Abstract
Peripheral blood smear (PBS) changes in coronavirus disease 2019 (COVID‐19) are diverse and have been reported in the literature in the form of case series with relatively smaller sample sizes and with a handful of studies showing the association between PBS and clinical severity. This study aims to highlight the numerical and morphological changes in peripheral blood of COVID‐19 patients and to compare the same in intensive care unit (ICU) and non‐ICU settings as well as with disease severity and outcome. The study included 80 COVID‐19 positive (41 ICU and 39 non‐ICU) patients and 32 COVID‐19 negative ICU patients. Complete blood counts (CBCs) and PBS findings were studied and scored by two pathologists blindfolded. Absolute lymphocyte count (ALC) and absolute eosinophil count (AEC) were significantly lower in COVID‐19 positive cases as compared to the COVID‐19 negative group (p = 0.001 and p = 0.001). COVID‐19 positive group showed significant left myeloid shift (p = 0.021), Dohle bodies (p = 0.025) with significant prominence of acquired pseudo–Pelger–Huët anomaly, ring‐shaped neutrophils, monolobate neutrophils, and plasmacytoid lymphocytes as compared to control group (p = 0.000, p = 0.009, p = 0.046, and p = 0.011, respectively). The overall mean white blood cell (WBC) counts were higher in COVID‐19 positive ICU patients as compared to non‐ICU COVID patients with significant shift to left, presence of ring‐shaped neutrophils, monocyte vacuolation, and large granular lymphocytes (p = 0.017, p = 0.007, p = 0.008, and p = 0.004, respectively). Deceased group showed significantly higher WBC count (p = 0.018) with marked neutrophilia (p = 0.024) and toxic granulation (p = 0.01) with prominence of monocyte vacuolization, ring‐shaped neutrophils, large granular lymphocytes, and reactive lymphocytes. Parameters like myeloid left shift, ring‐shaped neutrophils, monocyte vacuolation, and large granular lymphocytes emerged as highly sensitive markers of disease severity. Therefore, serial CBC with comprehensive PBS analysis should be done in every newly diagnosed hospitalized COVID‐19 patient which potentially predicts the course of the disease.
Keywords: COVID‐19, large granular lymphocytes, morphology, peripheral blood smear, ring‐shaped neutrophils
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) pandemic was first identified in Wuhan, Hubei province, China at the end of the year 2019. 1 As of November 15, 2021, there had been nearly 253 163 330 confirmed cases and 5 098 174 reported deaths globally. 2 It has been observed that the activation of the immune system with subsequent immune‐mediated inflammatory response plays an important role in the pathogenesis of COVID‐19. Laboratory findings, such as leukopenia, lymphopenia, neutrophilia, eosinopenia, and monocytosis, have been reported in the COVID‐19 patients. 3 , 4 , 5 In addition to these numerical changes, some studies have described morphological changes like atypical lymphocytes, cytoplasmic vacuolation, smudge cells, left myeloid shift, and defect in lobation of neutrophils. 6 , 7 , 8 However, literature on cell morphology is mostly confined to case reports, short discussions, and case series with relatively smaller sample sizes and with only a few studies showing association with disease severity and its course. 8 , 9
2. AIMS OF THE STUDY
To study the morphological spectrum of peripheral blood smear (PBS) changes in COVID‐19 positive patients and to compare these with COVID‐19 negative ICU patients.
To correlate these findings with the clinical severity of the disease and its course.
3. METHODS
This study was conducted over a period of 3 months from December 2020 to February 2021. Eighty (80) COVID‐19 cases were confirmed by reverse transcription‐polymerase chain reaction (RT‐PCR) on nasopharyngeal and oropharyngeal swabs sampling and 32 COVID‐19 negative ICU patients (which served as controls) were included in this study. The patients with a history of hematologic disorders (including leukemia, lymphoma, myelodysplasia/myeloproliferative neoplasm), autoimmune disorders, chronic use of immunosuppressants, and alcohol were excluded from the study. COVID‐19 positive group was composed of 41 intensive care unit (ICU) and 39 non‐ICU patients on the basis of disease severity. The control group was comprised of age and sex‐matched individuals admitted to ICU settings for conditions like pneumonia, ischemic heart disease, heart failure, renal failure, and sepsis. The patients were followed till 21 days after the admission. The data were collected from the patients' records at the time of diagnosis and included age, gender, relevant clinical and treatment history along with laboratory investigations. The data on disease severity (ICU and non‐ICU) and course (survived and deceased) were also collected.
Complete blood count (CBC) with PBS examination was performed in all of these patients at the time of admission to the hospital. The PBS were stained with May Grunwald Giemsa stain (MGG) and were screened by two pathologists who were blinded as to their identity.
