Abstract
Aquaporin channels facilitate bidirectional water flow in all cells and tissues. AQP4 is highly expressed in astrocytes. In the CNS, it is enriched in astrocyte endfeet, at synapses, and at the glia limitans, where it mediates water exchange across the blood–spinal cord and blood–brain barriers (BSCB/BBB), and controls cell volume, extracellular space volume, and astrocyte migration. Perivascular enrichment of AQP4 at the BSCB/BBB suggests a role in glymphatic function. Recently, we have demonstrated that AQP4 localization is also dynamically regulated at the subcellular level, affecting membrane water permeability. Ageing, cerebrovascular disease, traumatic CNS injury, and sleep disruption are established and emerging risk factors in developing neurodegeneration, and in animal models of each, impairment of glymphatic function is associated with changes in perivascular AQP4 localization. CNS oedema is caused by passive water influx through AQP4 in response to osmotic imbalances. We have demonstrated that reducing dynamic relocalization of AQP4 to the BSCB/BBB reduces CNS oedema and accelerates functional recovery in rodent models. Given the difficulties in developing pore-blocking AQP4 inhibitors, targeting AQP4 subcellular localization opens up new treatment avenues for CNS oedema, neurovascular and neurodegenerative diseases, and provides a framework to address fundamental questions about water homeostasis in health and disease.
Keywords: water channel, regulation, traumatic brain and spinal cord injury, neurodegeneration
Introduction
The control of water homeostasis is crucial in maintaining normal CNS function. Dysregulation results in rapid and potentially life-threatening increases in intracranial or intraspinal pressure,1,2 or the accumulation of toxic waste products.3 Of the three aquaporins described in the CNS (AQP1, 4 and 9), AQP4 is the most abundant. It is found in astrocytes and is enriched at the blood–spinal cord and blood–brain barriers (BSCB/BBB), tripartite synapses, ventricle lining and the glia limitans beneath the meninges (Fig. 1). Studies in transgenic mice have established that AQP4 is a major regulator of CNS water homeostasis,4,5 where it controls the exchange of CSF with brain interstitial fluid and facilitates the development (and may also facilitate the clearance) of CNS oedema.6
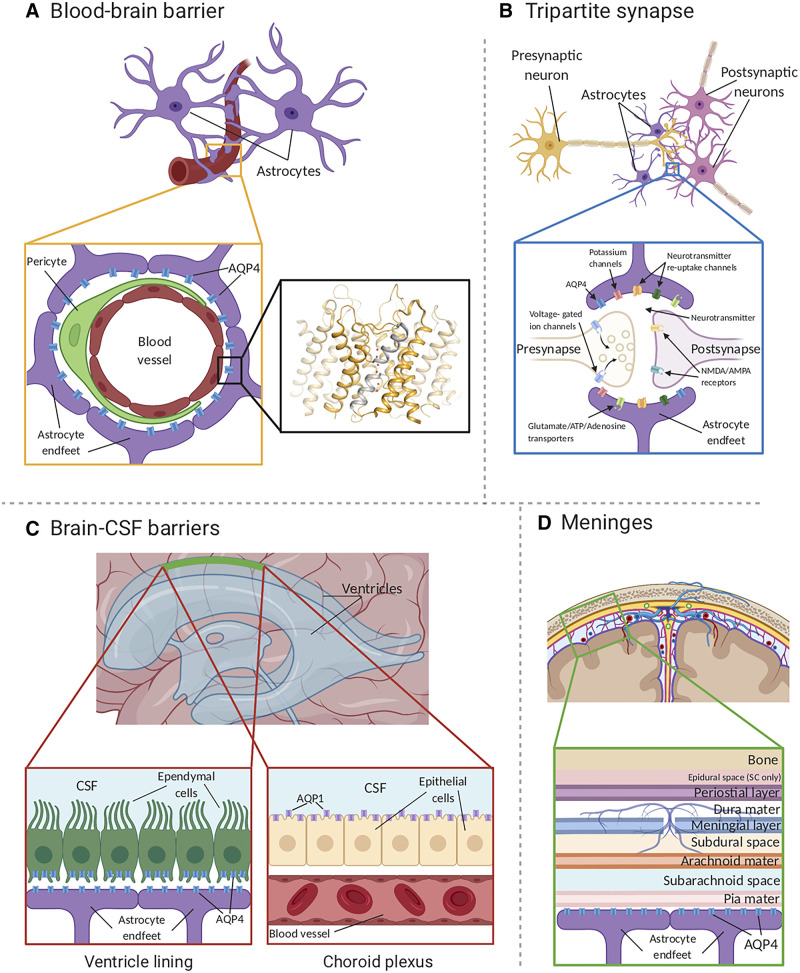
Figure 1.
