Abstract
Infection is associated with the occurrence, recurrence, and progression of atrial fibrillation (AF), and is also closely related to poor prognosis. However, studies of the relationship between infectivity and severe complications of coronavirus infectious disease‐19 (COVID‐19) with a history of AF are limited. To estimate infectivity and severity of complications in COVID‐19 patients with a history of AF, this study was done. From the Korean nationwide COVID‐19 dataset, 212 678 participants with at least one severe acute respiratory syndrome coronavirus 2 (COVID‐19) test were included between January 1 and June 4, 2020. AF was defined according to at least two outpatient hospital visits or one admission with an ICD‐10 code of “I48” before the COVID‐19 test. To investigate the association of AF with infectivity and severe complications of COVID‐19, 1:4 ratio propensity score matching (PSM) was performed. Severe complications of COVID‐19 were defined as a composite outcome of mechanical ventilation, intensive care unit admission, and death within 2 months after COVID‐19 diagnosis. Among 212 678 participants who underwent the COVID‐19 test, there were 7713 COVID‐19 positive patients. After PSM, COVID‐19 PCR positivity did not show a significant difference according to the presence of AF (odds ratio [OR]: 0.79, 95% confidence interval [CI]: [0.60–1.04]). Of 7713 COVID‐19 patients, 62 (0.8%) had a history of AF and severe complications occurred in 444 (5.7%) patients. After PSM, AF was associated with the development of severe complications (OR: 2.04, 95% CI: [1.10–3.79]) and mortality (OR: 2.09, 95% CI: [1.01–4.31]) of COVID‐19. We found that AF was associated with an increased risk of severe complications in COVID‐19 infected patients.
Keywords: atrial fibrillation, COVID‐19, infectivity, intensive care unit, mechanical ventilation, mortality, prognosis
RESEARCH HIGHLIGHTS
It has been known that the presence of comorbidities is associated with worse outcomes in COVID‐19 infection.
Based on the Korean nationwide COVID‐19 dataset, 212 678 participants underwent the COVID‐19 real‐time reverse transcription‐polymerase chain reaction test (January 1–June 4, 2020), we evaluated the infectivity and severe complications of COVID‐19 according to the presence of atrial fibrillation.
COVID‐19 positivity rate did not differ according to the presence of atrial fibrillation.
Among patients infected with COVID‐19, the presence of atrial fibrillation was significantly associated with increased risk for severe complications and mortality within 2 months after COVID‐19 diagnosis.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a positive sense, single‐stranded RNA coronavirus in genetic sequence, which is contagious to humans and is the cause of coronavirus infectious disease‐19 (COVID‐19). Most patients infected with COVID‐19 complain of asymptomatic or mild flu‐like symptoms, but respiratory insufficiency or hypoxemia is also accompanied in some cases. 1 Additionally, a COVID‐19 infection may require mechanical ventilation treatment, hospitalization in an intensive care unit (ICU), or may lead to death. 1 Therefore, it is essential to differentiate and treat patients likely to develop severe complications due to COVID‐19. Until recently, several studies have suggested factors, such as diabetes mellitus, heart failure, cancer, allergic diseases, and chronic kidney diseases that are associated with a poor prognosis after COVID‐19 infection. 2 , 3 , 4
Infection is associated with the occurrence, recurrence, and progression of atrial fibrillation (AF), and is also closely related to poor prognosis. 5 , 6 Recently, a relationship between COVID‐19 infection and AF has been proposed, 7 with the likelihood of AF being increased in COVID‐19 patients. 8 In addition, there have been some meta‐analyses on the mortality in COVID‐19 patients with or without AF. 9 , 10 , 11 However, most individual studies are retrospective, some meta‐analyses did not use the adjusted odds ratio, which could cause biased results. In addition, other important severe complications of COVID‐19 such as mechanical ventilation or ICU admission have rarely been investigated and statistical methods to reduce bias such as propensity score matching (PSM) have rarely been applied in previous studies.
Therefore, the purpose of our study was to investigate the relationship of AF with infectivity and severe complications of COVID‐19, including mechanical ventilation, ICU admission, and mortality, in a nationwide population‐based COVID‐19 dataset.
