Abstract
The efficacy of the inactivated severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccine has not been fully elucidated across the whole spectrum of patients on kidney replacement therapy. We aimed to characterize the long‐term antibody response of inactivated SARS‐CoV‐2 vaccine administered in kidney transplant recipients (KTRs) and hemodialysis (HD) patients. We performed this prospective observational study in 50 HD, 64 KTR, and 41 healthy control groups (HG) given two doses of CoronaVac. We measured anti‐Spike antibodies after 28 days of every vaccine dose, 3rd and 6th months after the first dose, and compared them between cohorts. After two doses, an anti‐spike immunoglobulin G of ≥50 AU/ml was present in HD, KTR, and HG as 44%, 7.2%, and 58.5%, respectively (p < 0.001). Furthermore, the proportion of antibody titers peaked at 86.5%, 23%, and 97.6% (p < 0.001) at the 3rd month and decreased significantly at the 6th month in most HD and HG participants, whereas this effect was not observed in KTRs from basal until the 6th month (p < 0.001). During the follow‐up, the incidence of coronavirus disease 2019 disease was higher (p < 0.003) in KTRs compared to the other groups, but there was no requirement for an intensive care unit and no death was recorded. We found a negative correlation between antibody seroconversion and age (p < 0.016). The antibody response following inactivated vaccine in dialysis patients is almost comparable to controls for 6 months. In contrast, kidney transplant patients have a poor response. These findings reinforce the need to discuss the vaccination strategy in immunocompromised patients, including the third dose with homologous or heterologous vaccines.
Keywords: COVID‐19, dialysis, inactivated vaccine, kidney disease, kidney transplantation, SARAS‐CoV‐2
Highlights
Incativated COVID‐19 vaccine has been shown to be effective in the normal population. However, its effectiveness in uremic patients and kidney transplant recipients is controversial.
Based on our results, inactivated COVID‐19 vaccine is safe and effective in the short and mid‐term in hemodialysis patients as well as in the normal population, but not in kidney recipients.
The incidence of COVID‐19 disease after CoronaVac was significantly higher in kidney transplant recipients compered with hemodialysis and normal population.
These findings reinforce the need to discuss the vaccination strategy in immunocompromised patients, including the third dose with homologous or heterologous vaccines.
1. INTRODUCTION
The new type of coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) threatens global public health by undergoing different mutations over time. Before the emergence of COVID‐19 vaccines, wearing face masks, social distancing, and contact tracing were the cornerstones of health policies to reduce or prevent viral spread among people. 1 , 2 With these measures, it has been clearly demonstrated that the COVID‐19 disease can be controlled after the emergence of different effective and safe vaccines in the normal population. 3 , 4 So far, messenger RNA (mRNA)‐based COVID‐19 vaccines have proven to be the most protective vaccine type against COVID‐19 disease in the normal population and in different chronic kidney diseases (CKDs). 3 , 5 , 6 , 7 CKD and kidney transplantation are important independent risk factors for severe COVID‐19 disease. 8 , 9 , 10 In addition, COVID‐19 disease‐related mortality rates are higher in patients receiving renal replacement therapy (RRT) than in the normal population. 11 , 12 , 13 Therefore, health authorities emphasize that vaccination approaches against COVID‐19 should be a priority in CKD and kidney recipients. However, the efficacy of COVID‐19 vaccines in immunocompromised kidney transplant patients was found to be significantly lower than in other populations. 5 , 6 At the beginning of the COVID‐19 pandemic, it was not understood how effective the vaccines were in these patient groups, as the phase III studies against COVID‐19 did not include CKD, dialysis, or kidney recipients and focused only on normal individuals. However, in studies conducted after the administration of vaccines over time, it has been found that antibody responses are weaker in uremic and immunosuppressive patients. 14 Studies have reported varying results in antibody response rates of approximately 5%–50% after two doses of mRNA vaccine in kidney recipients and up to >90% in dialysis patients for short periods of time. 5 , 15 The long‐term efficacy rates of the antibody response of the mRNA vaccine in dialysis patients were found at 1, 3, and 6 months after the second vaccine dose, respectively; 94%, 78%, and 73%. 16 Studies investigating the long‐term efficacy of mRNA vaccine in RRT patients are ongoing (ClinicalTrials.gov NCT04741386). 17
With respect to inactivated vaccines; anti‐spike immunoglobulin G (IgG) antibody response was detected in more than 80% of dialysis patients, but lower than 20% of kidney recipients after two doses of CoronaVac vaccines. 18 , 19 , 20 These results reflect an 8‐week time course and lead to debates about antibody response and its protection in RRT patients in the long term.
