Abstract
Background
Statins are a commonly used class of drugs, and reports have suggested that their use may affect COVID‐19 disease severity and mortality risk.
Objective
The purpose of this analysis was to determine the effect of discontinuation of previous atorvastatin therapy in patients hospitalized for COVID‐19 on the risk of mortality and ventilation.
Methods
Data from 146,413 hospitalized COVID‐19 patients were classified according to statin therapy. Home + in hospital atorvastatin use (continuation of therapy); home + no in hospital atorvastatin use (discontinuation of therapy); no home + no in hospital atorvastatin use (no statins). Logistic regression was performed to assess the association between atorvastatin administration and either mortality or use of mechanical ventilation during the encounter.
Results
Continuous use of atorvastatin (home and in hospital) was associated with a 35% reduction in the odds of mortality compared to patients who received atorvastatin at home but not in hospital (odds ratio [OR]: 0.65, 95% confidence interval [CI]: 0.59–0.72, p < .001). Similarly, the odds of ventilation were lower with continuous atorvastatin therapy (OR: 0.70, 95% CI: 0.64–0.77, p < .001).
Conclusions
Discontinuation of previous atorvastatin therapy is associated with worse outcomes for COVID‐19 patients. Providers should consider maintaining existing statin therapy for patients with known or suspected previous use.

INTRODUCTION
As the COVID‐19 pandemic has progressed, various drugs have been proposed for repurposing as potential treatment candidates. Candidates drawn from experience with other coronaviruses or mechanistic hypotheses have been tested for performance against COVID‐19 primarily in observational studies, with reports indicating that hundreds of different medications have been administered to these patients in attempt to identify agents that can potentially reduce the severity of COVID‐19 disease and modify patient outcomes. 1
Statins are among the most widely use class of drugs, and have been used since the late 1980s for their cholesterol‐lowering effects and impact on the risk of coronary heart disease. These drugs are also known to have anti‐inflammatory and immunomodulatory properties, and this has led to the repurposing of this drug for certain infectious diseases, including COVID‐19.The anti‐inflammatory effects of statins have been linked to possible decreases in influenza‐related hospitalizations and deaths. 2 Alternatively, or additionally, statin treatment may improve vascular and endothelial function in patients with severe COVID‐19 infection through upregulation of the ACE2 receptor. 3 , 4
Observational studies have indicated a possible association between statin intake and a beneficial effect on COVID‐19 clinical symptoms in susceptible populations. 5 , 6 Observational studies have linked statin use to lower mortality among patients with COVID‐19, however these studies highlighted possible variation in the effect based on statin dosage, patient characteristics, or COVID‐19 disease severity. 7 , 8 , 9 Yet not all studies have shown these beneficial effects on mortality or disease severity. 10 Current guidelines recommend continuation of statins in patients who were previously taking these medications, based primarily on the known effects of statins on ACE2. 11 Questions remain about the specific effects of the discontinuation of statin therapy in patients with COVID‐19. In this analysis, we used data from nearly 150,000 COVID‐19 patients admitted to facilities affiliated with a large healthcare system in the United States to compare the effect of receipt of continued or interrupted atorvastatin therapy on patient outcomes.
METHODS
Data were collected from the electronic health records from patients admitted to facilities affiliated with HCA Healthcare. Facilities within this system are located in 21 US states, with a concentration in the southern portion of the country. 12 Facilities are primarily community hospitals; the system also includes tertiary referral facilities and medical centers. Bed sizes range from 28 to 996 (mean = 279, median = 251). Collectively, these facilities provide care for 32 million patient encounters annually. Electronic health record data collected during the course of clinical care are collated in a standardized manner in an enterprise data warehouse for analysis purposes. To account for variation in data collection, we limited this analysis to facilities using the same electronic health record vendor (approximately 90% of facilities within the healthcare system).
Patients were included if ≥18 years of age, admitted as inpatients and discharged and final billed as of July 26, 2021. Only patients with laboratory‐confirmed COVID‐19 before or during the qualifying encounter were included. Other laboratory test data related to COVID‐19 disease progression were not available or inconsistently available and thus not used in this analysis.
