Abstract
There is growing evidence that angiotensin‐converting enzyme 2 is highly expressed on endothelial cells, endothelial dysfunction plays a critical role in coronavirus disease 2019 (COVID‐19) progression, but laboratory evidence is still lacking. This study established a multicenter retrospective cohort of 966 COVID‐19 patients from three hospitals in Wuhan, China. We found that male (62.8% vs. 46.5%), old age [72 (17) vs. 60.5 (21)], and coexisting chronic diseases (88.5% vs. 60.0%) were associated with poor clinical prognosis in COVID‐19. Furthermore, the deteriorated patients exhibited more severe multiorgan damage, coagulation dysfunction, and extensive inflammation. Additionally, a cross‐sectional study including 41 non‐COVID‐19 controls and 39 COVID‐19 patients assayed endothelial function parameters in plasma and showed that COVID‐19 patients exhibited elevated vascular cell adhesion molecule‐1 (VCAM‐1) (median [IQR]: 0.32 [0.27] vs. 0.17 [0.11] μg/ml, p < 0.001), E‐selectin (21.06 [12.60] vs. 11.01 [4.63] ng/ml, p < 0.001), tissue‐type plasminogen activator (tPA) (0.22 [0.12] vs. 0.09 [0.04] ng/ml, p < 0.001), and decreased plasminogen activator inhibitor‐1 (0.75 [1.31] vs 6.20 [5.34] ng/ml, p < 0.001), as compared to normal controls. Moreover, VCAM‐1 was positively correlated with d‐dimer (R = 0.544, p < 0.001); tPA was positively correlated with d‐dimer (R = 0.800, p < 0.001) and blood urea nitrogen (R = 0.638, p < 0.001). Our findings further confirm the strong association between endothelial dysfunction and poor prognosis of COVID‐19, which offers a rationale for targeting endothelial dysfunction as a therapeutic strategy for COVID‐19.
Keywords: COVID‐19, endothelial dysfunction, laboratory evidence, prognosis
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pneumonia epidemic has spread rapidly around the world since its occurrence. It poses an even more severe threat to global public health with the emergence of “super variants.” 1 , 2 , 3 , 4 , 5 , 6 A proportion of patients experience rapid disease exacerbation and even death during hospitalization for reasons that have not been fully elucidated. Angiotensin‐converting enzyme 2 (ACE2) is a primary host target of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which is widely expressed on the vascular endothelial cells of the lung, kidney, heart, and intestine. 7 A growing body of research has proposed that endothelial dysfunction may be an essential factor in the rapid progression of COVID‐19, but laboratory evidence is still lacking. 8 , 9 , 10
The endothelium plays a crucial role in maintaining the dynamic balance between procoagulants and fibrinolytic factors in the vascular system. Resting endothelial cells maintain vascular homeostasis by expressing antiplatelet and anticoagulant agents to inhibit platelet aggregation and fibrin formation. Events such as SARS‐CoV‐2 infection could activate the endothelium, which releases more procoagulant factors, triggering fibrin formation, as well as platelet adhesion and aggregation, promoting and exacerbating diffuse microvascular and macrovascular thrombosis. 9 , 11 Klok et al. 12 reported a 31% incidence of thrombotic complications in the intensive care unit patients with COVID‐19. Autopsy findings from many locations reported the formation of deep vein thrombosis and pulmonary thromboembolism in COVID‐19 decedents. 13 , 14 , 15 , 16 Activated endothelial cells also release leukocyte adhesion molecules, pro‐inflammatory cytokines, and chemokines which induce COVID‐19‐associated endotheliitis. 8 Varga et al. 17 have revealed the accumulation of inflammatory cells associated with endothelium and apoptotic bodies in the lung, heart, kidney, liver, and small intestine in autopsies of COVID‐19 patients. The presence of thrombosis and endotheliitis in different organs probably explains the clinical manifestations of multiorgan failure in patients with severe COVID‐19. Endothelial adhesion molecules that mediate vascular inflammation, including E‐selectin, p‐selectin intercellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), pro‐inflammatory cytokines (tumor necrosis factor‐α, interleukin 6 [IL‐6]) and pro‐inflammatory chemokines (IL‐8), and pro‐coagulation factors, including von Willebrand factor, plasminogen activator inhibitor‐1 (PAI‐1), play important roles in the aforementioned inflammation and coagulation dysfunction associated with COVID‐19, suggesting that these soluble mediators may serve as biomarkers of endothelial activation and endotheliitis. 8
To investigate the relationship between endothelial disorders and disease progression in patients with COVID‐19, we first compared the demographic characteristics of COVID‐19 patients with different outcomes. We then analyzed their multiple laboratory indicators, including organ function (liver, kidney, and heart), coagulation function, inflammatory status, and hematological indicators. Finally, we examined endothelial function parameters in plasma of COVID‐19 patients versus non‐COVID‐19 controls by enzyme‐linked immunosorbent assay (ELISA) and explored the correlation of these parameters with disease severity. This study is expected to provide further laboratory evidence for the connection between endothelial dysfunction and disease progression in COVID‐19.
