Abstract
The administration of intravenous vitamin C (IV‐VC) in treating patients with coronavirus disease 2019 (COVID‐19) is still highly controversial. There have been no previous studies on the effect of IV‐VC on the severity and mortality of COVID‐19. Hence, we conducted a systematic review and meta‐analysis to compare the disease severity and mortality in patients with COVID‐19 who promptly received IV‐VC treatment vs those who did not.
We performed a comprehensive systematic search of seven health science databases, including PubMed, Embase, Cochrane Library, MEDLINE, Web of Science, China National Knowledge Infrastructure, and Wanfang Data, up to June 23, 2021. We identified a total of seven related articles, which were included in this study.
This meta‐analysis showed that IV‐VC treatment did not affect disease severity compared with placebo treatment or usual care (odds ratio [OR], 0.70; 95% CI, 0.45 to 1.07; P = 0.10). In addition, no statistically significant difference in mortality was observed between patients who received IV‐VC treatment and those who did not (OR, 0.64; 95% CI, 0.41 to 1.00; P = 0.05). Moreover, the adjusted meta‐analysis revealed that the use of IV‐VC did not influence disease severity (OR, 0.67; 95% CI, 0.34 to 1.31; P = 0.242) or mortality (OR, 1.02; 95% CI, 0.75 to 1.40; P = 0.877) in comparison with a control group.
The results of this meta‐analysis demonstrated that short‐term IV‐VC treatment did not reduce the risk of severity and mortality in patients with COVID‐19.
Keywords: COVID‐19, critical illness, disease severity, meta‐analysis, mortality, SARS‐CoV‐2, vitamin C
INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has affected >100 million individuals worldwide, with ∼3 million deaths reported. 1 The clinical manifestations of COVID‐19 have ranged from absent or mild symptoms to severe respiratory illness or death. 2 Many previous studies have reported that cytokine storm, oxidative stress, and endothelial dysfunction are the main potential pathophysiological mechanisms of COVID‐19, which may lead to multiple organ failure and death. 3 , 4
Vitamin C (VC) is a water‐soluble vitamin with antioxidant, anti‐inflammatory, and immunomodulatory properties. 5 Consequently, VC has possible benefits in treating viral infections and inflammation. 6 VC has been clinically used for more than two centuries. 7 VC treatment can dramatically shorten the duration of stay in the intensive care unit (ICU) in patients with acute respiratory distress syndrome (ARDS). 8 Intravenously administered VC can also reduce 28‐day mortality in patients with sepsis by inhibiting the inflammatory reaction. 9 As for COVID‐19, some studies suggested that intravenous (IV) VC therapy could reduce mortality and improve prognosis. 10 , 11 Contrastingly, other studies revealed that IV‐VC treatment might have no discernible reduction in severity and mortality in patients with COVID‐19. 12 , 13 , 14 Hence, the definite effect of IV‐VC treatment of COVID‐19 remains controversial. Therefore, this study aimed to perform a systematic review and meta‐analysis to assess the effect of IV‐VC therapy on the clinical outcomes of patients with COVID‐19.
METHODS
We performed this meta‐analysis by following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 15 We conducted a comprehensive search of PubMed, Embase, Cochrane Library, MEDLINE, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Data to identify all relevant literature from January 1, 2019, to June 23, 2021. Search terms used, with no language restrictions, were as follows: (“2019‐nCoV” or “2019 novel coronavirus” or “coronavirus disease 2019” or “SARS‐CoV‐2” or “COVID‐19”) AND (“vitamin C” or “ascorbic acid” or “VC” or “l‐ascorbic acid” or “intravenous infusion” or “intravenous drip”). Details of the search strategy for each database are available in the online Supporting Information. We also scrutinized the reference lists, included articles of all eligible studies, and undertook a manual search of related articles to identify potential publications. Two independent investigators (Y.Y. and G.A.) performed the initial screening of titles and abstracts. They retrieved full‐length articles of all potential studies. Afterward, a screening using the eligibility criteria was conducted, in which studies were only included if they (1) enrolled patients with a diagnosis of COVID‐19; (2) provided a comparison of the clinical outcomes of patients treated with IV‐VC vs those who were not; (3) provided an odds ratio (OR) with 95% CI for outcomes of interest or data, such as overall survival or relevant clinical events, from which they could be calculated. We excluded studies if they were abstracts, conference presentations, editorials, or reviews. All decisions regarding eligibility were made according to prespecified selection criteria. Any discrepancies were resolved through consensus or discussion with a third investigator (X.Q.).
