Abstract
Vaccines against COVID‐19 provide immunity to deter severe morbidities associated with the infection. However, it does not prevent infection altogether in all exposed individuals. Furthermore, emerging variants of SARS‐CoV‐2 impose a threat concerning the competency of the vaccines in combating the infection. This study aims to determine the variability in adverse events and the extent of breakthrough infections in the Indian population. A retrospective study was conducted using a pre‐validated questionnaire encompassing social, demographic, general health, the status of SARS‐CoV‐2 infection, vaccination, associated adverse events, and breakthrough infections in the Indian population. Informed consent and ethical approval were obtained as per Indian Council of Medical Research (ICMR) guidelines. Participants, who provided the complete information, were Indian citizens, above 18 years, and if vaccinated, administered with either Covishield or Covaxin, were considered for the study. Data have been compiled in Microsoft Excel and analyzed for statistical differences using STATA 11. The responses from 2051 individuals fulfilling the inclusion criteria were analyzed. Among 2051, 1119 respondents were vaccinated and 932 respondents were non‐vaccinated. Among 1119 vaccinated respondents, 7 were excluded because of missing data. Therefore, out of 1112 vaccinated, 413 experienced adverse events with a major fraction of younger individuals, age 18–40 years, getting affected (74.82%; 309/413). Furthermore, considerably more females than males encountered adverse consequences to vaccination (p < 0.05). Among vaccinated participants, breakthrough infections were observed in 7.91% (88/1112; 57.96% males and 42.04% females) with the older age group, 61 years and above (odds ratio, 3.25 [1.32–8.03]; p = 0.011), and males were found to be at higher risk. Further research is needed to find the age and sex‐related factors in determining vaccine effectiveness and adverse events.
Keywords: adverse‐events, breakthrough infections, COVID‐19, SARS‐CoV‐2
Highlights
Significant higher adverse events following COVID‐19 vaccination in females in comparison to males.
Breakthrough infections among Indian population was found to be 7.91%.
Older people and males were found to be at high risk for getting breakthrough infections.
1. INTRODUCTION
India's Central Drugs Standard Control Organization (CDSCO) had given restricted emergency use authorization to seven COVID‐19 vaccines; the Oxford‐AstraZeneca adenovirus‐vectored recombinant vaccine‐AZD1222 and Covishield (ChAdOx1 nCoV‐ 19), whole‐virion inactivated corona virus vaccine‐Covaxin (BBV152) in January 2021, recombinant adenovirus‐vectored vaccine‐Sputnik V (Gam‐COVID‐Vac) in April 2021, Moderna's mRNA‐1273 vaccine (June 2021), Zydus Cadila's DNA vaccine‐ ZyCov‐D in August 2021 and Janssen's Ad26.COV2.S (August 2021). All these vaccines were rolled out to be given in two doses for optimum efficacy except Janssen's Ad26.COV2.S and ZyCov‐D that required one and three doses, respectively. 2 , 3 , 4
Only Covishield and Covaxin were rolled out in India by the time the current study was conducted. The efficacy of Covishield was found to be around 93%, after both the doses, based on the VIN‐WIN cohort study carried out on over 1.59 million healthcare and frontline workers of the Indian Armed Forces. 5 Kulkarni et al. 6 reported that SII‐ChAdOx1 nCoV‐19 has a good safety profile and is highly immunogenic in the adult Indian population in comparison to AZD122. Desai et al. 7 showed 50% efficacy of two doses of BBV152 (Covaxin) against symptomatic RT‐PCR confirmed SARS‐CoV‐2 during the second wave in India. The effectiveness of these vaccines has been reported in reducing the rate of infection, hospitalization incidences, the severity of disease, and fatalities pertaining to COVID‐19 disease.
