Abstract
Background
Nucleic acid amplification (NAA) tests rapidly detect Mycobacterium tuberculosis complex directly from clinical specimens, providing valuable results for those evaluated for tuberculosis.
Methods
We analyzed characteristics of cases with NAA testing performed, compared cases with positive and negative NAA test results, and calculated turnaround time and time to treatment for all verified cases reported to the National Tuberculosis Surveillance System in the United States during 2011–2017.
Results
Among 67082 verified tuberculosis cases with NAA testing information, 30820 (45.9%) were reported as not having an NAA test performed; the proportion without NAA testing declined annually, from 60.5% in 2011 to 33.6% in 2017. Of 67082 verified cases, 27912 (41.6%) had positive, 8215 (12.2%) had negative, and 135 (0.2%) had indeterminate NAA test results. Among the 33937 cases with an acid-fast bacilli (AFB) smear-positive result, 24093 (70.9%) had an NAA test performed; 11490 of the 30244 (38.0%) with an AFB smear-negative result had an NAA test performed. Although sputum was the most common specimen type tested, 79.8% (7023/8804) of nonsputum specimen types had a positive NAA test result. Overall, 63.7% of cases with laboratory testing had NAA test results reported <6 days following specimen collection; for 13891 cases not yet on treatment, median time to treatment after the laboratory report date was 2 days.
Conclusions
Our analyses demonstrate increased NAA test utilization between 2011 and 2017. However, a large proportion of cases did not have an NAA test performed, reflecting challenges in broader uptake, suggesting an opportunity to expand use of this diagnostic methodology.
Keywords: nucleic acid amplification testing, NAA, tuberculosis
Tuberculosis (TB), a disease caused by Mycobacterium tuberculosis complex (MTBC), remains a global public health challenge. With 8916 new cases reported to the National Tuberculosis Surveillance System (NTSS) in 2019, TB in the United States (US) has steadily declined to a rate of 2.7 cases per 100000 persons [1], due to a national strategic focus on preventing MTBC transmission, improving case management [2], and treating latent TB infection [3]. The rate of TB is consistently higher among non-US-born persons than those born in the US [4].
Effective control of MTBC transmission hinges upon early diagnosis of infectious TB cases and rapid public health response [5, 6]. Microbiologically, the standard of practice is the testing of 3 sputum specimens collected at least 8–24 hours apart [6, 7]. Acid-fast bacilli (AFB) smear microscopy is typically the first laboratory test performed, followed by culture and drug susceptibility testing [6, 7]. However, AFB smear results can be nonspecific (ie, are also positive for other mycobacteria), and, due to MTBC’s slow growth, culture and susceptibility results can take weeks. In contrast, nucleic acid amplification (NAA) testing can rapidly identify MTBC directly from clinical specimens and, with some assays, simultaneously detect drug resistance (eg, Cepheid Xpert MTB/RIF, hereafter “Xpert MTB/RIF”) [8].
In recent years, NAA testing has become an increasingly valuable tool. NAA test results can help release patients from airborne infection isolation, prevent delays in treatment, and minimize inappropriate treatment [9–14]. Compared to traditional reliance on serial AFB smear results, alternative decision-making strategies can reduce duration of isolation from a median of 68 hours using smear microscopy to a median of 20.8 or 41.2 hours based on Xpert MTB/RIF testing of 1 or 2 sputum specimens, respectively [11], and potentially save an estimated $2278 per admission [12].
In 2009, the US Centers for Disease Control and Prevention (CDC) updated NAA test guidelines to recommend routine NAA testing for any patient being evaluated for pulmonary TB, for whom a diagnosis has not been established and an NAA test result would affect case management [15]. The objective of this analysis was to examine subsequent NAA testing among US TB cases, including the demographic and microbiological factors associated with use of this diagnostic methodology.
METHODS
We conducted a retrospective cohort analysis of verified US TB cases reported to the CDC NTSS during 2011–2017. NTSS collects data from all US states and the District of Columbia on individual cases of TB using a standardized form, the Report of a Verified Case of Tuberculosis (RVCT). The RVCT collects data regarding risk and clinical factors, laboratory information, and demographics [16]. A case is considered verified if it meets the 2009 national TB surveillance case definition [17] or is determined to be a verified TB case by a healthcare provider.
For inclusion, the reported case’s NAA test result had to be recorded as positive, negative, indeterminate, or not done. The RVCT form used during this study period (ie, before an RVCT update to allow serial results starting in 2020) allowed 1 NAA test result per case report. Jurisdictions were instructed to report NAA testing of only specimens collected before TB treatment began and that any positive result superseded all other available results; a negative NAA test result means there were no positive results on that patient. Cases either missing (n=63) or with unknown (n=158) NAA test results were excluded from analysis.
