To the Editor,
Coronavirus disease‐2019 (COVID‐19) vaccines are considered one of the primary strategies for countering the pandemic. Inactivated severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines are being manufactured in China and India. In general, they have shown better tolerability in trials. 1 Here, we report the first case of refractory hypereosinophilia associated with seropositive rheumatoid arthritis (RA) with rheumatoid nodules, following inactivated COVID‐19 vaccine (BBV152, COVAXIN) administration.
An elderly female in her late 50s with a medical history of hypertension, developed acute onset facial puffiness, dysphagia, tongue, and neck swelling, with onset 1 week after the first dose of the BBV152 vaccine. Leukocytosis with eosinophilia in her general blood picture (Table 1) prompted a referral to our center. There was no history of substance abuse, periodontitis, psychiatric illness, or COVID‐19 in the past. She had no personal or family history of an autoimmune or allergic disorder. The swelling was observed on her face, tongue, and neck. Rest of the physical examination was non‐contributory. Further workup was performed focusing on rising eosinophil counts (>30%). Her serum immunoglobulin E (IgE) was high while stool examination was negative for parasitic ova, cysts, or atypical organisms. Despite no cardiac symptoms, the B‐type natriuretic peptide levels were elevated. An upper gastrointestinal endoscopy and biopsy performed as part of evaluation for dysphagia revealed no abnormalities. A cardiac magnetic resonance imaging performed in view of multiple cases of post‐vaccination myocarditis observed by us recently was also within normal limits. The patient was started empirically on oral prednisolone at a low dose of 10 mg once daily, considering a provisional diagnosis of vaccine‐induced eosinophilia with asymptomatic myocarditis. This was tapered to 5 mg/day within 4 weeks and continued. Furthermore, an empirical course of albendazole‐ivermectin and diethylcarbamazine was administered considering the endemicity of worm infestation and filariasis in India. Her initial complaints improved over the next 2 months while on prednisolone, but she developed a few bony nodules near both her elbow joints (Figure S1). This was followed by severe pain and swelling of the bilateral elbow, wrist, metacarpophalangeal, and proximal interphalangeal joints, sparing the distal interphalangeal joints. Serology for collagen vascular diseases revealed a high titer of rheumatoid factor and anticyclic citrullinated peptide antibody. Other autoantibodies were not detected. The patient's eosinophilia persisted, rising up to 55% on the differential count. A bone marrow aspirate was performed which revealed a hypercellular marrow with morphologically normal eosinophils, and no blasts (Figure 1). Biopsy of the elbow nodules showed palisading granulomas with cores of necrobiotic collagen, consistent with rheumatoid nodules (Figure 2A,B). The progression of the disease, medications received for short courses from local general practitioners, and sequence of investigations is shown in Table 1. The patient was diagnosed as a case of RA. Her RA classification criteria score (American College of Rheumatology/European League Against Rheumatism) was 9/10 (breakup in Table 1). She was started on weekly methotrexate 7.5 mg, daily prednisolone 20 mg, and indomethacin 75 mg for pain control. The initial response in pain was significant. The patient is on follow‐up and her eosinophil counts have normalized after 1 month of methotrexate therapy (absolute eosinophil count 430/µl). She was advised not to receive the second dose of the vaccine in view of the adverse event.
Table 1.
