Abstract
We assessed the circulation of severe acute respiratory syndrome coronavirus‐2 variants amongst vaccinated military personnel in Bogotá, Colombia to evaluate the mutations of certain variants and their potential for breakthrough infection in vaccinated subjects. We observed that in vaccinated individuals the most frequent infecting lineage was Mu (B.1.621 and B.1.621.1). The above is possibly associated with specific mutations that confer it with vaccine‐induced immune escape ability. Our findings highlight the importance of how genomic tracking coupled with epidemiological surveillance can assist in the study of novel emerging variants (e.g., Omicron) and their impact on vaccination efforts worldwide.
Keywords: RT‐PCR, SARS‐CoV‐2, sequencing, variants
1. INTRODUCTION
After the first reports of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2)‐associated pneumonia in Wuhan, China, in 2019 the coronavirus disease (COVID‐19) has spread globally, causing over five million deaths worldwide. 1 Colombia ranks fourth as the country with the highest number of COVID‐19‐related deaths in Latin America, only preceded by Brazil, Mexico, and Peru. 2 To overcome the effects of the pandemic, a number of vaccine alternatives have been implemented, including traditional live attenuated/killed virus vaccines, viral vectored, and recombinant/peptide vaccines, as well as more novel approaches such as messenger RNA (mRNA)‐based vaccines. 3 Nevertheless, the ever‐evolving nature of the virus has led to the emergence of variants with changing mutational landscapes conferring the ability to evade neutralizing antibodies and possibly compromise current vaccine effectiveness. 4
Most countries throughout the world have concentrated efforts on achieving herd immunity by maintaining steadily increasing vaccination rates. In Latin America, as of December 2021, estimates indicate that about half of the population has completed vaccination schemes. 5 Similarly, 49% (25.028.663 people) of Colombia's population have already completed their vaccination scheme, while 24% (31.457.653 people) have received their first dose. 6 For this country, vaccines have shown the efficacy of 69.9% in preventing hospitalizations and 79.4% in COVID‐19‐related deaths. 7 Even though all vaccines have proven effective against SARS‐CoV‐2, studies have demonstrated variability in their effectiveness, thus translating into partial protection against infection based on the vaccine formulation, dose, and the infecting virus variant. 8
In particular, mutations in the antigenic regions of the receptor‐binding protein are important in determining transmission capacity and immune evasion. Taking into consideration the immune escape capacity of certain SARS‐CoV‐2 variants and their potential for breakthrough infection in vaccinated subjects, we sequenced nasopharyngeal samples from COVID‐19‐positive patients by quantitative polymerase chain reaction (qPCR) aiming to track the circulation of SARS‐CoV‐2 variants amongst vaccinated military personnel in Bogotá, Colombia.
2. METHODS
We sequenced 57 nasopharyngeal swabs taken during the third epidemiological wave (between May and August 2021) from COVID‐19‐positive vaccinated military personnel by qPCR using Oxford Nanopore. Nanopore sequencing has been broadly used as a reliable system to support the current COVID‐19 pandemic due to its ability to generate long reads and rapid sequencing for an effective public health response. 9 The vaccination status of the patients corresponded to first dose (n = 48), complete scheme (n = 8), and single dose (n = 1). Vaccine formulations administered to individuals with first dose included Pfizer (n = 27, 56.2%) and Sinovac (n = 21, 43.7%). Second dose vaccination also included Pfizer (n = 1, 12.5%) and/or Sinovac (n = 7, 87.5%), while those receiving single dose corresponded to individuals vaccinated with the Janssen vaccine. We also evaluated the days elapsed between vaccinations and positive SARS‐CoV‐2 reverse transcription‐polymerase chain reaction (RT‐PCR) result, as well as the relationship between vaccine types and the identified lineages.
3. RESULTS
Under the assumption that vaccination is equally effective against all variants, we expected a similar proportion of cases with either variant in vaccinated people. Our results showed that in vaccinated individuals (regardless of the number of doses), the most frequent infecting lineage was Mu (B.1.621 and B.1.621.1) (85.96%, n = 49), followed by Lambda (C.37) (5.36%, n = 3), Gamma (P.1) (5.36%, n = 3) and B.1 (1.79%, n = 1) (Figure 1A,B). Then, considering that the effectiveness of the vaccines depends not only on the vaccine type but also on the infecting variant, we evaluated the relationship between these variables. We did not find a significant relationship between vaccine types and the identified lineages (Pearson's χ 2 test, p value = 0.2932). However, we were surprised by the fact that for both types of vaccines, the predominant lineage associated with breakthrough infection was Mu.
