 to 31 stimuli, so that the mean stimulation rate was ramped up from 1 to 15.5 Hz over 1 min. Stimulus cycles with the test stimulus alone were recorded before and after the end of the frequency ramp with 10 and 15 cycles, respectively, and each at 0.5 Hz. The evaluated frequency ramp parameters were Lat(15 Hz)First and Lat(15 Hz)Last (latency to the first and last response in train at 15 Hz), and Lat(30 Hz)First (latency to the first response in train at 30 Hz).
Figure 1.
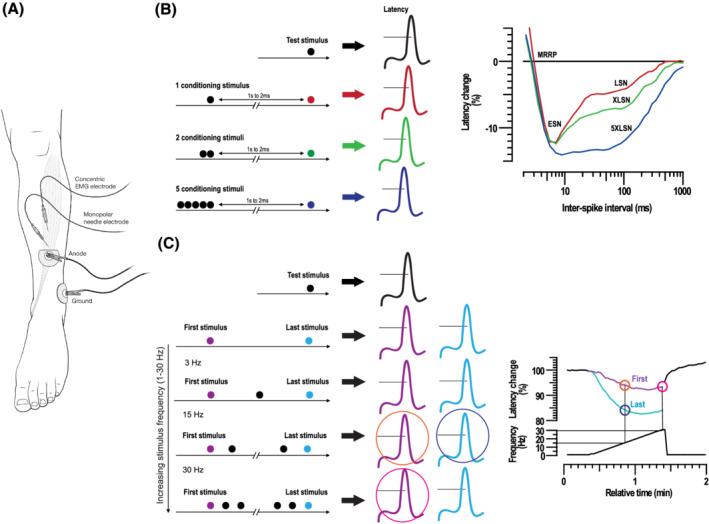
Illustration of muscle excitability measurements. (A) Electrode arrangement for muscle excitability measurements. A monopolar needle serving as cathode was inserted perpendicular to a depth of about 10 mm into the distal third of the tibialis anterior muscle. A surface electrode acting as anode was placed further distally. Recordings were made with a concentric EMG needle that was placed approximately 20 mm proximal to the stimulating needle along the assumed course of the muscle fibres. The surface ground electrode was placed above the malleolus lateralis. (B) (left side) Illustration of the technique of recording multi‐fibre muscle velocity recovery cycles (MVRC); (right side) illustration of a recovery cycle with percentage changes in latency due to 1 (red line) 2, (green line) and 5 (blue line) conditioning stimuli, plotted as a function of interstimulus interval. MRRP, muscle relative refractory period; ESN, early supernormality; LSN, late supernormality; XLSN, late supernormality with 2 stimuli; 5XLSN, late supernormality with 5 stimuli. (C) (left side) Illustration of the technique of recording frequency ramps. Test stimuli are preceded by 1 s trains of conditioning stimuli that increase linearly in frequency (in 1 Hz steps) to a maximum of 30 Hz over 1 min. During this period, the average rate of stimulation is increased from 0.5 to 15.5 Hz. In the end, a 30 s period of test stimulation was delivered at 0.5 Hz; (right side) on the top, latency of response to first (purple) and last (light blue) stimulus in conditioning train expressed as percentage of baseline. On the bottom, mean stimulation frequency. The evaluated frequency ramp parameters are Lat(15 Hz)First (orange), Lat(15 Hz)Last (dark blue), and Lat(30 Hz)First (pink).
Conventional neurophysiological examination (Day 10)
Conventional motor and sensory nerve conduction studies were performed along the standard approach. One nerve each at the upper and lower extremity was assessed. Repetitive motor nerve stimulation either of the median or ulnar nerve, depending on the accessibility, was used to screen for dysfunction of the neuromuscular junction. Needle EMGs were performed to screen for spontaneous activity and depending on patients' cooperation spontaneous motor unit potential analysis was performed. According to the diagnostic criteria, 1 CMAP amplitudes <80% of the lower limit of normal in two nerves without signs of conduction block and sensory nerve action potential amplitudes >80% of the lower limit of normal in parallel to typical findings in EMG (short‐duration, low amplitude motor unit potentials with or without fibrillation potentials) were considered relevant for diagnosis of CIM.
Fine needle muscle biopsy (Day 10)
Muscle biopsy from the tibialis anterior muscle was performed with a 16 Gauge soft tissue semi‐automated biopsy disposable needle instrument (Temno Evolution®) and immediately frozen (−80°C). Myosin:actin ratios were determined as described previously. 25 , 26 In brief, tissues in lithium dodecyl sulfate buffer (pH 8.5) underwent polyacrylamide gel electrophoresis followed by Coomassie staining. Gels were imaged using an iBright CL750 Imaging System (Thermo Fischer Scientific, MA, USA). Myosin and actin bands were quantified by densitometry using ImageJ.
Clinical examination (Days 1, 2, 5, and 10)
The clinical state of patients was scored according to the FDS and included assessment of deep tendon reflexes (0 = normal, 1 = reduced, 2 = absent), muscle weakness (0 = normal muscle force, 2 = mild, 3 = moderate and 4 = severe muscle weakness), muscle atrophy (0 = absent, 1 = present), and muscle fasciculation (0 = absent, 1 = present). 27 The muscle strength sum score was assessed at Day 10 by summarizing muscle force measured with the Medical Research Council (MRC) scale for three functional muscle groups on the upper (shoulder abduction, elbow flexion, and wrist extension) and lower extremities (hip flexion, knee extension, and ankle dorsiflexion). 2
Statistical analysis
The statistical analysis was performed using R, version 3.6.1 or higher, through R studio interface. Descriptive statistics were performed using frequencies and percentages for categorical variables and mean (± standard deviation) or median (interquartile range [IQR]) for continuous variables. Muscle excitability data of COVID‐19 patients were compared with an existing age‐matched group of healthy subjects (N = 31) using independent‐samples t‐tests. The main analysis was based on the comparison between patients with COVID‐19 who developed CIM (CIM+) and those who did not (CIM−). For muscle excitability and clinical data, two‐way repeated‐measures analysis of variance (ANOVA) was used to compare the two groups and residuals of the mixed model, using QQ plots to check the normality of the data. The group effect was estimated without the interaction over time if the latter was not significant. Post hoc comparison using independent‐samples t‐test for the singular days was performed for those parameters showing a significant difference in the repeated‐measures ANOVA. Chi‐square test or Fisher's exact test and independent‐samples t‐test or Mann–Whitney U‐test were used to compare all other categorical and continuous variables, respectively. Multiple comparisons of muscle excitability data were addressed by adjusting P values using false discovery rate (FDR) correction. Because for laboratory data and medication record the analysis was exploratory, no FDR correction was performed. Receiver operating characteristic (ROC) analysis was carried out for the most discriminative muscle excitability variables. Due to the low number of missing data and the limited sample size, only complete cases where included in the comparisons, and no formal multiple imputation method was applied. A two‐sided P value lower than 0.05 was considered significant.