2. METHODS
2.1. Study design and participants
This is a retrospective observational study using a nationwide COVID‐19 dataset from South Korea. South Korea has a public single‐payer health insurance system, the National Health Insurance Service (NHIS). The NHIS had a national health insurance claims database of hospital visits, diagnoses (recorded by International Statistical Classification of Diseases and Related Health Problems [ICD]‐10 codes), medical procedures, prescriptions, and mortality for the whole South Korean population. 12 , 13 In the face of the COVID‐19 pandemic, the Korea Disease Control and Prevention Centers and NHIS released a nationwide COVID‐19 dataset consisting of Koreans who underwent real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) assays of nasal and pharyngeal swabs to COVID‐19 from January 1 to June 4, 2020 for academic research. 14 The RT‐PCR assays followed World Health Organization guidelines and were validated by the Korea Disease Control and Prevention Centers. 15
Due to its nature as a retrospective analysis based on a fully anonymized dataset, this study was approved by the Institutional Review Board of our institution (Seoul Hospital Ewha Woman's University College of Medicine 2020‐10‐021), and the requirement for informed consent was waived.
2.2. Atrial fibrillation
In this study, AF was defined by at least two outpatient hospital visits or one admission with the diagnostic code of “I48” before the COVID‐19 RT‐PCR test. 16 The diagnostic accuracy of AF using this algorithm was 94.1% of the positive predictive value. 16 , 17
2.3. Study outcomes
The current study evaluated the risk of infectivity to COVID‐19 and prognosis after COVID‐19 infection by AF. In an analysis for COVID‐19 infectivity, the study outcome was the positivity in patients who underwent the COVID‐19 test. In an analysis for COVID‐19 prognosis, the study outcome was the development of severe complications in COVID‐19 patients. Severe complications of COVID‐19 were defined as a composite of mechanical ventilation, admission to the ICU, and mortality during the 2‐month period after COVID‐19 diagnosis. Mechanical ventilation was identified using claim codes of mechanical ventilation (M5850, M5857, M5858, and M5860). 18 Admission to the ICU was defined using the related claims codes (AH110, AH150, AH180‐95 AH190‐5, AH210, AH250, AH280‐9, AH28A, AH290‐9, AH380‐9, AH38A, AH390‐9, AH501, AJ001–AJ011, AJ020‐1, AJ031, AJ100‐390, AJ2A0, AJ3A0, AJ500‐590, V5100, V5200, V5210‐20, V5500‐5520). Mortality and time of death data were provided by NHIS and were previously validated. 19 , 20
2.4. Covariates
We acquired demographic information regarding sex, age, and household income level (tertiles). In the nationwide COVID‐19 dataset, age is presented in 10‐year intervals to preserve privacy. Therefore, we divided age categories by the median value and dichotomized data based on a cutoff age of 60 years. The definition of comorbidities regarding hypertension, diabetes mellitus, stroke, heart failure, coronary artery disease, asthma, chronic kidney disease, and malignancy was described in Supporting Information Methods. 21
2.5. Statistical analysis
To investigate the association of AF with infectivity and severe complications of COVID‐19, 1:4 ratio PSM was performed with the Greedy nearest‐neighbor algorithm to compare samples with and without AF, to balance the baselines of both groups and to reduce potential confounding. To determine whether PSM was appropriate, standardized mean differences were used. If the standardized mean differences were less than 0.1, we considered PSM to be appropriate.
Logistic regression analysis was performed to investigate infectivity and severe complications of COVID‐19 according to AF history in unmatched cohorts and cohorts balanced after PSM. The results are expressed as odds ratio (OR) and 95% confidence interval (CI). Secondary outcome analysis was performed for individual components of severe complications of COVID‐19 including mechanical ventilation, admission to ICU, and death.
There are prior reports that smoking is an important risk factor for worse outcomes of COVID‐19. 22 Therefore, we performed subgroup analysis with 3934 COVID‐19 infected patients whose smoking history (current smoking or not) was available from the national health screening check‐up program results (2015–2018). 12 Of them, 1:4 ratio PSM was performed to match patients with AF and without AF, and current smoking was additionally included as a covariate in the PSM.
Statistical analyses were executed using R software, version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria), and SAS 9.4 version (SAS Inc.). Two‐sided p values less than 0.05 were considered significant.