Several studies reported 13.1%–19% breakthrough COVID‐19 infection among healthcare workers postvaccination. 8 , 9 , 10 Farinholt et al. 11 in their research suggested that the SARS‐CoV‐2 Delta variant might possess the potential of immune evasion in fully vaccinated individuals and project the highest risk in comparison to other circulating virus strains. While hustling toward mass vaccination, surveillance in the community with respect to vaccination response and breakthrough infections represent a dire need during vaccination rollout. The SARS‐CoV‐2 immunity and reinfection evaluation (SIREN) survey among healthcare workers in the United Kingdom scrutinized the performance of the vaccines at various phases of rollout, linked the data to already available health records, and hence, helped in establishing a correlation of lower vaccine coverage with previous infection, job, age, gender, and ethnicity besides providing the efficacy of each vaccine in the native population in a real scenario. 12 However, such studies take time to come to fruition, online survey platforms are easier and cost‐effective as data sources.
Current research is a retrospective study aimed to investigate the adverse events and breakthrough COVID‐19 infections observed in the Indian population following each dose of COVID‐19 vaccines and estimate the cases of breakthrough infections postvaccination. The survey was conducted on both virtual and physical platforms. Collectively responses of 2064 participants were registered in the period spanning May 2021 to August 2021. The study was aimed to estimate the adverse events and breakthrough infections in vaccinated individuals and to draw comparisons on the basis of gender, age‐groups, and status of comorbidities.
2. MATERIALS AND METHODS
2.1. Data source
A questionnaire was designed and pre‐validated in two steps, first by following a pilot study using 200 volunteers, modified based on the inputs, and again 50 volunteers belonging to research and healthcare workers were recruited to validate the questionnaire. This validated questionnaire (see Supporting Information) was approved by the Institutional ethics committee (ACBR/IHEC/DS‐09/08‐2021) and was used to conduct the retrospective study.
2.1.1. Inclusion criterion
The survey was administered to subjects of India and above the age of 18 years with informed consent.
2.1.2. Exclusion criterion
Subjects below the age of 18 years and nonresidents of India were excluded. Also, people given vaccines other than Covishield and Covaxin were excluded. Participants who did not furnish complete details in the form were excluded.
The questionnaire encompasses social, demographic, general health, status of SARS‐CoV‐2 infection, vaccination, associated adverse events, and breakthrough infections in the Indian population. The survey was conducted via two different means, namely online Google form and offline survey along with consent form conducted with the help of trained healthcare workers (ASHA [Accredited Social Health Activist]) who were under the supervision of doctors affiliated with the Delhi Government hospital. These ASHA workers were chosen because they are trained females in maternal and child care, are cognizant about infectious and noninfectious diseases, able to identify symptoms, provide home visits, door to door surveys, first aid, immunization sessions, data maintenance, community health planning, and COVID‐19 related duties which include visiting patients at their homes and conducting surveys in the containment zone. For the present survey, they were explained to include people above 18 years of age, check the vaccination status/certificates, and were explained about the comorbidities and adverse events associated with COVID‐19 vaccination. The different zones of Delhi, coming under the Delhi Government hospital, were assigned to ASHA workers. To ensure the randomness of the study, data was collected from all households in the zone assigned to them.
In addition, the snowball sampling approach was utilized to increase the number of responses to the online survey. The Google survey form link was sent through WhatsApp, Facebook, emails, and other social media to the college students and colleagues of the authors and co‐authors, friends, family members, and relatives. Further, they were requested to roll out the online questionnaire to as many people as possible ensuring randomization. The data collected was a mix of nonvaccinated and partially or fully vaccinated individuals.
People who participated in the survey were requested to fill in the honest and appropriate information to the best of their knowledge and understanding. The study has no hidden or apparent agenda of intruding into participants' personal space and our objectives are strictly academic and research‐oriented. People were given free will whether they want to choose to participate or not and no incentives were given for participation. The identity of the participants was kept hidden and only one reply per participant was accepted.
2.2. Data analysis
A total of 2064 people from India participated in the online and offline surveys. After the exclusion of 13 responses with insufficient information, the remaining 2051 responses were analyzed. Figure 1 as per STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, the flow chart illustrating the total quantity of data, missing data exclusion, and the data fulfilling the inclusion criteria which were further utilized to analyze adverse events and breakthrough infections. The data was compiled in Microsoft Excel and checked for statistical differences by STATA 11 using a 5% level of significance for all statistical comparisons. Descriptive analyses were used for sociodemographic and categorical data. Multivariate logistic regression analysis was performed to find the correlation of adverse events and of breakthrough infections with sex, age, body mass index (BMI), and comorbidities. Chi‐square and Fisher's exact tests were performed to obtain the p value to determine the significance by more than two authors independently.