We did not find any comprehensive research investigating the effectiveness of inactivated COVID‐19 vaccines administered to different kidney patients for a long time. Our aim was to characterize the long‐term antibody response of inactivated SARS‐CoV2 vaccine administered in kidney transplant recipients (KTRs) and hemodialysis (HD) patients.
2. MATERIALS AND METHODS
Patients who preferred only inactivated SARS‐CoV‐2 vaccine (CoronaVac®) between January 30 and June 30, 2021, were included in this study. Other inclusion criteria; (1) being >18 years old, (2) No history of COVID‐19 disease, (3) History of kidney transplant or HD for at least 6 months, and (4) Approval of informed consent to the study. As exclusion criteria; (1) being <18 years old, (2) having a history of COVID‐19 disease, (3) having a history of malignancy, (4) having a previous history of allergy to any inactivated vaccine, and (5) having an unexplained 37.5°C fever or any symptoms of infection has been determined. CoronaVac® vaccine was administered intramuscularly in two doses, 28 days apart, in all participants.
The study was approved by the Ethical Committee of Sakarya University Faculty of Medicine Ethics Committee (Approval Number: E‐71522473‐050.01.04‐597811) on 29 January 2021. Written informed consents had been obtained from all patients before the blood samples were taken.
Demographic characteristics of patients and healthy volunteers such as age, gender, comorbid disease status, duration of dialysis, duration of kidney transplantation, immunosuppressive regimens, and any previous allergic condition were recorded. In addition, detailed physical examination and biochemical parameters such as complete urinalysis, hemogram, liver, and kidney function tests were examined before serum collection and before each vaccination. Also, we evaluated the vital signs of all participants and questioned possible local and systemic side effects before each vaccination. Serum samples were collected on day 28 of the first and second dose, 3rd and 6th months from the first vaccination procedures. At each collection, samples were centrifuged at room temperature (23°C), and the sera samples were kept at –80°C condition until the tests were studied.
2.1. Statistical analyses
Statistical analyses were performed using SPSS version 22 software. The suitability of the variables to normal distribution was examined using visual (histogram and probability graphs) and analytical methods (Kolmogorov–Smirnov). The continuous variables were expressed as mean and standard deviation or as median and interquartile range (IQR), depending on the normality of their distribution. Categorical variables were described as frequencies and percentages. Categorical features and relationships between groups were assessed using an appropriate chi‐square test. Variables that were not normally distributed were compared using the Kruskal–Wallis test. When binary comparisons were required, the Mann–Whitney U test was used. Normally distributed variables were compared using a one‐way analysis of variance test. When an overall significance was observed, pairwise post hoc tests were performed using Tukey's test. Levene test was used to assess the homogeneity of the variances. Whether there is a difference between the binary groups (such as groups formed according to MFF doses) in terms of numerical variables; If parametric test conditions were fulfilled, independent groups were examined by t test and if not, Mann–Whitney U test was used. While investigating the associations between nonnormally distributed and/or ordinal variables, the correlation coefficients and their significance were calculated using the Spearman test. The statistically significant two‐tailed p value was considered as <0.05.
2.2. SARS‐CoV‐2 antibody testing
The tests were performed blindly by the only authorized microbiologist in the laboratory of our university. After the peripheral blood samples taken from the patient were centrifuged at 4000 rpm/10 min, the serums were stored at −80°C until the quantitative SARS‐CoV‐2 IgG test was run. Quantitative SARS‐CoV‐2 IgG test (SARS‐CoV‐2 IgG II Quant; Abbott Diagnostics) was performed in the Abbott Architect device (Abbott Diagnostics) in accordance with the manufacturer's recommendations. This antibody test is based on the principle of chemiluminescence microparticle immunoassay test, binding of SARS‐CoV‐2 antigen‐coated paramagnetic microparticles to IgG antibodies that attach to the spike protein of the virus in human serum and plasma sample, and measuring the light unit as a result of the reaction. Quantitative results are given in AU/ml (arbitrary unit/ml). Samples with AU/ml ≥50 are considered positive for SARS‐CoV‐2 IgG antibodies.