To analyze NAA test results by specimen type, we dichotomized sputum from nonsputum (ie, any other respiratory or nonrespiratory specimens). Turnaround time (TAT) was defined as number of days between specimen collection date and laboratory report date. We focused on <6 or ≥6 days to explore alignment with the CDC National Tuberculosis Indicators Project objective that aims to increase the proportion of NAA test results reported within 6 days of specimen collection [18]. We included cases with NAA test results reported both before and after treatment began. For those with results reported before treatment began, time to treatment (TTT) was the time interval between laboratory report date and treatment start date. Cases with >365-day TAT were presumed to be misreported and excluded from analysis (n=74).
To assess NAA test utilization patterns, we stratified cases by whether an NAA test was performed (if positive, negative, and indeterminate results) or not performed (if NAA test reported as not done). Among TB cases where an NAA test was performed, we excluded those with indeterminate results and compared those with a positive result to those with a negative result. For comparisons, data were stratified by clinical characteristics, patient demographics, and risk factors associated with TB. Nativity was categorized based on US Census Bureau definitions: US-born for persons eligible for US citizenship at birth and non-US-born for all other persons, regardless of current immigration or citizenship status. NTSS defines homelessness and substance use on the basis of the 12 months before diagnosis. Contact with an infectious TB case was defined as a known exposure within the 2 years before diagnosis. Except for recent contact, any variables that were missing or reported as “unknown” were excluded from analysis.
We calculated odds ratios (ORs) with 95% confidence intervals (CIs) using logistic regression models to evaluate the associations between case characteristics and odds of having an NAA test performed, of a positive test result, and of TAT in <6 days. SAS version 9.4 software was used for all analyses, and Microsoft Excel was used to generate graphs.
Because these data were collected and analyzed as part of routine public health surveillance, CDC determined that this analysis did not constitute human subjects research and thus did not require approval by an institutional review board.
RESULTS
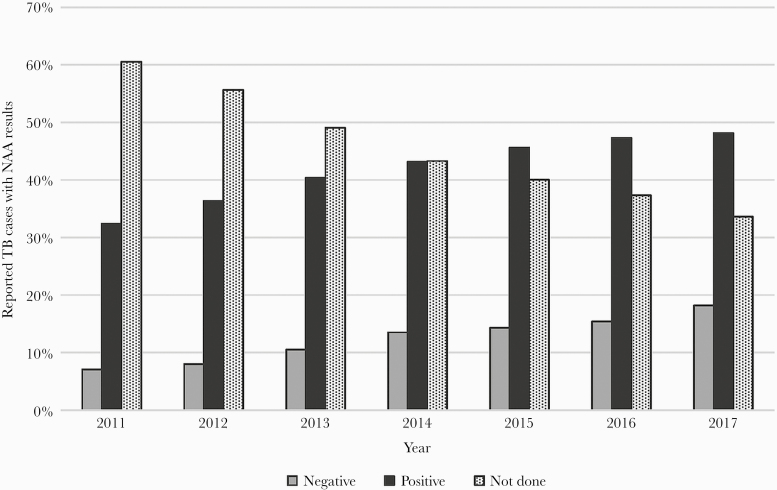
The 67082 TB cases reported in the US during 2011–2017 that had any reported value for the NAA test variable were included in this analysis: 27912 (41.6%) positive, 8215 (12.2%) negative, 135 (0.2%) indeterminate, and 30820 (45.9%) as NAA test not done. Overall, NAA testing increased during this time frame (Figure 1). The proportion of cases without a positive or negative NAA test result declined a mean of 4.5 percentage points annually, from 60.5% in 2011 to 33.6% in 2017.
Figure 1.
Nucleic acid amplification (NAA) test results among reported tuberculosis (TB) cases by year, United States (US), 2011–2017. Data displayed are percentages of verified cases of TB in the US reported to the National Tuberculosis Surveillance System with a positive, negative, or not done value for NAA testing during 2011‒2017 (N=66947). Indeterminate results are excluded due to the low number (n=135 over the 7-year study period).