3. RESULTS
3.1. COVID‐19 infectivity by AF
In the national COVID‐19 dataset, there were 212 678 individuals aged older than 20 years who underwent at least one SARS‐CoV‐2 test between January 1 and June 4, 2020 (Figure 1). Among 212 678 individuals, 208 958 individuals without AF, and 3720 (1.7%) individuals with AF were identified in the unmatched cohort (Table 1). There were 7713 with positivity for the COVID‐19 RT‐PCR test. Then, we applied 1:4 ratio PSM for 14 880 individuals without AF and 3720 individuals with AF, which were appropriately matched (Figure 1 and Table 1). After PSM, COVID‐19 PCR positivity did not show a significant difference according to AF history (OR: 0.79, 95% CI: (0.60–1.04), p = 0.087) (Table 2).
Figure 1.
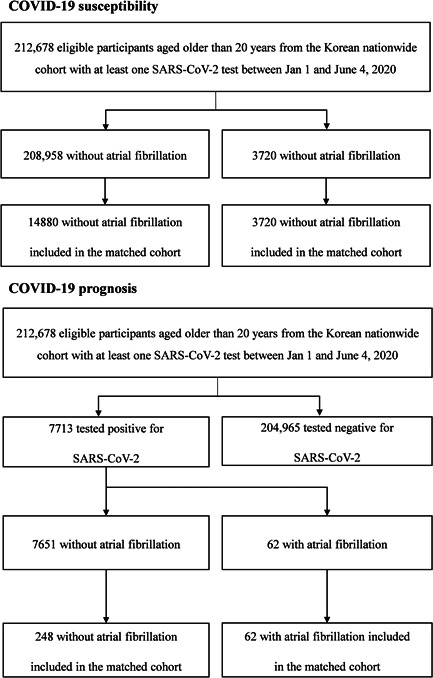
Study flow of a nationwide cohort study of COVID‐19 and atrial fibrillation between January 1 and June 4, 2020. Atrial fibrillation was identified by at least two outpatient hospital visits or one admission with International Classification of Diseases 10th Revision (ICD‐10) code of “I48” before COVID‐19 real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) test
Table 1.
Baseline characteristics of participants who underwent COVID‐19 test with and without atrial fibrillation before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | SMDa | ||
|---|---|---|---|---|---|
| Without AF | With AF | Without AF | With AF | ||
| N | 208 958 | 3720 | 14 880 | 3720 | |
| Sex, male | 97 856 (46.83) | 2182 (58.66) | 8770 (58.94) | 2182 (58.66) | 0.006 |
| Age, years | 0.006 | ||||
| <60 | 141 001 (67.48) | 428 (11.51) | 1739 (11.69) | 428 (11.51) | |
| ≥60 | 67 957 (32.52) | 3292 (88.49) | 13 141 (88.31) | 3292 (88.49) | |
| Household income | <0.001 | ||||
| T1, lowest | 70 979 (33.97) | 1254 (33.71) | 5007 (33.65) | 1254 (33.71) | |
| T2 | 70 013 (33.51) | 940 (25.27) | 3779 (25.4) | 940 (25.27) | |
| T3, highest | 67 966 (32.53) | 1526 (41.02) | 6094 (40.95) | 1526 (41.02) | |
| Medical history | |||||
| Hypertension | 62 230 (29.78) | 3345 (89.92) | 13 434 (90.28) | 3345 (89.92) | 0.012 |
| Diabetes mellitus | 27 772 (13.29) | 1219 (32.77) | 4866 (32.70) | 1219 (32.77) | 0.001 |
| Stroke | 10 853 (5.19) | 802 (21.56) | 3189 (21.43) | 802 (21.56) | 0.003 |
| Heart failure | 18 075 (8.65) | 2439 (65.56) | 9736 (65.43) | 2439 (65.56) | 0.003 |
| Coronary artery disease | 13 723 (6.57) | 931 (25.03) | 3822 (25.69) | 931 (25.03) | 0.015 |
| Asthma | 14 022 (6.71) | 618 (16.61) | 2423 (16.28) | 618 (16.61) | 0.009 |
| Chronic kidney disease | 18 431 (8.82) | 1098 (29.52) | 4351 (29.24) | 1098 (29.52) | 0.006 |
| Malignancy | 29 553 (14.14) | 1055 (28.36) | 4218 (28.35) | 1055 (28.36) | <0.001 |
Note: Data are presented as numbers with percentages.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; SMD, standard mean difference; T, tertile.
A standardized difference in the matched cohort. All standardized mean difference values were less than 0.10 in the propensity score‐matched cohort.
Table 2.