3. RESULTS
The study included 50 HD patients, 64 KTRs, and 41 HG. Forty‐five patients were excluded due to antibody positivity in the serum samples obtained before vaccination or having exclusion criteria set in the study. All HD patients were undergoing HD three times a week. All KTRs were receiving triple maintenance therapy with corticosteroids, tacrolimus, and mycophenolate mofetil (MMF). The median (IQR) age of the individuals was 54 (44.75–58), 47 (37–55.75), and 40 years (26.5–50.5) (p < 0.001), respectively, with a significant difference between KTR versus HG (p = 0.011) and HD versus HG (p < 0.001) were detected. There was no difference between the groups in terms of gender distribution (p = 0.056). There was no significant difference between the groups in terms of comorbid diseases such as diabetes mellitus, hypertension, atherosclerotic heart disease, and chronic obstructive pulmonary disease (p > 0.05). Other descriptive demographic characteristics and baseline laboratory results are summarized in Table 1. The median (IQR) HD duration in HD patients was 37.5 (24–52.25) months and ranged from 13 to 133 months. In the KTR group, the median (IQR) time between the first vaccine and transplantation was 18.9 (10.7–34.0) months and ranged from 0.7 to 208.8 months (min–max). The rate of living kidney transplantation among KTRs was 86% (no = 55).
Table 1.
Comparison of baseline clinical features and laboratory results
| HD (n = 50) | KTRs (n = 64) | HG (n = 41) | p | |
|---|---|---|---|---|
| Age, years | 54 (44.7–58) | 47 (37–55.8) | 40 (26.5–50.5) | <0.001a |
| Gender, male (%) | 34 (68) | 33 (51.6) | 18 (43.9) | 0.056 |
| Body mass index, kg/m2 | 25.7 ± 5.3 | 27.4 ± 4.7 | 25.2 ± 4.1 | 0.059 |
| Smoking, n (%) | 19 (38) | 3 (4.7) | 9 (22.0) | <0.001b |
| White blood cell count, 103/mm3 | 7.7 ± 1.8 | 7.8 ± 2.2 | 7.0 ± 2.1 | 0.130 |
| Absolute neutrophil count, 103/mm3 | 4.9 ± 1.9 | 5.4 ± 3.4 | 4.1 ± 1.5 | 0.069 |
| Absolute lymphocyte count, 103/mm3 | 1.9 (1.5–2.3) | 2.1 (1.5–2.5) | 2.2 (1.6–2.6) | 0.243 |
| Serum creatinine, mg/dl | 7.2 ± 2.4 | 1.1 ± 0.3 | 0.7 ± 0.2 | <0.001c |
| Serum uric acid, mg/ml | 5.5 ± 1.6 | 5.9 ± 1.3 | 5.0 ± 1.3 | 0.004d |
| Sedimentation, mm/h | 50.1 ± 28.2 | 11.9 ± 10.4 | 12.7 ± 10.3 | <0.001c |
| C‐reactive protein (CRP), mg/L | 8.3 (3.3–22.6) | 3.8 (3.3–9.1) | 3.3 (3.3–3.3) | <0.001c |
| 25‐OH‐vitamin D3, ng/ml | 8.8 ± 2.2 | 14.2 ± 10.6 | 19.2 ± 12.3 | 0.003d |
| Serum Albumin, gr/L | 4 (3.8–4.1) | 4.3 (4.1–4.5) | 4.4 (4.3–4.5) | <0.001e |
| Parathyroid hormone, pg/ml | 335 (225–738) | 119 (85–162) | 61 (46–83) | <0.001e |
| Thyroid‐stimulating hormone, mIU/L | 1.3 (0.8–1.7) | 1.5 (1.1–2.3) | 1.4 (1.1–1.6) | 0.200 |
Note: Descriptive results for continuous variables were expressed as mean and standard deviation or as median and interquartile range, depending on the normality of their distribution.
Abbreviations: HD, hemodialysis; HG, healthy group; KTR, kidney transplant recipient.