Sputum was the most frequently reported specimen type to undergo NAA testing (ie, 26352 of 36262 [72.7%] with results). When we further stratified data by anatomic code to examine testing for specimen types other than sputum, we determined that of the remaining 8710 nonsputum samples, 5079 (58.3%) were reflective of testing performed on lymph node (1091 [12.5%]), bronchial fluid (3105 [35.6%]), and lung tissue (883 [10.1%]) combined (data not shown). In our analysis, 4442 of 13781 (32.2%) of extrapulmonary only cases had NAA performed. Additionally, 1136 of 67082 (1.7%) of all verified cases had a positive NAA test result only (no culture confirmation) for laboratory criteria for diagnosis and the site of disease available. Of these, 344 of 1136 (30.3%), or 0.5% of our overall dataset, had extrapulmonary disease only.
Having an NAA test performed was associated with a positive AFB smear (OR, 4.0 [95% CI, 3.87–4.13]) and culture (OR, 2.39 [95% CI, 2.30–2.49]) result (Table 1). However, we noted a steady increase in NAA testing among AFB smear-negative specimens over time. Among the 33937 cases with an AFB smear-positive result, 70.9% (24093) had an NAA test performed, whereas 11490 of the 30244 (38.0%) cases with an AFB smear-negative result had an NAA test performed. Among the 51909 culture-positive cases, 59.8% (31028) had a reported NAA test performed, whereas 4851 of the 12657 (38.3%) culture-negative cases had an NAA test performed.
Table 1.
Characteristics of Tuberculosis Cases With and Without Nucleic Acid Amplification Tests Performed, US National Tuberculosis Surveillance System, 2011–2017 (N=67082)
| Characteristic | NAA Test Performed, No. | NAA Test Performed, % | No NAA Test Performed, No. | No NAA Test Performed, % | Unadjusted OR, Performed vs Not Performed | 95% CI |
|---|---|---|---|---|---|---|
| AFB smear | ||||||
| Negativea | 11490 | 32.3% | 18754 | 65.6% | … | … |
| Positive | 24093 | 67.7% | 9844 | 34.4% | 4.00 | 3.87–4.13 |
| Culture | ||||||
| Negativea | 4851 | 13.5% | 7806 | 27.2% | … | … |
| Positive | 31028 | 86.5% | 20881 | 72.8% | 2.39 | 2.30–2.49 |
| CXR | ||||||
| Normala | 4001 | 11.4% | 7056 | 24.1% | … | … |
| Abnormal | 30989 | 88.6% | 22186 | 75.9% | 2.46 | 2.36–2.57 |
| HIV status | ||||||
| Negativea | 30685 | 93.5% | 24300 | 93.8% | … | … |
| Positive | 2134 | 6.5% | 1587 | 6.2% | 1.07 | 1.00–1.14 |
| Nativity | ||||||
| US-borna | 11748 | 32.4% | 10736 | 34.9% | … | … |
| Non-US-born | 24549 | 67.6% | 20063 | 65.1% | 1.12 | 1.08–1.16 |
| Sex | ||||||
| Femalea | 13429 | 37.0% | 12801 | 41.5% | … | … |
| Male | 22893 | 63.0% | 18016 | 58.5% | 1.21 | 1.17–1.25 |
| Age, y | ||||||
| 0–14a | 876 | 2.4% | 2373 | 7.7% | … | … |
| 15–24 | 3945 | 10.9% | 2736 | 8.9% | 3.91 | 3.56–4.28 |
| 25–44 | 11451 | 31.5% | 9216 | 29.9% | 3.37 | 3.10–3.65 |
| 45–64 | 11720 | 32.3% | 9168 | 29.7% | 3.46 | 3.19–3.76 |
| ≥65 | 8328 | 22.9% | 7325 | 23.8% | 3.08 | 2.83–3.35 |
| Homelessness | ||||||
| Noa | 33881 | 93.9% | 29275 | 95.6% | … | … |
| Yes | 2196 | 6.1% | 1333 | 4.4% | 1.42 | 1.33–1.53 |
| Injection drug use | ||||||
| Noa | 35325 | 98.4% | 30069 | 98.8% | … | … |
| Yes | 558 | 1.6% | 371 | 1.2% | 1.28 | 1.12–1.46 |
| Noninjection drug use | ||||||
| Noa | 33071 | 92.2% | 28624 | 94.1% | … | … |
| Yes | 2807 | 7.8% | 1808 | 5.9% | 1.34 | 1.26–1.43 |
| Excess alcohol use | ||||||
| Noa | 31562 | 88.0% | 27697 | 91.1% | … | … |
| Yes | 4291 | 12.0% | 2721 | 8.9% | 1.38 | 1.32–1.46 |
| Previous episode of TB | ||||||
| Noa | 34276 | 94.8% | 29262 | 95.4% | … | … |
| Yes | 1870 | 5.2% | 1396 | 4.6% | 1.14 | 1.07–1.23 |
| Recent contact to infectious TB case | ||||||
| Unknowna | 33557 | 92.4% | 27976 | 90.8% | … | … |
| Yes | 2768 | 7.6% | 2844 | 9.2% | 0.81 | .77–.86 |
Denominators for each percentage exclude missing data points for that variable.