AQP4 localization in the CNS. (A) AQP4 (blue) is located within astrocyte endfeet processes surrounding blood vessels in both brain tissue and the BBB. The inset shows the crystal structure of human AQP4 (PDB code 3GD8). AQP4 assembles as a tetramer with each monomer comprising six transmembrane helices and two half-helices (grey). The two half helices harbour the aquaporin signature motif (NPA) as well as part of the aromatic-arginine (ar/R) motif that functions as a selectivity filter. Within the pore, water molecules (red spheres) align in a single file. (B) AQP4 is localized at the astrocyte component of the tripartite synapse. During neurotransmission, neurons release mediators and neurotransmitters from synaptic nerve terminals (affecter cells) into the synaptic cleft to communicate with other neurons (effector cells). This synaptic activity induces an increase in intracellular Ca2+ concentration, which is accompanied by changed water and solute concentrations in astrocytes, leading to the release of glutamate and other gliotransmitters. This gliotransmission results in negative feedback to the presynaptic neurons to modulate neurotransmission. AQP4 plays an essential role in maintaining water homeostasis during this process. (C) In ventricles, AQPs are present within ependymal cells lining the brain-CSF interfaces (left inset). AQP4 is localized to the basolateral membrane of ependymal cells and the endfeet of contacting astrocytes (right inset). AQP1 (purple) is localized to the apical membrane of the choroid plexus epithelium.6,30 (D) CSF within the subarachnoid and cisternal spaces flows into the brain specifically via periarterial spaces and then exchanges with brain interstitial fluid facilitated by AQP4 water channels that are positioned within perivascular astrocyte endfoot processes.
Aquaporin channels facilitate the bidirectional flow of water and small uncharged solutes, whose membrane permeability is controlled by aquaporin abundance.7,8 The structural biology of aquaporin transmembrane domains is well-established9: six membrane-spanning α-helices and two half-helices stack around the family’s signature Asn-Pro-Ala (NPA) motifs (located in the middle of the membrane) to form the water pore (Fig. 1A, inset). Members of the aquaporin family can be selective for water (e.g. AQP4) or also permit the transport of small neutral solutes such as glycerol and urea (e.g. AQP9).10 The substrate traverses the pore in single file, charged species are excluded by the channel electrostatics, and protons are excluded by the orientation of water molecules within the pore preventing proton diffusion along the hydrogen bond network via the Grotthuss mechanism. Less is known about the structures of the intracellular amino- and carboxy-termini, which are not usually resolved in crystallography studies,9 but where many key regulatory interactions are known to occur. Aquaporins are homotetramers, with each monomer containing an independent water pore. The functional relevance of the tetramer is unclear, although we have shown that AQP4 mutants that do not tetramerize are also unable to relocalize to the plasma membrane.11
AQP4 exists in two major isoforms, namely AQP4-M1 and AQP4-M23 (indicating the position of the initiating methionine residue). The shorter AQP4-M23 isoform can be derived from an alternatively-spliced transcript,12 or by leaky-scanning of the M1 transcript whereby the 40S ribosome skips the first (M1) start codon and initiates translation at the second (M23).13 AQP4-M23 forms square arrays in the astrocyte plasma membrane, known as orthogonal arrays of particles (OAPs).14 These OAPs can be observed directly by freeze fracture electron microscopy.15 OAP size depends upon the ratio between AQP4-M1 and AQP4-M23, with higher levels of AQP4-M1 composition reducing OAP size. Notably, OAP disintegration and changes in the ratio between AQP4-M1 and AQP4-M23 are observed early after stroke,16-18 although the (patho)physiological consequences of these changes are yet to be defined. Recent work suggests that OAP stability can impact astrocyte process motility and local synaptic activity.19 A better understanding of OAPs may be possible in the future with the development of a novel mouse lacking the OAP-forming AQP4-M23 isoform.20,21 An AQP4 isoform (AQPex) has also been reported that has an extended carboxy-terminus containing a conserved perivascular localization signal generated by translational read-through.22,23 The consequences of this carboxy-terminal extension are yet to be established.