The difference is both between the healthy controls and the kidney transplant recipients (p = 0.011) and between the healthy controls and the dialysis patients (p < 0.001).
The difference is due to the group of kidney transplant recipients.
The difference is due to the group of dialysis patients.
The difference is only between dialysis patients and healthy controls.
The three groups are different from each other.
The antibody seroconversion rates after the first and second vaccination were compared between the groups. After the first vaccine dose, there was no significant increase in anti‐spike IgG antibody levels between the groups (p = 0.440). However, after the second vaccine dose, in HD, KTR, and HG, respectively; 44%, 7.2%, and 58.5% higher anti‐spike IgG antibody levels were detected (p < 0.001). The significant difference was due to transplant recipients and was not significant between HD patients and HG. Likewise, the seroconversion rates of anti‐spiked IgG antibodies at the 3rd month after vaccination peaked, respectively; 86%, 23.4%, and 97.6% (p < 0.001) and at 6th month decreased to 78%, 28.6%, and 85.4%, respectively (p < 0.001), with the difference again due to transplant recipients (Figure 1). Median (IQR) anti‐spike IgG antibody values of HD, KTR, and HG, respectively; post 1st vaccination [1.8 AU/ml (0.8–4.0) vs. 2.2 (0.9–8.6) vs. 1.6 (0.5–3.3), p = 0.336], post 2nd vaccination [27.4 AU/ml (7.8–161.5) vs. 1.8 (0.5–11.6) vs. 74.9 (24.6–270.1) p < 0.001], 3rd month [320.4 AU/ml (77.9–932.3) vs. 3.1 (1.3–22.1) vs. 652.1 (407.7–1305.9), p < 0.001] and 6th month [116.3 AU/ml (52.1–661.5) vs. 4.2 (2.0–165.4) vs. 153.6 (82.2–431.4), p < 0.001] (Figure 1).
Figure 1.
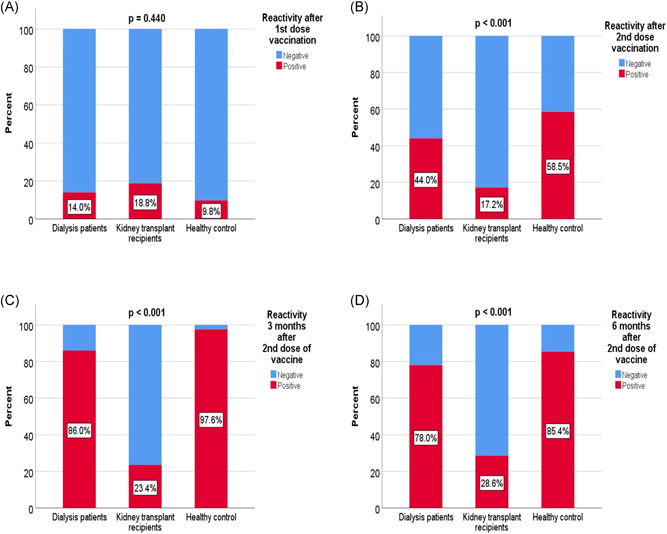
Comparison of seroconversion rates after vaccination between groups. (A) After first dose vaccination, (B) After second dose vaccination, (C) At 3rd month, (D) At 6th month
When the patient groups were compared with respect to whether the use of full‐dose and reduced‐dose of MMF played a significant role in antibody seroconversion; anti‐spike IgG antibodies appeared after the 1st vaccine dose (p = 0.152), the 2nd vaccine dose (p = 0.214), the 3rd month (p = 0.473) and the 6th month (p = 0.452) showed no significant difference in any of the groups. The median (IQR) anti‐spike IgG antibody values of the full‐dose and reduced‐dose MMF groups were after the 1st vaccine [2.85 AU/ml (0.78–44.1) vs. 1.9 AU/ml (1.1–2.7), p = 0.318], after the 2nd vaccine [2.1 AU/ml (0.8–28.3) vs. 1.7 AU/ml (1.0–2.9), p = 0.755], the 3rd month [3.6 AU/ml (1.0–123.3) vs. 2.3 AU/ml (1.4–14.1), p = 0.740] and the 6th month [4.2 AU/ml (2.1–183.9) vs. 6.2 AU/ml (1.9–82.3), p = 0.537].
