Abstract
Coronavirus disease 2019 (COVID‐19) is spreading worldwide; there is a need to address its sequelae known as Long COVID. This study evaluated postvaccination changes in symptoms and antibody titers in patients with Long COVID. Patients visiting the outpatient department specializing in Long COVID at our hospital were enrolled. Changes in symptoms were evaluated before and 14–21 days after first vaccination. Antibody titers were measured using ARCHITECT SARS‐CoV‐2 IgG II Quant at the same time. This study included 42 patients (median age: 45 years; 17 [40.5%] men). Median pre‐ and postvaccination antibody titers were 456 and 28,963 AU/ml, respectively. Postvaccination symptoms (fatigue, joint pain, and taste and olfactory abnormalities) were relieved, worsened, and unchanged in 7 (16.7%), 9 (21.4%), and 26 (61.9%) patients, respectively. Ratios of pre‐ and postvaccination antibody titers were 53, 40, and 174 in the unchanged, relief, and worsened groups, respectively. The worsened group had the significantly highest antibody titer ratio (p = 0.02). The higher increased rate of the antibody titer in the worsened group than in the nonworsened group suggests an excessive immune response to vaccination associated with worsening of sequelae. Although patients with Long COVID should be vaccinated, additional concerns should be addressed.
Keywords: antibody titer, Long COVID, vaccine
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) continues to spread worldwide, with >1.7 million people being infected in Japan. 1 The vaccination rate is >75%, with a decrease in the incidence of new cases. 2 However, there has been increasing attention on COVID‐19 sequelae, which have been termed as post‐COVID‐19 or Long COVID. The World Health Organization defined COVID‐19 sequelae as follows: “A condition that occurs in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, usually 3 months from the onset of COVID‐19 with symptoms, and that lasts for ≥2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction, and others; moreover, it generally affects everyday functioning.” 3
According to a report from Wuhan, China, 73% of patients had sequelae after discharge, which persisted after 12 months in 49% of patients. 4 , 5 In Japan, Morioka et al. 6 reported that female sex, young age, and low body mass index were risk factors for multiple sequelae symptoms and that some patients had long‐term sequelae, although they had mild acute symptoms. Few studies have investigated the pathophysiology and treatment of the sequelae of COVID‐19. However, Antonelli et al. 7 reported that after two vaccinations, there was a decrease in sequelae in terms of breakthrough infections. Additionally, several studies have demonstrated vaccine effectiveness in patients with Long COVID. Sherwood reported that vaccination relieved more than half of the symptoms 8 ; furthermore, Arnold et al. 9 evaluated postvaccination changes in each sequelae symptom using a case series. There has been increasing attention on the relationship between Long COVID and vaccination.
Vaccination is recommended for preventing reinfection in patients with previous SARS‐CoV‐2 infection. 10 Cavanaugh et al. 11 reported that full vaccination provides additional protection against reinfection among persons with previous SARS‐CoV‐2 infection. Contrastingly, adverse reactions to vaccination have been reported among these patients. 12 It remains unclear whether full vaccinations (two or three doses) can be safely administered to patients with Long COVID. On January 18, 2021, a specialized outpatient clinic for patients with Long COVID was started, with approximately 300 patients visiting the hospital by the end of November 2021. From April 2021, when vaccination began in Japan, vaccination was recommended in patients with Long COVID. Some patients showed strong reactions to the first vaccination dose and hesitated to take the second dose; moreover, other patients presented with worsening sequelae conditions and refused the second vaccination dose.
Antibody titers against spike proteins are negatively correlated with the risk of COVID‐19 infection. 13 Worldwide, the administration of the third vaccination has begun. Kaneko et al. 14 examined postvaccination changes in antibody titers. The median serum levels of anti‐spike receptor‐binding domain (RBD) immunoglobulin G (IgG) were 529.1 and 18 836.9 AU/ml on Days 14 and 28 (7 days after the second vaccination) after the first vaccination, respectively.