Laboratory and radiological investigations in the patient, medications prescribed, and diagnosis
| Investigation | Vaccination date— early June 2021 | Onset of symptoms—Day 5 (post‐vaccination) | Day 14 | Day 80 | Day 116 | Days 136–146 (referral to our center) | Days 160–172 | Day 198 (severe joint pain and tenderness; nodules) | Day 206 (methotrexate initiated) |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 9.3 | 11 | 9 | 10.7 | 10.7 | 10.1 | |||
| Total leukocyte count (/μl) | 10 800 | 18 180 | 18 800 | 18 700 | 27 000 | 15 630 | |||
| Differential leukocyte count (%) | N72 L8 E8 | N67 L20 E8 | N78 L11 E10 | N54 L21 E23 | N26 L15 E55 | N49 L17 E31 | |||
| Absolute eosinophil count (/μl) | 4400 | 14 850 | |||||||
| C‐reactive protein (mg/L) (normal <6) | 47 ↑↑ | Positive (qualitative) | |||||||
| Immunoglobulin E (IU/ml) (normal <200) | >1000 ↑↑ | ||||||||
| SARS‐COV‐2 anti‐spike IgG antibody (AU/ml) (negative < 0.8) | 144 ↑↑ | ||||||||
| T3 (ng/dl)/T4 (µg/dl)/TSH (µIU/ml) | 83/13/4 (normal) | 99.7/9.5/6.9 ↑ | |||||||
| Rheumatoid arthritis factor (IU/ml) (normal <14) | 68.3 ↑ | ||||||||
| Anti‐CCP (U/ml) (normal <17) | 279.3 ↑↑ | ||||||||
| ANA/anti‐ds‐DNA Ab/ANCA (IU/ml) | Negative | ||||||||
| Anti‐centromere/anti‐Scl 70/anti SSA/anti SSB (IU/ml) | Negative | ||||||||
| Anti Jo‐1/anti Smith/anti‐U1‐RNP (IU/ml) | Negative | ||||||||
| Procalcitonin | Normal | ||||||||
| Stool microscopy for ova/cyst/uncommon parasites | Negative | Negative | |||||||
| Electrocardiogram | Normal | ||||||||
| 2D echocardiography | No regional wall motion abnormality, normal left ventricular ejection fraction, Grade 1 diastolic dysfunction | ||||||||
| Cardiac magnetic resonance imaging | Normal | ||||||||
| Creatine kinase‐MB (ng/ml) (normal <4.3) | 2.2 | ||||||||
| Troponin I (ng/ml) (normal <0.4) | <0.01 | ||||||||
| B‐type natriuretic peptide (pg/ml) (normal <100) | 337 ↑ | ||||||||
| General blood picture | Leukocytosis, increased count of morphologically normal eosinophils | ||||||||
| Upper gastrointestinal endoscopy and histopathological examination of the esophageal biopsy specimen | Normal | ||||||||
| Medications prescribed/being consumed | Irregular intake of telmisartan‐amlodipine‐hydrochlorothiazide over the past 2 years for hypertension | Glyceryl trinitrate (sustained release), diltiazem, telmisartan, betahistine, rabeprazole, domperidone, escitalopram, clonazepam (by a local general practitioner)— indications unclear; prescribed for only 7 days; duration of consumption unclear | |||||||
| Amitriptyline, amlodipine‐hydrochlorothiazide combination, telmisartan, pregabalin, methyl cobalamin, cefixime (prescribed by another practitioner)–indications unclear except for antihypertensives; duration of consumption short and unclear | |||||||||
| Ceftriaxone‐sulbactam, amikacin (prescribed by another practitioner)—indication unclear; duration—5 days | |||||||||
| ACR‐EULAR classification criteria score | 9/10 | ||||||||
| Joint distribution: 5 | |||||||||
| Serology: 3 (anti‐CCP> 3X ULN) | |||||||||
| Duration: 0 (<6 weeks‐ confounded by early diagnosis) | |||||||||
| Acute phase reactants: 1 (C‐reactive protein elevated) | |||||||||
Note: The top row mentions the timeline of vaccination, the onset of symptoms, the first referral to our center, and the onset of rheumatoid arthritis symptoms. Some investigations were performed over a range of days and have been mentioned as such. Investigation results of interest are marked in bold.
Measuring units and standard reference values from the concerned lab are mentioned in brackets beside each investigation.
Other parameters (platelet count, mean cell volume, random blood glucose, routine microscopy and culture of urine, and serum levels of urea, creatinine, sodium, potassium, uric acid, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, total and direct bilirubin, alkaline phosphatase, calcium, phosphate) were within normal limits when measured at different time points. These values are not shown.