Possible factors that could affect the results of the 3rd month after vaccination, in which the highest seroconversion rates were obtained in KTRs, were evaluated with logistic regression analysis. Using the univariate analysis, we did not find significant effects in terms of age (≥45 years), gender, transplant duration (≥1 year), absolute lymphocyte count, neutrophil/lymphocyte ratio, serum 25‐OH‐vitD3, serum albumin, serum PTH levels, and MMF usage at the 3rd month (p > 0.05).
After receiving two doses of vaccine, 24 (15.5%) of the patients had COVID‐19 disease. The mean time between the second dose of vaccine and COVID‐19 infection time was 4.3 ± 2.6 months. The rates of COVID‐19 disease were 4.0% (n = 2) in HD patients, 26.6% (n = 17) in KTRs, and 12.2% (n = 5) in HG, respectively, with significant differences between the groups (p = 0.003). The significant difference was between HD patients and the KTR group (Figure 2, 3). During the follow‐up, none of the COVID‐19 positive patients in the HG and HD groups required hospitalization or intensive care unit (ICU). However, 5 (29.4%) of the patients who were positive for COVID‐19 in the KTR group were hospitalized in the ward but did not require an ICU. Fortunately, no deaths were recorded in any group.
Figure 2.
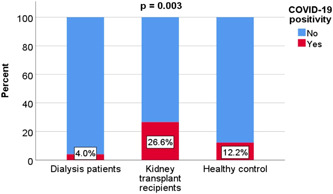
Comparison of the incidence of COVID‐19 disease after vaccination between groups. COVID‐19, coronavirus disease 2019
Figure 3.
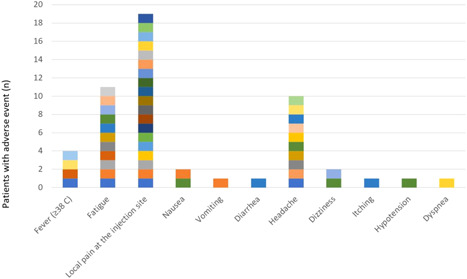
Side effects were observed after the first vaccination in all participants
After the first dose of vaccination, local pain at the injection site in 19 (12.3%) cases (12 HD; 7 HG), fatigue in 11 (7.1%) cases (4 HD; 7 HG), headache in 10 (6.5%) cases (2 HD; 1 KTR; 7 HG), fever in 4 (2.6%) cases (2 HD; 2 KTR), dizziness in 2 (1.3%) cases (HG), nausea in 2 (1.3%) cases (1 HD; 1 HG), vomiting 1 (HD) in (0.6%), diarrhea in 1 (0.6%) patient (HG), pruritus in 1 (0.6%) patient (HG), dyspnea in 1 patient (KTR), and hypotension in 1 (0.6%) patient (HG) reversible side effects were observed (Figure 2, 3).
After the second dose of vaccination, reversible side effects such as abdominal pain, hypotension, sore throat, hoarseness, diarrhea, difficulty swallowing, and vomiting were not observed in any of the participants, while headache in 6 patients (3 HD; 3 HG), weakness in 6 patients (3 HD; 3 HG), Dizziness was observed in 2 patients (HG), nausea in 1 patient (HG), and fever in 1 patient (HG). In addition, tenderness at the injection site was observed in 15 (9 HD; 6 HG) patients, redness at the injection site in 2 (HG) patients, and itching at the injection site in 1 (HG) patient, while shortness of breath (the patient who developed shortness of breath after the first vaccination) was observed in only 1 patient in the KTR group.
The relationship between antibody titer after the 2nd dose and age, body mass index (BMI), lymphocyte count, neutrophil‐lymphocyte ratio, vitamin D dose, 3rd‐month antibody titer, and 6th‐month antibody levels were analyzed by Spearman correlation analysis; there was a significant positive correlation between the 3rd and 6th‐month anti‐spike antibody titers (p < 0.001) and negative correlation with age (p < 0.016). The relationship between the other parameters was not significant (Table 2). There was no significant correlation between transplantation time and anti‐spike IgG antibody levels in the KTR group.
Table 2.