Contrastingly, the median serum levels of anti‐spike RBD IgG increased to 16 353 AU/ml after 21–25 days after the first vaccination in patients with previous SARS‐CoV‐2 infection. 15 Annapaola recommended a single messenger RNA vaccine for achieving sufficient immunity in patients with previous SARS‐CoV‐2 infection. 16
In Japan, there has been a recent increase in the number of commercial and accommodation facilities that require full vaccination. Patients who cannot be completely vaccinated due to worsening sequelae symptoms may not access services of these facilities.
In case the antibody titer after a single vaccination in patients with Long COVID is similar or higher than that in full vaccinated noninfected persons, patients with Long COVID may receive services from facilities using certificates confirming single vaccination and healing. However, the relationship between postvaccination antibody titers and symptom changes after a single vaccination in patients with Long COVID remains unclear. This study aimed to evaluate changes in symptoms and antibody titers after a single vaccination and assess the relationship in patients with Long COVID.
2. MATERIALS AND METHODS
Patients who visited the outpatient department specializing in Long COVID in our hospital were enrolled. The patients presented with several sequelae symptoms (fever, malaise, dyspnea, cough, taste abnormality, olfactory abnormality, hair loss, sore throat, joint pain, numbness of limbs, muscle pain, headache, chest pain, vomiting, diarrhea, decreased motivation, sleeplessness, anxiety, depressed mood, forgetfulness, and skin symptoms) after >2 months since the onset of the COVID‐19 diagnosed using a polymerase chain reaction test or antigen test.
For patients who requested vaccination and provided consent to participate in this study, antibody titers were measured before vaccination and approximately 2 weeks after the single vaccination. The patients were informed about the results and their interpretation. Quantitative anti‐spike RBD IgG antibody responses were measured using the Abbott SARS‐CoV‐2 IgGII Quant assay (cut‐off ≥ 50 AU/ml).
Moreover, three self‐assessments of postvaccination changes in the main sequelae symptoms were confirmed based on the patient's response as follows: unchanged, relief, and worsened.
Based on the results, patients chose whether to undergo the second vaccination. This study was approved by the ethics committee of St. Marianna University School of Medicine (Approval number: 5321). All the patients provided informed consent for each procedure and publication.
2.1. Statistical analysis
Data regarding age, sex, pneumonia complications, underlying diseases, employment status, smoking history, and main sequelae symptoms were collected. The prevaccination blood sampling date, first vaccination date, and postvaccination duration after the onset of COVID‐19 were recorded. Continuous variables were compared using the Kruskal–Wallis test. Categorical variables were compared using the χ 2 test and Fisher's exact test. The ratio of antibody titers before and after the first vaccination was calculated. Based on subjective postvaccination symptoms, patients were divided into three groups (unchanged, relief, and worsened groups) and two groups (worsened and nonworsened [unchanged + relief] groups). Antibody titers before and after the first vaccination were compared using the Mann–Whitney test and Kruskal–Wallis test. If the Kruskal–Wallis test was significant, multiple comparisons were performed using the Dunn's test. Antibody titers after the first and second vaccinations were not compared because of the small number of patients who underwent the second vaccination. Statistical significance was set at 0.05. All statistical analyses were performed using STATA V.15.0 (2019; STATA Corp.).
3. RESULTS
Forty‐two patients were enrolled. Table 1 shows the among‐group comparisons of patient characteristics and antibody titers. The median age was 45 (interquartile range: 32–55) years. Furthermore, there were 17 (40.5%) male patients. Postvaccination symptoms were relieved, worsened, and unchanged in 7 (16.7%), 9 (21.4%), and 26 (61.9%) patients, respectively. The nonworsened group had more young people than the worsened group (Kruskal–Wallis test, p = 0.02; Dunn test, unchanged group vs. relief group, p = 0.04; unchanged group vs. worsened group, p = 0.01; relief group vs. worsened group, p = 0.33). The postvaccination changes in antibody titers in the relief, worsened, and unchanged groups were 20 965 (14 711–30 137), 37 967 (30 710–71 098)L, 23 861 (12 725–35 171) AU/ml, respectively. Figure 1 shows the among‐group comparisons of antibody titers before and after vaccination.