Medication administration data were collected for all drugs within the statins class. Inpatient statin data were collected via medication administration records for the inpatient encounter. Statin use in‐hospital was defined as binary with a threshold of one dose. The majority of patients received atorvastatin (93%). To simplify the analysis and reduce confounding by specific cholesterol‐modifying medication, only patients who received atorvastatin were included in this analysis. Patients with documented receipt of any other statin class or fenofibrate class medication in the inpatient environment were excluded from the data set (Figure 1). To assess the effect of the discontinuation of existing atorvastatin therapy among admitted patients, we assessed home medication lists of the identified patient population and identified those patients with previous home use of atorvastatin. Home medication data were collected via medication reconciliation on admission and medication prescribed on discharge. There was a high degree of certainty regarding medications that were documented in these lists (i.e., medications that appear on the list reflect administrations), but these data on home medications can be incomplete for a variety of reasons. Thus, patients with hospital administration of statins but no documented home use may represent a mixed population of new users and current users and therefore this population was excluded in the current analysis. Duration of outpatient medication use is not captured at admission and therefore unavailable for this analysis.
Figure 1.
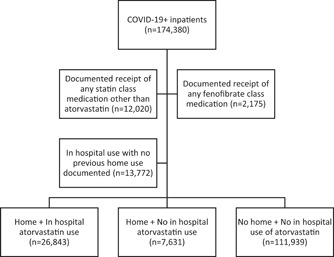
Diagram of analysis population
Logistic regression was performed to assess the association between atorvastatin administration and either mortality or use of mechanical ventilation during the encounter. Separate models were created to compare the effect of discontinuation of atorvastatin (home + in hospital vs. home + not in hospital) and the effect of these use patterns versus no known statin use. Variables included in the models were as follows: age at admission, gender, race (Asian/Black/White/other), ethnicity (Hispanic/not Hispanic/other), use of mechanical ventilation during encounter (for mortality model only), intensive care unit (ICU) at admission (at or within 8 h of admission time), Elixhauser score, coronary artery disease, lipidemias, atherosclerosis, body mass index, administration of select COVID‐19 related treatments (i.e., hydroxychloroquine, remdesivir, tocilizumab,), and administration of atorvastatin per groups defined above. The Elixhauser (van Walraven weighted) summary score was calculated using the comorbidity package for R Studio. 13 This method categorizing comorbidities of patients was based on the International Classification of Diseases diagnosis codes and includes 31 comorbidities. Each comorbid condition has an associated weight, and the sum of all weights results in a single comorbidity score per patient. Analyses were conducted using R Studio (version 1.4.1717).
RESULTS
A total of 174,380 COVID‐19+ inpatients were identified for possible inclusion in this analysis. After excluding patients who had in hospital or home use of statin class medications other than atorvastatin or fenofibrates, there were 160,185 eligible patients. To isolate the effect of discontinuation of atorvastatin from de novo use, patients with no documented atorvastatin use prior to admission (i.e., documented in list of home medications) but in hospital administration were excluded. The resulting analytical sample contained 146,413 patients comprised of three groups (Figure 1): Continuation of therapy (home + in hospital atorvastatin use); discontinuation of therapy (home + no in hospital atorvastatin use); no statins (no home + no in hospital atorvastatin use).
Patients with no statin use tended to be younger than those who received atorvastatin, with a difference in median age of 11 years (Table 1). In addition, patients with no statin use had a shorter median length of stay (7.8 vs. 9.6 days). There were fewer patients in the no statins group with cardiovascular comorbidities (coronary artery disease, lipidemias, atherosclerosis) or diabetes present on admission, as expected.
Table 1.