The notable localization of AQP4 to perivascular astrocyte endfoot processes results from its association with the dystrophin-associated complex (DAC), which anchors AQP4 intracellularly to the cytoskeleton and extracellularly to the cerebrovascular basal lamina. Deletion of the Dmd and Snta1 genes (which encode the DAC proteins, dystrophin and α-syntrophin), or of Agrn (which encodes the basal lamina protein, agrin) in mice results in the loss of this perivascular AQP4 localization.24–27 A recent study also suggests a potential role for β-syntrophin in AQP4 anchoring.28 Changes in perivascular localization of AQP4 have been reported across myriad pathological conditions, including CNS tumours, neurovascular disorders, such as ischaemic stroke and traumatic brain injury, and in the setting of neurodegenerative disease.29 Perivascular localization of AQP4 may also be regulated by differential regulation of AQP4-M1 versus AQP4-M23 expression, with AQP4-M23-enriched OAPs localizing to perivascular astroglial endfoot processes.20,31 The degree of enrichment of AQP4 to perivascular membranes differs between brain regions, although the molecular basis and physiological consequences of these differences remains incompletely understood.32
While studies of AQP4 function have historically focused on this cell-level localization to perivascular processes, more recent work from our group suggests that dynamic subcellular relocalization of AQP4, from intracellular vesicles to the plasma membrane, may play a crucial role in the regulation of AQP4 function.7 The plasma membrane abundance of most mammalian aquaporins has been shown to respond to distinct cellular or environmental triggers, such as hormones or changes in tonicity.33 This is best described for AQP2, for which trafficking in response to the pituitary hormone arginine vasopressin (AVP) involves regulated exocytosis of AQP2-containing storage vesicles in the kidney collecting duct principal cells.34 Although the specific triggers will vary between isoforms and cell types, studies indicate that the dynamic subcellular relocalization of human aquaporins share several features: (i) a trigger causing a signalling cascade leading to site-specific aquaporin phosphorylation; (ii) the subsequent movement of aquaporin-containing vesicles along the microtubule network; and (iii) vesicle fusion with the plasma membrane.35 Our recent work has shown that AQP4 plasma membrane abundance is tightly and dynamically regulated at the subcellular level by relocalization to and from intracellular vesicular pools in response to non-hormonal stimuli in astrocyte cultures.7,36,37 These include the changes in local oxygen tension and osmolality that are caused by traumatic injury and stroke. Targeting this regulatory mechanism is a viable anti-oedema therapy in rodent models of spinal cord injury, traumatic brain injury and stroke.7,38
Pore-blocking molecules for aquaporins remain difficult to develop. The small diameter of the aquaporin pore (water molecules traverse the pore in single file), the fact that interactions are limited to hydrogen bonding39 and a lack of in vitro assays suitable for screening and validating the pharmacological regulation of aquaporin function40 are all factors. This lack of tool compounds to modulate aquaporin function means that many fundamental questions about water homeostasis remain unanswered. Here we review the physiological and pathophysiological roles of AQP4 in the CNS, with a focus on novel insights into the mechanisms of glymphatic clearance in the maintenance of brain water homeostasis and new approaches to drug discovery that can be derived from the discovery of dynamic AQP4 subcellular relocalization.
Physiological roles of AQP4: the glymphatic pathway
Since 2012,41 AQP4 has been implicated as a key determinant of glymphatic function (Fig. 2). The glymphatic system (recently and comprehensively reviewed by Rasmussen and colleagues42) is a brain-wide network of perivascular pathways along which CSF enters the brain and interstitial solutes are cleared.43,44 Glymphatic exchange is driven by arterial pulsation,45,46 is active primarily during sleep,47–49 and contributes to the clearance of interstitial amyloid-β,41,49 tau50,51 and other solutes such as lactate,52 and inflammatory cytokines.53
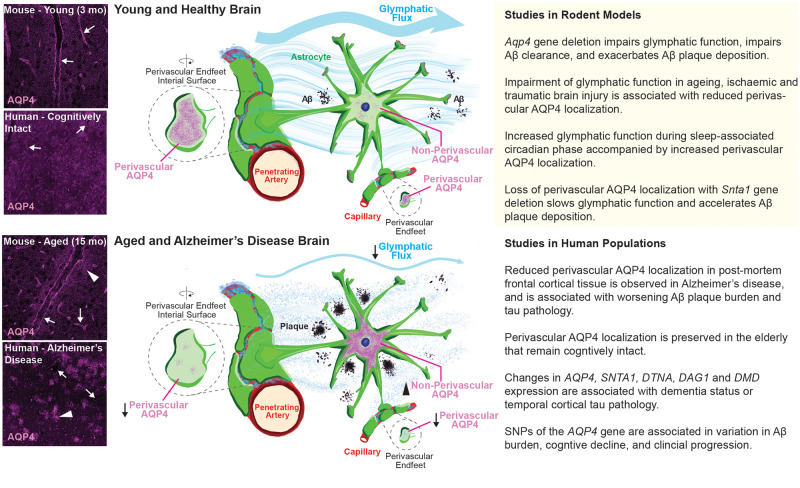
Figure 2.