Abbreviations: 2D, two dimensional; ACR‐EULAR, American College of Rheumatology‐European League Against Rheumatism; ANA, antinuclear antibody; ANCA, anti‐neutrophil cytoplasmic antibody; anti‐ds‐DNA Ab, anti‐double‐stranded DNA antibody; CCP, cyclic citrullinated peptide; COVID‐19, coronavirus disease‐2019; IgG, immunoglobulin G; SARS‐COV‐2, severe acute respiratory syndrome coronavirus 2; TSH, thyroid‐stimulating hormone; ULN, upper limit of normal.
Figure 1.
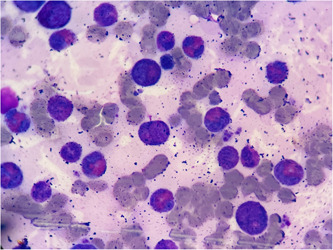
Bone marrow aspirate. ×100 magnification; Leishman‐stained smear showing increased counts of morphologically normal eosinophils and precursors.
Figure 2.
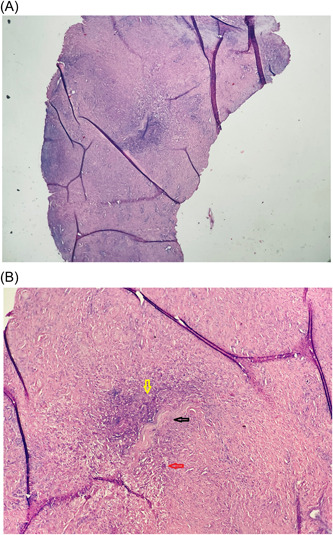
(A) Original magnification ×4 and (B) original magnification ×10; hematoxylin and eosin‐stained section showing subcutaneous necrobiotic collagen bundle (black arrow), fibrinoid degeneration with palisaded histiocytic inflammation (yellow arrow), and vascular granulation tissue (red arrow).
Postapproval, rare but serious adverse events including vaccine‐induced thrombosis and thrombocytopenia, and myocarditis have been reported with adenoviral vectored and messenger RNA‐based vaccines. 2 , 3 , 4 The inactivated vaccines in general have displayed a favorable safety profile in controlled settings. 1 In rare circumstances, however, cardiac, and new‐onset musculoskeletal phenomena have been reported. 5 The patient in the current case had not been diagnosed as having RA in the past, although on leading questioning, a history of intermittent mild pain in bilateral knee joints was obtained. The characteristic severe pain and tenderness in both small and large joints in a symmetric distribution and development of rheumatoid nodules were observed post the BBV152 vaccine. The associated hypereosinophilia was refractory to steroid therapy. Though the patient had elevated brain natriuretic peptide levels, there were no symptoms or signs of cardiac involvement. However, subclinical myocarditis cannot be ruled out. Since there was a delay in the patient being referred to our center, the possibility of a myocarditis event resolving by that time also cannot be ruled out. The lady had a background history of controlled hypertension. Previously, women and those with pre‐existing hypertension each have been shown to have a two times higher odds of development of adverse events following immunization. 6 The mechanisms of autoimmune induction by inactivated vaccines need to be delineated. It can be related to the aluminum hydroxide adjuvant present in the vaccine. Autoimmune/inflammatory syndromes induced by adjuvant was first described in 2011. 7 Aluminum in adjuvants is known to cause preferential Th2‐based interleukin‐5 (IL‐5) stimulation that can explain hypereosinophilia and autoantibodies seen in the patient. The musculoskeletal inflammation can also be explained by the NOD‐like receptor protein family 3 driven release of IL‐1 and IL‐18 by aluminum. 7 Autoantibodies generated as a response to viral proteins due to cross‐reactivity, and direct toxicity by the Spike or other viral proteins maybe other tentative pathogenetic pathways. 8 In other cases of postvaccination adverse events reported by our group, we have observed a moderate to high serum level of SARS‐CoV‐2 anti‐Spike IgG antibody. 3 , 9 This was also observed in the current case. Although the exact implications of this are not clear at present, there may be a link with a cross‐reactive antibody mechanism causing such events.