Relevant details from each screened article were independently extracted by two reviewers (Y.W. and Y.Y.). The following details were elicited from each study if available: first author's name, year of publication, study design, country of origin, number of participants, ages of patients, numbers of male and female participants, adjusted variables, intravenous administration of VC, and outcomes of interest. Estimation of disease severity was based on the definition in the individual study and depended mainly on severity of symptoms, hospitalization, ICU admission, intubation or mechanical ventilatory support, or deterioration into ARDS. The primary end point was the effect of IV‐VC on mortality and disease severity of COVID‐19. A study quality assessment was performed using the Jadad scale for randomized controlled trials (RCTs). 16 For non‐RCTs, a 9‐item Newcastle‐Ottawa scale (NOS) was used independently by two investigators (Y.W. and G.A.) to assess the quality of the studies. Consequently, we classified the NOS items for cohort studies into three dimensions: selection, comparability, and outcomes. The list of items included representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at the start of the study, comparability of cohorts based on the design or analysis, assessment of outcome, length of follow‐up adequate for outcomes to occur, and adequacy of follow‐up of cohorts. The overall NOS scores were classified into three levels: high (8–9 stars), medium (6–7 stars), and low (1–5 stars) quality. 17 Any discrepancy was resolved by reevaluation and consensus among the authors.
We used RevMan 5.3 (Cochrane Collaboration, London, UK) and Stata 12.0 (StataCorp, LLC, College Station, TX, USA) to conduct the statistical analysis. Unadjusted ORs and adjusted hazard ratios with 95% CIs were used as the summary statistic for dichotomous outcomes. We combined overall risk estimates in which unadjusted and adjusted dichotomous data were calculated using the Mantel‐Haenszel test and inverse‐variance methods, respectively. Statistical heterogeneity of all included studies was evaluated by Cochrane Q test and I 2 statistic. A Q‐statistic I 2 > 50% or a P < 0.05 suggested high heterogeneity. If I 2 > 50%, we used a random‐effect model to assess the impact of an intervention, whereas a fixed‐effect model was implemented for cases with an I 2 < 50%. We then conducted sensitivity analysis and subgroup analysis based on IV‐VC use, study design, country of origin, and all other factors that may cause heterogeneity. If >10 studies were included in this analysis, publication bias would have been assessed. 18 A P‐value < 0.05 was considered statistically significant. This research is registered with the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42021264847.
RESULTS
Figure 1 demonstrates a summary of the study retrieval process. A total of seven articles met our inclusion criteria and were subsequently included in this meta‐analysis. 10 , 11 , 12 , 13 , 14 , 19 , 20 Three of those articles were RCTs 14 , 20 and four were observational studies. 10 , 11 , 12 , 13 , 19 The detailed characteristics of each study are shown in Table 1. Among included articles, two studies enrolled patients with severe COVID‐19, 12 , 14 two enrolled critically ill patients in the ICU, 13 , 20 and three included patients with different stages of COVID‐19. 10 , 11 , 19 In addition to standard therapy for COVID‐19, patients included in the studies were treated with 2–24 g of IV‐VC/day for 3–7 days following admission. The sample size ranged from 32 to 323 patients. Three studies were from China, and the remaining four studies were from different countries. Some studies used propensity‐score matching to adjust for factors such as age, gender, and chronic medical conditions. The quality of the included articles is displayed in Table S1. All studies included in our meta‐analysis were of high quality (observational studies with an NOS score ≥ 6 and RCTs with a Jadad score ≥ 2).
Figure 1.
Flow diagram of literature search and study selection. IVC, intravenous vitamin C
Table 1.