Figure 1.
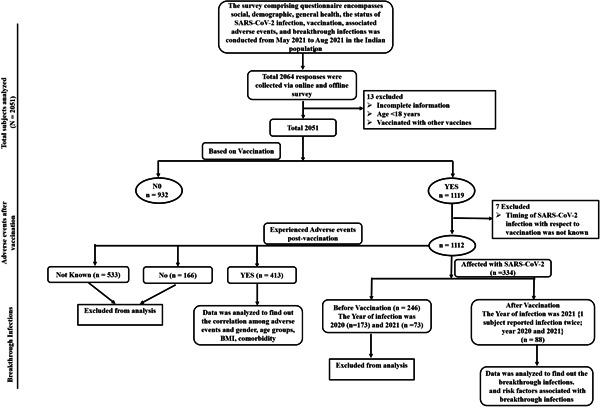
STROBE flow chart showing the COVID‐19 survey methodology
3. RESULTS
3.1. Study sample characteristics
Amongst 2051 subjects included in the study, 1018 (49.63%) were females and 1033 (50.37%) were males. Participants on the basis of age group were reckoned at 1456 (70.99%) between 18 and 40 years, 487 (23.74%) between 41 and 60 years, and 108 (5.27%), 61 years and above. A total of 761 (37.10%) of the study participants had experienced COVID‐19, whereas 1290 (62.90%) participants could steer clear of it. Table 1 summarizes the basic characteristics of the study participants. Among the 761 COVID‐19 cases, 754 responded to provide details of the time when they got infected. Out of 754 respondents, 34.08% (257/754) contracted the infection during the first wave of COVID‐19, whereas 65.92% (497/754) contracted the infection during the second wave, with 5 people getting the infection twice. Infection cases were classified as mild (with symptoms such as headache, loss of smell and taste, myalgia, etc., but no fever); moderate (infection cases with fever as one of the symptoms but not requiring oxygen support or hospitalization); whereas cases requiring oxygen support or hospitalization were considered severe. Out of 761 COVID‐19 cases, 758 responded to the question “was it symptomatic?”. Out of 758 respondents, 17.68% (134/758) were asymptomatic, whereas 82.32% (624/758) were symptomatic. Among 624 symptomatic respondents, 41.83% (261/624), 29.97% (187/624), and 28.21% (176/624) experienced mild, moderate, and severe COVID‐19 symptoms, respectively.
Table 1.
Demographic characteristics of study participants (N = 2051)
| Variable | n (%) |
|---|---|
| Age group (years) | |
| 18–40 | 1456 (70.99) |
| 41–60 | 487 (23.74) |
| 61 and above | 108 (5.27) |
| Gender | |
| Female | 1018 (49.63) |
| Male | 1033 (50.37) |
| Occupation category | |
| Academician | 429 (37.76) |
| Frontline workers | 178 (15.67) |
| Professionals and managerial | 367 (32.31) |
| Housewife/retired/unemployed | 162 (14.26) |
| 1. COVID affected (ever have experience with COVID‐19) | |
| Yes | 761 (37.10) |
| No | 1290 (62.90) |
| 1a. If COVID‐19 positive, when did you catch COVID‐19? | |
| 2020 | 257 (34.08) |
| 2021 | 497 (65.92) |
| 1b. Was it symptomatic? | |
| Yes | 624 (82.32) |
| No | 134 (17.68) |
| 1c. If it was symptomatic COVID‐19, what was severity of your symptoms? | |
| Mild | 261 (41.83) |
| Moderate | 187 (29.97) |
| Severe | 176 (28.21) |
| COVID‐19 vaccinated | |
| Yes | 1119 (54.56) |
| No | 932 (45.44) |
3.2. Data of COVID‐19 vaccination
Among the 2051 respondents included in the study, 932 (45.44%) were nonvaccinated and 1119 (54.56%) gave a nod to have received at least one COVID‐19 vaccine shot with 616 (55.05%) males and 503 (44.95%) females. We removed 7 respondents from further analysis since a total of 1112 people responded to the question “when did they catch COVID‐19 (before or after vaccination).”