Figure 1.
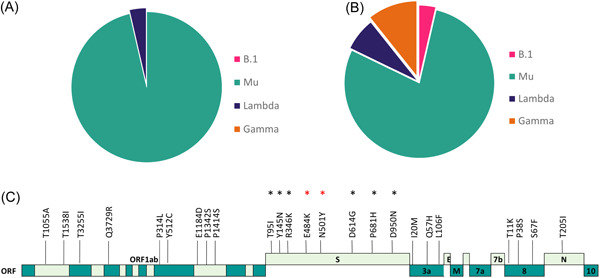
(A) SARS‐CoV‐2 lineages in patients vaccinates with Pfizer and (B) Sinovac. (C) Mutational profile of genomes of Mu variant isolated from military personnel vaccinated. In black asterisks, mutations of interest are shared with other variants of concerns and variants of interest. In red, mutations involved in immune escape.
We then evaluated the days elapsed between vaccinations and positive SARS‐CoV‐2 RT‐PCR results. For Sinovac (median 19.5 days), the number of days was greater than with Pfizer (10.5 days) (Wilcoxon's rank‐sum test, p value = 0.00177). No differences were evident when assessing the mean days between the evaluated vaccines and second dose status (Welch two‐sample t test, p = 0.5335), despite the mean being higher in the Pfizer group (30.75 days) than in Sinovac (22 days).
4. DISCUSSION
The monitoring of variants with immune evasion capacity is essential in evaluating the effectiveness of vaccination against these variants of the virus and as a strategy to tackle this pandemic. Our results showed that in vaccinated individuals, the most frequent infecting variant was Mu, probably associated with its vaccine‐induced immune escape ability. 10 Mutations in the spike protein such as T19R, L452R, T478K, D614G, and P681R (present in VOCs as delta) have been linked to the potential reduction in vaccine efficacy. 8 In fact, nine mutations in the Spike protein of Mu variant have been reported to be shared with other VOI and VOC, namely: E484K, N501Y, D614G, T95I (shared with Iota and some with Omicron), P681H (shared with Alpha), D950N (shared with Delta), and the most novel, Y144S, Y145N, and R346K. 11 Some of these mutations were found here and are known to affect the receptor recognition site of the spike protein, a region known to be a key target for neutralizing antibodies (Figure 1C). For example, it is known that the N501Y increases binding to the angiotensin‐converting enzyme 2 receptor, while E484K mutation confers resistance to various monoclonal antibodies while demonstrating a slight decrease in the efficacy of certain vaccines. 10 In this regard, an investigational study was carried out in the United Kingdom by Lopez Bernal et al. 12 to compare vaccine effectiveness against symptomatic disease caused by SARS‐CoV‐2 Delta and Alpha variants. Results demonstrated that effectiveness was greater against the Delta variant after receiving two doses of the BNT162b2 vaccine (88%) compared to the ChAdOx1 nCoV‐19 vaccine (67%). When assessing performance against the Alpha variant under the same conditions, vaccine efficacy after two doses of the BNT162b2 vaccine was 93.7% as opposed to ChAdOx1 nCoV‐19 vaccine, which revealed a 74.5% efficacy, thus highlighting the importance of promoting vaccine coverage with two doses in those populations with concurrent circulation of diverse virus variants. 12
When evaluating the days elapsed between vaccinations and positive SARS‐CoV‐2 RT‐PCR results, we found that for Sinovac the number of days was greater than with Pfizer. These findings would suggest that vaccine type may somehow impact SARS‐CoV‐2 patterns of infection, most probably as a result of the vaccine strategy employed. Yet, due to the observational nature of our work, further studies are needed to validate this correlation. mRNA‐based vaccines such as Pfizer are known to elicit rapid increases IgG and IgA levels in serum. However, the level of IgA falls below IgG levels, which has shown to be less effective for neutralizing variants harboring mutations in the spike such as P681H (present in Mu). 12 Although Pfizer has shown higher efficacy (95%) in preventing symptomatic disease than Sinovac (~50.65%), results may vary between regions as a reflection of the impact of new variants. 