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; CXR, chest radiograph; HIV, human immunodeficiency virus; NAA, nucleic acid amplification; OR, odds ratio; TB, tuberculosis; US, United States.
Reference group used for OR calculations.
The odds of having an NAA test performed were higher for cases with an abnormal chest radiograph (OR, 2.46 [95% CI, 2.36–2.57]). Other characteristics associated with NAA testing were being non-US-born (OR, 1.12 [95% CI, 1.08–1.16]), male sex (OR, 1.21 [95% CI, 1.17–1.25]), and age ≥15 years (ORs, 3.08–3.91 [95% CIs, 2.83–4.28]) (Table 1). Only 27.0% (876 of 3249) of TB cases in children aged <15 years were reported as having NAA testing performed. Recent contact with an infectious TB case was associated with not having an NAA test performed (OR, 0.81 [95% CI, .77–.86]).
We next examined factors associated with having a positive NAA test result among cases with NAA testing performed. Although sputum was the most common specimen type tested, that specimen type was less likely to have a positive NAA test result: 76.9% (20200/26269) of sputum specimens, compared with 79.8% (7023/8804) of nonsputum specimen types, had a positive result (OR, 0.84 [95% CI, .80–.90]) (Table 2). A positive AFB smear was strongly associated with a positive NAA test result (OR, 13.17 [95% CI, 12.41–13.98]); however, 47.0% (5358 of 11409) of AFB smear-negative cases tested by NAA had a positive NAA test result.
Table 2.
Characteristics of Tuberculosis Cases With Positive and Negative Nucleic Acid Amplification Test Results, US National Tuberculosis Surveillance System, 2011–2017 (N=36127)
| Characteristic | Positive NAA Test Result, No. | Positive NAA Test Result, % | Negative NAA Test Result, No. | Negative NAA Test Result, % | Unadjusted OR, Positive vs Negative | 95% CI |
|---|---|---|---|---|---|---|
| Specimen type | ||||||
| Nonsputuma | 7023 | 25.8% | 1781 | 22.7% | … | … |
| Sputum | 20200 | 74.2% | 6069 | 77.3% | 0.84 | .80–.90 |
| AFB smear | ||||||
| Negativea | 5358 | 19.5% | 6051 | 76.1% | … | … |
| Positive | 22109 | 80.5% | 1896 | 23.9% | 13.17 | 12.41–13.98 |
| Culture | ||||||
| Negativea | 920 | 3.3% | 3904 | 44.3% | … | … |
| Positive | 26775 | 96.7% | 4904 | 55.7% | 27.75 | 25.65–30.03 |
| CXR | ||||||
| Normala | 2381 | 8.5% | 1593 | 20.0% | … | … |
| Abnormal | 25531 | 91.5% | 6340 | 80.0% | 2.59 | 2.41–2.77 |
| HIV status | ||||||
| Negativea | 23526 | 93.4% | 7005 | 93.8% | … | … |
| Positive | 1655 | 6.6% | 466 | 6.2% | 1.06 | .95–1.18 |
| Nativity | ||||||
| US-borna | 9231 | 33.1% | 2444 | 29.8% | … | … |
| Non-US-born | 18660 | 66.9% | 5765 | 70.2% | 0.86 | .81–.90 |
| Age, y | ||||||
| 0–14a | 392 | 1.4% | 469 | 5.7% | … | … |
| 15–24 | 2915 | 10.4% | 1004 | 12.2% | 3.47 | 2.98–4.04 |
| 25–44 | 8679 | 31.1% | 2705 | 32.9% | 3.84 | 3.33–4.42 |
| 45–64 | 9150 | 32.8% | 2518 | 30.7% | 4.35 | 3.78–5.01 |
| ≥65 | 6772 | 24.3% | 1518 | 18.5% | 5.34 | 4.62–6.17 |
| Sex | ||||||
| Femalea | 9926 | 35.6% | 3426 | 41.7% | … | … |
| Male | 17984 | 64.4% | 4789 | 58.3% | 1.30 | 1.23–1.36 |
| Homelessness | ||||||
| Noa | 25972 | 93.7% | 7730 | 94.7% | … | … |
| Yes | 1756 | 6.3% | 432 | 5.3% | 1.21 | 1.09–1.35 |
| Injection drug use | ||||||
| Noa | 27142 | 98.4% | 8004 | 98.5% | … | … |