2. MATERIALS AND METHODS
2.1. Study design and data source
A multicenter retrospective cohort study was established in three hospitals (Tianyou Hospital, Wuhan University of Science and Technology; Puren Hospital, Wuhan University of Science and Technology; and Wugang Hospital) from Wuhan, China. Adult patients (older than 18 years) hospitalized with laboratory‐detected SARS‐CoV‐2 infection (confirmed by reverse‐transcription polymerase chain reaction) were recruited from January 13 to March 9, 2020. Participant information (including demographic data, medical history, exposure history, pre‐existing complications, symptoms, laboratory findings, chest computed tomography scans, and treatment) was retrospectively obtained and reviewed from the electronic medical records by two doctors (Li H. and Long H.), as shown in Table 1. Laboratory findings were tested within the first 3 days following admission (Table 2). The endpoint of this study was 14 days after access (including patients who were discharged or died during this period). The cross‐sectional study including 39 COVID‐19 patients and 41 age‐ and sex‐matched non‐COVID‐19 subjects was established in Tianyou Hospital. Blood specimens were collected at the time of the patient's initial diagnosis of COVID‐19 or physical examination. The clinical characteristics of these participants are described in Table 3. The flow chart of this study is shown in Figure 1. All subjects provided broad informed consent for research use of their biological samples. The Medical Ethics Review Board of Wuhan University of Science and Technology approved this study (No. 202009).
Table 1.
Demographic characteristics of COVID‐19 patients in this study
| Characteristics | Recovered/improved (n = 888) | Deteriorated/died (n = 78) |
|---|---|---|
| Age (years) | 60.5 (21) | 72 (17) |
| Sex | ||
| Male | 413 (46.51%) | 49 (62.82%) |
| Female | 475 (53.49%) | 29 (37.18%) |
| Symptoms on admission | ||
| Cough | 587 (66.10%) | 49 (62.82%) |
| Fever | 618 (69.59%) | 55 (70.51%) |
| Fatigue | 198 (22.30%) | 29 (37.18%) |
| Coexisting chronic diseases | 527 (59.95%) (n = 879) | 69 (88.46%) (n = 78) |
Note: Data are number (%) or median (IQR). Coexisting chronic diseases include cancer, cardiovascular diseases, diabetes, chronic kidney diseases, chronic pulmonary diseases, autoimmune diseases, etc.
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Table 2.