Results
Of the 44 included patients, 13 patients (30%) were transferred to another hospital (N = 12) or died (N = 1) before completion on Day 10. The total sample size with a full data set regarding the primary outcome therefore included 31 patients (70%). Patient characteristics of COVID‐19 patients are summarized in Table 1 . Of the 31 patients with a full data set, 17 received diagnosis of definite CIM at Day 10 (55%). COVID‐19 patients and healthy subjects did not differ significantly regarding age [60.9 (8.7) vs. 62.4 (9.8) years, P = 0.531]. Patients of the CIM+ group stayed longer in the ICU (19.6 vs. 13.2 days, P = 0.032) and had higher mortality during ICU stay (53% vs. 7%, P = 0.009) than patients of the CIM− group. There were no differences regarding age, sex, weight, height, body mass index, and the Charlson co‐morbidity index between the two groups.
Table 1.
Patient characteristics and clinical data
| CIM+ (N = 17) | CIM− (N = 14) | P value | |
|---|---|---|---|
| Age (years) | 62.2 (±9.3) | 62.7 (±10.8) | 0.882 |
| Sex (female) | 5.9% (N = 1) | 36% (N = 5) | 0.067 |
| Weight (kg) | 88.8 (±22.4) | 88.9 (±18.9) | 0.996 |
| Height (m) | 1.8 (±0.1) | 1.7 (±0.1) | 0.11 |
| BMI | 28.6 (±7.5) | 31.0 (±6.4) | 0.361 |
| ICU stay (days) | 19.6 (±10) | 13.2 (±5.6) | 0.032 |
| Mechanical ventilation (h) | 444.0 (±214.1) | 229.2 (±123.7) | 0.003 |
| ECMO (days) | 3.9 (±7.5) | 0 (±0) | 0.030 |
| Deceased in ICU | 53% (N = 9) | 7.1% (N = 1) | 0.009 |
| Charlson co‐morbidity index | 3.8 (±2.5) | 3.2 (±2) | 0.746 |
| APACHE‐II | 33.0 [29.0;39.0] | 27.0 [26.0;31.5] | 0.008 |
| SOFA | |||
| Day 1 | 12.0 [9.0;15.0] | 11.0 [8.2;11.0] | 0.190 |
| Day 2 | 11.0 [10.0;13.0] | 10.0 [9.2;11.0] | 0.098 |
| Day 5 | 11.0 [9.0;14.0] | 8.0 [5.2;9.0] | 0.001 |
| Day 10 | 11.0 [8.0;13.0] | 6.0 [2.8;7.8] | 0.000 |
| FDS | |||
| Day 1 | 5.0 [5.0;5.0] | 5.0 [5.0;5.0] | 0.054 |
| Day 2 | 5.0 [5.0;6.0] | 5.0 [5.0;5.0] | 0.065 |
| Day 5 | 5.0 [5.0;6.0] | 5.0 [5.0;5.8] | 0.047 |
| Day 10 | 6.0 [5.0;6.0] | 5.0 [4.0;5.0] | 0.005 |
| Muscle strength sum score | 0.0 [0.0;12.0] | 21.0 [16.5;38.2] | 0.000 |
APACHE‐II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; CIM, critical illness myopathy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; FDS, Functional Disability Score.
All patient characteristics are reported as mean (±standard deviation), and all clinical scores are reported as median [interquartile range]. Comparison of sex and deceased during ICU was analysed by Fisher's exact test; for comparison of ICU stay, duration of mechanical ventilation and ECMO, and Charlson co‐morbidity index, Mann–Whitney U‐test was implemented; in all other comparisons, independent‐samples t‐tests were used. Muscle strength sum score was assessed using the Medical Research Council scale. Two‐way repeated measures ANOVA for the SOFA and FDS score, respectively, showed a significant effect for time (F = 39.02, P < 0.001; F = 13.24, P = 0.004) as well as a significant time‐group interaction (F = 14.54, P = 0.002; F = 13.49, P = 0.004).
Comparison of muscle excitability measurements of COVID‐19 patients and healthy subjects showed increased MRRP, decreased ESN and 5XLSN, and increased Lat(15 Hz)Last and Lat(30 Hz)First in patients on all examination days. The data and results are shown in Table 2 . Mean MVRCs and ramp latency curves for CIM+, CIM−, and healthy subjects are shown in Figure 2 . Two‐way repeated measures ANOVA for muscle excitability data revealed a significant group effect for MRRP (F = 8.11, P = 0.013), ESN (F = 16.32, P < 0.001), 5XLSN (F = 8.9, P = 0.013), Lat(15 Hz)Last (F = 11.74, P = 0.002) and Lat(30 Hz)First (F = 16.24, P < 0.001). There were no significant effects for time and no significant interactions for any of the variables. The results of pairwise analyses and ROC analyses for MRRP, ESN, Lat(15 Hz)Last, and Lat(30 Hz)First are reported in Table 3 and illustrated in Figure 3 . In summary, the further analyses showed that on Day 10 all included parameters discriminate between patients who developed CIM and patients who did not with a diagnostic precision 75–90% (AUC). Furthermore, regarding early changes of muscle excitability, there were significant group differences on Day 1 of the same parameters with diagnostic precision of 68–73%, and on Day 2 regarding Lat(15 Hz)Last and Lat(30 Hz)First with diagnostic precision of 77–82%.
Table 2.
Comparison of muscle excitability data between healthy subjects and critically ill COVID‐19 patients
| COVID‐19 patients (N = 31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy subjects (N = 31) | Day 1 | P value | Day 2 | P value | Day 5 | P value | Day 10 | P value | |
| MRRP | 3.5 (±0.4) | 5.8 (±2.0) | <0.001 | 5.3 (±1.8) | <0.001 | 5.3 (±2.3) | <0.001 | 5.3 (±2.3) | <0.001 |
| ESN | 11.3 (±1.8) | 8.9 (±4.0) | 0.005 | 9.3 (±3.7) | 0.012 | 7.8 (±4.1) | <0.001 | 7.8 (±4.1) | <0.001 |
| LSN | 3.7 (±1.0) | 3.3 (±1.2) | 0.204 | 3.5 (±2.4) | 0.682 | 3.6 (±2.6) | 0.842 | 3.6 (±2.6) | 0.126 |
| XLSN | 2.5 (±0.6) | 2.2 (±1.1) | 0.288 | 2.1 (±1.3) | 0.115 | 2.2 (±1.1) | 0.180 | 2.2 (±1.1) | 0.087 |
| 5XLSN | 7.2 (±1.1) | 5.9 (±2.1) | 0.004 | 5.8 (±2.6) | 0.010 | 5.4 (±2.2) | <0.001 | 5.4 (±2.2) | 0.001 |
| Lat(15 Hz)Last | 85.3 (±2.6) | 88.6 (±5.1) | 0.002 | 87.6 (±5.7) | 0.050 | 89.0 (±8.2) | 0.027 | 89.1 (±6.2) | 0.003 |
| Lat(15 Hz)First | 94.9 (±1.8) | 96.6 (±2.8) | 0.004 | 97.2 (±2.7) | <0.001 | 96.7 (±6.4) | 0.164 | 97.6 (±3.2) | <0.001 |
| Lat(30 Hz)First | 96.3 (±2.2) | 100.4 (±4.4) | <0.001 | 100.3 (±4.7) | <0.001 | 100.3 (±8.1) | 0.015 | 100.7 (±6.1) | 0.001 |
Comparisons using independent samples t‐tests are presented as mean (±standard deviation). MRRP is given in ms; all other values are given in per cent.