| Yes | 433 | 1.6% | 121 | 1.5% | 1.06 | .86–1.29 |
| Noninjection drug use | ||||||
| Noa | 25239 | 91.5% | 7661 | 94.3% | … | … |
| Yes | 2335 | 8.5% | 462 | 5.7% | 1.53 | 1.38–1.70 |
| Excess alcohol use | ||||||
| Noa | 23920 | 86.9% | 7476 | 91.9% | … | … |
| Yes | 3619 | 13.1% | 656 | 8.1% | 1.72 | 1.58–1.88 |
| Previous episode of TB | ||||||
| Noa | 26327 | 94.8% | 7762 | 94.8% | … | … |
| Yes | 1436 | 5.2% | 427 | 5.2% | 0.99 | .89–1.11 |
| Recent contact to infectious TB case | ||||||
| Unknowna | 25988 | 93.1% | 7394 | 90.0% | … | … |
| Yes | 1924 | 6.9% | 821 | 10.0% | 0.67 | .61–.73 |
Denominators for each percentage exclude missing data points for that variable.
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; CXR, chest radiograph; HIV, human immunodeficiency virus; NAA, nucleic acid amplification; OR, odds ratio; TB, tuberculosis; US, United States.
Reference group used for OR calculations.
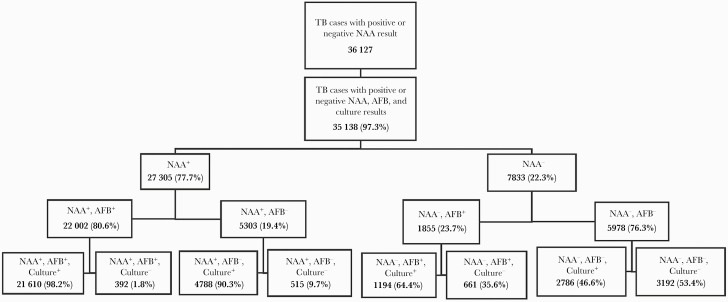
We then examined the concordance between NAA test and other laboratory test results. Culture-positive cases were much more likely to have a report of positive NAA result (OR, 27.75 [95% CI, 25.65–30.03]) (Table 2). Among culture-positive cases, 84.5% (26775/31679) were NAA positive, whereas only 19.1% (920/4824) of culture-negative cases were NAA positive (Table 2). Among the 35138 cases with complete positive or negative NAA test, AFB smear, and culture results, 27305 (77.7%) had a positive and 7833 (22.3%) had a negative NAA test result (Figure 2). Among the 22002 NAA test-positive cases that were also AFB smear positive; 1.8% (n=392) were reported as culture negative. Among the 5303 NAA test-positive cases that were AFB smear negative, 9.7% (n=515) were reported as culture negative. Among the 1855 NAA test-negative cases that were AFB smear positive, 64.4% (n=1194) were reported as culture-positive and accounted for 15.2% (1194/7833) of NAA test-negative cases. Among the 5978 of cases that were both NAA test negative and AFB smear negative, 46.6% (n=2786) were reported as culture positive (Figure 2). The sensitivity of NAA tests in smear-negative, culture-positive cases in this sample set was 63.2% (4788/7574), compared to 94.8% (21610/22804) in smear-positive, culture-positive cases. Among NAA test-negative, culture-positive cases, 27.3% (1117/4089) had extrapulmonary disease only. For cases with NAA test positivity, 13.4% (284/2118) of those with extrapulmonary disease only had a negative culture result in comparison to 2.5% (565/22750) of those with pulmonary disease only having a negative culture result (data not shown). For those aged <15 years, 87.6% (332/379) of cases with a positive NAA test result also had a positive culture vs 96.8% (26439/27312) of those in older age groups (data not shown).
Figure 2.
Distribution of tuberculosis (TB) nucleic acid amplification (NAA), acid-fast bacilli (AFB), and culture results. Stratified data include verified US cases from the National Tuberculosis Surveillance System that had completeness of reporting for all 3 laboratory tests: a reported positive or negative NAA test result, AFB smear microscopy result, and Mycobacterium tuberculosis complex culture result (N=35138 during 2011‒2017).