Laboratory findings on admission for different groups
| Variables | Recovered/improved | Deteriorated/died | p Value |
|---|---|---|---|
| Liver function markers | n = 858 | n = 75 | |
| ALT (U/L) | 24.00 (19.00) | 27.00 (21.00) | 0.036 |
| AST (U/L) | 25.00 (15.90) | 43.00 (34.60) | <0.001 |
| GGT (U/L) | 25.00 (22.00) | 33.00 (41.00) | 0.005 |
| ALP (U/L) | 70.00 (30.80) | 81.00 (56.00) | 0.005 |
| TBIL (μmol/L) | 10.00 (5.00) | 12.00 (9.00) | 0.010 |
| DBIL (μmol/L) | 3.00 (3.00) | 5.00 (5.00) | <0.001 |
| TP (g/L) | 65.00 (8.00) | 64.00 (8.00) | 0.878 |
| ALB (g/L) | 40.00 (7.00) | 35.50 (6.00) | <0.001 |
| GLB (g/L) | 25.00 (7.00) | 30.00 (6.00) | <0.001 |
| TBA (μmol/L) | 3.20 (3.09) | 3.50 (4.50) | 0.058 |
| Renal function markers | n = 826 | n = 67 | |
| BUN (mmol/L) | 4.20 (2.10) | 7.80 (7.90) | <0.001 |
| Cr (μmol/L) | 63.00 (24.00) | 79.00 (57.00) | <0.001 |
| Cys_C (mg/L) | 0.94 (0.32) | 1.29 (0.72) | <0.001 |
| Cardiac function markers | n = 593 | n = 38 | |
| CK (U/L) | 79.00 (86.00) | 121.50 (365.00) | 0.006 |
| LDH (U/L) | 214.00 (123.00) | 376.00 (269.5) | <0.001 |
| Inflammation markers | n = 605 | n = 58 | |
| Procalcitonin (μg/L) | 0.03 (0.05) | 0.14 (0.89) | <0.001 |
| SAA (mg/L) | 72.00 (195.80) | 200.00 (83.80) | <0.001 |
| CRP/hsCRP (mg/L) | 6.80 (32.40) | 69.00 (119.50) | <0.001 |
| Routine blood markers | n = 711 | n = 57 | |
| WBC (×109/L) | 5.20 (2.54) | 8.62 (6.87) | <0.001 |
| L (×109/L) | 1.23 (0.83) | 0.61 (0.50) | <0.001 |
| N (×109/L) | 3.33 (2.02) | 7.44 (6.88) | <0.001 |
| Coagulation parameters | n = 806 | n = 71 | |
| PT (s) | 12.10 (1.50) | 12.80 (2.00) | <0.001 |
| APTT (s) | 29.20 (7.90) | 31.00 (14.40) | 0.006 |
| TT (s) | 15.10 (2.30) | 15.60 (2.90) | 0.055 |
| FIB (g/L) | 3.70 (2.00) | 4.90 (2.15) | <0.001 |
| INR | 1.02 (0.13) | 1.08 (0.19) | <0.001 |
| d‐dimer (mg/L) | 0.33 (0.54) | 0.96 (3.92) | <0.001 |
Note: Data are median (IQR).
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; Cr, creatinine; CRP, C‐reactive protein; Cys_C, cysteine C; DBIL, direct bilirubin; FIB, fibrinogen; GGT, gamma‐glutamyl transpeptidase; GLB, globulin; hsCRP, high‐sensitivity C‐reactive protein; INR, international normalized ratio; IQR, interquartile range; L, lymphocyte counts; LDH, lactate dehydrogenase; N, neutrophil counts; PT, prothrombin time; SAA, serum amyloid A; TBA, total bile acid; TBIL, total bilirubin; TP, total protein; TT, thrombin time; WBC, white blood cell.
Table 3.
Demographic characteristics of participates in endothelial function detection assay
| Characteristics | COVID‐19 patients (n = 39) | Non‐COVID‐19 subjects (n = 41) |
|---|---|---|
| Age (years) | 57 (26) | 53 (21.5) |
| Sex | ||
| Male | 21 (53.85%) | 20 (48.78%) |
| Female | 18 (46.15%) | 21 (51.22%) |
| Coexisting chronic diseases | 22 (56.41%) | 22 (53.66%) |
Note: Data are number (%) or median (IQR). Coexisting chronic diseases include cancer, cardiovascular diseases, diabetes, chronic kidney diseases, chronic pulmonary diseases, autoimmune diseases, etc.
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Figure 1.
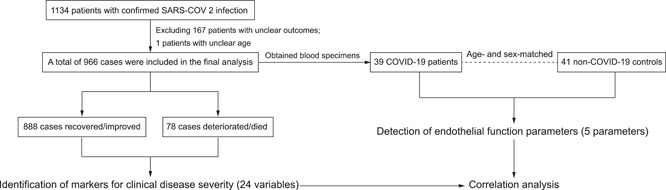
Study flow chart
2.2. Endothelium dysfunction indicators detection assay
Contemporaneous blood specimens were obtained from age‐ and sex‐matched patients with COVID‐19 (n = 39) and non‐COVID‐19 subjects (n = 41) to investigate endothelial function. In all cases, a fasting blood sample was collected into test tubes containing EDTA in the morning and centrifuged at 3000g and 4°C for 10 min. Then, the blood plasma was collected and inactivated, portioned into 0.5 ml aliquots, and stored at −80°C until the assays were performed. Endothelin‐1 (R&D Systems), VCAM‐1 (Solarbio), E‐selectin (Solarbio), PAI‐1 (Solarbio), and tissue‐type plasminogen activator ([tPA] Solarbio) were measured in plasma samples by ELISA according to the manufacturers' recommended protocols. All experiments with inactivated samples were done in a biosafety level (BSL) 2+ laboratory with BSL‐3 protection.