Correlation analysis between the level of antibody (IgG) formed after the second vaccination and different variables
| r value | p | |
|---|---|---|
| 3rd‐month antibody level, mg/dl | 0.827 | <0.001 |
| 6th‐month antibody level, mg/dl | 0.723 | <0.001 |
| Age, years | −0.192 | 0.016 |
| Body mass index, kg/m2 | −0.097 | 0.232 |
| Absolute lymphocyte count, 103/mm3 | 0.031 | 0.700 |
| Neutrophil lymphocyte ratio | −0.092 | 0.259 |
| 25‐OH‐vitamin D3, ng/ml | −0.015 | 0.875 |
Abbreviation: IgG, immunoglobulin G.
4. DISCUSSION
This comparative observational study prospectively investigated the effectiveness of inactivated SARS‐CoV‐2 vaccine in different kidney diseases compared to healthy individuals. Inactivated SARS‐CoV‐2 vaccine was approved by the Ministry of Health in the first quarter of 2021 in our country. We observed that anti‐spike IgG antibody responses were quite high in healthy individuals and HD patients after two doses of inactivated SARS‐CoV‐2 vaccine and remained high in most of the participants until the 6th month, but we did not observe this effect in KTRs.
The response of antibodies after various COVID‐19 vaccines have been shown in almost all normal populations, whereas contradictory results have been detected in different kidney diseases. 3 , 21 The antibody response of mRNA‐based COVID‐19 vaccines has proven high efficacy in CKD and dialysis patients (>80%), but not in kidney recipients 22 , 23 , 24 However, the efficacy of the inactivated SARS‐CoV‐2 vaccine in different kidney diseases has not been fully elucidated. In a study on HD patients, the seroconversion rates (80%) and neutralizing antibody levels (median 39.8 BAU/ml) were significantly lower in patients who received inactivated vaccines, whereas mRNA vaccines had better immunogenicity. However, both vaccines could be protected from symptomatic COVID‐19 disease when seropositivity was achieved. 19 In another study, IgG antibody response after two doses of inactivated vaccine administered to HD and peritoneal dialysis patients was 88%, whereas it was 100% in the healthy group. The results of whether antibody titers remained high after the 2‐month period were not reported. 20 To the best of our knowledge, we have not found any study investigating the long‐term antibody response of inactivated COVID‐19 vaccine in patients undergoing RRT. In the present study, antibody response after two doses of CoronaVac was 97.6% in the healthy group and 86.0% in HD patients consistent with the literature. In addition, we observed that the antibody seropositivity in the study population peaked at the 3rd month but decreased significantly from the 3rd month to the 6th month. Similarly, a study in the normal population showed that the initial antibody response from two doses of CoronaVac declined at or below the lower limit of seropositivity after 6 months. 24 Therefore, it is important to administer a third dose 3–6 months after the second dose in these patients.
On the other hand, we found significantly lower antibody responses in KTRs compared to HD and HG groups. KTRs, as with other inactivated viral vaccines, are expected to have low antibody responses to COVID‐19 vaccines because they have impaired humoral and cellular immunity, depending on the type and number of immunosuppressive agents. 25 , 26 Post mRNA vaccine, KTRs who received anti‐metabolite maintenance immunosuppression therapy were less likely to develop an antibody response than those who did not receive these agents. 26 , 27 However, in our study, we did not find any significant relationship between the use of MMF and the probability of developing antibodies after CoronaVac vaccination. Moreover, we found that during the study period, using full‐dose or low‐dose MMF did not affect the antibody response. Randomized controlled studies are needed to examine the results of these two different types of vaccines.
At follow‐up in our KTR group, the risk of developing COVID‐19 disease after two vaccinations were higher in the KTR group, as expected. However, none of the patients died or required an ICU. Although the seroconversion rate of CoronaVac was low in KTRs, the mean time between the second vaccine dose and the duration of COVID‐19 infection was 4.3 ± 2.6 months. After such a long time, a third vaccination is already recommended to protect against COVID‐19 disease. Perhaps in line with these results, it is extremely important to administer more than two COVID‐19 mRNA vaccines recommended for these patients. Kidney transplant patients who received a total of three doses of mRNA vaccine had raised spike‐specific IgG seroconversion significantly from 44.3% (n = 27) after the second dose to 62.3% (n = 38) after the third dose (p < 0.05). 15 , 28 As an important observation; a significant antibody response was observed in 49% of cases when a third dose of vaccine was administered to kidney recipients without anti‐Spike IgG antibody response after two doses of mRNA vaccine. 29 However, we need controlled studies investigating the efficacy of more than two inactivated COVID‐19 vaccines, especially in kidney transplant patients.