5XLSN, extra late supernormality due to five conditioning stimuli; ESN, early supernormality; Lat(15 Hz)First, latency to the first response in train at 15 Hz; Lat(15 Hz)Last, latency to the last response in train at 15 Hz; Lat(30 Hz)First, latency to the first response in train at 30 Hz; LSN, late supernormality; MRRP, muscle relative refractory period; XLSN, extra late supernormality due to two conditioning stimuli.
Figure 2.

Illustration of the recorded muscle excitability data. (A) (left side) Illustration of mean MVRCs with 1, 2, and 5 conditioning stimuli in healthy subjects (empty black dots) compared with COVID‐19 patients developing CIM (CIM+; filled red dots) and patients without CIM (CIM−; filled cyan dots), respectively, on Day 1. (A) (right side) Illustration of mean frequency ramps in healthy subjects (black lines) compared with COVID‐19 patients developing CIM (CIM+; red lines) and patients without CIM (CIM−; cyan lines), respectively, on Day 1. The same is shown in (B), (C), and (D) for Days 2, 5, and 10, respectively. CIM, critical illness myopathy; MVRC, muscle velocity recovery cycle.
Table 3.
Results of post hoc comparison and ROC analysis of muscle excitability data of COVID‐19 patients with and without critical illness myopathy
| CIM+ (N = 17) | CIM− (N = 14) | P value | Adj. P value | AUC (95% CI) | Cut‐off | Sensitivity | Specificity | Accuracy | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | ||||||||||
| MRRP (ms) | 6.6 (±2.3) | 5.0 (±1.3) | 0.025 | 0.049 | 0.721 (0.536; 0.906) | 6.065 | 0.588 | 0.929 | 0.742 | |
| ESN | 7.6 (±3.1) | 10.5 (±4.6) | 0.049 | 0.049 | 0.679 (0.470; 0.887) | 7.990 | 0.706 | 0.714 | 0.710 | |
| Lat(15 Hz)Last | 90.5 (±5.0) | 86.3 (±4.4) | 0.022 | 0.022 | 0.718 (0.534; 0.903) | 87.600 | 0.647 | 0.714 | 0.677 | |
| Lat(30 Hz)First | 102.2 (±4.0) | 98.4 (±4.1) | 0.013 | 0.022 | 0.734 (0.550; 0.919) | 102.650 | 0.562 | 0.857 | 0.700 | |
| Day 2 | ||||||||||
| MRRP (ms) | 5.9 (±1.9) | 4.6 (±1.4) | 0.056 | 0.056 | 0.752 (0.552; 0.953) | 4.765 | 0.875 | 0.769 | 0.828 | |
| ESN | 8.0 (±3.9) | 10.8 (±3.0) | 0.044 | 0.056 | 0.764 (0.566; 0.962) | 8.855 | 0.812 | 0.769 | 0.793 | |
| Lat(15 Hz)Last | 90.2 (±5.8) | 84.4 (±3.5) | 0.004 | 0.005 | 0.820 (0.652; 0.987) | 88.600 | 0.750 | 0.923 | 0.828 | |
| Lat(30 Hz)First | 102.5 (±4.2) | 97.7 (±3.9) | 0.005 | 0.005 | 0.777 (0.603; 0.951) | 99.750 | 0.800 | 0.692 | 0.750 | |
| Day 5 | ||||||||||
| MRRP (ms) | 5.5 (±2.6) | 4.9 (±1.9) | 0.476 | 0.476 | 0.625 (0.403; 0.847) | 4.620 | 0.625 | 0.692 | 0.655 | |
| ESN | 6.9 (±3.3) | 9.0 (±4.8) | 0.174 | 0.348 | 0.597 (0.377; 0.817) | 12.185 | 1.000 | 0.308 | 0.700 | |
| Lat(15 Hz)Last | 90.5 (±9.3) | 86.8 (±6.3) | 0.249 | 0.337 | 0.672 (0.466; 0.877) | 92.550 | 0.471 | 0.917 | 0.655 | |
| Lat(30 Hz)First | 101.5 (±10.1) | 98.6 (±3.8) | 0.337 | 0.337 | 0.728 (0.529; 0.927) | 101.200 | 0.706 | 0.833 | 0.759 | |
| Day 10 | ||||||||||
| MRRP (ms) | 6.2 (±1.7) | 4.3 (±1.1) | 0.002 | 0.002 | 0.824 (0.669; 0.978) | 4.350 | 0.941 | 0.643 | 0.806 | |
| ESN | 5.1 (±2.8) | 11.3 (±3.8) | 0.000 | 0.000 | 0.908 (0.799; 1.000) | 9.940 | 1.000 | 0.714 | 0.871 | |
| Lat(15 Hz)Last | 91.6 (±5.2) | 86.2 (±6.0) | 0.014 | 0.014 | 0.768 (0.579; 0.957) | 87.250 | 0.875 | 0.714 | 0.800 | |
| Lat(30 Hz)First | 104.2 (±5.9) | 97.4 (±4.3) | 0.002 | 0.004 | 0.857 (0.704; 1.000) | 100.350 | 0.846 | 0.857 | 0.852 | |
Data of post hoc comparisons using independent samples t‐tests are presented as mean (±standard deviation). Values are reported in percent if not differently indicated. P values were adjusted for multiple comparisons of parameters within one recording for all days separately (e.g. for MRRP and ESN of the muscle velocity recovery cycle on Day 1, and for MLat15 and MLatF30 of the frequency ramp on Day 1) using FDR method, and considered significant if <0.05.
AUC, area under the curve; CI, confidence interval; CIM, critical illness myopathy; ESN, early supernormality; Lat(15 Hz)Last, latency to the last response in train at 15 Hz; Lat(30 Hz)First, latency to the first response in train at 30 Hz; MRRP, muscle relative refractory period; ROC, receiver operator characteristic.