The changes in the white blood cell (WBC) morphology were reported as “positive” if they were noted in more than 5% of the cells on PBS. With regard to granulocytes, the variations included the presence of toxic changes (toxic granulation, Dohle bodies, and cytoplasmic vacuolation), smudged neutrophils, apoptotic neutrophils, myeloid left shift, hypersegmentation, monolobation, acquired pseudo–Pelger–Huët anomaly (APHA), and ring‐shaped neutrophils (ring‐shaped/doughnut‐shaped nuclei). Toxic granulation was graded as 1 (scattered granules in the cytoplasm with an increase in the staining intensity), 2 (increased number of granules in the cytoplasm with an increase in stain intensity), 3 (abundance of granules with intense blue‐black staining), and 4 (numerous coarse granules crowding the cytoplasm). 10 Shift to left and hypersegmentation were graded on a 4‐point scale as 0 (present in <5% of cells), 1 (present in 5%–10% of cells), 2 (present in 11%–25% of cells), and 3 (present in >25% of cells). 7 The variations in lymphocytes included the presence of reactive lymphocytes (including plasmacytoid forms), atypical lymphocytes, large granular lymphocytes (above the normal range), and cytoplasmic vacuolation. The morphological changes for monocytes included the presence of coalescing cytoplasmic vacuoles. The morphologic changes in RBCs and platelets were also noted.
All of the above numerical and morphological parameters were subjected to analysis between COVID‐19 positive and COVID‐19 negative groups as well as between COVID‐19 positive ICU and COVID‐19 positive non‐ ICU groups. Due to limited genomic sequencing, the study of hematologic parameters in different SARS‐CoV‐2 strains could not be performed.
The data analysis was performed using SPSS v26 software and Microsoft excel. The numerical and morphological data were compared using the Χ 2 test and the p value of ≤0.05 was considered significant.
4. RESULTS
The age group of the COVID‐19 positive ICU patients ranged from 30 to 88 years with a mean age of 61.1 years (male to female ratio 1.1:1). In the COVID‐19 positive non‐ICU group, mean age was 48.3 years (range: 19–75 years) with male preponderance (M:F ratio 1.9:1). The control group (17 males and 15 females) had mean age of 53.5 years (range 18–89 years). The most common presenting symptoms of COVID‐19 positive ICU patients were breathlessness (74.3%, n = 29) followed by fever (53.4%, n = 20). In non‐ICU COVID‐19 patients, fever was the most common symptom (56.0%, n = 23) followed by breathlessness (51.2%, n = 21) and cough (24.3%, n = 10).
Majority of COVID‐19 positive ICU patients (74.3%, n = 29) had underlying comorbidities with most common being diabetes mellitus (41.0%, n = 16) followed by hypertension (35.9%, n = 14), chronic kidney disease (5.1%, n = 2), cardiovascular disease (2.5%, n = 1), asthma (2.5%, n = 1), and others like thyroid disorders and liver disease. Out of 41 patients of non‐ICU COVID‐19 positive group, 20 patients (48.7%) had associated comorbidities like hypertension (21.9%, n = 9) and diabetes mellitus (19.5%, n = 8). The non‐COVID ICU group showed similar comorbidities in about 56.2% (n = 18) of the patients.
4.1. Comparison of hematological parameters and cellular morphology between COVID‐19 positive and control groups (Table 1)
Table 1.
Age and sex distribution, CBC, and morphological parameters in COVID positive (ICU and non‐ICU) patients and COVID negative ICU patients.
| COVID+ (ICU and non‐ICU) (n = 80) | COVID‐ (ICU) (n = 32) | Univariate analysis | ||
|---|---|---|---|---|
| Mean | Mean | T value | p value | |
| Age | 52.3 years | 56.10 years | 2.457 | 0.39 |
| M:F | 1.5:1 | 1:1.1 | 1.190 | 0.25 |
| Hb (g/dl) | 10.81 | 10.73 | 0.151 | 0.880 |
| TLC (/µl) | 11 807.50 | 13 456.25 | 1.150 | 0.253 |
| ANC (/µl) | 9962.30 | 10 688.97 | 0.585 | 0.253 |
| ALC (/µl) | 1232.30 | 1876.41 | 3.511 | 0.001 * |
| AMC (/µl) | 273.44 | 234.56 | 0.935 | 0.342 |
| AEC (/µl) | 80.28 | 165.75 | 3.317 | 0.001 * |
| Platelets (/µl) | 2.3 × 105 | 2.1 × 105 | 0.972 | 0.333 |
| Morphological parameters | ||||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade = 1 | Grade 0 | Grade = 1 | Odds ratio | p value | |
| Left shift | 49 | 31 | 12 | 20 | 9.73 | 0.021 * |
| Toxic granules | 48 | 32 | 14 | 18 | 4.5 | 0.212 |
| Neutrophil vacuolation | 57 | 23 | 16 | 16 | 4.54 | 0.033 * |
| Monocyte vacuolation | 28 | 52 | 9 | 23 | 0.48 | 0.48 |
| Lymphocyte vacuolation | 68 | 12 | 27 | 5 | 0.007 | 0.934 |
| Pseudo–Pelger–Huët anomaly | 23 | 57 | 26 | 6 | 25.6 | 0.00 * |
| Hypersegmented neutrophils | 37 | 44 | 16 | 16 | 0.673 | 0.880 |
| Smudged neutrophils | 55 | 25 | 22 | 10 | 0 | 1.000 |
| Apoptotic neutrophils | 58 | 22 | 28 | 4 | 2.885 | 0.089 |
| Ring neutrophils | 41 | 39 | 25 | 7 | 6.821 | 0.009 * |
| Dohle bodies | 64 | 16 | 31 | 1 | 5.056 | 0.025 * |
| Monolobated neutrophils | 41 | 39 | 23 | 9 | 3.97 | 0.046 * |
| Reactive lymphocytes | 23 | 57 | 4 | 28 | 3.299 | 0.069 |
| Plasmacytoid lymphocytes | 66 | 14 | 52 | 0 | 6.4 | 0.011 * |
| Large granular lymphocytes | 46 | 34 | 20 | 12 | 0.236 | 0.627 |
| Atypical lymphocytes | 69 | 11 | 30 | 2 | 1.253 | 0.263 |
| Large platelets | 66 | 14 | 23 | 9 | 1.581 | 0.209 |
Abbreviations: AEC, absolute eosinophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; CBC, complete blood count; Hb, hemoglobin; ICU, intensive care unit; PBS, peripheral blood smear; TLC, total leukocyte count.