Demographics
| No statins (no home + no in hospital use of atorvastatin) (n = 111,939) | Continuous therapy (home + in hospital atorvastatin use) (n = 26,843) | Discontinued therapy (home + no in hospital atorvastatin use) (n = 7631) | |
|---|---|---|---|
| Age, median years (SD) | 60.0 (19.0) | 71.0 (12.56) | 70.0 (13.37) |
| Gender (%) | |||
| Female | 50.4% | 44.7% | 43.9% |
| Male | 49.6% | 55.3% | 56.1% |
| Race (%) | |||
| White | 57.7% | 63.9% | 61.7% |
| Black | 17.6% | 18.3% | 17.3% |
| Asian | 2.6% | 2.9% | 4.1% |
| Other | 18.4% | 12.3% | 14.0% |
| Unknown | 3.7% | 2.6% | 2.8% |
| Ethnicity (%) | |||
| Hispanic/Latinx | 29.0% | 20.9% | 24.0% |
| Not Hispanic/Latinx | 63.9% | 73.6% | 69.8% |
| Unknown | 7.1% | 5.5% | 6.1% |
| Elixhauser score, median (SD) | 5(8.6) | 10 (9.2) | 9 (9.4) |
| Diabetes (%) | 33.3% | 64.4% | 61.5% |
| Coronary artery disease (%) | 8.8% | 39.9% | 28.1% |
| Lipidemias (%) | 21.6% | 78.2% | 70.6% |
| Atherosclerosis (%) | 0.5% | 1.6% | 1.0% |
| BMI, median (SD) | 30.9 (8.8) | 30.4 (8.0) | 30.2 (8.1) |
| ICU at admission | 17.6% | 13.4% | 21.0% |
| Length of stay, median days (SD) | 4.8 (9.6) | 6.2 (9.5) | 5.2 (10.0) |
| Discharge disposition expired (%) | 11.8% | 9.2% | 15.0% |
| Highest level of service (%) | |||
| On vent | 11.0% | 10.7% | 15.5% |
| In ICU | 28.5% | 27.5% | 34.3% |
| Treatment receipt (%) | |||
| Remdesivir | 35.8% | 35.3% | 34.2% |
| Hydroxychloroquine | 3.4% | 3.2% | 3.4% |
| Tocilizumab | 2.5% | 1.8% | 2.4% |
| Steroids | 67.9% | 71.6% | 73.9% |
Abbreviations: BMI, body mass index; ICU, intensive care unit.
In the primary analysis, we investigated the odds of mortality for (1) continuous therapy (patients with in home and hospital atorvastatin) versus discontinued therapy (patients with in home use only), and (2) continuous or discontinuous statin therapy versus patients with no documented statin use. After adjusting for covariates, continuous use of atorvastatin was associated with a decrease in the odds of mortality in COVID‐19 patients in comparison to the discontinuation of atorvastatin therapy group (Table 2). Patients who were received continuous atorvastatin therapy had a 38% reduction in the odds of mortality compared to patients had discontinued therapy (odds ratio [OR]: 0.65, 95% confidence interval [CI]: 0.59–0.72, p < .001). In comparison to patients with no known statin use (Table 3), patients with continuous (home and in hospital) atorvastatin administration had 28% reduction in the odds of mortality (OR: 0.72, 95% CI: 0.68–0.77, p < .001) and patients with home use only had no change in the odds of mortality (OR: 1.01, 95% CI: 0.92–1.10).
Table 2.
Effect of discontinuation of atorvastatin on odds of mortality
| Category | Variable | OR (95% CI) | p Value |
|---|---|---|---|
| Age | 1.04 (1.04, 1.04) | <.001 | |
| Gender (ref = male) | |||
| Female | 0.74 (0.68, 0.81) | <.001 | |
| Race (ref = White) | |||
| Asian | 1.46 (1.15, 1.86) | <0.01 | |
| Black | 1.31 (1.15, 1.48) | <.001 | |
| Other | 0.95 (0.82, 1.1) | ns | |
| Unknown | 1.01 (0.6, 1.71) | ns | |
| Ethnicity (ref = not Hispanic/Latinx) | |||
| Hispanic/Latinx | 1.65 (1.46, 1.86) | <.001 | |
| Elixhauser score | 1.03 (1.03, 1.04) | <.001 | |
| Coronary artery disease | 1.12 (1.02, 1.22) | <.05 | |
| Lipidemias | 0.99 (0.89, 1.11) | ns | |
| Atherosclerosis | 0.66 (0.47, 0.92) | <.05 | |
| BMI | 1.01 (1.01, 1.02) | <.001 | |
| Mechanical ventilation | 14.7 (13.34, 16.21) | <.001 | |
| ICU at admission | |||
| COVID‐19 therapies | |||
| Hydroxychloroquine | 1.46 (1.2, 1.79) | <.001 | |
| Remdesivir | 1.7 (1.55, 1.87) | <.001 | |
| Tocilizumab | 1.51 (1.22, 1.87) | <.001 | |
| Statin treatment (ref = home only) | |||
| Home and hospital | 0.65 (0.59, 0.72) | <.001 | |
Abbreviations: BMI, body mass index; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Table 3.