Characteristics of included studies
| IV‐VC | Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Study design | Sample size | Age a | Male (%) | Age a | Male (%) | Definition of severity used | Types of patients | Usage of IV‐VC | Adjusted variables |
| Gao | China | Retrospective | 76 | 63 (54–71) | 21 (45.7) | 57 (49–67) | 14 (46.7) | Noninvasive positive pressure ventilation | A total of 48 (63.2%) patients had a diagnosis of moderate COVID‐19, and 28 (36.8%) severe or critical disease | Loading dose of 6 g, per 12 h on the first day, and 6 g once for the following 4 days | NR |
| Kumari | Pakistan | RCT | 150 | 52 ± 11 | NR | 53 ± 12 | NR | Mechanical ventilation | Severe COVID‐19 infection | 50 mg/kg/day | NR |
| Li | America | Retrospective | 32 | 64.1 ± 8.3 | 3 (37) | 64.9 + 11.8 | 9 (37) | NR | Critically ill ICU patients | 1.5 g, every 6 h for up to 4 days | Age, gender, diabetes, and hypertension |
| Siahkali | Iran | RCT | 60 | 57.53 ± 18.27 | 15 (50) | 61 ± 15.90 | 15 (50) | Intubation | Severe COVID‐19 infection | 1.5 g, every 6 h for 5 days | Age, gender, laboratory results, and underlying diseases |
| Suna | Turkey | Retrospective | 323 | 60.16 ± 13.65 | 102 (66.7) | 64.27 ± 14.49 | 102 (60) | ICU | Mild, moderate, severe, and critical patients | 2 g/day for a median duration of 3 days | NR |
| Zhang | China | RCT | 56 | 66.3 ± 11.2 | 15 (55.6) | 67.0 ± 14.3 | 22 (75.9) | Invasive mechanical ventilation | Critically ill ICU patients | 12 g, every 12 h for 7 days | Age, gender, general condition, and comorbidities |
| Zhao | China | Retrospective | 110 | 36 (31–47) | 33 | 36 (31–46) | 35 | Disease aggravation from moderate to a final diagnosis of severe or critical COVID‐19 | Mild, moderate, severe, and critical patients | 12 g, every 12 h for 7 days | Age and gender |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; IV‐VC, intravenous vitamin C; NR, not reported; RCT, randomized controlled trial.
aAge data presented as median (interquartile range) or mean (SD).
This meta‐analysis showed that IV‐VC therapy did not affect disease severity compared with placebo treatment or usual care (OR, 0.70; 95% CI, 0.45 to 1.07; P = 0.10; I 2 = 26%) (Figure 2A). In addition, no statistically significant difference was observed in mortality between patients who received IV‐VC treatment and those who did not (OR, 0.64; 95% CI, 0.41 to 1.00; P = 0.05; I 2 = 0%) (Figure 2B). Furthermore, the adjusted analysis revealed that IV‐VC treatment had no impact on disease severity (OR, 0.67; 95% CI, 0.34 to 1.31; P = 0.242; I 2 = 34.5%) (Figure 2C) or mortality (OR, 1.02; 95% CI, 0.75 to 1.40; P = 0.877; I 2 = 7.8%) (Figure 2D) compared with a control group. Heterogeneity across the studies was low (I 2 < 50%). Subgroup analysis based on countries, IV‐VC use, and study design did not significantly alter the overall estimates. Sensitivity analysis, performed by excluding one study at a time, did not significantly alter the results.
Figure 2.