Statistically significant values (p ≤ 0.05).
Among the CBC parameters, both groups showed anemia (mean, 10.73 ± 2.4 vs. 10.81 ± 2.38 gm%, p = 0.88), mild leucocytosis (mean, 13.45 ± 0.6 vs. 11.80 ± 0.6 ×103/μl, p = 0.25) neutrophilia (mean, 10.68 ± 0.9 vs. 9.96 ± 1.0 ×103/μl, p = 0.56) and normal platelet count (mean, 2.14 ± 1.2 vs. 2.39 ± 1.1 lac/μl, p = 0.33) (Figure 1A). Absolute lymphocyte count (ALC) and absolute eosinophil count (AEC) were significantly lower but in normal range in COVID‐19 positive patients as compared to control group (ALC, 1.23 ± 0.7 vs. 1.87 ± 1.1 ×103/μl, p = 0.001; AEC, 0.08 ± 0.01 vs. 0.16 ± 0.01 ×103/μl, p = 0.001).
Figure 1.
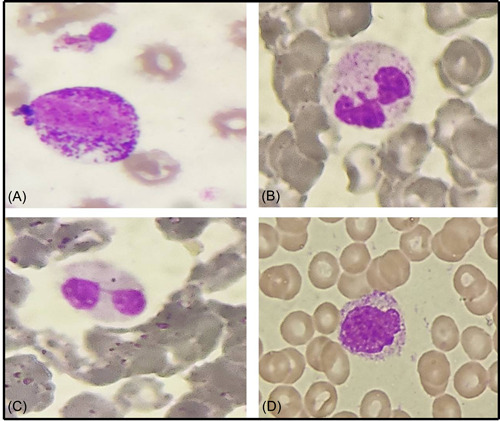
Peripheral blood smear from different COVID patients depicting: (A) myeloid left shift, (B) Dohle bodies, (C) pseudo–Pelger–Huët neutrophil, and (D) monolobated neutrophil (Leishman, ×400).
The PBS of the COVID‐19 positive group showed a significant left myeloid shift and Dohle bodies as compared to the control group (p = 0.021 and p = 0.025, respectively; Figure 1). However, the presence of severe toxic granules, hypersegmentation, and vacuolization was not significant in the COVID‐19 positive group. There was statistically significant prominence of acquired pseudo–Pelger–Huët anomaly (APHA; 71.25%, 57/80), ring‐shaped neutrophils (48.75%, 39/80), and monolobate neutrophils (48.75%, 39/80) in COVID‐19 patients as compared to control group (p = 0.000, p = 0.009, and p = 0.046, respectively; Figure 1). Smudged and apoptotic neutrophils were seen in 31.75% (25/80) and 27.50% (22/80) of the patients in the COVID‐19 positive group; however, the difference was not significant (Figure 2). Reactive lymphocytes were present in both groups, but only the presence of plasmacytoid lymphocytes was significant in the COVID‐19 positive group as compared to controls (p = 0.011; Figure 3). Monocytic vacuolization was seen in both COVID‐19 positive and negative groups (65.0%, 52/80 and 71.8%, 23/32, respectively), however, no significant difference was noted (Figure 4). In both groups, the majority of patients had normocytic normochromic RBC morphology with one COVID‐19 positive ICU case showing a leucoerythroblastic blood picture and presence of schistocytes (Figure 4). No significant difference was noted in platelet morphology in terms of hypogranularity, large granules, and presence of giant platelets between the two groups.
Figure 2.
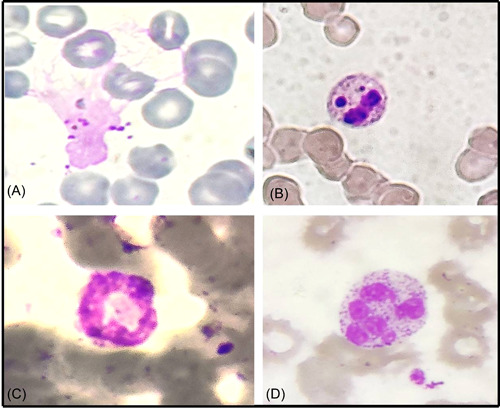
Morphological changes in neutrophils in peripheral smear from different COVID patients: (A) smudged neutrophil, (B) apoptotic neutrophil, (C) ring‐shaped neutrophil, and (D) hypersegmented neutrophil (Leishman, ×400).