Proportion of COVID‐19 infection in participants who underwent COVID‐19 test with and without atrial fibrillation before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| Without AF (N = 208 958) | With AF (N = 3720) | OR [95% CI] | Without AF (N = 14 880) | With AF (N = 3720) | OR [95% CI] | |
| COVID‐19 | ||||||
| Negative (−) | 201 307 (96.34) | 3658 (98.33) | Ref | 14 566 (97.89) | 3658 (98.33) | Ref |
| Positive (+) | 7651 (3.66) | 62 (1.67) | 0.45 [0.35–0.57] | 314 (2.11) | 62 (1.67) | 0.79 [0.60–1.04] |
Note: Data are presented as numbers with percentages.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; OR, odds ratio.
3.2. COVID‐19 prognosis by AF
Among 7713 patients with COVID‐19, 62 (0.8%) had a history of AF in the unmatched cohort (Figure 1 and Table 3). During 2 months after the diagnosis of COVID‐19, the overall primary outcome occurred in 444 (5.7%) patients, including 171 (2.2%) cases of mechanical ventilation, 265 (3.4%) cases of ICU admission, and 224 (2.9%) cases of death (Table 4). In the unmatched cohort, severe complications of COVID‐19 were more frequently noted in individuals with AF (overall p < 0.001) (Table 4). We applied PSM for 248 individuals without AF and 62 individuals with AF who were appropriately matched (Figure 1 and Table 3). After PSM, history of AF was associated with severe complications of COVID‐19 (OR: 2.04, 95% CI: [1.10–3.79], p = 0.025) and mortality (OR: 2.09, 95% CI: (1.01–4.31), p = 0.048), while the frequency of mechanical ventilation and ICU admission did not differ according to AF history (Table 4).
Table 3.
Baseline characteristics of COVID‐19 patients with and without atrial fibrillation before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | SMDa | ||
|---|---|---|---|---|---|
| Without AF | With AF | Without AF | With AF | ||
| N | 7651 | 62 | 248 | 62 | |
| Sex, male | 3016 (39.42) | 32 (51.61) | 122 (49.19) | 32 (51.61) | 0.048 |
| Age, years | <0.001 | ||||
| <60 | 5486 (71.7) | 6 (9.68) | 24 (9.68) | 6 (9.68) | |
| ≥60 | 2165 (28.3) | 56 (90.32) | 224 (90.32) | 56 (90.32) | |
| Household income | 0.013 | ||||
| T1, lowest | 3315 (43.33) | 32 (51.61) | 129 (52.02) | 32 (51.61) | |
| T2 | 2192 (28.65) | 8 (12.90) | 33 (13.31) | 8 (12.90) | |
| T3, highest | 2144 (28.02) | 22 (35.48) | 86 (34.68) | 22 (35.48) | |
| Medical history | |||||
| Hypertension | 1672 (21.85) | 56 (90.32) | 230 (92.74) | 56 (90.32) | 0.087 |
| Diabetes mellitus | 752 (9.83) | 16 (25.81) | 71 (28.63) | 16 (25.81) | 0.063 |
| Stroke | 285 (3.73) | 13 (20.97) | 43 (17.34) | 13 (20.97) | 0.092 |
| Heart failure | 432 (5.65) | 37 (59.68) | 144 (58.06) | 37 (59.68) | 0.033 |
| Coronary artery disease | 290 (3.79) | 19 (30.65) | 70 (28.23) | 19 (30.65) | 0.053 |
| Asthma | 338 (4.42) | 6 (9.68) | 21 (8.47) | 6 (9.68) | 0.042 |
| Chronic kidney disease | 421 (5.50) | 16 (25.81) | 75 (30.24) | 16 (25.81) | 0.099 |
| Malignancy | 489 (6.39) | 8 (12.90) | 36 (14.52) | 8 (12.90) | 0.047 |
Note: Data are presented as numbers with percentages.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; SMD, standard mean difference; T, tertile.
A standardized difference in the matched cohort. All standardized mean difference values were less than 0.10 in the propensity score‐matched cohort.
Table 4.