13 , 14 For example, a study carried out by Serrano‐Coll et al. 15 found that Sinovac showed 94.3% effectiveness against mild disease while 99.9% against severe infection in a rural population of the Colombian Amazon. Although the study mentioned above does not evaluate other vaccines and the SARS‐CoV‐2 variants infecting the people, it is interesting that the high efficacy in preventing symptomatic disease is likely associated with the achievement of herd immunity through mass vaccination. In contrast, no differences were evident when assessing the mean days between the evaluated vaccines and second dose status (Welch two‐sample t test, p = 0.5335), despite the mean being higher in the Pfizer group (30.75 days) than in Sinovac (22 days). However, an interesting finding of our study when contrasting vaccine efficacy was the observation that breakthrough infection to a diverse number of lineages (B.1, Mu, Lambda, Gamma) (Figure 1A) was more common in individuals vaccinated with the Sinovac vaccine than with Pfizer, who only allowed infection by two lineages (Mu and Lambda) (Figure 1B). Why Sinovac was permissive to a larger number of variants of breakthrough infection remains to be deciphered. However, a possible hypotheses to this phenomenon relies on the more stable immunogenic nature of mRNA‐based vaccines when compared to the more ancestral CZ02 strain‐inactivated based vaccine.
To date, Colombia has experienced a series of three COVID‐19 waves characterized by an increase in the number of cases, as well as the circulation of variants of interest/concern such as Alpha (B.1.1.7), Gamma (P. 1), Delta (B.1.617.2), Mu (B.1.621), and recently Omicron (B. 1.1. 529). 6 However, since its first appearance back in January 2021, Mu stood out for its dominance over other circulating variants, until it was formally recognized as the main driving force behind the third epidemiological wave affecting the country. 11 , 16 This, prompted efforts to increase vaccination rates in Colombia allowing the administration of over 58 million doses of vaccines by December 2021. 2 Our findings suggest that Mu can not only reinfect but also circumvent vaccine‐acquired immunity, possibly by carrying the N501Y and E484K mutations, which have been linked not only to increased transmissibility but also to the vaccine and infection‐induced immune evasion capabilities. Although these results are observational and limited by the inability to quantify neutralizing antibodies in these patients at that time, the higher prevalence of Mu during a period in which the number of administered vaccines was low underscores the importance of vaccination as a strategy to tackle this pandemic, while reminding us that natural immunity as well as vaccine‐induced immunity does not elicit absolute protection against COVID‐19. This also opens the debate about how key mutations associated with increased transmissibility versus immune escape may impact COVID‐19 epidemiology. Our results show that spike mutations associated with immune escape can adversely influence the absolute effectiveness of vaccines. The recent emergence of the Omicron variant, now reaching its period of epidemic effervescence in Colombia, seems to have stereotypically followed the path of Mu, thus emphasizing the notion of how emerging variants may quickly evolve to escape vaccine and natural infection‐induced immunity. In fact, with more than 30 mutations in key regions of its genome, Omicron stands out as a risk against the performance of current vaccines against COVID‐19. In this sense, the data suggest that to protect people against serious diseases, at least in the short term, a third dose, or booster, of vaccines based on messenger RNA is necessary. 17 However, even with a third dose, studies show effectiveness against hospitalization drops from 92% to 83% in just 10 weeks, raising the question of the need for an Omicron vaccine. 18 In this respect, our findings highlight the importance of how genomic tracking coupled with epidemiological surveillance can assist in the study of novel emerging variants and their impact on vaccination efforts. Even so, against infection by SARS‐CoV‐2, vaccines have proven to be an effective strategy in reducing hospitalizations and deaths, even against infections caused by new variants (e.g., Omicron). However, it is essential to monitor the appearance of new mutations and how they can contribute to evading neutralizing antibodies to guide the best strategies against this pandemic.