Out of 1112 vaccinated individuals, only 199 (17.90%) received Covaxin, whereas a huge majority of 913 (82.10%) received the Covishield vaccine. Among 1112 vaccinated individuals, 60.07% (n = 668), 31.21% (n = 347), and 8.72% (n = 97) belonged to the age groups 18–40, 41–60, and 61 years and above, respectively. About 55.13% (n = 613) were males and 44.87% (n = 499) were females. Since the vaccination drive in India focussed to immunize the population chronologically, from the elderly to the young, a higher proportion of the elderly population was reported to have been vaccinated than the younger (Figure 2).
Figure 2.
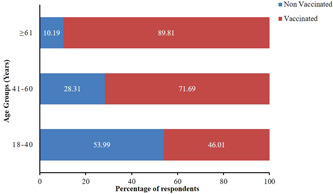
100% stacked bar of the vaccination status of participants belonging to various age groups. Different colors in one bar represent the fraction of vaccinated and nonvaccinated respondents belonging to that age group
Among 932 nonvaccinated individuals, 84.12% (n = 784), 14.70% (n = 137) and 1.18% (n = 11) belonged to age groups 18–40, 41–60, and 61 years and above, respectively. About 44.74% (n = 417) were male and 55.26% (n = 515) were female.
3.3. Adverse events of vaccination
Out of 1112 vaccinated participants, 579 responded to the question “Did you experience any adverse events postvaccination?.” Among 579 respondents, 413 developed adverse events as a result of their vaccination. Interestingly, the fraction of people developing adverse events varied as a correlate of not only sex but also age (Figure 3). Among females who experienced adverse events, 80.10% (165/206), 16.99% (35/206), and 2.91% (6/206) belonged to the age groups of 18–40, 41–60, and 61 years and above, respectively. However, 69.57% (144/207), 25.60% (53/207), and 4.83% (10/207) were males belonging to age groups of 18–40, 41–60, and 61 years and above, respectively, who developed adverse events after vaccination (Figure 3). Furthermore, the younger age group of 18–40 years, as well as the female sex were found to be more positively correlated with adverse events associated with the vaccinations concerned (Table 2). However, we found no correlation between comorbidity, BMI, and the occurrence of adverse events.
Figure 3.
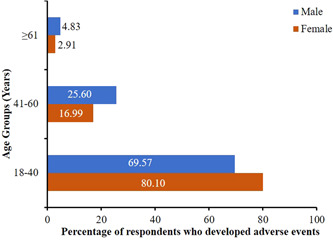
Percentage of vaccinated males and females belonging to various age brackets who developed adverse events after vaccination
Table 2.
Multivariate logistics regression – Adverse events after vaccination
| Independent variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age group (years) | ||
| 18–40 | 1.00 | |
| 41–60 | 0.51 (0.32–0.81) | 0.004* |
| >60 | 0.32 (0.13–0.74) | 0.008* |
| Gender | ||
| Female | 1.00 | |
| Male | 0.37 (0.24–0.58) | <0.0001* |
| BMI (kg/m 2 ) | 0.99 (0.95–1.05) | 0.964 |
| Comorbidity present | 1.47 (0.86–2.50) | 0.160 |
Abbreviations: BMI, body mass index; CI, confidence interval; p value, under 0.05 indicates a significant outcome.
3.4. Breakthrough infections
Out of 932 nonvaccinated respondents, 45.06% (420/932) contracted the COVID‐19 infection, with 333 symptomatic cases and 87 asymptomatic cases. Among 333 symptomatic cases, 45.05% (n = 150), 25.23% (n = 84), and 29.73% (n = 99) developed mild, moderate, and severe (hospitalization and/or oxygen support) symptoms, respectively.
Among 1112 vaccinated respondents, 334 individuals contracted the SARS‐CoV‐2 infection. However, only 7.91% (88/1112) of 334 people contracted infection postvaccination (breakthrough infections), including one subject that was infected twice in both the years 2020 and 2021. Out of 88 postvaccinated SARS‐CoV‐2 breakthrough infections, 80 (90.91%) were symptomatic cases (Figure 4), and the remaining 8 (9.09%) were asymptomatic. Among symptomatic breakthrough infection (n = 80), 31.25% (n = 25), 43.75% (n = 35), and 25.0% (n = 20) were mild, moderate, and severe infection, respectively.