Of the 34774 cases with an evaluable NAA test result TAT, 63.7% (n=22160) had laboratory test results reported in <6 days and 36.3% (n=12614) in ≥6 days, including 2648 with a TAT of ≥30 days. During 2011–2017, the proportion of NAA test results reported in <6 days increased a mean of 2.5 percentage points annually (data not shown). In this analysis, sputum specimens and AFB smear-positive cases were more likely to have TAT of <6 days (Table 3). The mean reported TAT for all NAA test results was 8.7 days, with a median of 4 days. For cases with sputum specimens tested by NAA, the mean TAT was 7.4 days with a median of 4.0 days compared to a mean of 14.5 days with a median of 6 days for nonsputum specimens. For sputum specimens, 25% of NAA test results were reported in 2 days after specimen collection. The mean NAA test TAT for AFB-positive cases was 7.3 days with a median of 4.0 days while AFB-negative cases had a mean TAT of 13.2 days with a median of 5.0 days.
Table 3.
Nucleic Acid Amplification Test Result Turnaround Time by Specimen Type, US National Tuberculosis Surveillance System, 2011–2017 (N=34774)
| Characteristic | <6-day TAT, No. | <6-day TAT, % | ≥6-day TAT, No. | ≥6-day TAT, % | Unadjusted OR ≥6 day vs <6-day TAT | 95% CI |
|---|---|---|---|---|---|---|
| Specimen type | ||||||
| Nonsputuma | 4051 | 18.7% | 4410 | 36.2% | … | … |
| Sputum | 17662 | 81.3% | 7771 | 63.8% | 0.40 | .38–.43 |
| AFB smear | ||||||
| Negativea | 5778 | 26.6% | 4853 | 39.2% | … | … |
| Positive | 15955 | 72.4% | 7525 | 60.8% | 0.56 | .54–.59 |
| Culture | ||||||
| Negativea | 2810 | 12.8% | 1495 | 12.0% | … | … |
| Positive | 19094 | 87.2% | 10973 | 88.0% | 1.08 | 1.01–1.16 |
Denominators for each percentage exclude missing data points for that variable.
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; OR, odds ratio; TAT, turnaround time.
Reference group used for OR calculations.
To assess how NAA testing affected TTT, we stratified NAA test results by those reported before and after treatment initiation. Of the 34166 cases with an evaluable TTT, 59.3% (n=20275) began treatment before an NAA test result was reported by the laboratory (data not shown); 80.4% of these (n=16296) later had a positive NAA test result. When treatment was initiated beforehand, the median TTT was 5 days before the NAA test result report date. Among the 40.7% (n=13891) whose treatment began afterward, median TTT after the NAA test result report date was 2 days, and 77.2% (n=10721) of those had a positive NAA test result.
DISCUSSION
Our analysis shows a clear increase in NAA test utilization among TB cases in the US during 2011–2017. Several factors potentially contributed to this observed increase. In 2009, updated CDC guidance recommended NAA testing as standard practice, [15] and CDC targeted funding support to public health laboratories to increase access to NAA testing. Additionally, in 2013, the US Food and Drug Administration authorized a novel type of NAA test, the Xpert MTB/RIF, that also assesses for mutations associated with rifampin resistance [8, 19, 20]. However, a large proportion of cases overall (45.9%) did not have an NAA test performed, reflecting challenges in broader uptake. Barriers include few commercially available options, resource limitations prohibiting universal testing, and empiric treatment that could limit the utility of NAA testing (eg, some methods are validated only for cases on treatment for ≤3 days).
As expected, we found that sputum was the specimen type most likely to be used for NAA testing. In 2019, 79% of US TB cases had pulmonary involvement [1]. Laboratories may be reluctant or unable to validate nonsputum specimen types for commercially available or laboratory-developed tests [21]. Interestingly, we found that nonsputum specimens, compared to sputum specimens, were more likely to have positive NAA test results, suggesting value of NAA testing with these specimen types. Additionally, we observed that a third of cases with positive NAA test results as the sole laboratory criteria for diagnosis had extrapulmonary disease only, indicating the potential importance of testing other tissue types, including fixed tissue samples, when additional microbiological testing may not be possible.
As in other studies, we found a greater association between AFB smear-positive results and any reported NAA result [14, 22]. A common testing algorithm among US laboratories is to perform an NAA test for every newly AFB smear-positive specimen but only for AFB smear-negative specimens on request [23]. However, we noted a steady increase in NAA testing among AFB smear-negative specimens over time. Given the sensitivity of NAA testing among smear negatives in this study (63.2%), use of NAA testing may well be considered including for patients who may not have a classic presentation of TB.