Figure 3.
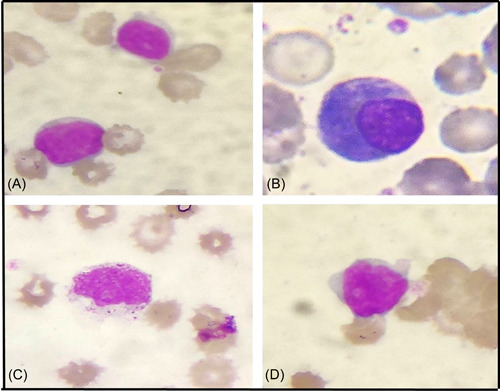
Morphological changes in lymphocytes in a peripheral blood smear from different COVID patients: (A) reactive lymphocyte, (B) plasmacytoid lymphocyte, (C) large granular lymphocyte, and (D) atypical lymphocyte with high N:C ratio and prominent nucleoli (Leishman, ×400).
Figure 4.
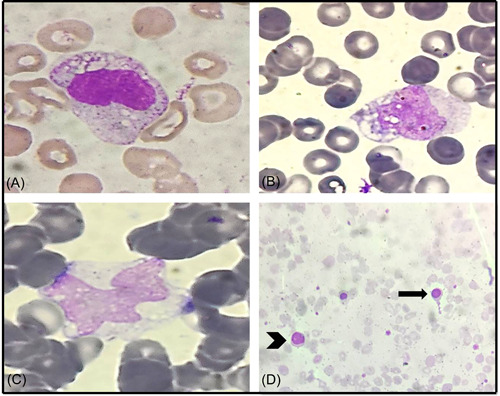
Vacuolation in lymphocyte (A) and monocytes (B) in peripheral blood smear (PBS) of COVID ICU patients, (C) monocyte vacuolization in PBS of COVID non‐ICU patient, (D) PBS of COVID ICU patient showing leucoerythroblastic picture with nucleated red blood cell (arrow) and left shift (arrowhead; Leishman, ×400). ICU, intensive care unit.
Taking into consideration only pneumonia and sepsis patients in the control group (n = 18), significant statistical difference was noted in AEC (p = 0.001), pseudo–Pelger–Huët anomaly (p = 0.00), and ring‐shaped neutrophils (p = 0.03) between COVID‐19 positive and the control group.
4.2. Comparison of hematological parameters and cellular morphology between COVID‐19 positive ICU and non‐ICU groups (Table 2)
Table 2.
Age and sex distribution, CBC, and morphological parameters in COVID positive ICU and COVID positive non‐ICU patients.
| COVID+ (non‐ICU) (n = 41) | COVID+ (ICU) (n = 39) | Univariate analysis | ||
|---|---|---|---|---|
| Mean | Mean | T value | p value | |
| Age | 48.3 years | 61.1 years | 1.930 | 0.120 |
| M:F | 1.9:1 | 1.1:1 | 1.150 | 0.230 |
| Hb (g/dl) | 10.92 | 9.80 | 0.232 | 0.998 |
| TLC (/µl) | 10.8 × 103 | 12.4 × 103 | 1.150 | 0.25 |
| ANC (/µl) | 5.3 × 103 | 10.6 × 103 | 2.432 | 0.02 * |
| ALC (/µl) | 1.8 × 103 | 1.2 × 103 | 2.327 | 0.01 * |
| AMC (/µl) | 0.27 × 103 | 0.23 × 103 | 0.963 | 0.35 |
| AEC (/µl) | 0.10 × 103 | 0.08 × 103 | 0.934 | 0.33 |
| Platelets (/µl) | 2.2 × 105 | 1.8 × 105 | 0.290 | 0.782 |
| Morphological parameters | ||||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade =1 | Grade 0 | Grade =1 | Odds ratio | p value | |
| Left shift | 28 | 13 | 21 | 18 | 8.12 | 0.017 * |
| Toxic granules | 28 | 13 | 20 | 19 | 2.48 | 0.289 |
| Neutrophil vacuolation | 32 | 9 | 25 | 14 | 1.89 | 0.168 |
| Monocyte vacuolation | 31 | 10 | 21 | 18 | 7.020 | 0.008 * |
| Lymphocyte vacuolation | 34 | 7 | 34 | 5 | 0.284 | 0.594 |
| Pseudo–Pelger–Huët anomaly | 14 | 27 | 9 | 30 | 1.196 | 0.274 |
| Hypersegmented neutrophils | 21 | 20 | 16 | 23 | 3.89 | 0.276 |
| Smudged Neutrophils | 32 | 9 | 23 | 16 | 3.38 | 0.066 |
| Apoptotic neutrophils | 30 | 11 | 28 | 11 | 0.019 | 0.890 |
| Ring neutrophils | 27 | 14 | 14 | 25 | 7.179 | 0.007 * |
| Dohle bodies | 36 | 5 | 28 | 11 | 3.20 | 0.074 |
| Monolobated neutrophils | 21 | 10 | 20 | 19 | 0.00 | 0.996 |
| Reactive lymphocytes | 10 | 31 | 13 | 26 | 0.720 | 0.377 |
| Plasmacytoid lymphocytes | 32 | 9 | 34 | 5 | 1.164 | 0.283 |
| Large granular lymphocytes | 30 | 11 | 16 | 23 | 9.451 | 0.004 * |
| Atypical lymphocytes | 33 | 8 | 36 | 3 | 2.355 | 0.125 |
| Large platelets | 35 | 6 | 31 | 8 | 0.478 | 0.489 |
Abbreviations: AEC, absolute eosinophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; CBC, complete blood count; Hb, hemoglobin; ICU, intensive care unit; PBS, peripheral blood smear; TLC, total leucocyte count.