Table 1.
The characteristics of study participants and anti‐spike RBD IgG of three groups
| Unchanged (n = 26) | Relief (n = 7) | Worse (n = 9) | p Value | Test | |
|---|---|---|---|---|---|
| Age (years) (median [interquartile range]) | 40 (30–47) | 53 (29–58) | 50 (48–55) | 0.02 | Kruskal–Wallis |
| Sex (n [%]) | 0.45 | Fisher's exact | |||
| Male | 12 (46.2) | 3 (42.9) | 2 (22.2) | ||
| Complication of pneumoniae (n [%]) | 0.72 | Fisher's exact | |||
| No | 14 (56.0) | 3 (42.9) | 3 (33.3) | ||
| + | 6 (24.0) | 3 (42.9) | 4 (44.4) | ||
| No examination | 5 (20.0) | 1 (14.3) | 2 (22.2) | ||
| Past history (n [%]) | 0.08 | Fisher's exact | |||
| + | 16 (61.5) | 5 (71.4) | 9 (100.0) | ||
| Working situation (n [%]) | 0.83 | Fisher's exact | |||
| Continued | 16 (62) | 4 (57) | 4 (44) | ||
| Changed work type and continued | 3 (12) | 1 (14) | 3 (33) | ||
| Temporary leave from work | 6 (23) | 2 (29) | 2 (22) | ||
| Resignation | 1 (4) | 0 (0) | 0 (0) | ||
| Smoking history (n [%]) | 0.26 | Fisher's exact | |||
| Never | 16 (62) | 5 (71) | 7 (78) | ||
| Quit smoking | 8 (31) | 0 (0) | 1 (11) | ||
| Current smoker | 2 (8) | 2 (29) | 1 (11) | ||
| Symptom (n [%]) | |||||
| Fatigue | 15 (55.6) | 5 (18.5) | 4 (14.8) | ||
| Joint pain | 2 (7.4) | 0 (0) | 2 (7.4) | ||
| Taste and olfactory abnormality | 5 (18.5) | 0 (0) | 0 (0) | ||
| Numbness | 0 (0) | 0 (0) | 1 (3.7) | ||
| Sore throat | 0 (0) | 0 (0) | 1 (3.7) | ||
| Dizziness | 0 (0) | 1 (3.7) | 0 (0) | ||
| Memory impairment | 1 (3.7) | 0 (0) | 0 (0) | ||
| Palpitations | 0 (0) | 1 (3.7) | 0 (0) | ||
| Cough | 1 (3.7) | 0 (0) | 0 (0) | ||
| Headache | 0 (0) | 0 (0) | 1 (3.7) | ||
| Chest ache | 1 (3.7) | 0 (0) | 0 (0) | ||
| Anxiety | 1 (3.7) | 0 (0) | 0 (0) | ||
| Onset‐before vaccination (days) | 196 (110–238) | 146 (58–338) | 173 (136–227) | 0.84 | Kruskal–Wallis |
| Blood sampling date before vaccination‐vaccination date (days) | 44 (24–77) | 30 (19–39) | 36 (35–94) | 0.25 | Kruskal–Wallis |
| Vaccination date‐postvaccination blood sampling (day) | 15 (14–17) | 24 (14–40) | 15 (13–17) | 0.21 | Kruskal–Wallis |
| Before vaccination SIgG (AU/ml) | 456 (217–918) | 773 (163–930) | 360 (219–807) | 0.94 | Kruskal–Wallis |
| After the first vaccination SIgG (AU/ml) | 25 717 (13 171–36 824) | 21 787 (14 846–30 910) | 38 186 (30 979–71 458) | ||
| Ratio of antibody titer | 53 (29–94) | 40 (18–110) | 174 (115–198) | 0.06 | Kruskal–Wallis |
Abbreviations: IgG, immunoglobulin G; RBD, receptor‐binding domain.