Severe complication in COVID‐19 patients with and without atrial fibrillation before and after propensity score matching
| Outcomes | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Without AF (N = 7651) | With AF (N = 62) | OR [95% CI] | p value | Without AF (N = 248) | With AF (N = 62) | OR [95% CI] | p value | ||
| Severe complication in COVID‐19 | |||||||||
| Mechanical ventilation | 165 (2.16) | 6 (9.68) | 4.86 [2.07–11.44] | <0.001 | 17 (6.85) | 6 (9.68) | 1.46 [0.55–3.86] | 0.450 | |
| Intensive care unit admission | 254 (3.32) | 11 (17.74) | 6.28 [3.24–12.20] | <0.001 | 28 (11.29) | 11 (17.74) | 1.70 [0.79–3.63] | 0.174 | |
| Death | 211 (2.76) | 13 (20.97) | 9.36 [5.00–17.51] | <0.001 | 28 (11.29) | 13 (20.97) | 2.09 [1.01–4.31] | 0.048 | |
| Compose of outcome | 424 (5.54) | 20 (32.26) | 8.12 [4.72–13.95] | <0.001 | 47 (18.95) | 20 (32.26) | 2.04 [1.10–3.79] | 0.025 | |
Note: Data are presented as numbers with percentages.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; OR, odds ratio.
3.3. Subgroup analysis for prognosis in COVID‐19 patients with available smoking history
We performed a subgroup analysis with COVID‐19 infected patients whose smoking history was available from the national health screening check‐up data because smoking history is an important issue in COVID‐19 prognosis. 12 , 38 Among 3934 COVID‐19 infected patients with available smoking history, 40 (1.0%) had a history of AF, and 299 (7.6%) were current smokers in the unmatched cohort (Figure S1 and Table S1). In the unmatched cohort, severe complications of COVID‐19 such as mechanical ventilation (15.0%), ICU admission (27.5%), and death (22.5%) were more frequent in individuals with AF (all p < 0.001) as shown in Table S2. After 1:4 PSM, 160 individuals without AF and 40 individuals with AF were matched and all covariates, including current smoking, were balanced appropriately (Table S1). In the matched cohort (Table S2), a history of AF was significantly associated with ICU admission (OR: 3.41, 95% CI: [1.44–8.11], p = 0.005) and compose of outcome (OR: 2.89, 95% CI: [1.37–6.10], p = 0.005) with COVID‐19 infection (Table 5).
Table 5.
The results of previous meta‐analysis on outcome in COVID‐19 patients with and without atrial fibrillation
| Author, year | N | Outcome | OR [95% CI] | p value | Problems in the analysis |
|---|---|---|---|---|---|
| Yang, et al. (2021) 11 | 23 | Unfavorable outcome | 1.14 [1.03–1.26] | 0.01 | There is no definition of unfavorable outcome |
| Yang, et al. (2021) 11 | 23 | Death | 1.13 [1.02–1.25] | <0.05 | – |
| Romiti, et al. (2021) 9 | 14 | Mortality | 3.97 [2.76–5.71] | <0.05 | This is the result of meta‐analysis of univariate OR |
| Zuin, et al. (2021) 10 | 12 | Death in the short‐term period | 2.22 [1.47–3.36] | <0.0001 | This is the result of meta‐analysis of unadjusted OR |
Abbreviations: CI, confidence interval; N, number of included study; OR, odds ratio.
3.4. The results of the previous meta‐analyses on COVID‐19 prognosis by AF
There have been three meta‐analyses on COVID‐19 prognosis by AF. Yang et al. 11 showed that AF was significantly associated with an increased risk of unfavorable outcomes among COVID‐19 patients (pooled effect size = 1.14, 95% CI: 1.03–1.26, p = 0.01; I 2 = 63.9%, random‐effects analysis). However, there was no definition of unfavorable outcome in this study. When they limited the outcome to death, there was a significant relation between AF and COVID‐19 mortality (pooled effect size = 1.13, 95% CI: 1.02–1.25). 11 Romiti et al. 9 analyzed the 14 studies reporting on all‐cause mortality among COVID‐19 patients and according to AF status, which showed there was a fourfold higher risk of death compared to patients without AF (OR: 3.97, 95% CI: 2.76–5.71), but the heterogeneity among studies was high (I 2 = 78%) and they used univariate ORs. Zuin et al. 10 also demonstrated that preexisting AF was significantly associated with a higher risk of death in the short‐term period (OR: 2.22, 95% CI: 1.47–3.36, p < 0.0001), but similarly, they did not use adjusted ORs.