AUTHOR CONTRIBUTIONS
Juan David Ramírez and Marina Muñoz designed the study. Angie L. Ramirez, Nicolas Luna, Luz H. Patiño, Sergio Castañeda, and Nathalia Ballesteros performed the experiments. Julie Perez, Camilo A. Correa‐Cárdenas, Maria Clara Duque, Claudia Mendez, and Carolina Oliveros provided the samples. Angie L. Ramirez, Juan David Ramírez, and Alberto Paniz‐Miondolfi wrote the manuscript. All authors read and approved the final version of the manuscript,
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We thank the Dirección de Investigación e Innovación from Universidad del Rosario. This project was funded by the Universidad del Rosario in the framework of its strategic plan RUTA2025. Thanks to the President and the University Council for leading the strategic projects.
Ramirez AL, Luna N, Patiño LH, et al. Impact of SARS‐CoV‐2 Mu variant on vaccine effectiveness: a comparative genomics study at the peak of the third wave in Bogota, Colombia. J Med Virol. 2022;94:3988‐3991. 10.1002/jmv.27808
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information Material of this article.
REFERENCES
- 1. COVID‐19 coronavirus pandemic . Worldometer. December, 2021. Accessed December 5, 2021. https://www.worldometers.info/coronavirus/
- 2.Número de personas fallecidas a causa del coronavirus (COVID‐19) en América Latina y el Caribe al 2 de diciembre de, por país. Statista. December, 2021. Accessed December 5, 2021. https://es.statista.com/estadisticas/1105336/covid-19-numero-fallecidos-america-latina-caribe/
- 3. Tatsi EB, Filippatos F, Michos A. SARS‐CoV‐2 variants and effectiveness of vaccines: a review of current evidence. Epidemiol Infect. 2021: 1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porcentaje de vacunados y dosis administradas contra el coronavirus (COVID‐19) en América Latina y el Caribe a 2 de diciembre de, por país. Statista . December, 2021. Accessed December 5, 2021. https://es.statista.com/estadisticas/1258801/porcentaje-y-numero-vacunados-contra-covid-19-en-latinoamerica-por-pais/
- 6. Covid en Colombia . INS. December, 2021. Accessed December 5, 2021. https://www.ins.gov.co/Noticias/Paginas/coronavirus-casos.aspx
- 7.Efectividad de las vacunas contra el COVID‐19 en Colombia. MinSalud. December, 2021. Accessed December 5, 2021. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/MET/estudio-efectividad-vacunas-colombia-msps.pdf
- 8. Harder T, Koch J, Vygen‐Bonnet S, et al. Efficacy and effectiveness of COVID‐19 vaccines against SARS‐CoV‐2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26(28):2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freed NE, Vlková M, Faisal MB, Silander OK. Rapid and inexpensive whole‐genome sequencing of SARS‐CoV‐2 using 1200 bp tiled amplicons and Oxford Nanopore Rapid Barcoding. Biol Methods Protoc. 2020;5(1):bpaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahimi F, Kamali N, Bezmin Abadi AT. The Mu strain: the last but not least circulating ‘variant of interest’ potentially affecting the COVID‐19 pandemic. N Engl J Med. 2021;385:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gutierrez B, Márquez S, Prado‐Vivar B, et al. Emergence of lineage B. 1.621 in Latin America and the Caribbean. Virologic; [Google Scholar]
- 12. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid‐19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;385:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinovac says COVID‐19 vaccine effective in preventing hospitalization, death . Reuters. December, 2021. Accessed December 5, 2021. https://www.reuters.com/article/us-health-coronavirus-sinovac-biotech-idUSKBN2A52Q6
- 14. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID‐19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serrano‐Coll H, Miller H, Guzmán C, et al. Effectiveness of the CoronaVac® vaccine in a region of the Colombian Amazon, was herd immunity achieved? Trop Dis Travel Med Vaccines. 2022;8(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laiton‐Donato K, Franco‐Munoz C, Alvarez‐Diaz DA, et al. Characterization of the emerging B. 1.621 variant of interest of SARS‐CoV‐2. medRxiv. 2021;95:105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waltz E. Does the world need an Omicron vaccine? What researchers say. Nature. 2022;602(7896):192‐193. [DOI] [PubMed] [Google Scholar]
- 18. SARS‐CoV‐2 variants detected in the UK . UK Health Security Agency. December, 2021. Accessed December 5, 2021. https://www.gov.uk/government/news/covid-19-variants-identified-in-the-uk#:%7E:text=In%20the%20UK,%2038%20cases,of%20these%20are%20in%20France
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information Material of this article.