Effect of atorvastatin therapy on odds of mortality
| Category | Variable | OR (95% CI) | p Value |
|---|---|---|---|
| Age | 1.05 (1.05, 1.06) | <.001 | |
| Gender (ref = male) | |||
| Female | 0.74 (0.71, 0.78) | <.001 | |
| Race (ref = White) | |||
| Asian | 1.25 (1.09, 1.43) | <.001 | |
| Black | 1.12 (1.05, 1.2) | <.001 | |
| Other | 1.06 (0.99, 1.13) | ns | |
| Unknown | 0.81 (0.62, 1.07) | ns | |
| Ethnicity (ref = not Hispanic/Latinx) | |||
| Hispanic/Latinx | 1.4 (1.32, 1.49) | <.001 | |
| Elixhauser score | 1.01 (1.01, 1.02) | <.001 | |
| Coronary artery disease | 1.01 (0.96, 1.08) | ns | |
| Lipidemias | 0.82 (0.78, 0.87) | <.001 | |
| Atherosclerosis | 0.67 (0.53, 0.84) | <.001 | |
| BMI | 1.02 (1.02, 1.03) | <.001 | |
| Mechanical ventilation | 15.4 (14.63, 16.22) | <.001 | |
| ICU at admission | 1.47 (1.4, 1.55) | <.001 | |
| COVID‐19 therapies | |||
| Hydroxychloroquine | 0.93 (0.84, 1.03) | ns | |
| Remdesivir | 1.14 (1.09, 1.19) | <.001 | |
| Tocilizumab | 1.32 (1.2, 1.46) | <.001 | |
| Statin treatment (ref = no known use) | |||
| Home and hospital | 0.72 (0.68, 0.77) | <.001 | |
| Home only | 1.01 (0.92, 1.1) | ns | |
Abbreviations: BMI, body mass index; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
In the secondary analysis, we investigated the odds of ventilation for the same comparisons as above. After adjusting for covariates, continuous use of atorvastatin was associated with a decrease in the odds of ventilation in COVID‐19 patients in comparison to the discontinuation of atorvastatin therapy (Table 4). Patients who were received atorvastatin both in the hospital and at home had a 30% reduction in the odds of ventilation compared to patients who received atorvastatin at home but not in hospital (OR: 0.70, 95% CI: 0.64–0.77, p < .001). In comparison to patients with no known statin use (Table 5), patients with continuous (home and in hospital) atorvastatin administration had 24% reduction in the odds of ventilation (OR: 0.76, 95% CI: 0.72–0.8, p < .001) and patients with home use only had no change in the odds of ventilation (OR: 1.06, 95% CI: 0.97–1.15, ns).
Table 4.