Statistically significant values (p ≤ 0.05).
No significant difference was noted in the CBC parameters between COVID‐19 positive ICU and non‐ICU groups. However, the overall mean WBC counts were higher in patients with ICU settings as compared to non‐ICU patients (WBC, 12.43 ± 1.5 vs. 10.8 ± 1.5 ×103/μl, p = 0.25) with absolute neutrophilia (mean ANC, 10.60 ± 1.3 vs. 5.32 ± 1.4 ×103/μl, p = 0.24). Absolute monocyte count (AMC) and AEC were lower but in normal range in patients with ICU settings as compared to non‐ICU group (AMC, 0.23 ± 0.04 vs. 0.27 ± 0.04 ×103/μl, p = 0.37; AEC, 0.08 ± 0.02 vs. 0.10 ± 0.02 ×103/μl, p = 0.53).
A significant number of the COVID‐19 positive ICU patients (66.66%, 26/39) showed leucocytosis with shift to left (46.15%, 18/39) as compared to non‐ICU group (p = 0.038 and p = 0.017, respectively). WBC series showed most striking morphological changes in COVID‐19 positive patients. The most common morphological findings were APHA (ICU, 76.92%, 30/39 and non‐ICU, 65.85%, 27/41), ring‐shaped neutrophils (ICU, 64.1%, 25/39 and non‐ICU, 34.14%, 14/41), monolobate neutrophils (ICU, 48.71%, 19/39 and non‐ICU, 24.3%, 10/41), smudged neutrophils (ICU, 41.02%, 16/39 and non‐ICU, 21.95%, 9/41), reactive lymphocytes (ICU, 66.66%, 26/39 and non‐ICU, 63.26%, 31/41), large granular lymphocytes (ICU, 58.97%, 23/39 and non‐ICU, 26.82%, 11/41) and monocyte vacuolization (ICU, 46.15%, 18/39 and non‐ICU, 24.30%, 10/41; Figures 1, 2, 3, 4).
Interestingly, ring‐shaped neutrophils, monocyte vacuolization, and large granular lymphocytes were more prevalent in patients with ICU settings as compared to non‐ICU group (p = 0.007, p = 0.008, and p = 0.004, respectively)
4.3. Comparison of hematological parameters and cellular morphology between COVID‐19 positive ICU survived and deceased patients (Table 3)
Table 3.
Age and sex distribution, CBC, and morphological parameters in COVID positive ICU survived patients and COVID positive ICU deceased patients.
| Survived (n = 25) | Deceased (n = 14) | Univariate analysis | ||
|---|---|---|---|---|
| Mean | Mean | T value | p value | |
| Age | 53.8 years | 61.58 years | 1.843 | 0.210 |
| M:F | 1.2:1 | 1:1.3 | 1.965 | 0.27 |
| Hb (g/dl) | 11.07 | 10.40 | 0.938 | 0.354 |
| TLC (/µl) | 10 460.0 | 17 364.29 | 2.470 | 0.018 * |
| ANC (/µl) | 8926.16 | 14 856.93 | 2.357 | 0.024 * |
| ALC (/µl) | 1082.08 | 1486.00 | 2.074 | 0.045 * |
| AMC (/µl) | 205.60 | 309.79 | 2.005 | 0.052 |
| AEC (/µl) | 62.20 | 70.36 | 0.247 | 0.806 |
| Platelets (/µl) | 2.2 × 105 | 2.4 × 105 | 0.486 | 0.636 |
| Morphological parameters | ||||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade =1 | Grade 0 | Grade =1 | Odds ratio | p value | |
| Left shift | 16 | 7 | 5 | 4 | 5.175 | 0.075 |
| Toxic granules | 17 | 8 | 3 | 11 | 9.232 | 0.010 * |
| Neutrophil vacuolation | 17 | 8 | 8 | 6 | 0.46 | 0.408 |
| Monocyte vacuolation | 6 | 19 | 2 | 12 | 0.519 | 0.471 |
| Lymphocyte vacuolation | 22 | 3 | 12 | 2 | 0.042 | 0.838 |
| Pseudo–Pelger–Huët anomaly | 4 | 21 | 5 | 9 | 1.965 | 0.161 |
| Hypersegmented neutrophils | 11 | 14 | 5 | 9 | 3.477 | 0.324 |
| Smudged neutrophils | 13 | 12 | 10 | 4 | 1.40 | 0.237 |
| Apoptotic neutrophils | 18 | 7 | 10 | 4 | 0.001 | 0.970 |
| Ring Neutrophils | 11 | 14 | 3 | 11 | 1.987 | 0.159 |
| Dohle bodies | 19 | 6 | 9 | 5 | 0.608 | 0.435 |
| Monolobated neutrophils | 12 | 13 | 8 | 6 | 0.30 | 0.584 |
| Reactive lymphocytes | 9 | 16 | 4 | 10 | 0.283 | 0.637 |
| Plasmacytoid lymphocytes | 23 | 2 | 11 | 3 | 1.448 | 0.229 |
| Large granular lymphocytes | 12 | 13 | 4 | 10 | 1.40 | 0.237 |
| Atypical lymphocytes | 23 | 2 | 13 | 1 | 0.009 | 0.923 |
| Large platelets | 20 | 5 | 11 | 3 | 0.011 | 0.960 |
Abbreviations: AEC, absolute eosinophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; CBC, complete blood count; Hb, hemoglobin; ICU, intensive care unit; PBS, peripheral blood smear; TLC, total leucocyte count.