A small number (n=1194) of the overall TB cases in this analysis were AFB smear positive and culture positive yet NAA test negative; yet, these represented 15.2% of all NAA test-negative samples. This is a notable finding given the smear and culture positivity and the high percentage of NAA test positivity observed for these samples in our analysis. However, specimens from these individuals could have contained inhibitory substances impacting NAA testing or included nontuberculous mycobacteria (ie, AFB positive) with few MTBC. We also noted that almost 10% of AFB smear-negative, NAA test-positive cases were culture negative, underscoring the benefit of NAA testing in AFB smear-negative cases. The potential lack of culture positivity in these cases could have been due to testing of different specimens by each method or empiric treatment of patients prior to laboratory evaluation, therefore impacting MTBC viability.
While our findings suggest an increase in NAA testing over time, at least 2 limitations should be acknowledged. First, the NTSS dataset comprise only verified TB cases; we lack information about the broader context of NAA testing among persons who were evaluated for TB but ultimately determined not to have a verified case of TB to report. Without that other group, we cannot assess overall NAA test utilization nationwide. Second, the NTSS allowed for only 1 NAA test result, 1 AFB smear result, and 1 culture result to be reported per TB case during 2011–2017 (with instructions to report the first positive result for each test if there were positive results on any of the specimens). Therefore, when our analysis compared NAA test, AFB smear, and culture results, we were not necessarily comparing results from the same specimen. The 2020 RVCT will allow reporting of multiple results for these laboratory tests [24]. Nevertheless, this analysis advances our understanding of the use of NAA testing in the US in recent years.
During 2011–2017, TB cases among non-US-born individuals ranged from 63% to 71% of all US TB cases [1], correlating with the higher proportion of NAA testing within this group (Table 1). A study by Marks et al that included individuals being evaluated for pulmonary TB, not just reported TB cases, found that NAA testing was also common for non-US-born individuals but was used more often for those with smear-positive vs smear-negative disease [14]. NAA testing may be used more often for non-US-born individuals due to a higher index of clinical suspicion for TB due to country of birth or other factors including travel history to areas with higher rates of TB than the US.
Compared with other age groups, those aged <15 years had the lowest proportion of NAA testing performed, even though nearly half (392/861) had positive results (Table 2). NAA tests generally have a lower sensitivity in children compared to adults [25, 26] due to difficulties in obtaining specimens from children and the paucibacillary nature of TB in this population. These challenges may limit use of NAA tests for pediatric cases. However, 12.4% of those aged <15 years who had positive NAA test results were culture negative, highlighting the value of NAA testing in this group for laboratory diagnosis of TB.
Improvement in TAT <6 days from 2011 to 2017 could be due to changes in NAA test algorithms, improved understanding of the surveillance variable, and some standardizing of laboratory report formats. During the timeframe of this surveillance analysis, educational efforts by CDC focused on timely NAA testing and reporting. Given the need for rapid detection of pulmonary TB to prevent ongoing transmission, it was reassuring that cases with sputum specimen tested, AFB-positive disease, and abnormal chest radiograph were less likely to have long TAT (≥6 days) for NAA testing. Longer TAT for nonsputum specimen types could be due to limited onsite laboratory validation of NAA testing for other specimen types and delays associated with transport to another laboratory for testing. Treatment was frequently initiated before an NAA test result was reported, suggesting a high clinical suspicion of TB that the subsequent NAA test result generally supported. However, it should be noted in our analysis that among cases where the positive or negative NAA, AFB smear, and culture results were available, more than half of cases with a negative NAA test result were culture positive for MTBC, highlighting the continued need for specimen culture. Because AFB smear status is often the first laboratory result available, treatment could have been initiated based on smear positivity before NAA test results are received, in accordance with treatment guidelines [6].
To summarize, NAA testing was increasingly used in the diagnostic evaluation of TB cases in the US between 2011 and 2017. However, nearly half of cases did not have an NAA test performed, suggesting an opportunity to continue to expand use of this valuable diagnostic methodology for nonsputum specimens and for cases with AFB smear-negative TB.
Notes
Author contributions. A. M. S. and T. L. D. conceived of the study concept. A. M. S. oversaw overall direction and writing. A. W. developed the analytic plan and performed initial analyses. T. L. D., R. L., and L. R. A. provided guidance on analytic approaches and provided critical feedback. V. K. performed analyses and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Patient consent statement. Patient consent was not required because these data were collected and analyzed as part of routine public health surveillance.