Figure 1.
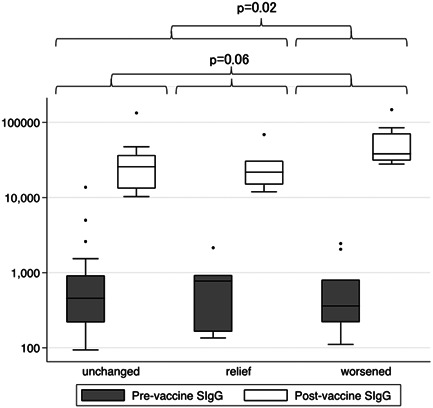
This figure shows the among‐three group (unchanged, relief, and worsened) comparisons of antibody titers before and after vaccination. The ratios of pre‐ and postvaccination antibody titers show no significant differences (Kruskal–Wallis test; p = 0.06). The worsened group shows a significantly higher antibody titer ratio than the non‐worsened group (Mann–Whitney test; p = 0.02). SIgG, anti‐spike RBD IgG
The ratios of pre‐ and postvaccination antibody titers were 53, 40, and 174 in the unchanged, relief, and worsened groups, respectively, without significant among‐group differences (Kruskal–Wallis test; p = 0.06).
However, the worsened group showed a significantly higher antibody titer ratio than the non‐worsened group (Mann–Whitney test; p = 0.02). There were 12 (29%) patients who did not receive the second vaccination.
4. DISCUSSION
This study showed no postvaccination changes in the symptoms of Long COVID; moreover, the symptom relief rate was lower than that reported in a previous study. 8 Furthermore, the worsened group showed a significantly higher change ratio in the antibody titer than the nonworsened group.
In most patients, prevaccination antibody titers are equal or less than those after a single vaccination in uninfected persons. 15 Lack of vaccination increases the risk of reinfection even in patients with previous SARS‐CoV‐2 infection. 11 Therefore, vaccination is necessary for preventing reinfection in patients with Long COVID. However, in our study, 29% of patients did not undergo the second vaccination.
Patients refused to undergo the second vaccination because of worsening Long COVID symptoms, concerns about strong side effects, and satisfaction with the antibody titers after the first vaccination. Regarding antibody titers, studies have reported that sufficient antibody titers can be obtained by a single vaccination for patients with previous SARS‐CoV‐2 infection. 15 , 16
Our findings showed that even in patients with Long COVID, a sufficient antibody titer can be obtained with a single vaccination. A small‐scale study in real‐world settings reported no significant difference in the occurrence of reinfection between infected individuals with only one vaccination and without vaccination. 11 Therefore, future studies are warranted.
The third vaccination program has started; however, there is a need to address worries regarding additional vaccinations among patients with Long COVID. The higher increased rate of the antibody titer in the worsened group than in the nonworsened group suggests that an excessive immune response to vaccination may be associated with worsening of sequelae. Numerous autoantibodies are produced after COVID‐19 infection and cause various symptoms 17 ; moreover, immune disorders are associated with the pathophysiology of sequelae. 18
In case an immune overreaction to vaccination worsens the sequelae, the sequelae may result from an immune disorder. Gracia‐Abellán et al. 19 reported a relationship between low antibody titers and sequelae. Antibodies are involved in the regulation of inflammatory responses through activation of Fc‐γ receptors, Toll‐like receptors, and complements, which induce the secretion or suppression of various proinflammatory and anti‐inflammatory mediators. Therefore, they suggested that low peak antibody titers worsened sequelae symptoms.
However, the previous findings cannot be directly compared with our findings. The previous study included admitted patients; contrastingly, most of our patients were in the mild acute phase. Vaccination of patients with Long COVID can be recommended without changes in sequelae, psychiatric symptoms, or quality of life. 20 Unvaccinated patients with Long COVID should be vaccinated to prevent reinfection. However, if the sequelae symptoms worsen after the first vaccination, the second or third vaccination should not be coercively administered.