4. DISCUSSION
The key findings of this study based on a nationwide COVID‐19 database is that COVID‐19 patients with AF are more likely to suffer severe complications, particularly mortality by propensity score matching analysis. In our study, OR for the risk of death in COVID‐19 patients with AF was 9.36 but it was decreased to 2.09 after propensity score matching, which suggests that unadjusted OR for this outcome is highly biased. Similarly, some important outcomes such as mechanical ventilation and admission to ICU, were statistically significant before propensity score matching, but those became nonsignificant after propensity score matching. In addition, infectivity for COVID‐19 infection was not different according to a history of AF.
During the pandemic period, the morbidity and mortality of COVID‐19 have continued to disrupt health systems around the world. This requires a concentration of medical resources for critically ill patients, and therefore, studies of risk factors that predict progression to severe COVID‐19 are being conducted. Cardiovascular diseases, including cardiac arrhythmia, are known risk factors for adverse outcomes and mortality in COVID‐19 infected patients. 7 , 23 A previous single‐center study showed that 7.2% of cardiac arrhythmias were documented during COVID‐19 hospitalization, detected a significant correlation between cardiac arrhythmia and disease severity, and identified risk factors for adverse outcomes including age, congestive heart failure, and troponin levels. 24 AF is the most common type of sustained cardiac arrhythmia and is a well‐known risk factor for other cardio‐cerebral vascular diseases, as well as the most commonly identified arrhythmia in previous COVID‐19 studies. 7 , 24 A recent meta‐analysis of new‐onset AF in COVID‐19 patients showed that patients with AF were at a 2.4‐fold higher risk of all‐cause mortality than patients without AF. 9 Our results were in line with these previous studies and suggested AF is a significant risk factor associated with poor prognosis in COVID‐19 infection.
It is difficult to demonstrate how AF worsens outcomes of COVID‐19 using the findings presented here, but there is some evidence for a possible explanation (Figure 2). First, in the closely connected cardio‐respiratory system, COVID‐19 patients with respiratory failure may be at risk of increased cardiac injury. Cardiac dysfunctions such as cardiomyopathy, cardiac arrhythmias, and hemodynamic instability are known as acute COVID‐19 cardiovascular syndrome. 25 SARS‐CoV‐2 can directly invade cardiomyocytes and pericytes through angiotensin‐converting enzyme‐2 and systemic inflammatory cytokines can also induce cardiac injury. 25 Respiratory failure in patients with COVID‐19 may also increase the cardiac burden in patients with AF who have cardiac remodeling, one of the key pathophysiological mechanisms of AF. Although the underlying mechanisms are unclear, patients who experience sepsis and acute respiratory distress syndrome with AF are at higher risk of severe respiratory failure and mortality than patients with no history of AF. 26 , 27 Second, systematic inflammatory response followed by SARS‐CoV‐2 infection may worsen outcomes in patients with AF. Higher C‐reactive protein (CRP) and interleukin‐6 (IL‐6) levels in AF patients support the increase of inflammation burden in AF patients. 28 The severity of inflammation during COVID‐19 is also associated with adverse clinical outcomes and mortality. Elevation of inflammation biomarkers including CRP, IL‐6, and lactate dehydrogenase can predict severe COVID‐19, and at the same time, early normalization of these markers has been observed in patients with better prognosis. 29 , 30 Third, thromboembolism (TE) is another potential mechanism since SARS‐Cov‐2 contagion can contribute to immunothrombosis. 31 , 32 As is well known, TE is one of the major cardiovascular complications in patients with COVID‐19, presumably due to the inflammatory response of SARS‐Cov‐2, with endothelial dysfunction observed in multiple organs and abnormal coagulation. 33 As AF is a risk factor for TE, TE may influence the poor prognosis of AF patients diagnosed with COVID‐19. In a previous study comparing seasonal influenza and COVID‐19, TE including pulmonary embolism was found to be significantly higher in COVID‐19 patients. 34 It is difficult for us to provide a clear rationale or cause for these results.
Figure 2.
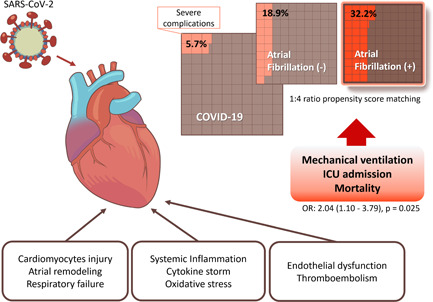
Summary of possible mechanisms and results of our study
In our study, the infectivity of COVID‐19 did not differ according to the history of AF. In the general population, information on the infectivity or prevalence of AF in COVID‐19 infection is lacking. Italy's national health data showed that 24.5% of people who died from COVID‐19 had a history of AF before COVID‐19 infection. 8 A small hospital‐based study reported that 75% of elderly COVID‐19 patients have AF. 35 Various factors such as design, population, duration of follow‐up, ethnicity, and comorbidity of the study may have induced these discrepancies between our results and those of other researchers. Therefore, it is necessary to refer to studies involving a larger number of people or considering other races.