In the present study, no irreversible CoronaVac adverse events were recorded among the participants. We found the most common side effects such as local pain at the injection site, fatigue, and headache. These results were found to be similar to the side effects seen in the normal population reported in the literature. 30 , 31
In one small study; old age, high ferritin level, and low absolute lymphocyte count were independently associated with poor humoral immune responses after two doses of inactivated vaccine in HD patients compared with the healthy control group. 20 Age and serum creatinine elevation in KTRs has been reported as independent factors for vaccine unresponsiveness. 18 We detected a significant negative correlation with age, but not with other factors. In logistic regression analysis, we did not find any predictive factor that affects poor antibody seroconversion after CoronaVac vaccination. We are of the opinion that randomized controlled and larger studies are needed on this subject.
The main strength of our study is the prospective design with the inclusion of different cohorts of kidney patients as well as a control cohort for a long time. The study also has some limitations. First, we included a relatively small number of patients and included different age groups, which could affect antibody titers between groups. However, the ages of all the cases included in the study were under 60 years old. Second, all participants received the same inactivated vaccine, which prevented obtaining and comparing results regarding response to other types of vaccines. In fact, different vaccines are being investigated in the same normal population to increase their efficacy. 32 The issue of administering different vaccines to the same RRT patients has not yet been clarified. Third, we excluded patients who previously experienced COVID‐19 to have a uniform, immunologically naïve patient group. However, we have not been able to learn the antibody responses we obtained, how much the post‐vaccine antibody responses increased after suffering COVID‐19 disease, and how long they lasted. As antibody responses were observed after COVID‐19 in patients receiving RRT, we could not learn that higher seroconversion rates can be obtained after additional vaccination in these patients. Fourth, cellular immunity was not evaluated in the present study. However, assessment of cellular immunity is unlikely to be routinely available in all centers in the near future. Fifth, we included patients with HD and transplantation periods of at least 6 months. This calls for caution in extrapolating these outcomes to patients who received RRT less than 6 months ago.
In conclusion, the rate of anti‐Spike antibody development was low in KTRs in the short and long term after vaccination. It was sufficient in HD patients and healthy individuals in the short term, whereas it was low in all groups in the long term; suggesting that KTRs require persistent isolation measures. These findings reinforce the need to discuss the vaccination strategy in immunocompromised populations, including the third dose with homologous or heterologous vaccines.
AUTHORS CONTRIBUTIONS
Hamad Dheir and Oguz Karabay: conceived of the presented idea. Hamad Dheir, Selcuk Yaylaci, Ahmed Cihad Genc, and Ahmed Bilal Genc: developed the theory and performed the computations. Mehmet Koroglu, Taner Demirci, and Hande Toptan: verified the analytical methods. Aysel Tocoglu and Taner Demirci: involved in data analysis. Hamad Dheir and Savas Sipahi: supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27714
ACKNOWLEDGEMENTS
I would like to thank our dialysis training nurses Peruze Aydin and Esen Aka and organ transplant coordinator Gulercan Senel for their devoted work and excellent contributions in collecting serum samples of the study and communicating with patients. Also, I would like to thank the Sakarya University of Scientific Research Projects Unit (Research Project number: BAPK 2020‐6‐23‐84) for their financial support.