Figure 3.
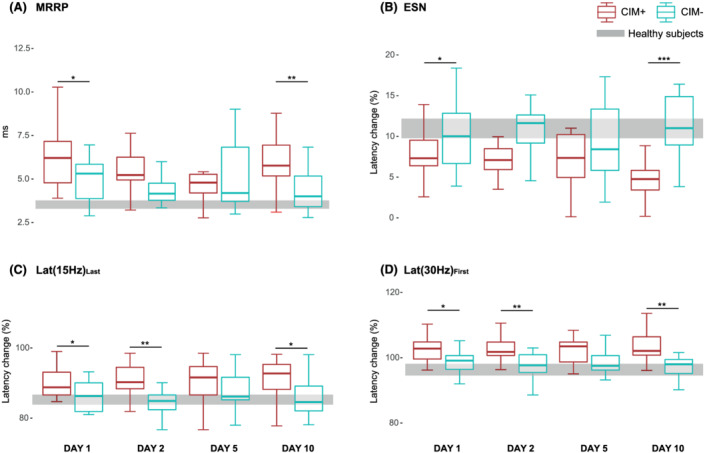
Results of post‐hoc comparison of muscle excitability data. (A) Boxplots of MRRP for COVID‐19 patients with CIM (CIM+) in red and patients without CIM (CIM−) in cyan on Days 1, 2, 5, and 10 after intubation. The same is shown in (B), (C), and (D) for ESN, Lat(15 Hz)Last, and Lat(30 Hz)First, respectively. The grey bar refers to the confidence interval of the corresponding data of healthy subjects. CIM, critical illness myopathy; MRRP, muscle velocity recovery cycles; ESN, early supernormality; Lat(15 Hz)Last, latency to the last response in train at 15 Hz; Lat(30 Hz)First, latency to the first response in train at 30 Hz. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Clinical scores of the CIM+ and CIM− groups are presented in Table 1 . The APACHE‐II score at admission was significantly higher in the CIM+ than in the CIM− group (P = 0.008). The SOFA score decreased from Day 1 to Day 10 by one point in the CIM+ and by five points in the CIM− group. Two‐way repeated measures ANOVA confirmed a significant effect for time (F = 8.80, P < 0.001) as well as a significant interaction (F = 4.85, P = 0.004). FDS decreased in CIM+ and remained stable in CIM− patients. Statistical analysis showed a significant interaction (F = 4.50, P = 0.006). On Day 10 28 patients in total (90%), 17 (100%) of the CIM+ and 11 (79%) of the CIM− group had a MRC muscle strength sum score <48. Patients with CIM scored significantly lower than patients without CIM (P < 0.001).
Comparisons of the cumulative dosage of medications and nutrition administered in the CIM+ versus CIM− group are reported in Table 4 . The analysis did not reveal any significant differences, with the exception that atracurium besilate was higher in patients with CIM by Day 10 (P = 0.034). There were also no group differences regarding percentage days of sedation (P = 0.215) and NMBAs (P = 0.131). Patients who developed CIM needed longer mechanical ventilation (100% vs. 90% of the first 10 days, P = 0.033; 444 h vs. 229 h in total, P = 0.003) and treatment with extracorporeal membrane oxygenation (3.9 vs. 0 days, P = 0.030) than patients who did not develop CIM.
Table 4.
Results of medication record
| CIM+ (N = 17) | CIM− (N = 14) | P value | |
|---|---|---|---|
| Day 1 | |||
| Rocuronium bromide (mg) | 200.0 [100.0; 380.0] | 230.0 [85.0; 368.0] | 0.470 |
| Atracurium besilate (mg) | 0.0 [0.0; 305.0] | 0.0 [0.0; 30.0] | 0.566 |
| Midazolam (mg) | 0.0 [0.0; 4.3] | 2.5 [0.0; 26.0] | 0.679 |
| Propofol (mg) | 5293.0 [1894.0; 7787.0] | 4095.0 [2132.0; 6693.2] | 0.557 |
| Dexmedetomidin (mcg) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.278 |
| Fentanyl (mg) | 1691.0 [750.0; 2370.0] | 1840.0 [819.5; 3212.0] | 0.269 |
| Norepinephrine (mcg) | 1251.0 [735.0; 6685.0] | 704.0 [371.8; 2376.5] | 0.686 |
| Insulin aspart (U) | 2.4 [0.0; 39.6] | 3.3 [0.0; 28.0] | 0.378 |
| Glucocorticoids (Yes) | 24% (N = 4) | 21% (N = 3) | 1.000 |
| Nutrition (kcal) | 196.6 [0.0; 463.8] | 380.7 [45.8; 553.3] | 0.615 |
| Day 2 | |||
| Rocuronium bromide (mg) | 280.0 [200.0; 410.0] | 280.0 [85.0; 407.5] | 0.364 |
| Atracurium besilate (mg) | 0.0 [0.0; 499.0] | 0.0 [0.0; 199.8] | 0.820 |
| Midazolam (mg) | 0.0 [0.0; 6.0] | 2.5 [0.0; 56.8] | 0.309 |
| Propofol (mg) | 12022.0 [9008.0; 15024.0] | 10797.5 [3956.5; 13001.5] | 0.441 |
| Dexmedetomidin (mcg) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.278 |
| Fentanyl (mg) | 4939.0 [2532.0; 5556.0] | 4486.0 [2957.5; 7732.8] | 0.507 |
| Norepinephrine (mcg) | 3265.0 [1505.0; 7411.0] | 2049.5 [460.2; 6479.8] | 0.514 |
| Insulin aspart (U) | 39.0 [6.4; 98.2] | 30.3 [8.7; 103.8] | 0.407 |
| Glucocorticoids (Yes) | 29% (N = 5) | 21% (N = 3) | 0.698 |
| Nutrition (kcal) | 1096.3 [858.5; 1959.1] | 1720.4 [1063.0; 2171.5] | 0.166 |
| Day 5 | |||
| Rocuronium bromide (mg) | 286.0 [200.0; 640.0] | 301.0 [92.5; 780.0] | 0.372 |
| Atracurium besilate (mg) | 180.0 [0.0; 1870.0] | 0.0 [0.0; 199.8] | 0.285 |
| Midazolam (mg) | 4.3 [0.0; 171.0] | 3.0 [0.0; 57.8] | 0.882 |
| Propofol (mg) | 23520.0 [19809.0; 31533.0] | 18229.0 [8378.5; 35689.0] | 0.317 |
| Dexmedetomidin (mcg) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.300 |
| Fentanyl (mg) | 12367.0 [6019.0; 19787.0] | 9675.0 [5585.8; 20260.5] | 0.569 |
| Norepinephrine (mcg) | 8999.0 [4619.0; 18347.0] | 3130.5 [1571.8; 10314.2] | 0.834 |
| Insulin aspart (U) | 123.9 [72.2; 448.3] | 170.2 [69.7; 374.7] | 0.361 |
| Glucocorticoids (Yes) | 29% (N = 5) | 29% (N = 4) | 1.000 |
| Nutrition (kcal) | 5105.6 [3962.7; 6056.2] | 5669.9 [4838.1; 6847.9] | 0.290 |
| Day 10 | |||
| Rocuronium bromide (mg) | 430.0 [200.0; 954.0] | 302.0 [150.0; 950.0] | 0.372 |
| Atracurium besilate (mg) | 942.0 [70.0; 2904.0] | 0.0 [0.0; 253.0] | 0.034 |
| Midazolam (mg) | 26.0 [0.0; 234.0] | 8.0 [0.0; 214.0] | 0.585 |