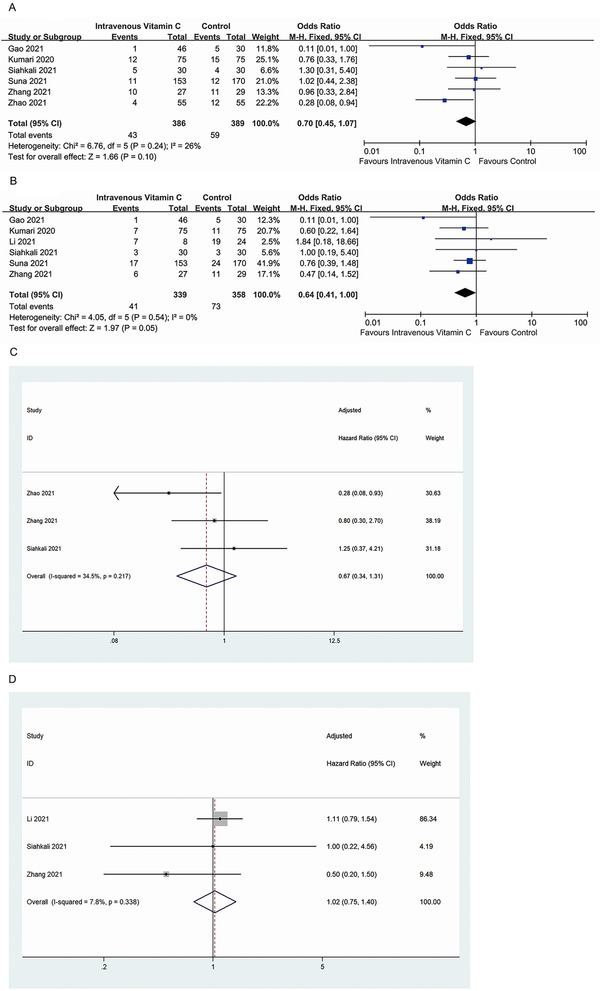
(A) Intravenous vitamin C exposure and risk of severity in patients with COVID‐19. (B) Intravenous vitamin C exposure and risk of mortality in patients with COVID‐19. (C) Intravenous vitamin C exposure and meta‐analysis of adjusted results of severity in patients with COVID‐19. (D) Intravenous vitamin C exposure and meta‐analysis of adjusted results of mortality in patients with COVID‐19. COVID‐19; coronavirus disease 2019; df, degree of freedom; M‐H, Mantel‐Haenszel
DISCUSSION
In this systematic review and meta‐analysis, we included three RCTs and four observational studies to evaluate the role of IV‐VC therapy in patients with COVID‐19. The overall results indicated that patients with COVID‐19 who were treated with IV‐VC did not manifest signs of improved prognosis. These results are consistent with those of a recent RCT, which showed that IV‐VC treatment did not reduce the mortality rate in patients with septic shock. 21
Respiratory failure due to ARDS is the primary cause of mortality in patients with COVID‐19. 22 Both cytokine storm and oxidative stress play an essential role in the progression of COVID‐19 to ARDS. 23 Previous studies have intimated the immunomodulatory, antioxidant, and anti‐inflammatory activities of VC. 24 , 25 , 26 VC has been widely used in treating several inflammatory diseases, especially ARDS and sepsis. 27 , 28 , 29 Severe inflammation and cytokine storm contribute to severe ARDS and subsequent mortality in COVID‐19. 30 The role of VC is limited in patients with mild to moderate COVID‐19 who are unlikely to develop severe inflammation or cytokine storm. 31 Several studies have suggested that VC can effectively inhibit numerous viruses, such as influenza type A, rhinovirus, avian virus H1N1, and poliovirus type 1. 32 , 33 , 34 Other studies have shown that IV‐VC use may promote better clinical outcomes for patients in the ICU. 8 , 35 A large RCT conducted in the United States, the CITRIS‐ALI trial, demonstrated that administration of 200 mg/kg/day of IV‐VC for 4 days did not significantly improve organ dysfunction scores or alter markers of inflammation and vascular injury. In contrast, the mortality rate of patients with sepsis and ARDS had decreased in the same trial. 9 The CITRIS‐ALI trial enrolled patients with sepsis and fully developed ARDS instead of those in the early stages of sepsis and implied that VC might exert better effect if given earlier in the course of the illness. Nevertheless, a recent systematic review of RCTs among critically ill patients with sepsis found that IV‐VC therapy might be associated with a trend toward reduction in overall mortality. 36 That finding is similar to our unadjusted analysis in patients with COVID‐19, indicating possible beneficial effects of VC in reducing inflammatory responses and oxidative stress. However, after considering the potential confounding factors, our pooled analysis of adjusted results revealed no difference in mortality between patients who received IV‐VC treatment and those who did not.