Figure 4.
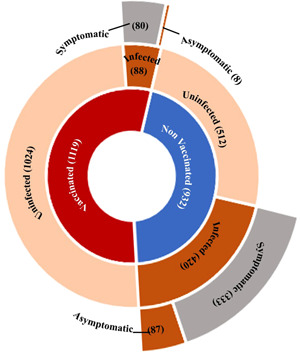
Sunburst chart depicting the status of vaccination among participants in the inner‐most circle. The middle circle represents the fraction of SARS‐CoV‐2 virus‐infected and uninfected, among both the vaccinated and nonvaccinated groups. The outer circle highlights the proportion of respondents who developed symptomatic COVID‐19 against asymptomatic cases
Among 88 individuals who contracted SARS‐CoV‐2 breakthrough infection, 57.96% (n = 51) were males and 42.04% (n = 37) were females. Among breakthrough infections, 44.31% (39/88) belonged to the young age group 18–40 years, 42.24% (37/88) were of age group 41–60 years, and only 13.63% (12/88) belonged to the age group 61 years and above. Notably, when we performed multivariate regression analysis, the older age group (61 years and above) and males were found to be at higher risk of acquiring breakthrough infections (Table 3). However, we found no link between comorbidity, BMI, and breakthrough infections.
Table 3.
Multivariate logistics regression – COVID‐19 infection after vaccination
| Independent variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age group (years) | ||
| 18–40 | 1.00 | |
| 41–60 | 1.24 (0.82–1.87) | 0.304 |
| >60 | 3.25 (1.32–8.03) | 0.011* |
| Gender | ||
| Female | 1.00 | |
| Male | 1.71 (1.20–2.43) | 0.003* |
| BMI (kg/m 2 ) | 0.98 (0.94–1.03) | 0.409 |
| Comorbidity present | 1.32 (0.84–2.08) | 0.227 |
Abbreviations: BMI, body mass index; CI, confidence interval; p value, under 0.05 indicates a significant outcome.
3.5. Comorbidity status among breakthrough infection
Out of 334 vaccinated and COVID‐19 infected respondents, 68 individuals had a history of at least one chronic illness. Among 68 comorbid and COVID‐19 positive individuals, only 23 respondents acquired infection postvaccination, whereas the remaining 45 contracted infection before vaccination. This accounts for 26.14% (23/88) of comorbid people among breakthrough infections. Diabetes, hypertension, obesity, and thyroid disease were the most common chronic illnesses reported by survey participants who became infected after receiving the vaccination, as shown in Figure S1. However, we did not find any correlation between breakthrough infections and the presence of chronic illness among patients, as shown in Table 3 (OR 1.32 [0.84–2.08]; p = 0.227).
4. DISCUSSION
In the current study with the data analyzed from 2051 individuals, apparently, 7.91% of the participants who were vaccinated with at least one dose of either Covaxin or Covishield still contracted the infection. Similar kinds of breakthrough infections have been reported by Kaur et al. (19%) and Satwik et al. (13.1%). 9 , 10 Most of the subjects caught the infection after the first dose of vaccination. Our results were consistent with another study carried out with healthcare workers in Delhi which reported breakthrough infections in 13.3% of the subjects. 8 It is noteworthy that among the participants in our study who contracted SARS‐CoV‐2 infection postvaccination, 90.91% of the participants reported experiencing symptomatic infection, while only 9.09% (8/88) reported the asymptomatic infection. The Delta variant of the SARS‐CoV‐2 virus was more prevalent during the second wave of the pandemic. This variant has been reported to be associated with more severe cases and it could be a major factor responsible for as high as 25.0% (20/88) of severe cases in symptomatic breakthrough infections. 13 Also, the number of participants with severely symptomatic infection was only 20, further study with a larger population size might be required to find the actual prevalence of the severe breakthrough cases in the population.