Our study has limitations. First, the current study had a retrospective observational design based on an already existing health claims database, causal relationships could not be explored. Due to the limitation of claims data, there are uncovered covariates that could be confounding factors on the association between AF and COVID‐19, such as physical activity or body mass index. 36 , 37 Second, it is difficult to generalize our results because our dataset represents the Korean general population. Third, detailed information regarding severity, type, or characteristics of AF could not be acquired because our dataset did not include the information, including the electrocardiograms. Fourth, our dataset did not include the information of COVID‐19 related symptoms that could be related to prognosis or infectivity in the participants at the time of RT‐PCR testing. Therefore, we could not confirm the individuals in this cohort had COVID‐19 symptoms or not. Last, it is unknown the medication history of anticoagulation after COVID‐19 infection in patients with AF. We could not figure out the answer to how many AF patients are on or quitted anticoagulants during COVID‐19. There is a need for further research on the relationship between anticoagulation and the prognosis of COVID‐19 patients with AF.
In conclusion, we demonstrated that a history of AF is associated with an increased risk of severe complications of COVID‐19. Patients with AF may have poor prognoses if infected with COVID‐19, therefore, careful management and monitoring of such patients is necessary.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Jinkwon Kim and Tae‐Jin Song: study design, manuscript writing, and review. Jin Park, Jae Il Shin, Jinkwon Kim, Tae‐Jin Song: study design, data collection, and manuscript writing. Dong‐Hyeok Kim, Junbeom Park, Jimin Jeon: data collection and statistical analysis. All authors read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27647
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2020R1I1A1A01060447 to JK, NRF‐2021R1F1A1048113 to T‐JS). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Park J, Il Shin J, Kim D‐H, et al. Association of atrial fibrillation with infectivity and severe complications of COVID‐19: A nationwide cohort study. J Med Virol. 2022;94:2422‐2430.
Jin Park, and Jae Il Shin contributed equally to this study as first authors.
Jinkwon Kim, and Tae‐Jin Song contributed equally to this work as corresponding authors.
Contributor Information
Jinkwon Kim, Email: antithrombus@gmail.com.
Tae‐Jin Song, Email: knstar@ewha.ac.kr.
DATA AVAILABILITY STATEMENT
The COVID‐19 dataset supporting the conclusions of this study is accessible from National Health Insurance System, but restrictions apply to the availability of the data. Use of the dataset is limited to the current research under license, therefore, it is not publicly available. Data are only available on the reasonable request and permission from the National Health Insurance System (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
REFERENCES
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA 2020;324(8):782‐793. [DOI] [PubMed] [Google Scholar]
- 2. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan RE, Adab P, Cheng KK. Covid‐19: risk factors for severe disease and death. BMJ 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 4. Kim HJ, Park MS, Shin JI, et al. Associations of heart failure with susceptibility and severe complications of COVID‐19: a nationwide cohort study. J Med Virol. 2021. 94(3):1138–1145. 10.1002/jmv.27435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(9):1064‐1070. [DOI] [PubMed] [Google Scholar]
- 6. Park CS, Choi EK, Kim B, et al. Association between atrial fibrillation, myocardial infarction, heart failure and mortality in patients with nontuberculous mycobacterial infection: a nationwide population‐based study. Sci Rep. 2019;9(1):15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatla A, Mayer MM, Adusumalli S, et al. COVID‐19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gawałko M, Kapłon‐Cieślicka A, Hohl M, Dobrev D, Linz D. COVID‐19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romiti GF, Corica B, Lip GYH, Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID‐19: a systematic review and meta‐analysis. J Clin Med. 2021;10(11):2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuin M, Rigatelli G, Bilato C, Zanon F, Zuliani G, Roncon L. Pre‐existing atrial fibrillation is associated with increased mortality in COVID‐19 Patients. J Interv Card Electrophysiol. 2021, 62:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang H, Liang X, Xu J, Hou H, Wang Y. Meta‐analysis of atrial fibrillation in patients with COVID‐19. Am J Cardiol. 2021;144:152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seong SC, Kim YY, Park SK, et al. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;7(9):e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new‐onset diabetes: a nationwide population‐based cohort study. Diabetologia 2020;63(5):924‐933. [DOI] [PubMed] [Google Scholar]