2.3. Statistics
Patients' clinical characteristics and laboratory findings were given the median (interquartile range) for continuous variables and the number (%) for categorical variables. The Wilcoxon rank‐sum test was used for quantitative data not conforming to a normal distribution. For categorical data, the χ 2 test was used. Pearson correlations examined relationships between variables, and false discovery rate was used to correct multiple comparisons. Graphical abstract was created using graphics from www.Biorender.com.
Missing data were omitted in the analysis of clinical indicators for different groups, and all statistical analyses were performed using SAS statistical software (version 9.4). We set the level of statistical significance at 5%, and all statistical tests were two‐tailed.
3. RESULTS
3.1. Demographic characteristics of COVID‐19 patients in this cohort
Between January 13 and March 9, 2020, a total of 966 patients with laboratory‐confirmed COVID‐19 were enrolled, 888 (91.93%) of these patients were recovered/improved, and 78 (8.07%) were deteriorated/died. The clinical characteristics of the study cohort at baseline are described in Table 1. As previously reported, males (62.82% vs. 46.51%) predominated in the deteriorated/died group compared with the recovered/improved group, and the patients in the deteriorated/died group were significantly older than those in the recovered/improved group [72 (17) vs. 60.5 (21)]. Coexisting chronic diseases had higher frequencies in the deteriorated/died group (88.46%) than in the recovered/improved group (59.95%). There was no significant difference in common COVID‐19 symptoms reported cough and fever between the groups.
3.2. Laboratory findings of patients in this cohort at admission
On admission, compared with the recovered/improved group, the deteriorated/died group had significantly higher white blood cell (WBC) and neutrophil counts and more pronounced lymphopenia. In addition, they also exhibited more severe multiorgan impairment, mainly manifested by significantly elevated serum transaminases (alanine aminotransferase [ALT], p = 0.036; aspartate aminotransferase [AST], p < 0.001) and bilirubin (total bilirubin [TBIL], p = 0.010; direct bilirubin [DBIL], p < 0.001) in liver function, creatine kinase and lactate dehydrogenase in cardiac function, and blood urea nitrogen (BUN, p < 0.001), creatinine (p < 0.001), and cysteine C (p < 0.001) in renal function. The deteriorated/dead patients also exhibited coagulation dysfunction, including significantly prolonged prothrombin time (PT, p < 0.001) and activated partial thromboplastin time (APTT, p = 0.006), as well as meaningfully elevated fibrinogen (p < 0.001) and d‐dimer (p < 0.001) levels. Apart from that, their C‐reactive protein (CRP, p < 0.001), procalcitonin (p < 0.001), and serum amyloid A (SAA, p < 0.001) levels (markers of the acute inflammatory response) were also significantly elevated. The differences in laboratory findings are detailed in Table 2. These results indicated that extensive multiorgan damage, coagulation dysfunction, and systemic inflammation are important reasons for the aggravation of COVID‐19 or even death.
3.3. COVID‐19 patients exhibited abnormal endothelial function parameters
To investigate whether the endothelial function is aberrant in COVID‐19 patients, we tested endothelial function‐related markers in the plasma of 39 COVID‐19 patients and 41 non‐COVID‐19 controls using ELISA. The results showed that plasma PAI‐1 (median [IQR], 0.75 [1.31] vs 6.20 [5.34] ng/ml, p < 0.001) was statistically significantly diminished in COVID‐19 patients compared to normal controls. At the same time, VCAM‐1 (0.32 [0.27] vs. 0.17 [0.11] μg/ml, p < 0.001), E‐selectin (21.06 [12.60] vs. 11.01 [4.63] ng/ml, p < 0.001), and tPA (0.22 [0.12] vs. 0.09 [0.04] ng/ml, p < 0.001) were augmented, and endothelin‐1 (1.40 [0.95] vs.1.31 [0.65] pg/ml, p = 0.127) tended to be increased but not meaningful (Figure 2), suggesting that COVID‐19 patients suffer from abnormal endothelial function. Correlation analysis showed that plasma levels of VCAM‐1 in COVID‐19 patients were significantly and positively correlated with d‐dimer (R = 0.544, p < 0.001); and tPA levels were positively correlated with d‐dimer (R = 0.800, p < 0.001) and BUN (R = 0.638, p < 0.001) (Figure 3). In brief, these parameters of endothelial function were significantly associated with markers of clinical disease severity (d‐dimer and BUN). These findings highlight the strong association of abnormal endothelial function with COVID‐19 disease progression.