| Propofol (mg) | 37245.0 [20985.0; 60789.0] | 26013.0 [11299.0; 54999.0] | 0.244 |
| Dexmedetomidin (mcg) | 0.0 [0.0; 384.0] | 0.0 [0.0; 0.0] | 0.464 |
| Fentanyl (mg) | 28592.0 [11194.0; 41980.0] | 15493.0 [6148.0; 42308.0] | 0.614 |
| Norepinephrine (mcg) | 21270.0 [9209.0; 37755.0] | 5056.0 [2233.0; 15321.0] | 0.553 |
| Insulin aspart (U) | 515.2 [246.0; 842.3] | 402.9 [291.6; 520.1] | 0.159 |
| Glucocorticoids (Yes) | 35% (N = 6) | 29% (N = 4) | 1.000 |
| Nutrition (kcal) | 12760.0 [10005.6; 14560.7] | 14454.3 [7064.7; 16035.5] | 0.693 |
| Days with sedation (%) | 100 [80; 100] | 100 [100; 100] | 0.215 |
| Days with NMBA (%) | 50 [30; 70] | 70 [40; 90] | 0.131 |
CIM, critical illness myopathy; NMBA, neuromuscular blocking agents.
All values are reported as median [interquartile range] with unadjusted P values. For variables with cumulative doses, independent‐samples t‐tests were implemented, for those with percentage days Mann–Whitney U‐test was carried out, and for glucocorticoids (yes/no) Fisher's exact test was used. The doses administered were corrected for body weight, except for insulin and nutrition, where the values are reported as absolute doses and calories. Days with sedation refers to the percentage of days patients received sedation (propofol and/or midazolam). Days with relaxation refers to the percentage of days patients received NMBA (rocuronium bromide and/or atracurium besilate).
Comparisons of laboratory analyses are shown in Table 5 . In summary, the analyses showed significantly higher potassium on Days 1 (P = 0.005), 2 (P = 0.031), and 10 (P = 0.003) in patients with CIM compared with patients without CIM. Further pairwise comparisons showed decreased pH on Days 1 (P = 0.040) and 10 (P = 0.005), decreased bicarbonate on Days 5 (P = 0.002) and 10 (P = 0.002), decreased arterial pCO2 on Days 2 (P = 0.025) and 5 (P = 0.007), decreased base excess on Days 5 (P = 0.004) and 10 (P = 0.001), and increased creatinine on Day 10 (P = 0.033) in CIM+ compared with CIM− patients. Results of glucose and potassium variability are shown in the Supporting Information, Table S1 . None of the glucose variability parameters revealed any significant group differences. Patients with CIM had higher mean, median, minimum, and maximum potassium values during the first 10 days in the ICU than patients without CIM. Ten patients (59%) in the CIM+ versus none in the CIM− group received hemofiltration by Day 10 (P < 0.001). In order to be as minimally invasive as possible and to avoid interference with the treatment, we only performed fine needle muscle biopsies. Unfortunately, due to the small amount of tissue taken, further histological analyses were not possible.
Table 5.
Results of laboratory analysis
| CIM+ (N = 17) | CIM− (N = 14) | P value | |
|---|---|---|---|
| Day 1 | |||
| Bicarbonate (mmol/L) | 24.1 [20.5; 27.4] | 27.6 [25.0; 29.1] | 0.204 |
| pCO2 (mmHg) | 44.0 [42.0; 52.0] | 46.0 [41.5; 47.8] | 0.928 |
| pO2 (mmHg) | 62.0 [44.0; 78.0] | 76.0 [71.2; 81.0] | 0.986 |
| Base excess (mmol/L) | −0.7 [−4.0; 2.8] | 2.0 [0.3; 4.9] | 0.094 |
| Lactate (mmol/L) | 1.3 [1.2; 1.6] | 1.3 [1.0; 1.4] | 0.578 |
| Glucose (mmol/L) | 8.4 [7.7; 9.5] | 8.1 [7.6; 9.6] | 0.908 |
| Potassium (mmol/L) | 4.5 [4.3; 4.9] | 4.1 [3.8; 4.2] | 0.005 |
| Sodium (mmol/L) | 139.0 [136.0; 142.0] | 138.0 [135.2; 139.8] | 0.170 |
| Chloride (mmol/L) | 107.0 [105.0; 109.0] | 106.5 [101.2; 108.8] | 0.315 |
| pH | 7.4 [7.3; 7.4] | 7.4 [7.4; 7.4] | 0.040 |
| Creatinine (μmol/L) | 88.0 [66.0; 141.0] | 87.5 [75.0;97.5] | 0.205 |
| Creatine kinase (U/L) | 267.5 [92.5;1848.8] | 119.0 [53.0;293.0] | 0.153 |
| C‐reactive protein (mg/L) | 179.5 [114.8;234.0] | 160.0 [104.8;208.5] | 0.569 |
| Day 2 | |||
| Bicarbonate (mmol/L) | 25.5 [23.0;28.6] | 30.1 [27.6;31.5] | 0.087 |
| pCO2 (mmHg) | 42.0 [38.0;47.0] | 47.5 [45.2;50.0] | 0.025 |
| pO2 (mmHg) | 68.0 [64.0;74.0] | 55.5 [38.0;70.8] | 0.194 |
| Base excess (mmol/L) | 1.1 [−0.7;4.6] | 4.6 [2.6;6.4] | 0.132 |
| Lactate (mmol/L) | 1.6 [1.1;1.8] | 1.4 [1.0;1.7] | 0.172 |
| Glucose (mmol/L) | 8.8 [8.2;10.3] | 9.3 [8.3;10.2] | 0.900 |
| Potassium (mmol/L) | 4.2 [3.9;4.3] | 3.9 [3.6;4.1] | 0.031 |
| Sodium (mmol/L) | 140.0 [138.0;144.0] | 140.0 [138.0;142.0] | 0.794 |
| Chloride (mmol/L) | 108.0 [106.0;109.0] | 105.5 [101.5;109.8] | 0.285 |
| pH | 7.4 [7.4;7.4] | 7.4 [7.4;7.5] | 0.967 |
| Creatinine (μmol/L) | 110.0 [90.2;165.8] | 106.0 [92.0;123.0] | 0.219 |
| Creatine kinase (U/L) | 1672.5 [1372.2;2068.8] | 159.0 [159.0;159.0] | 0.293 |
| C‐reactive protein (mg/L) | 215.0 [133.5;311.0] | 171.0 [136.0;203.0] | 0.381 |
| Day 5 | |||
| Bicarbonate (mmol/L) | 29.2 [27.9;31.4] | 34.6 [31.6;36.4] | 0.002 |
| pCO2 (mmHg) | 45.0 [42.0;48.0] | 51.5 [48.5;55.5] | 0.007 |
| pO2 (mmHg) | 69.0 [64.0;74.0] | 61.5 [59.2;77.8] | 0.848 |
| Base excess (mmol/L) | 6.0 [3.6;6.7] | 9.1 [7.2;11.7] | 0.004 |