Effect of discontinuation of atorvastatin on odds of ventilation
| Category | Variable | OR (95% CI) | p Value |
|---|---|---|---|
| Age | 0.99 (0.99, 0.99) | <.001 | |
| Gender (ref = male) | |||
| Female | 0.79 (0.73, 0.86) | <.001 | |
| Race (ref = White) | |||
| Asian | 1.56 (1.27, 1.91) | <.001 | |
| Black | 1.14 (1.03, 1.27) | <.05 | |
| Other | 1.17 (1.04, 1.33) | <.05 | |
| Unknown | 0.66 (0.42, 1.03) | <.01 | |
| Ethnicity (ref = not Hispanic of Latino) | |||
| Hispanic or Latino | 1.56 (1.4, 1.73) | <.001 | |
| Elixhauser score | 1.07 (1.07, 1.08) | <.001 | |
| Coronary artery disease | 1.03 (0.95, 1.11) | ns | |
| Lipidemias | 1.03 (0.93, 1.13) | ns | |
| Atherosclerosis | 1.44 (1.11, 1.87) | <.01 | |
| BMI | 1.02 (1.02, 1.03) | <.001 | |
| ICU at admission | 2.94 (2.71, 3.19) | <.001 | |
| COVID‐19 therapies | |||
| Hydroxychloroquine | 3.26 (2.77, 3.84) | <.001 | |
| Remdesivir | 2.61 (2.41, 2.82) | <.001 | |
| Tocilizumab | 4.54 (3.79, 5.44) | <.001 | |
| Statin treatment (ref = home only) | |||
| Home and hospital | 0.70 (0.64, 0.77) | <.001 | |
Abbreviations: BMI, body mass index; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Table 5.
Effect of atorvastatin therapy on odds of ventilation
| Category | Variable | OR (95% CI) | p Value |
|---|---|---|---|
| Age | 0.99 (0.99, 0.99) | <.05 | |
| Gender (ref = male) | |||
| Female | 0.76 (0.73, 0.79) | <.001 | |
| Race (ref = White) | |||
| Asian | 1.44 (1.28, 1.61) | <.001 | |
| Black | 1.08 (1.02, 1.14) | <.05 | |
| Other | 1.19 (1.12, 1.26) | <.001 | |
| Unknown | 0.69 (0.55, 0.86) | <.001 | |
| Ethnicity (ref = not Hispanic/Latinx) | |||
| Hispanic/Latinx | 1.47 (1.4, 1.55) | <.001 | |
| Elixhauser score | 1.08 (1.08, 1.09) | <.001 | |
| Coronary artery disease | 0.91 (0.86, 0.96) | <.001 | |
| Lipidemias | 1.25 (1.19, 1.3) | <.001 | |
| Atherosclerosis | 1.33 (1.11, 1.6) | <.01 | |
| BMI | 1.03 (1.03, 1.03) | <.001 | |
| ICU at admission | 3.98 (3.82, 4.15) | <.001 | |
| COVID‐19 therapies | |||
| Hydroxychloroquine | 2.99 (2.76, 3.25) | <.001 | |
| Remdesivir | 2.38 (2.28, 2.48) | <.001 | |
| Tocilizumab | 3.62 (3.33, 3.94) | <.001 | |
| Statin treatment (ref = no known use) | |||
| Home and hospital | 0.76 (0.72, 0.8) | <.001 | |
| Home only | 1.06 (0.97, 1.15) | ns | |
Abbreviations: BMI, body mass index; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
DISCUSSION
In this retrospective analysis of nearly 150,000 COVID‐19 patients, we found that continuous receipt of atorvastatin was associated with a decrease in the risk of mortality and a decrease in the risk of ventilation as compared to patients who had discontinuation of previous atorvastatin therapy during the hospital encounter. This decrease in the risk of mortality or ventilation persisted for patients with continuous atorvastatin therapy when compared to patients with no known statin use.
