Abstract
To date the optimal antiviral treatment against severe coronavirus disease 2019 (COVID‐19) has not been proven; remdesivir is a promising drug with in vitro activity against several viruses, but in COVID‐19 the clinical results are currently not definitive. In this retrospective observational study, we analyzed the clinical outcomes (survival analysis, efficacy, and safety) in a group of hospitalized patients with COVID‐19 treated with remdesivir in comparison with a control group of patients treated with other antiviral or supportive therapies. We included 163 patients treated with remdesivir and 403 subjects in the control group; the baseline characteristics were similar in the two groups; the mortality rate was higher in the control group (24.8% vs. 2.4%, p < 0.001), the risk of intensive care unit (ICU) admission was higher in the control group (17.8% vs. 9.8%, p = 0.008); hospitalization time was significantly lower in patients treated with remdesivir (9.5 vs. 12.5 days, p < 0.001). The safety of remdesivir was good and no significant adverse events were reported. In multivariate analysis, the remdesivir treatment was independently associated with a 34% lower mortality rate (odds ratio = 0.669; p = 0.014). In this analysis, the treatment with remdesivir was associated with lower mortality, lower rate of ICU admission, and shorter time of hospitalization. No adverse events were observed. This promising antiviral treatment should also be confirmed by other studies.
Keywords: antiviral therapy, COVID‐19, hospitalization, remdesivir, SARS‐CoV‐2
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease‐2019
- CS
corticosteroids
- ICU
intensive care unit
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is the novel pathogen responsible for the coronavirus disease‐19 (COVID‐19) which emerged in December 2019 in Wuhan. 1 Later, the World Health Organization would declare the COVID‐19 pandemic on March 11th, 2020. The greater mortality and hospitalization rate were mainly related to interstitial pneumonia with progression to critical hypoxemia and the development of acute respiratory distress syndrome (ARDS). 2
Due to the lack of a proven antiviral therapy, clinicians widely employed supportive treatment in advanced COVID‐19 phases with oxygen, ventilatory support, corticosteroids (CS), and low‐molecular‐weight heparin (LMWH). 3 , 4
A specific antiviral treatment useful in the first phase of COVID‐19, before the development of ARDS, has not yet been identified; despite the recent availability of oral antiviral molnupiravir and paxlovir is promising, other antivirals drugs with inhibition of viral protease or viral RNA synthetase have been repurposed for use against SARS‐CoV‐2 infection with conflicting results. 5 , 6
Remdesivir (GS‐5734; Gilead Sciences Inc.) is a nucleoside analog that inhibits RNA‐dependent RNA polymerase that was previously proposed for the treatment of Ebola, the Middle‐East respiratory syndrome coronavirus, and the SARS‐CoV. 7 , 8 , 9 The effectiveness of remdesivir use in COVID‐19 has not been definitively proven: in the first trial by Wang et al. 10 performed in Wuhan, remdesivir was not associated with a clinical or survival improvement; however, the small sample size of this study and the varying severity of the enrolled patients do not allow a definitive conclusion to be reached. In other recent studies, however, remdesivir treatment significantly improved survival and recovery both in early and in advanced disease, 11 , 12 , 13 but some studies reported a major impact on mortality and hospitalization when the remdesivir was administered in the early phase of viral infection, before the beginning of the “cytokine storm” (defined as hyperinflammation, dysregulation of cytokines, and immune response) or ARDS. 14 , 15 For this reason, the Italian Medicines Agency (AIFA) approved the use of remdesivir (Veklury®) in Italy in patients with the onset of symptoms within 10 days. 16
2. METHODS
2.1. Study design and definitions
This is a single‐center, observational, “real‐life,” retrospective study considering all the consecutive patients admitted at our Infectious Diseases unit of St. Andrea Hospital, Vercelli, Italy, between March 9, 2020 and May 20, 2021, with a confirmed diagnosis of SARS‐CoV‐2 infection with radiological evidence of interstitial pneumonia. All patients with COVID‐19 treated with remdesivir were enrolled in the study group; all patients treated with different therapies (lopinavir/ritonavir, darunavir/cobicistat, hydroxychloroquine [HCQ]) were included in the control group. Any patient in this cohort received anti‐interleukin therapies such as tocilizumab, baricitinib, or anakinra. Inclusion criteria of this retrospective analysis were: confirmed diagnosis of SARS‐CoV‐2 infection based on a positive result on a real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) of nasal or pharyngeal swab specimen; radiological confirmation of interstitial pneumonia; onset of symptoms within 10 days from hospital admission, estimated glomerular filtration rate (eGFR) ≥ 30 ml/min, and need for low‐flow oxygen at the time of admission. Exclusion criteria were: clinical diagnosis without confirmed RT‐PCR of SARS‐CoV‐2 infection, SARS‐CoV‐2 RT‐PCR positive without evidence of interstitial pneumonia, the onset of symptoms after 10 days from the hospital admission, eGFR < 30 ml/min, the need of continuous positive airway pressure support (CPAP), noninvasive ventilation (NIV), or orotracheal intubation at the time of admission. The clinical severity of patient at the hospital admission was evaluated through the “Modified Early Warning Score” (MEWS) with the following categories: 0–2 mild disease, uncritical patient; 3–5: moderate disease, potentially critical patient; and >5: severe disease, critical patient.