To the best of our knowledge, this is the first meta‐analysis focusing on IV‐VC use and risk of severity and mortality in COVID‐19. We analyzed pooled analyses of both unadjusted and adjusted results. A previous meta‐analysis demonstrated that regular supplementation of VC was more effective than starting it at the onset of illness in respiratory tract infections. 37 In our study, most patients began IV‐VC treatment after hospitalization or progressing to severe COVID‐19. Thus, initiation of IV‐VC was not early enough, and the treatment duration was relatively short. Time of initiation and duration of IV‐VC therapy are important in assessing its efficacy, considering that early and adequate therapy may be required to attenuate cytokine storm and inflammation. 38 Mode of administration, VC dosage, initiation time, treatment duration, disease type, and disease progression may explain why our results are discordant with those of previous studies.
This study has some inherent limitations described as follows. First, the number of eligible high‐quality studies was relatively small, which may have affected the accuracy of the results. Second, despite only mild heterogeneity observed in the analysis of COVID‐19 severity, the underlying clinical heterogeneity may cause a degree of statistical heterogeneity in the results. The definitions of COVID‐19 severity were inconsistent among the enrolled studies. Third, studies included in the meta‐analysis did not provide sufficient data regarding the effect of timing and duration of therapy on the outcome of interest. These studies used IV‐VC therapy for different durations and in different dosages. To that end, more robust RCTs are required to evaluate and optimize the timing, dosage, and duration of IV‐VC treatment to understand the precise effect of this intervention on prognosis in patients with COVID‐19.
CONCLUSION
This meta‐analysis indicated that short‐term IV‐VC treatment did not reduce the risk of severity and mortality in patients with COVID‐19.
CONFLICT OF INTEREST
None declared.
FINANCIAL DISCLOSURE
None declared.
AUTHOR CONTRIBUTIONS
Guangyu Ao participated in scientific direction, data collection, systematic review, and image analysis; Jing Li participated in data collection, data analysis, and article revision; Yang Yuan participated in the study design, data analysis, statistical analysis, and writing; Yushu Wang participated in data collection and writing of the article; Basma Nasr participated in English writing; Mulong Bao participated in data collection and data analysis; Ming Gao participated in data collection and data analysis; and Xin Qi participated in coordinating and directing the project. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
Supporting information
Supplementary Table 1 Observational studies using the Newcastle‐Ottawa Scale
Supplementary Information
Ao G, Li J, Yuan Y, et al. Intravenous vitamin C use and risk of severity and mortality in COVID‐19: A systematic review and meta‐analysis. Nutr Clin Pract. 2022;37:274‐281. 10.1002/ncp.10832
Guangyu Ao, Jing Li, Yang Yuan, and Yushu Wang contributed equally to this study.
REFERENCES
- 1. WHO Coronavirus Disease (COVID‐19) Dashboard . Accessed June 20, 2021. https://covid19.who.int/.
- 2. Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus disease (COVID‐19): comprehensive review of clinical presentation. Front Public Health. 2021;8:582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kouhpayeh S, Shariati L, Boshtam M, et al. The molecular basis of COVID‐19 pathogenesis, conventional and nanomedicine therapy. Int J Mol Sci. 2021;22(11):5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beltrán‐García J, Osca‐Verdegal R, Pallardó FV, et al. Oxidative stress and inflammation in COVID‐19‐associated sepsis: the potential role of anti‐oxidant therapy in avoiding disease progression. Antioxidants. 2020;9(10):936. [Google Scholar]
- 5. Carr AC, Rowe S. The emerging role of vitamin C in the prevention and treatment of COVID‐19. Nutrients. 2020;12(11):3286. [Google Scholar]
- 6. Holford P, Carr AC, Jovic TH, et al. Vitamin C‐an adjunctive therapy for respiratory infection, sepsis and COVID‐19. Nutrients. 2020;12(12):3760. [Google Scholar]
- 7. Carpenter KJ. The discovery of vitamin C. Ann Nutr Metab. 2012;61(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 8. Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta‐analysis. Nutrients. 2019;11(4):708. [Google Scholar]
- 9. Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS‐ALI randomized clinical trial. JAMA. 2019;322(13):1261‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao D, Xu M, Wang G, et al. The efficiency and safety of high‐dose vitamin C in patients with COVID‐19: a retrospective cohort study. Aging. 2021;13(5):7020‐7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao B, Liu M, Liu P, et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID‐19 pneumonia. A retrospective propensity matched before‐after study. Front Pharmacol. 2021;12:638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumari P, Dembra S, Dembra P, et al. The role of vitamin C as adjuvant therapy in COVID‐19. Cureus. 2020;12(11):e11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Ching TH, Hipple C, Lopez R, Sahibzada A, Rahman H. Use of intravenous vitamin C in critically ill patients with COVID‐19 infection. J Pharm Pract. 2021;8971900211015052. [Google Scholar]
- 14. Jamali Moghadam Siahkali S, Zarezade B, Koolaji S, et al. Safety and effectiveness of high‐dose vitamin C in patients with COVID‐19: a randomized open‐label clinical trial. Eur J Med Res. 2021;26(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178‐189. [DOI] [PubMed] [Google Scholar]
- 16. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1‐17. [DOI] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta‐analyses. Dept of epidemiology and community medicine, university of Ottawa. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed on May 1, 2021. [Google Scholar]
- 18. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook.