| Lactate (mmol/L) | 1.4 [1.1;1.8] | 1.4 [1.3;1.6] | 0.731 |
| Glucose (mmol/L) | 9.0 [8.2;10.3] | 8.3 [7.6;10.1] | 0.532 |
| Potassium (mmol/L) | 4.3 [4.0;4.6] | 4.0 [4.0;4.4] | 0.171 |
| Sodium (mmol/L) | 141.0 [138.0;145.0] | 142.0 [140.0;146.5] | 0.155 |
| Chloride (mmol/L) | 106.0 [104.0;109.0] | 105.5 [99.0;111.8] | 0.363 |
| pH | 7.4 [7.4;7.5] | 7.4 [7.4;7.5] | 0.625 |
| Creatinine (μmol/L) | 118.0 [97.0;169.5] | 88.0 [79.0;102.0] | 0.132 |
| Creatine kinase (U/L) | 154.5 [74.5;2823.2] | 543.0 [508.0;549.5] | 0.456 |
| C‐reactive protein (mg/L) | 136.0 [73.0;224.0] | 154.0 [63.2;252.0] | 0.973 |
| Day 10 | |||
| Bicarbonate (mmol/L) | 28.3 [24.8;32.0] | 32.5 [30.2;37.5] | 0.002 |
| pCO2 (mmHg) | 46.0 [40.0;53.0] | 45.5 [43.2;53.2] | 0.834 |
| pO2 (mmHg) | 68.0 [61.0;78.0] | 68.5 [65.2;78.0] | 0.123 |
| Base excess (mmol/L) | 3.4 [−1.0;6.1] | 8.9 [5.9;12.2] | 0.001 |
| Lactate (mmol/L) | 1.5 [1.3;1.7] | 1.5 [1.2;1.8] | 0.783 |
| Glucose (mmol/L) | 9.0 [8.0;9.8] | 8.7 [7.3;10.2] | 0.776 |
| Potassium (mmol/L) | 4.4 [4.2;4.7] | 4.0 [3.7;4.2] | 0.003 |
| Sodium (mmol/L) | 141.0 [138.0;144.0] | 142.5 [141.2;144.8] | 0.960 |
| Chloride (mmol/L) | 108.0 [103.0;110.0] | 106.0 [102.0;108.0] | 0.287 |
| pH | 7.4 [7.4;7.5] | 7.5 [7.4;7.5] | 0.005 |
| Creatinine (μmol/L) | 138.0 [113.0;204.0] | 81.5 [61.8;115.5] | 0.033 |
| Creatine kinase (U/L) | 443.0 [139.0;1419.8] | 222.0 [83.0;356.0] | 0.302 |
| C‐reactive protein (mg/L) | 68.0 [35.0;149.0] | 72.0 [36.8;94.5] | 0.350 |
CIM, critical illness myopathy.
All values are reported as median [interquartile range]. P values are unadjusted and refer to independent‐samples t‐tests.
Discussion
In this prospective observational study, 55% of the enrolled critically ill COVID‐19 patients with ARDS developed CIM (according to current diagnostic criteria 1 ) within the first 10 days of intensive care treatment. We were able to show that muscle excitability measurements can discriminate between patients who have developed CIM and those who did not, with a diagnostic precision of up to 90%. Furthermore, muscle excitability parameters, measured within 24 and 48 h after intubation, also discriminate with 68–73% and 75–82% diagnostic precision, respectively, between patients who will develop CIM and those who will not.
The CIM prevalence of 55% observed in this study is in line with previous observations in COVID‐19 cohorts. 28 , 29 The reported prevalence of CIM in non‐COVID‐19 populations varies widely between 9% and 86%, depending on the included cohort, the implemented diagnostic methods and the diagnostic criteria referred to. 30 More recently, systematic reviews have shown a mean prevalence of ICU‐acquired weakness of 33–40%. 30 , 31 However, the prevalence of ICU‐acquired weakness cannot be assumed to equate with the prevalence of CIM. ICU‐acquired weakness is a purely clinical diagnosis, which does not require electrophysiological examinations or muscle biopsy, and therefore includes both critical illness polyneuropathy (CIP) and CIM as well as other pathologies that cause muscle weakness. This indicates that the true prevalence of CIM can be assumed to be lower. This assumption is supported by our finding that 90% of all patients (100% of CIM+ and 79% of CIM− patients) would have been diagnosed with ICU‐acquired weakness by Day 10 if a purely clinical diagnostic approach had been taken. 2 Taken all together, the prevalence of CIM in critically ill COVID‐19 patients is probably higher than that reported in corresponding non‐COVID‐19 populations.
Several conclusions can be drawn from the muscle excitability recordings done in this study. First, on Day 10 post‐intubation the muscle excitability parameter ESN can discriminate between patients who had developed definite CIM and those who had not, with a diagnostic precision of 90%. This suggests that muscle excitability measurements may provide a reliable and more convenient alternative to the methods currently required for the diagnosis of CIM. The use of muscle excitability measurements would obviate the need for muscle biopsy and patient cooperation for quantitative EMG examinations. The current requirement of muscle biopsy for definite diagnosis of CIM has led to a trend in recent studies to focus on the purely clinical diagnosis of ICU‐acquired weakness and to omit differentiation into CIM and/or CIP. Because the frequency, prognosis and probably also pathophysiology of CIM and CIP differ vastly, 2 this can lead to a blurring of the results and affect the validity of these studies. Additionally, muscle excitability measurements are also able to distinguish between patients who are in the process of developing CIM and those who are not, and may therefore be suitable for early diagnosis, for estimating the risk for development of CIM, and for monitoring in future clinical trials with the goal of disease prevention and/or treatment.