Figure 2.
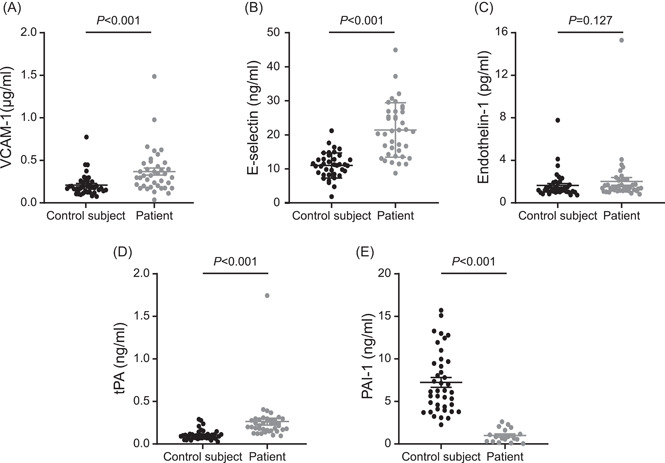
Patients with COVID‐19 exhibit endothelial dysfunction. (A) The plasma concentration levels of VCAM‐1 in patients with COVID‐19 and non‐COVID‐19 control subjects. (B) The plasma concentration levels of E‐selectin in patients with COVID‐19 and non‐COVID‐19 control subjects. (C) The plasma concentration levels of endothelin‐1 in patients with COVID‐19 and non‐COVID‐19 control subjects. (D) The plasma concentration levels of tPA in patients with COVID‐19 and non‐COVID‐19 control subjects. (E) The plasma concentration levels of PAI‐1 in patients with COVID‐19 and non‐COVID‐19 control subjects. All data presented as the mean ± SEM. Differences were tested using unpaired two‐tailed Mann–Whitney test. COVID‐19, coronavirus disease 2019; PAI‐1, plasminogen activator inhibitor‐1; tPA, tissue‐type plasminogen activator; VCAM‐1, vascular cell adhesion molecule‐1
Figure 3.
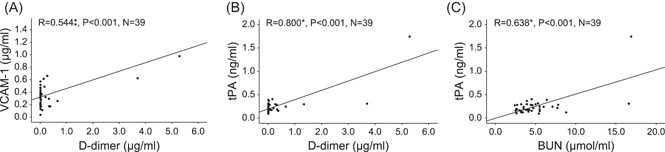
Positive correlations between indicators of endothelial function and clinical markers of disease severity. (A) Positive correlations between plasma concentrations of VCAM‐1 and clinical laboratory indices of d‐dimer. Positive correlations between plasma concentrations of tPA and clinical laboratory indices of (B) d‐dimer and (C) BUN. R for Pearson's correlation coefficient, *p < 0.001. BUN, blood urea nitrogen; tPA, tissue‐type plasminogen activator; VCAM‐1, vascular cell adhesion molecule‐1
4. DISCUSSION
In this retrospective study, we found that the poor prognosis of COVID‐19 patients may be associated with male, old age, chronic disease, multiorgan damage, extensive inflammation, and abnormal coagulation. Further, our laboratory data showed that COVID‐19 patients exhibit abnormal endothelial function compared to non‐COVID‐19 controls and endothelial function‐related parameters significantly correlate with COVID‐19 disease severity. These findings provide further evidence for the strong association between endothelial dysfunction and poor prognosis of COVID‐19 and provide a rationale for targeting endothelial dysfunction as a therapeutic strategy for COVID‐19.
An emerging body of research has indicated that abnormal coagulation and inflammation may be important reasons for the deterioration or death of COVID‐19 patients. 18 , 19 , 20 , 21 Masi et al. 18 reported that the systemic inflammatory response is a crucial contributor to COVID‐19‐associated coagulopathy. d‐dimer and consumptive coagulopathy are reported to be indicators of mortality. 20 This study found a significant increase in WBC counts and a decrease in lymphocyte counts in COVID‐19 patients who eventually deteriorated/died compared to those who recovered/improved. CRP and SAA, markers of the acute inflammatory response, were significantly elevated, which may be due to excessive activation and rapid depletion of lymphocytes in the acute phase. In addition, our findings revealed that compared with recovered/improved patients, the deteriorated/died patients had reduced platelet counts, prolonged PTs, and elevated d‐dimer and fibrinogen levels, exhibiting abnormal coagulation. Our findings further confirm that inflammation and coagulation are vital contributors to the poor prognosis of COVID‐19 patients.