- 14. Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID‐19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7(12):1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SW, Ha EK, Yeniova A.Ö, et al. Severe clinical outcomes of COVID‐19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut 2021;70(1):76‐84. [DOI] [PubMed] [Google Scholar]
- 16. Lee SS, Ae Kong K, Kim D, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38(34):2599‐2607. [DOI] [PubMed] [Google Scholar]
- 17. Jin MN, Yang PS, Song C, et al. Physical activity and risk of atrial fibrillation: a nationwide cohort study in general population. Sci Rep. 2019;9(1):13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Son M, Seo J, Yang S. Association between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19 infection in South Korea. Hypertension 2020;76(3):742‐749. [DOI] [PubMed] [Google Scholar]
- 19. Song TJ, Jeon J, Kim J. Cardiovascular risks of periodontitis and oral hygiene indicators in patients with diabetes mellitus. Diabetes Metab. 2021;47(6):101252. [DOI] [PubMed] [Google Scholar]
- 20. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the national health insurance service‐national sample cohort (NHIS‐NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. [DOI] [PubMed] [Google Scholar]
- 21. Chang Y, Woo HG, Lee JS, Song TJ. Better oral hygiene is associated with lower risk of stroke. J Periodontol. 2021;92(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 22. Patanavanich R, Glantz SA. Smoking is associated with worse outcomes of COVID‐19 particularly among younger adults: a systematic review and meta‐analysis. BMC Public Health. 2021;21(1):1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rav‐Acha M, Orlev A, Itzhaki I, et al. Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID‐19) patients: Prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int J Clin Pract. 2021;75(4):e13788. [DOI] [PubMed] [Google Scholar]
- 25. Hendren NS, Drazner MH, Bozkurt B, Cooper LT, Jr. Description and proposed management of the acute COVID‐19 cardiovascular syndrome. Circulation 2020;141(23):1903‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein Klouwenberg P.M.C., Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new‐onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195(2):205‐211. [DOI] [PubMed] [Google Scholar]
- 27. Ambrus DB, Benjamin EJ, Bajwa EK, Hibbert KA, Walkey AJ. Risk factors and outcomes associated with new‐onset atrial fibrillation during acute respiratory distress syndrome. J Crit Care. 2015;30(5):994‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zylla MM, Merle U, Vey JA, et al. Predictors and prognostic implications of cardiac arrhythmias in patients hospitalized for COVID‐19. J Clin Med. 2021;10(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng Z, Yu H, Chen H, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID‐19 from Wuhan, China. Crit Care. 2020;24(1):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smilowitz NR, Kunichoff D, Garshick M, et al. C‐reactive protein and clinical outcomes in patients with COVID‐19. Eur Heart J. 2021;42(23):2270‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicolai L, Leunig A, Brambs S, et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID‐19 from influenza pneumonia. J Thromb Haemost. 2021;19(2):574‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Dam LF, Kroft LJM, van der Wal LI, et al. Clinical and computed tomography characteristics of COVID‐19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet 2020;395(10235):1517‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID‐19 and seasonal influenza: a nationwide, population‐based retrospective cohort study. Lancet Respir Med. 2021;9(3):251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fumagalli S, Salani B, Gabbani L, Mossello E, Ungar A. Covid‐19 cases in a no‐Covid‐19 geriatric acute care setting. A sporadic occurrence? Eur J Intern Med. 2020;77:141‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS‐CoV‐2 infection, severe COVID‐19 illness and COVID‐19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021. [DOI] [PubMed] [Google Scholar]
- 37. Gao M, Piernas C, Astbury NM, et al. Associations between body‐mass index and COVID‐19 severity in 6·9 million people in England: a prospective, community‐based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID‐19: A nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The COVID‐19 dataset supporting the conclusions of this study is accessible from National Health Insurance System, but restrictions apply to the availability of the data. Use of the dataset is limited to the current research under license, therefore, it is not publicly available. Data are only available on the reasonable request and permission from the National Health Insurance System (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).