- 19. Suna K, Melahat UŞ, Murat Y, Figen ÖE, Ayperi Ö. Effect of high‐dose intravenous vitamin C on prognosis in patients with SARS‐CoV‐2 pneumonia. Med Clin. 2021;S0025‐7753(21)00252‐9. [Google Scholar]
- 20. Zhang J, Rao X, Li Y, et al. Pilot trial of high‐dose vitamin C in critically ill COVID‐19 patients. Ann Intensive Care. 2021;11(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujii T, Luethi N, Young PJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323(5):423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial dysfunction in COVID‐19: Lessons learned from coronaviruses. Curr Hypertens Rep. 2020;22(9):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. [Google Scholar]
- 25. May CN, Bellomo R, Lankadeva YR. Therapeutic potential of megadose vitamin C to reverse organ dysfunction in sepsis and COVID‐19. Br J Pharmacol. 2021;178(19):3864‐3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abobaker A, Alzwi A, Alraied AHA. Overview of the possible role of vitamin C in management of COVID‐19. Pharmacol Rep. 2020;72(6):1517‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before‐after study. Chest. 2017;151(6):1229‐1238. [DOI] [PubMed] [Google Scholar]
- 28. Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score‐based analysis of a before‐after cohort study. J Crit Care. 2018;47:211‐218. [DOI] [PubMed] [Google Scholar]
- 29. Hoang BX, Shaw G, Fang W, Han B. Possible application of high‐dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramasamy S, Subbian S. Critical determinants of cytokine storm and type I interferon response in COVID‐19 pathogenesis. Clin Microbiol Rev. 2021;34(3):e00299‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hiedra R, Lo KB, Elbashabsheh M, et al. The use of IV vitamin C for patients with COVID‐19: a case series. Expert Rev Anti Infect Ther. 2020;18(12):1259‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim Y, Kim H, Bae S, et al. Vitamin C is an essential factor on the anti‐viral immune responses through the production of interferon‐α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013;13(2):70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furuya A, Uozaki M, Yamasaki H, Arakawa T, Arita M, Koyama AH. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med. 2008;22(4):541‐545. [PubMed] [Google Scholar]
- 34. Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019;9(3):73‐79. [Google Scholar]
- 35. Hemilä H, Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta‐regression analysis. J Intensive Care. 2020;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sato R, Hasegawa D, Prasitlumkum N, et al. Effect of IV high‐dose vitamin C on mortality in patients sepsis: A systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2021;49(12):2121‐2130. [DOI] [PubMed] [Google Scholar]
- 37. Ran L, Zhao W, Wang J, et al. Extra dose of vitamin C based on a daily supplementation shortens the common cold: A meta‐analysis of 9 randomized controlled trials. Biomed Res Int. 2018;2018:1837634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Wajanaponsan N, Reade MC, Milbrandt EB. Steroids in late ARDS? Crit Care. 2007;11(4):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Observational studies using the Newcastle‐Ottawa Scale
Supplementary Information