The underlying chronic inflammatory features and comorbidities may lead to the upregulation of specific ACE2‐related molecules, potentially predisposing patients to SARS‐CoV‐2 infection. 22 Some individual factors, such as age and coexisting chronic diseases, also affect vascular endothelial function. 23 , 24 Previous studies have reported that ACE2‐mediated SARS‐CoV‐2 entry into the body may activate endothelial cells. 24 , 25 , 26 Endothelial cell activation leads to increased cytokines and adhesion molecules that trigger leukocyte homing, adhesion, and migration to the vascular endothelium. 27 VCAM‐1, E‐selectin, and endothelin‐1 are well‐known biomarkers of endothelial activation or impairment. Especially, E‐selectin is probably the most specific for endothelial activation, 28 and we found here that plasma E‐selectin concentrations were significantly higher in COVID‐19 patients than non‐COVID‐19 controls. Endothelial cell activation is also involved in procoagulation, marked by a disturbed tPA and PAI‐1 balance, promoting coagulation and platelet activation. This theory is mainly based on the clinical features of COVID‐19 patients and the current knowledge of the ACE2 target. However, there is only a limited amount of direct laboratory evidence to support this view. Previous studies have compared endothelial function parameters, such as VCAM‐1, in COVID‐19 patients with different clinical outcomes. 29 , 30 , 31 , 32 Still, the relationship of these parameters with other markers of clinical disease severity has not been investigated. The causal relationship between endothelial disorders and disease progression remains elusive. In the present study, we compared endothelial function in COVID‐19 patients and non‐COVID‐19 controls. We found that COVID‐19 patients exhibited abnormal endothelial function compared to controls, suggesting that abnormal endothelial function may be associated with the onset of COVID‐19. Moreover, there was a significant association between these endothelial function parameters and clinical markers of disease severity (d‐dimer and BUN), indicating that endothelial dysfunction may be involved in the progression of COVID‐19. These findings provide direct evidence for the strong correlation between abnormal endothelial function and exacerbation/death in COVID‐19. Abnormal activation of endothelial cells caused by viral infection (including variants) or other reasons may trigger vascular dysfunction in patients with COVID‐19, resulting in systemic inflammation, coagulation dysfunction, and multiorgan damage, ultimately leading to the aggravation of the disease or even death.
Several mutations in the “super variants” occur in the critical antigenic regions of the receptor‐binding protein. In contrast, the receptors for viral invasion into the host are usually unaltered, 33 , 34 suggesting that the pathogenesis of COVID‐19 is well conserved, and endothelial disturbances may be prevalent in infected individuals of various variants. Multiple therapies targeting endothelial disorders have been used to alleviate symptoms and protect multiorgan function in patients with COVID‐19, especially those with severe conditions and poor prognosis. 9 , 35 The renin–angiotensin–aldosterone system (RAAS) inhibitors, including ACE inhibitors and angiotensin receptor blockers, have been proven to improve endothelial dysfunction. 36 , 37 A UK cohort study revealed that RAAS inhibitors were associated with a lower incidence risk of COVID‐19. 38 A Spanish study found that the use of ACE inhibitors significantly reduced the risk of COVID‐19 hospital admissions, and those diabetic persons with RAAS inhibitors had a lower risk than nonusers. 39 Further, RAAS inhibitors were reported with a trend to reduce COVID‐19‐related mortality. 40 Statins are another group of drugs for the treatment of endothelial dysfunction and prevention of vascular damage. A retrospective cohort study from a Chinese population showed lower mortality in patients with COVID‐19 using statins versus non‐users. 41 Our data showed that COVID‐19 patients exhibit aggravated endothelial dysfunction compared to non‐COVID‐19 controls, which provides new laboratory evidence for therapeutic strategies targeting endothelial dysfunction in COVID‐19.