Dheir H, Tocoglu A, Toptan H, et al. Short and mid‐term SARS‐CoV‐2 antibody response after inactivated COVID‐19 vaccine in hemodialysis and kidney transplant patients. J Med Virol. 2022;94:3176‐3183. 10.1002/jmv.27714
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cheng H‐Y, Jian S‐W, Liu D‐P, et al. Contact tracing assessment of COVID‐19 transmission dynamics in Taiwan and Risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kucharski AJ, Klepac P, Conlan AJK, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS‐CoV‐2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20(10):1151‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS‐CoV‐2 Vaccine in Chile. N Engl J Med. 2021;385(10):875‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS‐CoV‐2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA‐1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quiroga B, Soler MJ, Ortiz A, et al. Safety and immediate humoral response of COVID‐19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant. 2021. Online ahead of print. 10.1093/ndt/gfab313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia P, Anand S, Han J, et al. COVID‐19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol. 2022;33(1):33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goffin E, Candellier A, Vart P, et al. COVID‐19‐related mortality in kidney transplant and haemodialysis patients: a comparative, prospective registry‐based study. Nephrol Dial Transplant. 2021;36(11):2094‐2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rother N, Yanginlar C, Lindeboom RGH, et al. Hydroxychloroquine inhibits the trained innate immune response to interferons. Cell Rep Med. 2020;1(9):100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID‐19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35(12):2083‐2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández AR, Sánchez‐Tarjuelo R, Cravedi P, Ochando J, López‐Hoyos M. Review: ischemia reperfusion injury‐A translational perspective in organ transplantation. Int J Mol Sci [Internet]. 2020;21(22):21. Available from 10.3390/ijms21228549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID‐19‐related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cravedi P, Mothi SS, Azzi Y, et al. COVID‐19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy S, Chitturi C, Yee J. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 15. Caillard S, Thaunat O. COVID‐19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17(12):785‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berar‐Yanay N, Freiman S, Shapira M, et al. Waning humoral response 3 to 6 months after vaccination with the SARS‐COV‐2 BNT162b2 mRNA vaccine in dialysis patients. J Clin Med Res. 2021;11(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kho MML, Reinders MEJ, Baan CC, et al.The RECOVAC IR study: the immune response and safety of the mRNA‐1273 COVID‐19 vaccine in patients with chronic kidney disease, on dialysis or living with a kidney transplant. Nephrol Dial Transplant. 2021;36(9):1761‐1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eren Sadioğlu R, Demir E, Evren E, et al. Antibody response to two doses of inactivated SARS‐CoV‐2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis. 2021;23(6):e13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murt A, Altiparmak MR, Yadigar S, et al. Antibody responses to the SARS‐CoV‐2 vaccines in hemodialysis patients: is inactivated vaccine effective? Ther Apher Dial [Internet]. 2021. Online ahead of print. 10.1111/1744-9987.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boongird S, Chuengsaman P, Setthaudom C, et al. Short‐term immunogenicity profiles and predictors for suboptimal immune responses in patients with end‐stage kidney disease immunized with inactivated SARS‐CoV‐2 vaccine. Infect Dis Ther. 2021;11(1):351‐365. 10.1007/s40121-021-00574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS‐CoV‐2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broseta JJ, Rodríguez‐Espinosa D, Rodríguez N, et al. Humoral and cellular responses to mRNA‐1273 and BNT162b2 SARS‐CoV‐2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders J‐SF, Bemelman FJ, Messchendorp AL, et al. The RECOVAC immune‐response Study: the immunogenicity, tolerability, and safety of COVID‐19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2021. Online ahead of print. 10.1097/TP.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two‐dose schedule, in healthy adults: interim results from two single‐centre, double‐blind, randomised, placebo‐controlled phase 2 clinical trials. Lancet Infect Dis [Internet]. 2021. Available from 10.1016/S1473-3099(21)00681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baluch A, Humar A, Eurich D, et al. Randomized controlled trial of high‐dose intradermal versus standard‐dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13(4):1026‐1033. [DOI] [PubMed] [Google Scholar]
- 26. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Correia AL, Leal R, Pimenta AC, et al. The type of SARS‐CoV‐2 vaccine influences serological response in kidney transplant recipients. Clin Transplant. 2022;Jan 8:e14585. [DOI] [PubMed] [Google Scholar]
- 28. Massa F, Cremoni M, Gérard A, et al. Safety and cross‐variant immunogenicity of a three‐dose COVID‐19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73:103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA [Internet]. 2021;326(11):1063‐1065. Available from 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tosun S, Ozkan Ozdemir H, Erdogan E, et al. Adverse events report of inactivated COVID‐19 vaccine from 4040 healthcare workers. Postgrad Med. 2021:1‐7. [DOI] [PubMed] [Google Scholar]
- 31. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime‐boost schedules with an adenoviral vectored and mRNA COVID‐19 vaccine (Com‐COV): a single‐blind, randomised, non‐inferiority trial. Lancet. 2021;398(10303):856‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