The patients received a 5‐day course of treatment with remdesivir as follows: 200 mg on Day 1, then 100 mg daily on Days 2–5.
The use of corticosteroids was not different in the two groups: we administered—according to national recommendations—a fixed dose of dexamethasone (20 mg/daily for the first 5 days, then 10 mg/daily for 5 days) in all subjects with evidence of pulmonary disease, the need of oxygen support and hyperinflammation laboratory signs.
The study protocol was approved by the local Ethics Committee “Comitato Etico Interaziendale ASL VC” (August 4, 2020; protocol number: 0026301). This study which involves human participants is in compliance with the 1964 Helsinki declaration and its later amendments. All included patients have signed the informed consent of this study.
2.2. Study endpoints
The primary endpoint was the comparison of in‐hospital mortality between patients receiving remdesvir treatment and the control group; secondary endpoints were the assessment of the length of hospitalization and the time to SARS‐CoV‐2 RT‐PCR negativization after the initiation of treatment between the two groups of patients. The PCR test was performed at the end of treatment (5 days in patients treated with remdesivir, 7–10 days in the control group) and repeated after 1 week if was still detectable. Other reported outcomes were: need for CPAP/NIV, need for intensive care unit (ICU) admission, and diagnosis of sepsis (defined as bloodstream documented infection—bacterial or fungal— with systemic signs of infection).
2.3. Statistical analysis
In descriptive statistics, continuous variables were summarized as median (interquartile range [IQR]: 25th–75th percentiles). Categorical variables were described as frequency and percentage. All data were assessed for normality using a Shapiro–Wilk test and categorical data were compared using the χ 2‐test or Fisher exact test. To investigate continuous data, a Spearman's rank correlation was utilized. Multivariate logistic regression analysis with stepwise forward selection was performed for mortality evaluation with p < 0.05 as the criteria for model inclusion. All p‐values were two‐tailed. p < 0.05 was considered statistically significant. Linear regression analysis was made to examine related factors with hospitalization time. Survival analysis was carried out comparing the two groups using the Kaplan–Meier plot and compared with the log rank (Mantel–Cox) test. Statistical analyses were conducted by using SPSS software package ver. 26.0.
3. RESULTS
3.1. Patient selection and baseline characteristics
In the study period, 696 patients with a confirmed diagnosis of SARS‐CoV‐2 infection were admitted to our center; 130 subjects were excluded according to the criteria reported above for the following reasons: 13 patients died within 24 h of hospital admission, 25 were directly admitted to the ICU, 71 required NIV or CPAP at admission, 18 had symptoms for more than 10 days, and 3 had no evidence of lung involvement. In the end, 566 subjects were enrolled in this analysis.
Table 1 shows the baseline characteristics of the patients enrolled. In the remdesivir group, we included 163 subjects, while there were 403 in the control group. No significant differences were observed in the baseline characteristics between the two groups according to age, sex, body mass index (BMI), days before the symptoms, MEWS score, or laboratory results. We reported a lower number of patients with chronic kidney disease in the remdesivir group (p = 0.027), and a lower number of subjects with the neoplastic disease in the control group (p = 0.020). Observed comorbidities included: cardiovascular diseases, chronic obstructive pulmonary disease, diabetes, neurological, psychiatric, neoplastic, and kidney diseases.