Although our results provide new laboratory evidence for the view that endothelial dysfunction correlates with poor prognosis in COVID‐19 patients, several limitations should be noted in interpreting the results. First, due to limited blood samples, we only compared the differences in endothelial function indicators between COVID‐19 patients and non‐COVID‐19 controls. We did not subgroup COVID‐19 patients to compare whether there were differences in endothelial function indicators between groups. Second, the patient cohort was recruited only from inpatients in one Chinese province, and whether these changes in endothelial function indicators can also be extended to other patients with different genetic and geographic backgrounds requires further validation. Finally, the high number of missing values for specific variables (e.g., markers of cardiac function) in our cohort may cause a bias in the results. However, the differences we found between the two groups were highly significant, making our results still convincing.
5. CONCLUSIONS
In summary, our study provides new laboratory evidence for the theory that endothelial dysfunction relates to multiorgan damage, extensive inflammation and coagulation disturbances, and eventual exacerbation/death in COVID‐19 patients. Our findings provide new insights into how to advance the understanding of the pathogenesis of this disease and provide a basis for targeting endothelial dysfunction as a therapeutic strategy for COVID‐19.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Nan Liu, Hui Long, Jianhua Sun, Bingshun Wang, Qingming Wu, and Likun Gong conceived and designed the study. Nan Liu wrote the first draft of the manuscript. Huan Li, Hui Long, and Qingming Wu were responsible for disease diagnosis and data collection. Nan Liu and Yunting He analyzed the data and performed statistical analyses under the supervision of Bingshun Wang. Likun Gong, Jianhua Sun, Qingming Wu, Kai Pan, and Nan Liu designed and performed the ELISA. Yongliang Tong assisted with the revised manuscript. Qingming Wu and Likun Gong contributed to the interpretation of the results. All authors revised and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the patients and their families or legal representatives for providing consent and assisting with the present study. Additionally, we want to express our gratitude to the hospital boards for their support. We are also very grateful to all the heads of the clinical departments and all the staff in the clinical biochemistry departments from the three hospitals mentioned in the article, without whose assistance this study could not have been completed. There was no funding source for this study.
Liu N, Long H, Sun J, et al. New laboratory evidence for the association between endothelial dysfunction and COVID‐19 disease progression. J Med Virol. 2022;94:3112‐3120. 10.1002/jmv.27693
Nan Liu and Hui Long contributed equally as co‐first authors.
[Correction added on 5 April 2022, after first online publication: Some results in the following sections were revised: “Abstract”, “2.3 Statistics” and “3.3 COVID‐19 patients exhibit abnormal endothelial function parameters.”]
Contributor Information
Jianhua Sun, Email: jhsun@cdser.simm.ac.cn.
Bingshun Wang, Email: wangbingshun@sjtu.edu.cn.
Qingming Wu, Email: wuhe9224@sina.com.
Likun Gong, Email: lkgong@cdser.simm.ac.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Galloway SE, Paul P, MacCannell DR, et al. Emergence of SARS‐CoV‐2 B.1.1.7 lineage—United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(3):95‐99. 10.15585/mmwr.mm7003e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . Threat assessment brief: emergence of SARS‐CoV‐2 B.1.617 variants in India and situation in the EU/EEA. Accessed May 11, 2021. https://www.ecdc.europa.eu/en/publications-data/threat-assessment-emergence-sars-cov-2-b1617-variants
- 3. Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med. 2020;383(27):2684‐2686. 10.1056/NEJMcibr2032888 [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Jackson CB, Mou H, et al. SARS‐CoV‐2 spike‐protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11(1):6013. 10.1038/s41467-020-19808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hou YJ, Chiba S, Halfmann P, et al. SARS‐CoV‐2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz‐Ordaz K, Keogh RH. Increased mortality in community‐tested cases of SARS‐CoV‐2 lineage B.1.1.7. Nature. 2021;593:270‐274. 10.1038/s41586-021-03426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scialo F, Daniele A, Amato F, et al. ACE2: the major cell entry receptor for SARS‐CoV‐2. Lung. 2020;198(6):867‐877. 10.1007/s00408-020-00408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Tecson KM, McCullough PA. Endothelial dysfunction contributes to COVID‐19‐associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020;21(3):315‐319. 10.31083/j.rcm.2020.03.126 [DOI] [PubMed] [Google Scholar]