Over 42% of the total female respondents encountered the infection whereas, among the total number of male respondents, more than 57% encountered the infection. The rationale behind it can be associated with several factors including physiological, genetic, and behavioral. One of the major reasons for males of different age groups showing higher rate of infection could be due to the differential expression of ACE‐2 receptors. In humans, Angiotensin‐converting enzyme‐2 (ACE‐2) receptors have been found to be the receptors responsible for the entry of SARS‐CoV‐2 in the host cells but the expression of these receptors gets downregulated following the entry of virus particles into the cells. This might prevent further viruses from entering into the cell but reduction in ACE‐2 levels drive elevation of angiotensin II by ACE thereby, inducing vasoconstriction, increased lung vascular permeability, and pro‐fibrosis, leading to acute lung injury. 14 , 15 , 16 , 17 Higher expression of ACE‐2 in females as compared to males renders them less vulnerable to severities of COVID‐19. 18 , 19 Both, the genes and the hormone, estrogen are responsible for providing this protection. Estrogen plays a role in upregulating the levels of ACE‐2 and in reducing ACE‐1 and consequently angiotensin II levels. Interestingly enough, the ACE‐2 genes are located at sites on the X‐chromosomes that are able to skip the process of X‐chromosome inactivation. By the virtue of two X chromosomes contributing to the pool of ACE‐2 enzyme, this becomes another reason why females have higher expression of ACE‐2 and thus, higher protection against the ongoing pandemic than males. 20 , 21 , 22
Female physiology, hormones, and genes have certainly helped them survive better in the pandemic but the role of behavioral response seems to also contribute. 23 A study conducted in Spain highlights that women showed higher compliance with the safety measures, washed hands regularly, maintained physical distance in public, and cared more to wear masks and hence, steer clear of the infection. 24
As reported, we also found a conspicuous difference in the fraction of males and females who developed adverse events after receiving the vaccine against COVID‐19. 25 Among the young females and males belonging to the age bracket of 18–40 years, 80.10% of females reported adverse events whereas only 69.57% of males complained of adverse events among vaccinated respondents. Rapidly induced higher TLR7 expression and subsequent higher pro‐inflammatory response upon vaccination has been reported among females belonging to reproductive age. 26 , 27 , 28 This comparatively higher pro‐inflammatory response of the activated innate immune system is plausibly responsible for this varied fraction of side‐effects observed between the two sexes. This sex bias is encountered not only in case of developing side‐effects but a higher adaptive immune response resulting in higher antibody titer as is observed in females upon stimulation with bacteria or virus‐associated vaccines. 28 A reverse order was observed in the age group 41–60 years where 25.60% males reported the adverse events and 16.99% female respondents reported the adverse events attributed to the decline in the levels of reproductive hormones, more specifically estradiol. 29 Regardless of the sex, adverse events in the vaccination group reduced consistently with respect to age as is expected with immunosenescence. 30 As immediate adverse events are indicative of rapid innate immune response, status of breakthrough infections can be a good indicator of active adaptive immunity gained through vaccination. 31 In the present study, a conspicuous difference in breakthrough infections could be seen among respondents aged 18–40 (44.31%), 41–60 (42.24%), and respondents aged 61 years and above (13.63%). Further, a higher percentage of respondents with comorbidities was noticed in vaccinated individuals with breakthrough infections with respect to nonvaccinated individuals who contracted the infection suggesting that people without comorbidities are more likely to bypass SARS‐CoV‐2 infection postvaccination. The most common comorbidities found in the respondents who contracted breakthrough infection were diabetes and hypertension which are again more prevalent in the older age group and thus, justifying the higher rate of breakthrough infection. It can be observed in our study as well that the age group of 61 years and above were found to be more positively (OR 3.25 [1.32–8.03]) and significantly (p = 0.011) associated with breakthrough infections than the younger age groups. Also, the role of immunosenescence is more dominant in breakthrough infections as the elderly population does not respond very efficiently to naïve or previously encountered pathogens. With aging, the immune system of the elderly is left with very few naïve lymphocytes but abundant dysfunctional memory cells along with primary lymphoid organs involutions and altered innate immune response, ultimately leading to reduced response to vaccination which is apparent in the study. 30
5. CONCLUSION
The adverse events and breakthrough infections with COVID‐19 vaccination have been shaking the trust of masses in stepping ahead for it inducing a seed of hesitancy. Also, encountering breakthrough infections seem to push the target of achieving herd immunity a little further. Preexisting knowledge about the differential performance of the immune system, expression of genes and milieu of hormones among males and females, and the change in their dynamics with age helped us provide a rationale for the pattern observed in the breakthrough infections. This opens up various avenues of fundamental and clinical research in deciphering the mechanisms responsible for the differential level of immunity gained via vaccination based on sex and age.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
ETHICS STATEMENT
The present study has been approved by the Institutional Human Ethics Committee of Delhi School of Public health, IoE (Institute of Eminence), and Dr. B.R. Ambedkar Center for Biomedical Sciences, University of Delhi, India as per ICMR guidelines. Participants were informed that their participation was voluntary, and consent was implied on the completion of the questionnaire.