Table 1.
Baseline characteristics of the study population and clinical outcomes
| Characteristics | Remdesivir group | Control group | p |
|---|---|---|---|
| (n = 163) | (n = 403) | ||
| Demographics | |||
| Age (median, IQR) | 69 [55–80] | 71 [58–84] | 0.219 |
| Male sex (n, %) | 113 (69.3) | 249 (62.1) | 0.661 |
| BMI (median, IQR) | 26.4 [24.3–30.8] | 25.6 [23.8–31.7] | 0.614 |
| Comorbidities (n, %) | 113 (69.3) | 313 (78) | |
| Cardiovascular disease | 25 (15.3) | 67 (16.6) | 0.344 |
| COPD | 16 (9.8) | 41 (10.2) | 0.286 |
| Chronic kidney disease | 11 (6.7) | 38 (9.4) | 0.027 |
| Diabetes | 28 (17.1) | 78 (19.3) | 0.110 |
| Neurological chronic disease | 12 (7.4) | 35 (8.7) | 0.090 |
| Psychiatric disease | 11 (6.7) | 39 (9.6) | 0.108 |
| Neoplastic disease | 10 (6.1) | 15 (3.7) | 0.020 |
| Days from the onset of symptoms (median, IQR) | 9.5 [4.5–10.5] | 8.4 [6.9–11.5] | 0.289 |
| MEWS score | 3.5 [2.8–5] | 3.8 [2.8–4.8] | 0.366 |
| Mild disease (0–2) | 64 (39.2) | 177 (43.9) | 0.144 |
| Moderate disease (3–5) | 99 (60.7) | 226 (56) | 0.178 |
| Treatment | |||
| Hydroxychloroquine | – | 177 (43.9) | |
| Lopinavir/ritonavir | – | 140 (34.7) | |
| Darunavir/cobicistat | – | 65 (16.1) | |
| No antiviral therapies | – | 21 (5.2) | |
| Corticosteroids | 125 (76.7) | 301 (74.7) | 0.415 |
| Low‐molecular‐weight heparins | 151 (92.6) | 378 (93.7) | 0.612 |
| Laboratory examinations | |||
| WBC (109/L) | 5.6 [4.3–7.8] | 5.9 [4.1–8.6] | 0.782 |
| Platelets (109/L) | 265 [181–318] | 284 [141–331] | 0.223 |
| CRP (mg/L) | 34.8 [21.5–88.9] | 41.4 [27.8–103.4] | 0.118 |
| Ferritin (ng/ml) | 998 [616–1408] | 1066 [884–3327] | 0.554 |
| d‐dimer (ng/ml) | 322 [121–2217] | 288 [178–1178] | 0.352 |
| Procalcitonin (ng/ml) | 1.4 [0.5–4.5] | 1.2 [0.8–6.9] | 0.712 |
| P/F (median, IQR) | 224 [211–286] | 210 [208–312] | 0.189 |
| Clinical outcomes | |||
| Death (n, %) | 4 (2.4) | 100 (24.8) | <0.001 |
| Sepsis (n, %) | 2 (1.2) | 60 (14.8) | <0.001 |
| Need of CPAP/NIV (n, %) | 54 (33.1) | 317 (78.6) | <0.001 |
| Need of ICU admission (n, %) | 16 (9.8) | 72 (17.8) | 0.008 |
| Length of treatment with CPAP/NIV (days) (median, IQR) | 5 [4–6.5] | 9 [8–11] | 0.012 |
| Days of hospitalization (median, IQR) | 9.5 [6–12] | 12.5 [9.5–16.2] | <0.001 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CRP, C‐reactive protein; ICU, intensive care unit; IQR,interquartile range;MEWS, modified early warning score; NIV, noninvasive ventilation; WBC, white blood cell.