This study has several limitations. First, this was a single‐center study with a small sample size. Changes in sequelae symptoms could not be evaluated. Moreover, it is possible that outpatient treatment for symptoms had begun and that self‐assessment of sequelae did not accurately reflect the relationship of the vaccine with the sequelae status. Future studies are required to evaluate changes in symptoms due to vaccination using a larger sample of patients with Long COVID.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design of study: Tomoya Tsuchida. Acquisition of data: Tomoya Tsuchida, Masanori Hirose, Yoko Inoue. Analysis and/or interpretation of data: Masanori Hirose, Tomoya Tsuchida. Drafting the manuscript: Tomoya Tsuchida, Hiroyuki Kunishima. Revising the manuscript critically for important intellectual content: Takehito Otsubo, Takahide Matsuda.
ETHICS STATEMENT
This study was approved by the ethics committee of St. Marianna University School of Medicine (Approval number: 5321). All the patients provided informed consent for each procedure and publication.
Tsuchida T, Hirose M, Inoue Y, Kunishima H, Otsubo T, Matsuda T. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J Med Virol. 2022;94:3416‐3420. 10.1002/jmv.27689
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Ministry of Health, Labour and Welfare . Domestic outbreaks, etc. Accessed December 11, 2021. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou.html
- 2. Prime Minister's Office of Japan . About the novel coronavirus vaccines in Japan. Accessed December 10, 2021. https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html
- 3. World Health Organization . A clinical case definition of post COVID‐19 condition by a Delphi consensus. October 6, 2021. Accessed November 21, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1pdf
- 4. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang L, Yao Q, Gu X, et al. 1‐year outcomes in hospital survivors with COVID‐19: a longitudinal cohort study. Lancet. 2021;398:747‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyazato Y, Tsuzuki S, Morioka S, et al. Risk factors associated with development and persistence of Long COVID. Medrxiv. 2021;7:ofaa507. 10.1101/2021.09.22.21263998 [DOI] [Google Scholar]
- 7. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID Symptom Study app: a prospective, community‐based, nested, case‐control study. Lancet Infect Dis. 2021;22:43‐55. 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Long CovidSOS . The impact of COVID vaccination on symptoms of Long Covid. An international survey of 900 people with lived experience. May 2021. Accessed November 21, 2021. https://3ca26cd7-266e-4609-b25f-6f3d1497c4cf.filesusr.com/ugd/8bd4fe_7301ed588cc44d1483e9fc8df7989a03.pdf
- 9. Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Symptoms after COVID‐19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med. 2021;174:1334‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pramesh CS, Babu GR, Basu J, et al. Choosing wisely for COVID‐19: ten evidence‐based recommendations for patients and physicians. Nat Med. 2021;27:1324‐1327. [DOI] [PubMed] [Google Scholar]
- 11. Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS‐CoV‐2 after COVID‐19 vaccination—Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneko S, Kurosaki M, Sugiyama T, et al. The dynamics of quantitative SARS‐CoV‐2 antispike IgG response to BNT162b2 vaccination. J Med Virol. 2021;93:6813‐6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS‐CoV‐2 infection on humoral and T‐cell responses to single‐dose BNT162b2 vaccine. Lancet. 2021;397:1178‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93:4612‐4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595:283‐288. [DOI] [PubMed] [Google Scholar]
- 18. Proal AD, VanElzakker MB. Long COVID or post‐acute sequelae of COVID‐19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia‐Abellan J, Padilla S, Fernandez‐Gonzalez M, et al. Antibody response to SARS‐CoV‐2 is associated with long‐term clinical outcome in patients with COVID‐19: a longitudinal study. J Clin Immunol. 2021;41:1490‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Are vaccines safe in patients with Long COVID? A prospective observational study. Medrxiv. 2021. 10.1101/2021.03.11.21253225 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