AUTHOR CONTRIBUTIONS
The first authorship is jointly contributed by Geetika Arora and Jyoti Taneja. Sunita Jetly, Daman Saluja, Jyoti Taneja, Priya Bhardwaj, and Sunita K. Yadav designed the survey form. Sunita Jetly, Daman Saluja, Jyoti Taneja, Shorya Goyal, Priya Bhardwaj, and Sunita K. Yadav collected the epidemiological data. Kumar Naidu, Geetika Arora, Sunita Jetly, Jyoti Taneja, and Priya Bhardwaj analyzed the data. Geetika Arora, Sunita Jetly, Daman Saluja, Jyoti Taneja, and Priya Bhardwaj drafted the manuscript. All authors read the manuscript and approved it.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors would like to acknowledge all the participants and volunteers for collecting offline data from door to door. The authors thank Prof. Ravi Toteja (Principal, Acharya Narendra Dev College, University of Delhi) and Prof. Savita Roy (Principal, Daulat Ram College, University of Delhi) for their logistic support and cooperation. The authors would like to thank ICMR for providing fellowship to GA.
Arora G, Taneja J, Bhardwaj P, et al. Adverse events and breakthrough infections associated with COVID‐19 vaccination in the Indian population. J Med Virol. 2022;94:3147‐3154. 10.1002/jmv.27708
G. Arora and J. Taneja are equal contributors.
Contributor Information
Daman Saluja, Email: dsalujach59@gmail.com, Email: dsalujach1959@gmail.com.
Sunita Jetly, Email: sunitajetly@andc.du.ac.in.
DATA AVAILABILITY STATEMENT
The raw data will be provided on request.
REFERENCES
- 1.India – COVID19 Vaccine Tracker [Internet]. [cited December 19, 2021]. https://covid19.trackvaccines.org/country/india/
- 2.Zydus Receives EUA from DCGI for ZyCoV‐D, the Only Needle‐free COVID Vaccine in the World. [cited December 19, 2021]. www.zyduscadila.com
- 3.Covid Vaccines FAQs, Prices, Doses: Covishield, Covaxin, Sputnik V, Johnson & Johnson – Coronavirus Outbreak News [Internet]. [cited December 19, 2021]. https://www.indiatoday.in/coronavirus-outbreak/story/covid-vaccines-india-price-availability-dose-gap-1821594-2021-07-01
- 4.Ministry of Health and Family Welfare. Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency Approval of COVID‐19 Virus Vaccine. 2021;2. https://pib.gov.in/PressReleseDetail.aspx?PRID=1685761
- 5. Ghosh S, Shankar S, Chatterjee K, et al. COVISHIELD (AZD1222) VaccINe effectiveness among healthcare and frontline Workers of INdian Armed Forces: interim results of VIN‐WIN cohort study. Med J Armed Forces India. 2021;77(Suppl 2):S264‐S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulkarni PS, Padmapriyadarsini C, Vekemans J, et al. A phase 2/3, participant‐blind, observer‐blind, randomised, controlled study to assess the safety and immunogenicity of SII‐ChAdOx1 nCoV‐19 (COVID‐19 vaccine) in adults in India. EClinicalMedicine. 2021;42:101218. 10.1016/j.eclinm.2021.101218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desai D, Khan AR, Soneja M, et al. Effectiveness of an inactivated virus‐based SARS‐CoV‐2 vaccine, BBV152, in India: a test‐negative, case‐control study. Lancet Infect Dis. 2021. 10.1016/S1473-3099(21)00674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tyagi K, Ghosh A, Nair D, et al. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr. 2021;15(3):1007‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 nCoV‐19 effectiveness during an unprecedented surge in SARS COV‐2 infections. Eur J Intern Med. 2021;93:112‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur U, Bala S, Ojha B, Jaiswal S, Kansal S, Chakrabarti SS. Occurrence of COVID‐19 in priority groups receiving ChAdOx1 nCoV‐19 coronavirus vaccine (recombinant): a preliminary analysis from North India. J Med Virol. 2022;94(1):407‐412. https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.27320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farinholt T, Doddapaneni H, Qin X, et al. Transmission event of SARS‐CoV‐2 delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021;19(1):1‐6. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-02103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall VJ, Foulkes S, Saei A, et al. COVID‐19 vaccine coverage in health‐care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725‐1735. https://pubmed.ncbi.nlm.nih.gov/33901423/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shastri J, Parikh S, Aggarwal V, et al. Severe SARS‐CoV‐2 breakthrough reinfection with delta variant after recovery from breakthrough infection by alpha variant in a fully vaccinated health worker. Front Med. 2021;8:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng H, Wang Y, Wang GQ. Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J Med Virol. 2020;92:726‐730. https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Wit E, Van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523‐534. https://pubmed.ncbi.nlm.nih.gov/27344959/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang SJ, Jung SI. Age‐related morbidity and mortality among patients with COVID‐19. Infect Chemother. 2020;52(2):154‐164. http://www.ncbi.nlm.nih.gov/pubmed/32537961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaur U, Acharya K, Mondal R, et al. Should ACE2 be given a chance in COVID‐19 therapeutics: a semi‐systematic review of strategies enhancing ACE2. Eur J Pharmacol. 2020;887:173545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bukowska A, Spiller L, Wolke C, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med. 2017;242(14):1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xudong X, Junzhu C, Xingxiang W, Furong Z, Yanrong L. Age‐ and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166‐2171. https://pubmed.ncbi.nlm.nih.gov/16303146/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bwire GM. Coronavirus: why men are more vulnerable to Covid‐19 than women? SN Compr Clin Med. 2020;2(7):874‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID‐19. Cell Death Discov. 2020;6(37). http://creativecommons.org/licenses/by/4.0/.Correspondence:ElenaOrtona [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. Int J Mol Sci. 2020;21:3474. www.mdpi.com/journal/ijms [DOI] [PMC free article] [PubMed]
- 23. Conti P, Younes A. Coronavirus COV‐19/SARS‐CoV‐2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2):339‐343. https://pubmed.ncbi.nlm.nih.gov/32253888/ [DOI] [PubMed] [Google Scholar]
- 24. Giefing‐Kröll C, Berger P, Lepperdinger G, Grubeck‐Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309‐321. 10.1111/acel.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaur U, Ojha B, Pathak BK, et al. A prospective observational safety study on ChAdOx1 nCoV‐19 coronavirus vaccine (recombinant) use in healthcare workers—first results from India. EClinicalMedicine. 2021;38:101038. 10.1016/j.eclinm.2021.101038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sex and Gender and COVID‐19 Vaccine Side Effects Yale School of Medicine [Internet]. [cited October 3, 2021]. https://medicine.yale.edu/news-article/sex-and-gender-and-covid-19-vaccine-side-effects/
- 27. Fink AL, Engle K, Ursin RL, Tang W‐Y, Klein SL. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci USA. 2018;11549:12477‐12482. https://www.pnas.org/content/115/49/12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626‐638. https://www.nature.com/articles/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 29. Gameiro C, Romao F. Changes in the immune system during menopause and aging. Front Biosci. 2010;2(4):1299‐1303. https://pubmed.ncbi.nlm.nih.gov/20515802/ [DOI] [PubMed] [Google Scholar]
- 30. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Sem Immunol. 2018;40:83‐94. [DOI] [PubMed] [Google Scholar]
- 31. Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva f. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4(1):1‐11. https://www.nature.com/articles/s41541-019-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The raw data will be provided on request.