Observational evidence has been growing to support in‐hospital use of statins in patients with COVID‐19 patients. Among over 13,000 COVID‐19 patients in China, approximately 1200 had in‐hospital statin use which was associated with a lower risk of all‐cause mortality; the mortality rate was 5.5% in the statin group and 6.8% in the nonstatin group. 9 Similar to our study group, the majority of the patients receiving statins in that study had received atorvastatin, however any in‐hospital use of statins was included in that analysis. A meta‐analysis of 13 observational studies found a 27% reduction in negative clinical outcomes, when adjusting for confounding variables, namely in‐hospital mortality or severe disease. 14 Another meta‐analysis confirmed the association of statin use with lower risk of all‐cause mortality and severe illness, but noted the limitations of sample size and heterogeneity between studies. 15 This suggests that further analysis and controlled trials are needed to better understand the potential of statins as a COVID‐19 treatment
The recently completed CORONADO study found that routine statin use was associated with increased morality in hospitalized COVID‐19 patients with type 2 diabetes mellitus. 16 In contrast, another retrospective cohort study found that while inpatient statin use was associated reduced mortality, especially among those with a history of diabetes mellitus. 17 In our analysis, we accounted for all comorbidities, including diabetes mellitus, through the Elixhauser score. When assessed separately, the presence of diabetes mellitus itself was associated with an increased odds of mortality in comparison with patients with no known statin use and no diabetes present on admission (OR: 1.24, 95% CI: 1.19–1.29). As we have previously shown that the management of hyperglycemia can affect mortality risk in COVID‐19 patients both with and without diabetes, the interaction of this with statins use is an area for future research. 18
Our analysis confirmed the observational evidence in support of the continued use of statins in COVID‐19 patients. In comparison to no statin use, patients with any atorvastatin use tended to be older with more severe comorbidities, which are known risk factors for more severe COVID‐19 disease and mortality. Rates of the major medications used for treatment of COVID‐19 at the time of this analysis were similar between the groups, suggesting that the use of statins was likely not a major factor in treatment decision‐making. Additional controlled studies will be needed to further understand the risk of discontinuation of statin therapy in hospitalized COVID‐19 patients and the potential benefit to beginning therapy in those who are statin‐naïve.
The association of the discontinuation of statins with the risk of worse outcomes is not unprecedented. Perioperative exposure to atorvastatin has been associated with a reduction in the risk of mortality and certain complications. 19 , 20 , 21 Furthermore, patients who stopped statin therapy after a myocardial infarction had a higher risk of mortality than patients who never used statins. 22 Recommendations from the American College of Cardiology/American Heart Association favor the continuation of statin therapy for patients undergoing surgery to reduce the risk of cardiac complications. 23 As there is an increased occurrence of cardiovascular events in COVID‐19 patients, continuous stain therapy may be providing protective effects through pleiotropic benefits on inflammation, oxidative stress, coagulation, and endothelial function. 24
In our data set, we attempted to identify those patients whose in‐hospital administration of atorvastatin was a continuation of their ongoing use of this medication for other conditions. Due to the nature of our data, we were limited to medication lists reported by the patient or their representative at admission. Such lists are known to often be incomplete or not supplied for a variety of reasons, such as communication challenges or patient condition at admission, and thus these results should be interpreted with caution. We excluded patients with in hospital atorvastatin administration but no previous in‐home use as we could not distinguish between de novo atorvastatin administration or a lack of documentation of home medication in these patients. In contrast, those who had indicated home use of atorvastatin but had no in‐hospital use are highly likely to represent a discontinuation of previous therapy, as medication administration data in our system have been independently verified for both research and billing purposes.
Our data set reflects the overall population in that those patients who had statin administration were older on average than those patients with no known statin exposure. Although this variable was taken into account as part of the regression, the younger age of the no‐statin group would favor reduced mortality, as age has been consistently identified as a risk factor for COVID‐19 mortality. The observation that statin use was associated with reduced mortality compared to no‐statin use could argue that our finding might have been even greater if the ages of these two groups was comparable.
Other limitations of this analysis are those common to studies using retrospective data, namely a lack of control of confounding variables and the inability to interpret a relationship beyond correlation. We simplified our analysis to atorvastatin‐only in order to control for any potential differences between individual medications; additional work is underway to confirm these findings for other commonly used statins. We also did not investigate how atorvastatin dose, timing, or days of therapy could affect mortality risk as these were beyond the scope of the current analysis; additional work on these questions is also underway.
Additional limitations are related to the nature of the data source itself. While the HCA enterprise data warehouse is powerful, it is limited in the fact that it receives data streams from >185 facilities, nearly all of which developed their EHR systems independently. A decade of effort to standardize/normalize data has allowed for much to be gained from this data source. However, local variation in data‐related processes is a known issue and there are still a few areas that are not yet at the quality needed for research, such as laboratory results, and thus unavailable for analysis in this way.