3.2. Clinical outcomes
In the remdesivir group, four patients died (2.4%), sepsis was observed in two subjects (1.2%); 54 (33.1%) required higher flow oxygen with CPAP or NIV, 16 (9.8%) were later admitted to the ICU; the median time on CPAP/NIV was 5 days, while the median hospitalization time was 9.5 days. In the control group, 100 patients died (24.8%), sepsis was observed in 60 (14.8%), 317 (78.6%) needed CPAP/NIV, and 72 (17.8%) needed ICU admission; the median time on CPAP/NIV was 9 days, and the median hospitalization time was 12.5 days. In the control group, treatment was given as follows: HCQ in 177 patients (43.9%), lopinavir/ritonavir in 140 (34.7%), and darunavir/cobicistat in 65 (16.1%); in 21 patients, no antiviral treatment was administered. According to the timing of remdesivir administration, we observed 90 patients (55.2%) with early treatment (<7 days from the symptoms' onset) and 73 patients (44.7%) who were treated within 7–10 days from the onset of symptoms.
Supportive treatment with corticosteroids was given in 125 patients in the remdesivir group (76.7%) and 301 in the control group (74.7%). Statistically significant differences were observed between the two groups according to mortality (p < 0.001), presence of sepsis (p < 0.001), need for CPAP/NIV (p < 0.001), need for ICU admission (p = 0.008), median time on CPAP/NIV (p = 0.012), and median hospitalization time (p < 0.001) (Figure 1). A rise in aminotransferases after the initiation of treatment was observed in 12 subjects in the remdesivir group (12.9%) and in 22 subjects in the control group (20%) (p = 0.075). Finally, we observed a bilirubin increase only in 16 patients (3.9%) and no one in remdesivir group.
Figure 1.
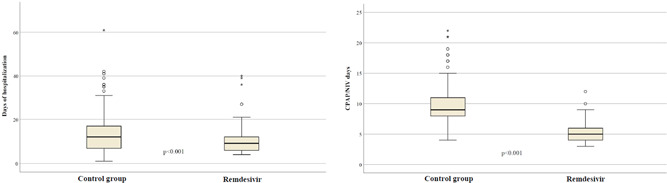
Different median time of hospitalization and length of treatment with continuous positive airway pressure (CPAP)/noninvasive ventilation (NIV) in patients receiving remdesivir and in the control group.
3.3. Univariate and multivariate analysis considering the mortality and hospitalization length in the study population
The following factors were considered in the univariate analysis: age >70 years, male sex, BMI > 25, MEWS score, presence of comorbidities, CPAP or NIV during the hospitalization, ICU admission, sepsis, corticosteroids, and remdesivir treatment (Table 2). The following factors were statistically significant for mortality and were considered in the multivariate analysis: age >70 years, comorbidities, CPAP or NIV, ICU admission, sepsis, and remdesivir treatment. In the multivariate analysis, the following were significantly associated with mortality: age >70 years (odds ratio [OR] = 3.092; 95% confidence interval [CI] = 2.078–4.552; p < 0.001), comorbidities (OR = 5.669; 95% CI = 4.218–17.864; p < 0.001), ICU admission (OR = 2.227; 95% CI = 1.390–4.921; p = 0.004), and remdesivir treatment (OR = 0.669; 95% CI = 0.523–0.941; p = 0.014). Remdesivir treatment was also significantly associated with a lower chance to ICU admission (OR = 0.135; 95% CI = 0.054–0.341; p < 0.001) and lower need of CPAP/NIV (OR = 0.776; 95% CI = 0.460–0.891; p = 0.011). In the linear regression analysis considering the hospitalization length, the same factors were considered; in the final multivariate analysis, the following were significantly associated with increased in‐hospital stay: age >70 years (β = 1.886; DS = 0.814; p < 0.001), CPAP/NIV (β = 3.289; DS = 0.796; p = 0.001), ICU admission (β = 2.938; DS = 1.176; p = 0.013), and remdesivir treatment (β = −4.179; DS = 0.814; p < 0.001) (Table 3).
Table 2.