Disclaimer. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC) or the US Department of Health and Human Services (HHS). Use of trade names is for identification only and does not constitute endorsement by the US HHS, the US Public Health Service, or the CDC.
Financial support. This project was supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2019. https://www.cdc.gov/tb/statistics/reports/2019/default.htm. Accessed 23 June 2021. [Google Scholar]
- 2.Armstrong LR, Winston CA, Stewart B, et al. Changes in tuberculosis epidemiology, United States, 1993–2017. Int J Tuberc Lung Dis 2019; 23:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020; 69:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis—United States, 2017. MMWR Morb Mortal Wkly Rep 2018; 67:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salinas JL, Mindra G, Haddad MB, et al. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep 2016; 65:273–8. [DOI] [PubMed] [Google Scholar]
- 6.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64:e1–33. [DOI] [PubMed] [Google Scholar]
- 7.Procop GW. Laboratory diagnosis and susceptibility testing for Mycobacterium tuberculosis. Microbiol Spectr 2016; 4. doi: 10.1128/microbiolspec.TNMI7-0022-2016. [DOI] [PubMed] [Google Scholar]
- 8.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 2010; 48:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Division of Microbiology Devices Office of In Vitro Diagnostics, Food and Drug Administration, Centers for Disease Control and Prevention. Revised device labeling for the Cepheid Xpert MTB/RIF assay for detecting Mycobacterium tuberculosis. MMWR Morb Mortal Wkly Rep 2015; 64:193. [PMC free article] [PubMed] [Google Scholar]
- 10.National Tuberculosis Controllers Association. Consensus statement on the use of Cepheid Xpert MTB/RIF assay in making decisions to discontinue airborne infection isolation in healthcare settings. 2016. https://www.aphl.org/programs/infectious_disease/tuberculosis/Documents/NTCA_APHL_GeneXpert%20Consensus%20Statement_Final.pdf. Accessed 23 June 2021.
- 11.Lippincott CK, Miller MB, Popowitch EB, et al. Xpert MTB/RIF assay shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis 2014; 59:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millman AJ, Dowdy DW, Miller CR, et al. Rapid molecular testing for TB to guide respiratory isolation in the U.S.: a cost-benefit analysis. PLoS One 2013; 8:e79669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CW, Wu YK, Lan CC, et al. Impact of nucleic acid amplification test on pulmonary tuberculosis notifications and treatments in Taiwan: a 7-year single-center cohort study. BMC Infect Dis 2019; 19:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks SM, Cronin W, Venkatappa T, et al. The health-system benefits and cost-effectiveness of using Mycobacterium tuberculosis direct nucleic acid amplification testing to diagnose tuberculosis disease in the United States. Clin Infect Dis 2013; 57:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Report of verified case of tuberculosis (RVCT) instruction manual. 2009. https://www.cdc.gov/tb/programs/rvct/InstructionManual.pdf. Accessed 23 June 2021. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Tuberculosis (TB) (Mycobacterium tuberculosis) 2009 Case Definition. Atlanta, GA: CDC; 2009. [Google Scholar]
- 18.Division of Tuberculosis Elimination, Centers for Disease Control and Prevention. National Tuberculosis Indicators Project (NTIP) user guide. 2015. https://www.cdc.gov/tb/programs/evaluation/pdf/ntipuserguide.pdf. Accessed 23 June 2021. [Google Scholar]
- 19.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62:821–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Cepheid. Xpert MTB-RIF [package insert]. 2019. https://www.cepheid.com/Package%20Insert%20Files/Xpert-MTB-RIF-ENGLISH-Package-Insert-301-1404-Rev-F.pdf. Accessed 23 June 2021.
- 22.Peralta G, Barry P, Pascopella L.. Use of nucleic acid amplification tests in tuberculosis patients in California, 2010-2013. Open Forum Infect Dis 2016; 3:ofw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourgi K, Patel J, Samuel L, et al. Clinical impact of nucleic acid amplification testing in the diagnosis of Mycobacterium tuberculosis: a 10-year longitudinal study. Open Forum Infect Dis 2017; 4:ofx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 2020 Report of Verified Case of Tuberculosis (RVCT): Instruction Manual. Atlanta, GA: Division of Tuberculosis Elimination, CDC; 2021. [Google Scholar]
- 25.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atherton RR, Cresswell FV, Ellis J, et al. Xpert MTB/RIF ultra for tuberculosis testing in children: a mini-review and commentary. Front Pediatr 2019; 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]