- 9. Nagele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID‐19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58‐62. 10.1016/j.atherosclerosis.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elyaspour Z, Zibaeenezhad MJ, Razmkhah M, Razeghian‐Jahromi I. Is it all about endothelial dysfunction and thrombosis formation? The secret of COVID‐19. Clin Appl Thromb Hemost. 2021;27:10760296211042940. 10.1177/10760296211042940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15(130):130. 10.1186/s12872-015-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buja LM, Wolf DA, Zhao B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID‐19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. 10.1016/j.carpath.2020.107233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wichmann D, Sperhake J‐P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173(4):268‐277. 10.7326/m20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adachi T, Chong J‐M, Nakajima N, et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID‐19, Japan. Emerging Infect Dis. 2020;26(9):2157‐2161. 10.3201/eid2609.201353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duarte‐Neto AN, Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement in COVID‐19 patients assessed with ultrasound‐guided minimally invasive autopsy. Histopathology. 2020;77(2):186‐197. 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. 10.1016/s0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masi P, Hékimian G, Lejeune M, et al. Systemic inflammatory response syndrome is a major contributor to COVID‐19‐associated coagulopathy: insights from a prospective, single‐center cohort study. Circulation. 2020;142(6):611‐614. 10.1161/CIRCULATIONAHA.120.048925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7(6):e438‐e440. 10.1016/s2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/s0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75(11):2829‐2845. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toda N. Age‐related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159‐176. 10.1016/j.pharmthera.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 24. Amraei R, Rahimi N. COVID‐19, renin‐angiotensin system and endothelial dysfunction. Cells. 2020;9(7):1652. 10.3390/cells9071652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khider L, Gendron N, Goudot G, et al. Curative anticoagulation prevents endothelial lesion in COVID‐19 patients. J Thromb Haemost. 2020;18(9):2391‐2399. 10.1111/jth.14968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar A, Narayan RK, Kumari C, et al. SARS‐CoV‐2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID‐19 patients. Med Hypotheses. 2020;145:110320. 10.1016/j.mehy.2020.110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285‐1295. 10.1161/CIRCULATIONAHA.106.652859 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Defelice AF, Hanig JP, Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug‐induced vascular injury. Toxicol Pathol. 2010;38(6):856‐871. 10.1177/0192623310378866 [DOI] [PubMed] [Google Scholar]
- 29. Tong M, Jiang Y, Xia D, et al. Elevated expression of serum endothelial cell adhesion molecules in COVID‐19 patients. J Infect Dis. 2020;222(6):894‐898. 10.1093/infdis/jiaa349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L, Huang M, Shen J, et al. Serum levels of soluble platelet endothelial cell adhesion molecule 1 in COVID‐19 patients are associated with disease severity. J Infect Dis. 2021;223(1):178‐179. 10.1093/infdis/jiaa642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Syed F, Li W, Relich RF, et al. Excessive matrix metalloproteinase‐1 and hyperactivation of endothelial cells occurred in COVID‐19 patients and were associated with the severity of COVID‐19. J Infect Dis. 2021;224(1):60‐69. 10.1093/infdis/jiab167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spadaro S, Fogagnolo A, Campo G, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID‐19 ICU patients. Crit Care. 2021;25(1):74. 10.1186/s13054-021-03499-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Q, Wu J, Nie J, et al. The impact of mutations in SARS‐CoV‐2 spike on viral infectivity and antigenicity. Cell. 2020;182(5):1284‐1294. 10.1016/j.cell.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yurkovetskiy L, Wang X, Pascal KE, et al. Structural and functional analysis of the D614G SARS‐CoV‐2 spike protein variant. Cell. 2020;183(3):739‐751. 10.1016/j.cell.2020.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and dysfunction in COVID‐19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. 10.1038/s41392-020-00454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta‐analysis of randomised controlled trials. Atherosclerosis. 2011;216(1):7‐16. 10.1016/j.atherosclerosis.2011.02.044 [DOI] [PubMed] [Google Scholar]
- 37. Li S, Wu Y, Yu G, Xia Q, Xu Y. Angiotensin II receptor blockers improve peripheral endothelial function: a meta‐analysis of randomized controlled trials. PLoS One. 2014;9(3):e90217. 10.1371/journal.pone.0090217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hippisley‐Cox J, Young D, Coupland C, et al. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503‐1511. 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Abajo FJ, Rodríguez‐Martín S, Lerma V, et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395(10238):1705‐1714. 10.1016/s0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058‐2066. 10.1093/eurheartj/ehaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang XJ, Qin JJ, Cheng X, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32(2):176‐187. 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