In total, our analysis supports the continuation of statin therapy for COVID‐19 patients. Providers should consider maintaining existing statin therapy for patients with known or suspected previous use. Additional study will be needed to elucidate the potential benefit of statin treatment among COVID‐19 patients with no history of previous use.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. The authors would like to acknowledge Charity Loput, PharmD.
Andrews L, Goldin L, Shen Y, et al. Discontinuation of atorvastatin use in hospital is associated with increased risk of mortality in COVID‐19 patients. J Hosp Med. 2022;17:169‐175. 10.1002/jhm.12789
REFERENCES
- 1. Fajgenbaum DC, Khor JS, Gorzewski A, et al. Treatments administered to the first 9152 reported cases of COVID‐19: a systematic review. Infect Dis Ther. 2020;9(3):435‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory‐confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 3. Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID‐19 infection. mBio. 2020;11(2):e00398‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wösten‐van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin‐(1‐7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618‐627. [DOI] [PubMed] [Google Scholar]
- 5. De Spiegeleer A, Bronselaer A, Teo JT, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21(7):909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3(12):e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lohia P, Kapur S, Benjaram S, Mir T. Association between antecedent statin use and severe disease outcomes in COVID‐19: A retrospective study with propensity score matching. J Clin Lipidol. 2021;15(3):451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Memel ZN, Lee JJ, Foulkes AS, Chung RT, Thaweethai T, Bloom PP. Statins are associated with improved 28‐day mortality in patients hospitalized with SARS‐CoV‐2 infection. J Infect Dis. 2021;225(1):19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X‐J, Qin J‐J, Cheng X, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32(2):176‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariyanto TI, Kurniawan A. Statin therapy did not improve the in‐hospital outcome of coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr. 2020;14(6):1613‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Institutes of Health . Considerations for certain concomitant medications in patients with COVID‐19. 2021. Accessed August 11, 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/concomitant-medications/
- 12. HCA Healthcare . HCA Healthcare Fact Sheet. 2021. Accessed November 30, 2021. https://hcahealthcare.com/util/documents//2021-Jun-HCA-Healthcare-Fact-Sheet-a.pdf
- 13. Gasparini A. Computing Comorbidity Scores. 2020. Accessed November 21, 2021. https://cran.r-project.org/web/packages/comorbidity/comorbidity.pdf
- 14. Scheen AJ. Statins and clinical outcomes with COVID‐19: meta‐analyses of observational studies. Diabetes Metab. 2020;47(6):101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kow CS, Hasan SS. The association between the use of statins and clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. Am J Cardiovasc Drugs. 2021;134:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cariou B, Goronflot T, Rimbert A, et al. Routine use of statins and increased COVID‐19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2021;47(2):101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lohia P, Kapur S, Benjaram S, et al. Statins and clinical outcomes in hospitalized COVID‐19 patients with and without diabetes mellitus: a retrospective cohort study with propensity score matching. Cardiovasc Diabetol. 2021;20(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morse J, Gay W, Korwek KM, et al. Hyperglycaemia increases mortality risk in non‐diabetic patients with COVID‐19 even more than in diabetic patients. Endocrinol Diabetes Metab. 2021;4(4):e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med. 2017;177(2):231‐242. [DOI] [PubMed] [Google Scholar]
- 20. Ma B, Sun J, Diao S, Zheng B, Li H. Effects of perioperative statins on patient outcomes after noncardiac surgery: a meta‐analysis. Ann Med. 2018;50(5):402‐409. [DOI] [PubMed] [Google Scholar]
- 21. Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta‐analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61(2):519‐532. [DOI] [PubMed] [Google Scholar]
- 22. Daskalopoulou SS, Delaney JAC, Filion KB, Brophy JM, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population‐based study. Eur Heart J. 2008;29(17):2083‐2091. [DOI] [PubMed] [Google Scholar]
- 23. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2014;130(24):e278‐e333. [DOI] [PubMed] [Google Scholar]
- 24. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]