The case is interesting as the first reported case of refractory hypereosinophilia associated with RA diagnosed following inactivated BBV152 vaccine administration. Using the World Health Organization causality assessment scale, the event was categorized as “Probable” (Supporting Information Data). The rapidity of disease progression with the appearance of rheumatoid nodules, refractory hypereosinophilia, and a possibility of subclinical myocarditis are other atypical features of this case. The patient's eosinophil counts normalized with the initiation of methotrexate therapy, suggesting that the patient's early symptoms and eosinophilia may have been a prodrome of her RA, and these may have been part of a common pathogenetic continuum. Focused research is needed on autoimmune complications resulting from COVID‐19 vaccines, particularly their association with aluminum‐adjuvanted vaccines. There is also a constant need to update guidelines for vaccinating patients with autoimmune conditions using diverse vaccine types, including the updating of potential contraindications.
AUTHOR CONTRIBUTIONS
Rohit Singh wrote the first draft of the paper. Upinder Kaur revised the paper for scientific accuracy and designed the table. Ankur Singh provided technical inputs in pathology. Sankha S. Chakrabarti conceptualized the paper and edited the final draft of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
ETHICS STATEMENT
No human experimentation was performed. All procedures were performed following the Declaration of Helsinki and its modifications. Written informed consent was taken from the patient's legal guardian for publication of the case.
Supporting information
Supplementary Figure 1. Rheumatoid nodule near elbow.
Supporting information.
Contributor Information
Upinder Kaur, Email: drupinder.bhu@gmail.com.
Sankha S. Chakrabarti, Email: sankha.geriatrics@gmail.com.
DATA AVAILABILITY STATEMENT
The complete data of the case is provided in the manuscript, table, and Supporting Information. Any additional data requests may be addressed to the corresponding author.
REFERENCES
- 1. Chen M, Yuan Y, Zhou Y, et al. Safety of SARS‐CoV‐2 vaccines: a systematic review and meta‐analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://idpjournal.biomedcentral.com/articles/10.1186/s40249-021-00878-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. http://www.nejm.org/doi/10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh R, Chakrabarti SS, Gambhir IS, et al. Acute cardiac events after ChAdOx1 nCoV‐19 corona virus vaccine: report of three cases. Am J Ther . 2022. https://journals.lww.com/10.1097/MJT.0000000000001472 [DOI] [PubMed]
- 4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202‐1206. https://jamanetwork.com/journals/jamacardiology/fullarticle/2781601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiwari A, Chakrabarti SS, Panda PK, Karna G, Kaur U. Hyper‐eosinophilic syndrome with myocarditis after inactivated SARS‐CoV‐2 vaccination: a case study. Res Sq . 2021. 10.21203/rs.3.rs-806335/v1 [DOI] [PubMed]
- 6. Kaur U, Ojha B, Pathak BK, et al. A prospective observational safety study on ChAdOx1 nCoV‐19 corona virus vaccine (recombinant) use in healthcare workers—first results from India. eClinicalMedicine. 2021;38:101038. https://linkinghub.elsevier.com/retrieve/pii/S2589537021003187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoenfeld Y, Agmon‐Levin N. “ASIA”—autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4‐8. https://linkinghub.elsevier.com/retrieve/pii/S0896841110000788 [DOI] [PubMed] [Google Scholar]
- 8. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS‐CoV‐2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:617089. https://www.frontiersin.org/articles/10.3389/fimmu.2020.617089/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakrabarti SS, Tiwari A, Jaiswal S, Kaur U. Rapidly progressive dementia with asymmetric rigidity following ChAdOx1 nCoV‐19 vaccination. Aging Dis . 2021. 10.14336/AD.2021.1102 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Rheumatoid nodule near elbow.
Supporting information.
Data Availability Statement
The complete data of the case is provided in the manuscript, table, and Supporting Information. Any additional data requests may be addressed to the corresponding author.