Univariate and multivariate logistic regression considering the mortality in the study population.
| Univariate analysis | |
|---|---|
| Factors | OR, 95% CI, p |
| Age >70 years | 5.744 (3.271–10.089), p < 0.001 |
| Sex male | 0.713 (0.462–1.101), p = 0.127 |
| BMI > 25 | 1.607 (1.018–2.535), p = 0.042 |
| MEWS score (mild vs. moderate) | 0.817 (0.715–2.444), p = 0.091 |
| Comorbidities | 7.932 (3.158–19.923), p < 0.001 |
| CPAP/NIV during hospital admission | 3.351 (1.782–5.328), p = 0.017 |
| ICU admission | 2.044 (1.200–3.479), p = 0.008 |
| Sepsis | 1.926 (1.729–3.029), p = 0.018 |
| Corticosteroids | 0.596 (0.341–1.041), p = 0.069 |
| Remdesivir | 0.760 (0.270–0.910), p < 0.001 |
| Multivariate analysis | |
| Factors | OR, 95% CI, p |
| Age >70 years | 3.092 (2.078–4.552), p < 0.001 |
| Comorbidities | 5.669 (4.218–17.864), p < 0.001 |
| CPAP/NIV during hospital admission | 1.437 (0.676–3.051), p = 0.346 |
| ICU admission | 2.227 (1.390–4.921), p = 0.004 |
| Sepsis | 1.005 (0.443–2.281), p = 0.991 |
| Corticosteroids | 0.712 (0.569–4.518), p = 0.103 |
| Remdesivir | 0.669 (0.523–0.941), p = 0.014 |
Note: Bold values are the statistically significant values.
Abbreviations: BMI, body mass index; CI, confidence interval; CPAP, continuous positive airway pressure; ICU, intensive care unit; MEWS, modified early warning score; NIV, noninvasive ventilation; OR, odds ratio.
Table 3.
Linear regression analysis considers the factors related to the length of hospitalization.
| Factors | β, DS, p |
|---|---|
| Age >70 years | 1.886, 0.814, p < 0.001 |
| Comorbidities | 1.621, 0.855, p = 0.059 |
| NIV or CPAP during hospital admission | 3.289, 0.796, p = 0.001 |
| ICU admission | 2.938, 1.176, p = 0.013 |
| Sepsis | 1.926, 0.922, p = 0.088 |
| Remdesivir | −4.179, 0.814, p < 0.001 |
Note: Bold values are the statistically significant values.
Abbreviations: CPAP, continuous positive airway pressure; ICU, intensive care unit; NIV, noninvasive ventilation.
3.4. Survival analysis
Survival analysis was carried out comparing the patients treated with remdesivir and the control group according to in‐hospital mortality (Figure 2) with a significant difference between the two groups (χ 2 = 15.185, p < 0.001). Figure 3 shows the probabilities of ICU admission and CPAP or NIV during hospitalization in the different groups; the probability of ICU admission was significantly higher in the control group (χ 2 = 9.159, p = 0.002) and the need of CPAP/NIV was more frequent in patients without remdesivir treatment (χ 2 = 14.766, p < 0.001).
Figure 2.
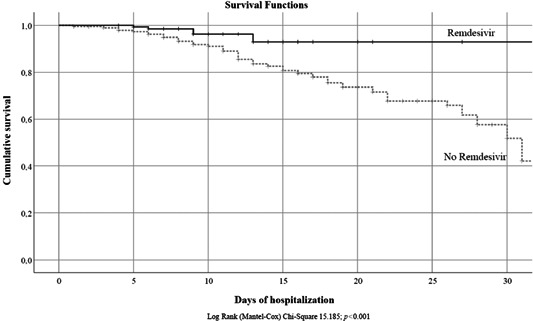
Survival analysis in patients treated with remdesivir and control group
Figure 3.
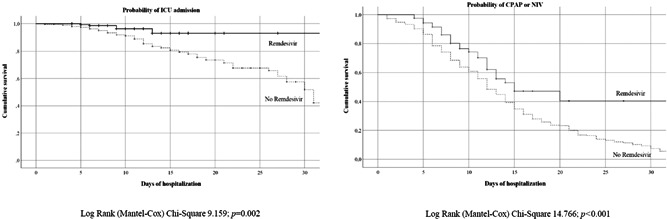
Probability of intensive care unit (ICU) admission and continuous positive airway pressure (CPAP)/noninvasive ventilation (NIV) in the study population according to received treatment.
3.5. Time to SARS‐CoV‐2 PCR negativization
This analysis was available for 462 subjects (81.6%); the PCR test was not repeated in deceased patients (n = 104).