Furthermore, muscle excitability measurements showed that on all examinations over the course of 10 days, COVID‐19 patients had prolonged MRRPs and reduced ESN when compared with a healthy population, which has been shown earlier to be indicative of muscle membrane depolarization. 18 This was not only true for the patient group who developed CIM; the COVID‐19 group who did not develop CIM also initially showed, although less pronounced, signs of membrane depolarization, which normalized over the course of the following days. These early and milder muscle membrane alterations in the CIM− group probably reflect to some extent pathophysiological processes related to disease severity. In contrast, the more pronounced muscle membrane alterations associated with the development of CIM point to additional specific processes that lead to muscle cell damage, and for which membrane depolarization seems to be an early indicator, or possibly even a contributory factor. This hypothesis is supported by previous observations in an animal model reporting alterations of muscle excitability parameters within 6 h after induction of experimental sepsis 21 and the findings of another research group that analysed serial measurements of compound muscle action potentials elicited by direct muscle stimulation in critically ill patients and showed that altered muscle membrane excitability occurs within 7.5 days of critical illness. 32 Changes of ESN and MRRP are, however, not only indicative of muscle depolarization as they can also occur due to increased sodium channel inactivation or reduced sodium channel availability, which has been postulated as a possible etiological factor for CIM. 19 , 33 In patients with CIP, sodium channel dysfunction has been shown to be a specific feature, whereas membrane depolarization of motor nerves can also be observed in critically ill patients without CIP. 34 , 35
One unexpected finding of this study is that many of the previously proposed risk factors for CIM could not be confirmed. Neither cumulative doses nor the number of days of sedative medication (especially propofol) or NMBAs differed significantly between patients who developed CIM and those who did not. The same was true for glucose levels as well as for glucose variability, administered nutrition and insulin. However, the CIM+ group had a higher APACHE‐II score at admission and consistently high SOFA scores, while SOFA scores in the CIM− group decreased over time. This suggests that the CIM+ group was sicker from the start and had longer lasting multi‐organ dysfunction. For example, none of the patients of the CIM− group compared with 59% of patients of the CIM+ group needed hemofiltration. The significantly higher potassium values in patients with CIM on Days 1, 2, and 10, and the higher potassium variability in the first 10 days may represent a consequence of either renal dysfunction, muscle fibre necrosis or a combination of both. As sicker patients generally require prolonged intensive care and more extensive pharmacological therapy, previous findings of studies that led to proposing sedation or NMBAs as risk factors for CIM may have been confounded by the underlying degree of disease severity. Patients who developed CIM were mechanically ventilated longer than those who did not. This may either provide further evidence for mechanical ventilation being a risk factor for CIM as it has been postulated earlier, 36 or it may be an indicator for CIM‐associated weaning failure.
Our study has several limitations. The study was done in a cohort of COVID‐19 patients, which limits the generalizability to non‐COVID‐19 populations. The cohort also included only a small number of female patients. As only complete cases were included into our analyses and the number of drop‐outs was considerable, the reported frequency of CIM must be interpreted with caution. The investigation into and the findings regarding early diagnosis of CIM using muscle excitability measurements or risk factors were not affected by the drop‐out rate. Additionally, we cannot exclude that CIM in COVID‐19 patients may be combined with other myopathic processes. 37 Furthermore, the cut‐off values determined by this study could not be internally validated. Although the trend was the same as for the other measurements, muscle excitability parameters measured on Day 5 did not reliably discriminate between patients with and without CIM. We attribute this at least partly to higher variability in the data compared with the other measurements. In future studies the presented results should therefore be validated in an independent sample of a COVID‐19 but also in a non‐COVID‐19 population.
In summary, our results have several important implications. Firstly, we report a 55% prevalence of definite CIM in a COVID‐19 population with ARDS. Secondly, we show that muscle excitability measurements may not only be an alternative method for CIM diagnosis as muscle excitability parameters discriminate between patients with and without CIM, but may possibly also be a tool for early diagnosis of CIM. Furthermore, the results indicate that muscle membrane depolarization may be an early sign of CIM and that in this case the pathophysiological process underlying CIM development starts as early as within 24 h after intubation.
Conflict of interest
H. Bostock receives royalties from University College London for sales of the QtracW software used in this study. The Department of Intensive Care Medicine has, or has had in the past, research & development/consulting contracts with Edwards Lifesciences Services GmbH, Phagenesis Limited and Nestlé. The money was paid into a departmental fund, and none of the authors received any financial gain. The Department of Intensive Care Medicine has received in the past unrestricted educational grants from the following organizations for organizing bi‐annual postgraduate courses in the fields of critical care ultrasound, management of ECMO and mechanical ventilation: Pierre Fabre Pharma AG (formerly known as RobaPharm), Pfizer AG, Bard Medica S.A., Abbott AG, Anandic Medical Systems, PanGas AG Healthcare, Orion Pharma, Bracco, Edwards Lifesciences AG, Hamilton Medical AG, Fresenius Kabi (Schweiz) AG, Getinge Group Maquet AG, Dräger Schweiz AG, Teleflex Medical GmbH. All other authors do not report any disclosures.
Funding
The study was supported by a grant of the Swiss Foundation for Research on Muscle Diseases and a grant of the University of Bern held by W.J. Z'Graggen.