Statistically significant values (p ≤ 0.05).
In COVID‐19 positive ICU patients, mortality was high (35.8% [14/39]) as compared to non‐ICU group where only 3 patients out of 41 (7.3%) died of the disease during the study period.
The WBC count was significantly high in the deceased group (17.36 ± 1.1 vs. 10.46 ± 0.6 ×103/μl, p = 0.018) with marked neutrophilia (14.85 ± 1.0 vs. 8.92 ± 0.5 ×103/μl, p = 0.024) and significant toxic granulation (78.57%, 11/14 vs. 32%, 8/25, p = 0.01) (Figure 5). ALC and absolute monocyte count were significantly lower but in the normal range in survivors as compared to the deceased group (p = 0.045 and p = 0.05, respectively). On PBS, the majority of the deceased group showed monocyte vacuolization (12/14), ring‐shaped neutrophils (11/14), large granular lymphocytes (10/14), and reactive lymphocytes (10/14) as compared to survivors. However, no significant difference was noted which could be due to the limited sample size.
Figure 5.
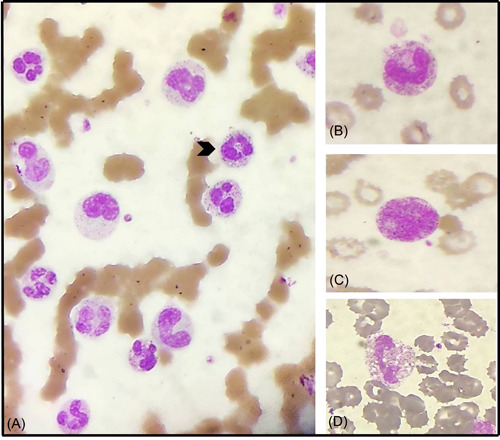
Toxic changes in the neutrophils in a peripheral blood smear from different COVID patients: (A) numerous neutrophils with occasional toxic granules Grade 1 (arrowhead), (B) toxic granules Grade 2, (C) toxic granules Grade 4, and (D) neutrophilic vacuolation (Leishman, x400)
5. DISCUSSION
Coronavirus disease (COVID‐19) is an infectious disease with both pulmonary and extra‐pulmonary manifestations. 11 Only a handful of published data have shown that COVID‐19 also affects blood cells and causes a spectrum of hematological and morphological changes which are distinct in patients depending on the disease severity. 7 , 8 , 9 , 11 , 12 The current study showed a significant difference in CBC and peripheral smear morphology between the COVID‐19 positive group and the control group as well as in COVID‐19 patients in ICU and non‐ICU settings.
This study proposes the use of basic and simple routine investigation, that is, CBC with peripheral smear examination in monitoring COVID‐19 patients and predicting disease severity and its course thus, ensuring medical interventions at the earliest in hospitalized patients.
In the current study, COVID‐19 positive ICU patients were about a decade older than the non‐ICU patients which are similar to that reported by Fan et al. 13 where the median age of the ICU patients was 54 years old while that of non‐ICU patients was 42 years old confirming that age was an independent risk factor for severity of COVID‐19 infection. 13
Clinical presentation with respiratory symptoms and underlying comorbidities of hypertension and diabetes mellitus was significantly associated with the severity of COVID‐19 disease in the current study which was in agreement with the studies of Hassan et al., Lian et al., and Gupta et al. 12 , 14 , 15 Therefore, patients with above symptoms and coexisting morbidities should be taken seriously and strict measures should be taken at the time of presentation to prevent the disease progression.
Till now, the laboratory findings reported in COVID‐19 patients include leucocytosis, leucopenia, lymphopenia, neutrophilia, monocytosis, and eosinopenia. 3 In the current study COVID‐19 positive patients had relatively lower ALC and AEC as compared to controls; however, the values were within the normal range which was in disagreement with other studies. Moreover, there was no significant difference noted in ALC among the two groups of COVID‐19 positive patients in this study. Tan et al. 16 reported lymphopenia as a predominant hematologic finding in COVID‐19 which predicts disease severity. Similar to the study of Fan et al., 13 lymphopenia was seen in 38.75% of COVID‐19 positive patients. 13 Various hypotheses exist for the mechanism of lymphopenia including cytokine‐induced lymphocyte apoptosis and viral‐induced direct lymphocyte inhibition. In severe COVID‐19 patients, AMC and AEC were significantly lower as compared to mild/moderate cases, that is, in non‐ICU settings. This was in concordance with the studies which have reported lower monocyte counts and eosinophils counts were associated with disease severity. 13 , 17 , 18 , 19 It has been postulated that due to significant elevation of CXCL10 and CCL2 in critically ill COVID‐19 positive patients, there may be the recruitment of monocytes to the sites of inflammation (i.e., lungs) leading to monocytopenia. 16 Higher viral load of SARS‐CoV‐2 in severe cases causes eosinopenia by depletion of CD8 T cells which contributes to eosinophil proliferation and activation by secreting interleukin‐5, and also virus triggered eosinophil granule protein consumption. 18
Interestingly, in the current study, COVID‐19 survivors had significantly lower ALC and absolute monocyte count as compared to the non‐survivors. This shows that not all the severe cases with lower ALC and AMC have a poor disease course.