Figure 4 shows the median time for SARS‐CoV‐2 PCR negativization according to different treatment groups: in the control group, the patient's median time was 18 days (IQR:15–23); in patients treated with remdesivir after 7 days from the onset of symptoms, it was 14.5 days (IQR: 12.5–18); in patients with “early” remdesivir treatment (within 7 days from the symptoms' onset), it was 9.5 days (IQR = 7.5–11) with significant differences among the three groups (p < 0.001).
Figure 4.
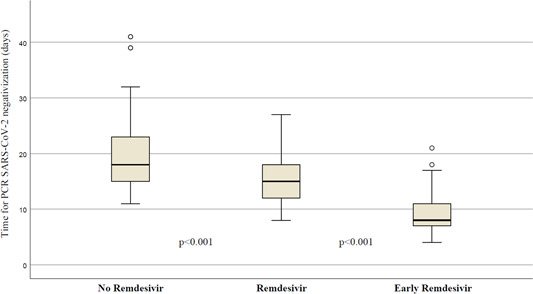
Different times of viral clearance were measured by reverse transcriptase‐polymerase chain reaction (PCR) severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) according to received treatment.
4. DISCUSSION
Due to the need for antiviral therapy against SARS‐Cov‐2 infection, great hope has been placed on the clinical effect of remdesivir in COVID‐19 patients. The preclinical and in vitro studies evidenced the inhibition of the SARS‐CoV‐2 replication. 17 However, the clinical effectiveness varied widely according to the populations of treated patients. In the study by Wang et al., 10 remdesivir use was not associated with a significant clinical benefit, but the study population included subjects on mechanical ventilation and with a longer duration of symptoms before the hospital admission; based on other clinical studies we believe that remdesivir treatment may be effective in the early phase of viral infection, before the “cytokine storm” and ARDS onset, in patients not requiring high‐flow oxygen or mechanical ventilation. 15 , 18 , 19 In Italy, the use of remdesivir in COVID‐19 was routinely approved by AIFA from September 18th, 2020 in subjects with a confirmed diagnosis of SARS‐CoV‐2 infection, initial interstitial pneumonia without the need for CPAP, NIV, or intubation, with a duration of symptoms shorter than 10 days. For this reason, we used the same criteria in the control group, including patients with homogeneous characteristics and disease severity to limit selection bias as far as possible. Some possible doubts maybe not only the distribution but the homogeneity of use in CS and LMWH; in fact, although the CS treatment does not differ between the two groups, the LMWH use may have been different in the dosing approach during the first phase of pandemic (with the prevalent use of preventive than therapeutic dose) with a possible negative effect on the clinical outcomes.
In our weighted logistic regression, remdesivir use was associated with a 34% lower adjusted odds of mortality rate in patients affected by COVID‐19 pneumonia; antiviral therapy with remdesivir was also significantly related to a lower probability of CPAP/NIV, ICU admission, and shorter hospitalization length. Interestingly, in the 54 subjects who needed the CPAP/NIV despite the remdesivir treatment, the median time of ventilation was 4 days shorter than in the control group (p < 0.001). This observation may be related to a preventive effect of remdesivir on pneumonia and ARDS development with a lower grade of inflammation and a higher rate of response to ventilation. The antiviral effect of remdesivir was also observed in the time of PCR negativization with significantly faster viral clearance in patients with “early” treatment in comparison to other groups. This effect can have two benefits: major clinical improvement with shorter hospitalization time and a rapid discharge of patients without the need for home isolation. Finally, we reported a good safety profile in the remdesivir group, without significant serious adverse events related to the antiviral treatment; despite a previously reported hepatotoxicity due to remdesivir treatment, 20 we observed only 12 patients (12.9%) with a rise in hepatic enzymes, but all were asymptomatic, not requiring clinical intervention or treatment interruption, and in all cases, the values of transaminases normalized after the end of treatment. The role of remdesivir can also be discussed considering its use in real‐life neutralizing monoclonal antibody treatments and news antivirals such as molnupiravir and paxlovir; however, none of these showed proven effectiveness after pulmonary involvement and required an early and prompt administration after the onset of symptoms; for this reason, these seem more a “preventive” than “therapeutic” approach and needed further investigations, despite the obvious advantages of some drugs with oral administration. There are also several limitations of this analysis: retrospective design with a different sample size of the two groups; a possible time bias in the enrollment period of the control group because a large portion of these patients was included during the first wave of the pandemic (March–June 2020) when remdesivir treatment was not available in Italy; the short follow‐up period may underestimate the overall mortality after hospital discharge.