Supporting information
Table S1. Glucose and potassium variability
Acknowledgement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. 38
Rodriguez B., Branca M., Gutt‐Will M., Roth M., Söll N., Nansoz S., Cameron D. R., Tankisi H., Tan S. V., Bostock H., Raabe A., Schefold J. C., Jakob S. M., and Z'Graggen W. J. (2022) Development and early diagnosis of critical illness myopathy in COVID‐19 associated acute respiratory distress syndrome, Journal of Cachexia, Sarcopenia and Muscle, 13, 1883–1895, 10.1002/jcsm.12989
References
- 1. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931–941. [DOI] [PubMed] [Google Scholar]
- 2. Schefold JC, Wollersheim T, Grunow JJ, Luedi MM, Z'Graggen WJ, Weber‐Carstens S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle 2020;11:1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N Engl J Med 2011;364:1293–1304. [DOI] [PubMed] [Google Scholar]
- 4. Hermans G, van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute Outcomes and 1‐Year Mortality of Intensive Care Unit‐acquired Weakness A Cohort Study and Propensity‐matched Analysis. Am J Respir Crit Care Med 2014;190:410–420. [DOI] [PubMed] [Google Scholar]
- 5. Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, et al. Joblessness and Lost Earnings after ARDS in a 1‐Year National Multicenter Study Abstracts. Am J Respir Crit Care Med 2017;196 (8):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schefold JC, Bierbrauer J, Weber‐Carstens S. Intensive care unit‐acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 2010;1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berger D, Bloechlinger S, von Haehling S, Doehner W, Takala J, Z'Graggen WJ, et al. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle 2016;7:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber‐Carstens S, Deja M, Koch S, Spranger J, Bubser F, Wernecke KD, et al. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care 2010;14:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Letter M, Schmitz PI, Visser LH, Verheul FA, Schellens RL, de Coul DA, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med 2001;29:2281–2286. [DOI] [PubMed] [Google Scholar]
- 10. Nanas S, Kritikos K, Angelopoulos E, Siafaka A, Tsikriki S, Poriazi M, et al. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand 2008;118:175–181. [DOI] [PubMed] [Google Scholar]
- 11. Lonnqvist PA, Lönnqvist PA, Bell M, Karlsson T, Wiklund L, Höglund AS, et al. Does prolonged propofol sedation of mechanically ventilated COVID‐19 patients contribute to critical illness myopathy? Br J Anaesth 2020;125:e334–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Aerde N, Van den Berghe G, Wilmer A, Gosselink R, Hermans G. Intensive care unit acquired muscle weakness in COVID‐19 patients. Intensive Care Med 2020;46:2083–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tankisi H, Tankisi A, Harbo T, Markvardsen LK, Andersen H, Pedersen TH. Critical illness myopathy as a consequence of Covid‐19 infection. Clin Neurophysiol 2020;131:1931–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bagnato S, Boccagni C, Marino G, Prestandrea C, DAgostino T, Rubino F. Critical illness myopathy after COVID‐19. Int J Infect Dis 2020;99:276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maury A, Lyoubi A, Peiffer‐Smadja N, de Broucker T, Meppiel E. Neurological manifestations associated with SARS‐CoV‐2 and other coronaviruses: A narrative review for clinicians. Rev Neurol (Paris) 2021;177:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bax F, Lettieri C, Marini A, Pellitteri G, Surcinelli A, Valente M, et al. Clinical and neurophysiological characterization of muscular weakness in severe COVID‐19. Neurol Sci 2021;42:2173–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boerio D, BoËRio D, Z'graggen WJ, Tan SV, Guetg A, Ackermann K, et al. Muscle velocity recovery cycles: Effects of repetitive stimulation on two muscles. Muscle Nerve 2012;46:102–111. [DOI] [PubMed] [Google Scholar]
- 18. Z'Graggen WJ, Bostock H. Velocity recovery cycles of human muscle action potentials and their sensitivity to ischemia. Muscle Nerve 2009;39:616–626. [DOI] [PubMed] [Google Scholar]
- 19. Z'Graggen WJ, ZGraggen WJ, Brander L, Tuchscherer D, Scheidegger O, Takala J, et al. Muscle membrane dysfunction in critical illness myopathy assessed by velocity recovery cycles. Clin Neurophysiol 2011;122:834–841. [DOI] [PubMed] [Google Scholar]
- 20. Tankisi A, Pedersen TH, Bostock H, Z'Graggen WJ, Larsen LH, Meldgaard M, et al. Early detection of evolving critical illness myopathy with muscle velocity recovery cycles. Clin Neurophysiol 2021;132:1347–1357. [DOI] [PubMed] [Google Scholar]
- 21. Ackermann KA, Bostock H, Brander L, Schröder R, Djafarzadeh S, Tuchscherer D, et al. Early changes of muscle membrane properties in porcine faecal peritonitis. Crit Care 2014;18. 10.1186/s13054-014-0484-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Z'Graggen WJ, Tankisi H. Critical Illness Myopathy. J Clin Neurophysiol 2020;37:200–204. [DOI] [PubMed] [Google Scholar]
- 23. Marrero H, Stålberg EV, Cooray G, Corpeno Kalamgi R, Hedström Y, Bellander BM, et al. Neurogenic vs. Myogenic Origin of Acquired Muscle Paralysis in Intensive Care Unit (ICU) Patients: Evaluation of Different Diagnostic Methods. Diagnostics (Basel) 2020;10. 10.3390/diagnostics10110966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am 2007;32:154–156. [DOI] [PubMed] [Google Scholar]
- 25. Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 1993;472:595–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derde S, Hermans G, Derese I, Güiza F, Hedström Y, Wouters PJ, et al. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit Care Med 2012;40:79–89. [DOI] [PubMed] [Google Scholar]
- 27. Druschky A, Herkert M, Radespiel‐Tröger M, Druschky K, Hund E, Becker CM, et al. Critical illness polyneuropathy: clinical findings and cell culture assay of neurotoxicity assessed by a prospective study. Intensive Care Med 2001;27:686–693. [DOI] [PubMed] [Google Scholar]
- 28. Cabanes‐Martinez L, Villadóniga M, González‐Rodríguez L, Araque L, Díaz‐Cid A, Ruz‐Caracuel I, et al. Neuromuscular involvement in COVID‐19 critically ill patients. Clin Neurophysiol 2020;131:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frithiof R, Rostami E, Kumlien E, Virhammar J, Fällmar D, Hultström M, et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID‐19 patients: A prospective study. Clin Neurophysiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Appleton RT, Kinsella J, Quasim T. The incidence of intensive care unit‐acquired weakness syndromes: A systematic review. J Intensive Care Soc 2015;16:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit‐acquired weakness in adults. Am J Respir Crit Care Med 2014;190:1437–1446. [DOI] [PubMed] [Google Scholar]
- 32. Weber‐Carstens S, Koch S, Spuler S, Spies CD, Bubser F, Wernecke KD, et al. Nonexcitable muscle membrane predicts intensive care unit‐acquired paresis in mechanically ventilated, sedated patients. Crit Care Med 2009;37:2632–2637. [DOI] [PubMed] [Google Scholar]
- 33. Haeseler G, Foadi N, Wiegand E, Ahrens J, Krampfl K, Dengler R, et al. Endotoxin reduces availability of voltage‐gated human skeletal muscle sodium channels at depolarized membrane potentials. Crit Care Med 2008;36:1239–1247. [DOI] [PubMed] [Google Scholar]
- 34. Koch S, Bierbrauer J, Haas K, Wolter S, Grosskreutz J, Luft FC, et al. Critical illness polyneuropathy in ICU patients is related to reduced motor nerve excitability caused by reduced sodium permeability. Intensive Care Med Exp 2016;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Z'Graggen WJ, Lin CS, Howard RS, Beale RJ, Bostock H. Nerve excitability changes in critical illness polyneuropathy. Brain 2006;129:2461–2470. [DOI] [PubMed] [Google Scholar]
- 36. Larsson L. Experimental animal models of muscle wasting in intensive care unit patients. Crit Care Med 2007;35:S484–S487. [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez B, Nansoz S, Cameron DR, Z'Graggen WJ. Is myopathy part of long‐Covid? Clin Neurophysiol 2021;132:1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Glucose and potassium variability