On PBSs review, COVID‐19 positive cases showed significant morphological variations mainly in the leukocytes in form of left myeloid shift, Dohle bodies, acquired pseudo–Pelger–Huët anomaly, ring‐shaped neutrophils, monolobate neutrophils, and plasmacytoid lymphocytes as compared to controls. These findings were similar to the studies by Zini et al., Singh et al., and Nazarullah et al. 5 , 6 , 8 Hyper segmentation of neutrophils was equally noted in both the groups (47.5% vs. 50.0%, p = 0.880). Salib et al. reported hypersegmented neutrophils in 88% of the COVID‐19 patients while Lakhdari N et al reported them in 66.66% of the COVID‐19 patients. 20 , 21 Previous studies have reported hypersegmented neutrophils in cases of acute respiratory distress syndrome, inflammation, and severe viral respiratory infection. 22 , 23 Since, the control group in the current study was of ICU settings therefore no significant difference was noted between the two groups. WBC morphology changes were also distinct among the two groups of COVID‐19 positive patients as well as between the survivor and deceased groups. Leucocytosis with a myeloid shift to left was significantly associated with disease severity in the current study. SARS‐CoV‐2 induced cytokines release, direct virus‐induced myelocytotoxicity, and overproduction of marrow in response to stress/inflammation causing mobilization of marrow reserves in the peripheral blood circulation are a few hypotheses explaining the reason for granulocytic left shift akin to bacterial infections in severe cases. Sharif et al, in their study, reported that neutrophilic leucocytosis was significantly associated with the development of acute respiratory distress syndrome, mortality, and need for a ventilator. 24 Most of the deceased patients in the current study showed significant leucocytosis with marked neutrophilia and toxic granulation as compared to survivors, indicating that these patients might have a secondary bacterial infection as an underlying cause of death.
A leukoerythroblastic blood picture was reported by Mitra et al in a 46‐year‐old COVID‐19 positive patient. 25 In our study, only one case showed peripheral blood leukoerythroblastosis which could be attributed to the marrow stress due to the viral infection.
A finding unique to this study was the significant association of ring‐shaped neutrophils with COVID‐19 disease and its clinical severity (p = 0.009 and p = 0.007, respectively). Only 7 out of 30 controls showed ring‐shaped neutrophils on peripheral smear. Langenhujsen et al. 26 found neutrophils with ring‐shaped nuclei in 12 out of 28 patients with myeloproliferative disease and only rarely in patients with toxemic granulocytosis. 26 Ring‐shaped neutrophils in a COVID‐19 patient were also observed by Singh et al. 8 Presence of ring‐shaped neutrophils can be an important morphological marker of abnormal granulopoiesis indicating progression of disease severity. However, the underlying pathophysiology and mechanism of this process need to be studied. There was also significant prominence of monocyte vacuolization and large granular lymphocytes in severe COVID‐19 positive cases as compared to those in non‐ICU settings. Zhang et al. 27 described the presence of large, atypical, vacuolated monocytes in circulation which correlated with the disease outcome. 27 LGLs play an important role in the cytotoxic response to specific antigens, especially in viral infections like Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). 28 Majority of these cells are derived from natural killer cells and few are cytotoxic T cells subsets. Nazarullah et al. 6 reported LGLs in 11 out of 12 COVID‐19 patients. 6
The deceased COVID‐19 patients in the current study showed a higher number of monocyte vacuoles (85.71%), ring‐shaped neutrophils (78.57%), and larger granular lymphocytes (71.42%) as compared to survivors. Previous studies have shown that the patients with the presence of monocytes having cytoplasmic vacuoles had a shorter hospitalization duration. 29 In this study, the presence of ring‐shaped neutrophils, monocyte vacuoles, and LGLs correlated with disease severity, however, it was not significantly associated with disease outcome. This might be due to the limited sample size or due to ICU settings of both deceased and survivor groups as mortality in the COVID positive non‐ICU group was very low. Thus we would like to propose further studies on a larger cohort of patients and with a serial follow‐up period to understand the underlying COVID‐19 pathogenesis and to establish the relationship between the above morphologic parameters and disease outcome.
6. CONCLUSION
COVID‐19 disease patients have a specific spectrum of hematological and peripheral smear morphological findings as compared to the control group. Among these parameters, myeloid left shift, ring‐shaped neutrophils, monocyte vacuolation, and large granular lymphocytes emerged as highly sensitive markers for COVID‐19 disease severity in hospitalized patients. Therefore, serial CBC with comprehensive PBS analysis for morphological parameters should be done in every newly diagnosed hospitalized COVID‐19 patient to potentially predict the course of the disease and its clinical severity.