5. CONCLUSION
To conclude, remdesivir treatment was associated with encouraging and promising results in patients with early‐stage SARS‐CoV‐2 infection with lung involvement without the need for ventilation. In this context, the antiviral treatment seems to be the best choice, because monoclonal antibodies against SARS‐CoV‐2 should be used before pneumonia development, reserving the anti‐cytokines drugs in the severe/critical patients with the hyperinflammatory syndrome. In our study lower mortality, shorter hospitalization time, and faster viral clearance were observed in patients treated with a 5‐day course of remdesivir in comparison to the control group. Other studies and real‐life data are urgently needed for the optimal use of remdesivir in SARS‐CoV‐2 infection.
AUTHOR CONTRIBUTIONS
All authors fulfill the criteria for authorship as defined by the ICMJE authorship criteria.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the Local Ethics Committee (August 4, 2020).
ACKNOWLEDGMENTS
The authors would like to thank the study participants: authors shared their suffering, isolation, fear of death, lack of affection, and finally, the hope of going home. It was an unforgettable experience, for better or for worse. The authors are also indebted to all the nursing staff of the Infectious Diseases Unit of Saint Andrea Hospital (Vercelli, Italy), who took care of them day and night with invaluable dedication. Open access funding provided by universita degli studi del piemonte orientale amedeo avogadro within the CRUI‐CARE Agreement.
Boglione L, Dodaro V, Meli G, et al. Remdesivir treatment in hospitalized patients affected by COVID‐19 pneumonia: a case‐control study. J Med Virol. 2022;94:3653‐3660. 10.1002/jmv.27768
DATA AVAILABILITY STATEMENT
The reported data underlying this article will be shared upon reasonable request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Ma X. Acute respiratory failure in COVID‐19: is it “typical” ARDS? Crit Care. 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anon . Documents—AIFA. Adaptive randomized trial for therapy of corona virus disease 2019 at home with oral antivirals (ARCO‐Home study). Accessed May 7, 2020. https://www.aifa.gov.it/documents/20142/1131319/covid-19_sperimentazioni_in_corso_09.05.2020.pdf/9d46f533-7406-8f7c-5e34-c119f7087d65
- 5. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID‐19: an overview. Eur J Pharmacol. 2020;889:173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS‐5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9:e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tchesnokov EP, Feng JY, Porter DP, Götte M. Mechanism of inhibition of ebola virus RNA‐dependent RNA polymerase by remdesivir. Viruses. 2019;11:E326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olender SA, Perez KK, Go AS, et al. Remdesivir for severe COVID‐19 versus a cohort receiving standard of care. Clin Infect Dis. 2021;73:e4166‐e4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antinori S, Cossu MV, Ridolfo AL, et al. Compassionate remdesivir treatment of severe Covid‐19 pneumonia in intensive care unit (ICU) and non‐ICU patients: clinical outcome and differences in post‐treatment hospitalisation status. Pharmacol Res. 2020;158:104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hillaker E, Belfer JJ, Bondici A, Murad H, Dumkow LE. Delayed initiation of remdesivir in a COVID‐19‐positive patient. Pharmacotherapy. 2020;40:592‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Y, Chen D, Cai D, Yi Y, Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID‐19 persons: a network meta‐analysis. J Med Virol. 2021;93:1171‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anon . Farmaci utilizzabili per il trattamento della malattia COVID‐19. Accessed July 16, 2021. https://aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19
- 17. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid‐19 patients: systematic review and meta‐analysis including network meta‐analysis. Rev Med Virol. 2021;31:e2187. [DOI] [PubMed] [Google Scholar]
- 19. Lai C‐C, Chen C‐H, Wang C‐Y, Chen K‐H, Wang Y‐H, Hsueh P‐R. Clinical efficacy and safety of remdesivir in patients with COVID‐19: a systematic review and network meta‐analysis of randomized controlled trials. J Antimicrob Chemother. 2021;76:1962‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus 2019. Clin Gastroenterol Hepatol. 2020;18:2835‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The reported data underlying this article will be shared upon reasonable request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