AUTHOR CONTRIBUTIONS
Concept: Swasti Jain, Rachana Meena, Vijay Kumar, Ranvinder Kaur. Design: Swasti Jain, Rachana Meena, Umesh Tiwari. Data collection and processing: Swasti Jain, Rachana Meena, Umesh Tiwari, Ranvinder Kaur. Analysis and interpretation: Swasti Jain, Rachana Meena. Literature review: Swasti Jain, Rachana Meena, Vijay Kumar. Writing: Swasti Jain, Rachana Meena, Vijay Kumar.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the institutional ethics committee of ABVIMS and RMLH (File number: 478(14/2021)/IEC/ABVIMS/RMLH).
Jain S, Meena R, Kumar V, Kaur R, Tiwari U. Comparison of hematologic abnormalities between hospitalized coronavirus disease 2019 positive and negative patients with correlation to disease severity and outcome J Med Virol. 2022;94:3757‐3767. 10.1002/jmv.27793
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus disease (COVID‐19) dashboard. WHO. Accessed November 15, 2021. Online Version, 2021.
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐ Ocampo E, et al. Clinical, laboratory and imaging features of COVID 19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020;95:870‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nazarullah A, Liang C, Villarreal A, Higgins RA, Mais DD. Peripheral blood examination findings in SARS‐CoV‐2 infection. Am J Clin Pathol. 2020;154:319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pozdnyakova O, Connell NT, Battinelli EM, Connors JM, Fell G, Kim AS. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID‐19 infection. Am J Clin Pathol. 2021;155(3):364‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh A, Sood N, Narang V, Goyal A. Morphology of COVID‐19‐affected cells in peripheral blood film. BMJ Case Rep. 2020;13:e236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadigh S, Massoth LR, Christensen BB, Stefely JA, Keefe J, Sohani AR. Peripheral blood morphologic findings in patients with COVID‐19. Int J Lab Hematol. 2020;42:e248‐e251. [DOI] [PubMed] [Google Scholar]
- 10. Vyver AVD, Delport EF, Esterhuizen M, Pool R. The correlation between C‐ reactive protein and toxic granulation of neutrophils in the peripheral blood. S Afr Med J. 2010;100:442‐444. [DOI] [PubMed] [Google Scholar]
- 11. Sayed AA, Allam AA, Sayed AI, Alraey MA, Joseph MV. The use of neutrophil‐to‐lymphocyte ratio (NLR) as a marker for COVID‐19 infection in Saudi Arabia. Saudi Med J. 2021;42:370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID‐19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12:e7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95:e131‐e134. [DOI] [PubMed] [Google Scholar]
- 14. Lian J, Jin X, Hao S, et al. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID‐19) from Zhejiang province in China. Influenza Other Respir Viruses. 2020;14:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta N, Agrawal S, Ish P, et al. Clinical and epidemiologic profile of the initial COVID‐19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90:1. [DOI] [PubMed] [Google Scholar]
- 16. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 18. Nair AP, Soliman A, Masalamani MAA, et al. Clinical outcome of eosinophilia in patients with COVID‐19: a controlled study. Acta Biomed. 2020;91(4):e2020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salib C, Feldstein JT. Hypersegmented granulocytes and COVID‐19 infection. Blood. 2020;135:2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakhdari N, Tablet B, Boudraham L, et al. Red blood cells injuries and hypersegmented neutrophils in COVID‐19 peripheral blood film. medRxiv . 10.1101/2020.07.24.20160101 [DOI]
- 22. Juss JK, House D, Amour A, et al. Acute respiratory distress syndrome neutrophils have a distinct phenotype and are resistant to phosphoinositide 3‐kinase inhibition. Am J Respir Crit Care Med. 2016;194(8):961‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortjens B, Ingelse SA, Calis JC, et al. Neutrophil subset responses in infants with severe viral respiratory infection. Clin Immunol. 2017;176:100‐106. [DOI] [PubMed] [Google Scholar]
- 24. Sharif F, Khan S, Junaid A, et al. Early hematological indicators of severe COVID‐19 disease in hospitalized patients: data from a South Asian population. Int J Lab Hematol. 2021;43(5):1237‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID‐19 infection. Am J Hematol. 2020;95:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langenhuijsen MM. Neutrophils with ring‐shaped nuclei in myeloproliferative disease. Br J Haematol. 1984;58:227‐230. [DOI] [PubMed] [Google Scholar]
- 27. Zhang D, Guo R, Lei L, et al. COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes. J Leukocyte Biol. 2021;109(1):13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zambello R, Semenzato G. Large granular lymphocyte disorders: new etiopathogenetic clues as a rationale for innovative therapeutic approaches. Haematologica. 2009;94:1341‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berberi I, Cagasar O, Sarici A, et al. Peripheral blood smear findings of COVID‐19 patients provide i̇nformation about the severity of the disease and the duration of hospital stay. Mediterr J Hematol Infect Dis. 2021;13(1):e2021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