The glymphatic pathway. The glymphatic system is a perivascular network that facilitates fluid exchange between the CSF and interstitial compartments, supporting the clearance of interstitial solutes. The function of the glymphatic system relies on perivascular astrocyte AQP4 expression. In the healthy young brain, AQP4 localizes to the astrocyte endfeet along the perivascular space (top left, arrows). In the context of ageing and Alzheimer’s disease, perivascular AQP4 levels are reduced while cellular AQP4 levels are increased (bottom left, arrows). The loss of AQP4 from perivascular astrocytic endfeet slows glymphatic clearance, which may accelerate amyloid-β accumulation and cognitive decline. The column on the right details specific findings from studies in rodents (top) and humans (bottom).
Both glymphatic influx of CSF and interstitial solute clearance are dependent upon perivascular AQP4. In the initial description of the glymphatic system,41 Aqp4 gene deletion was observed to slow CSF tracer influx and interstitial tracer efflux in mice. Similarly, deletion of the Aqp4 gene slowed the clearance of amyloid-β from the brain,41 and promoted the formation of amyloid plaques.54 Although one study failed to reproduce this effect of Aqp4 gene deletion on CSF tracer distribution,55 a subsequent study reporting data from five independent laboratories using five different transgenic mouse lines confirmed the role of AQP4 in perivascular glymphatic exchange.56 In that study, Stna1 gene deletion was observed to impair glymphatic function, demonstrating that perivascular localization of AQP4 plays a critical role in AQP4-dependent glymphatic exchange.
Under physiological conditions in mice, increasing perivascular AQP4 levels during rest and declining perivascular AQP4 levels during activity were associated with increased, and reduced glymphatic function, respectively.47 Pathologically, glymphatic function is impaired in ageing mice,57 following traumatic brain injury,50 and in rodent models of cerebrovascular disease.58–60 In each case, impairment of perivascular exchange was associated with a reduction in the cell-level localization of AQP4 to perivascular processes.57,61,62 When this perivascular localization of AQP4 is disrupted by deletion of the Snta1 gene, glymphatic function is similarly impaired.56 While these findings suggest that one of the roles of perivascular AQP4 is to facilitate the exchange of CSF and interstitial fluid along the axis of the cerebral vasculature, thereby supporting solute distribution and waste clearance, the mechanism controlling changes in the cell-level localization of AQP4 to perivascular endfeet under physiological and pathological conditions remains to be established. Importantly, these studies defining the role of perivascular AQP4 in glymphatic function have not clearly distinguished between AQP4 pools inserted into the endfoot plasma membrane and those in sub-membrane vesicles. The manner in which cell-level changes in perivascular AQP4 localization interact with the recently described dynamic subcellular changes in AQP4 abundance7 to govern glymphatic function remains to be explored.
Pathological roles of AQP4
Neurodegenerative disease
Ageing, cerebrovascular disease, prior exposure to traumatic brain injury, and sleep disruption are established and emerging risk factors for the development of neurodegenerative conditions, including Alzheimer’s disease. In animal models of each, glymphatic function is impaired.50,57–60,63 Given the role of perivascular glymphatic exchange in amyloid-β41,49 and tau50,51 clearance, impairment of glymphatic pathway function is now proposed to be important in the development of these conditions.64 While imaging of glymphatic function using dynamic contrast-enhanced MRI (DCE-MRI) has only recently begun,43,65 early studies demonstrate that glymphatic function in humans is impaired in normal-pressure hydrocephalus44,66 and in the presence of small vessel disease.67
The role of glymphatic impairment in the development of other neurodegenerative diseases has not yet been directly evaluated, but emerging data from studies in human populations suggest a role for AQP4 in these conditions. In a post-mortem case series,68 reduced perivascular AQP4 abundance was observed in the frontal cortex of subjects diagnosed with Alzheimer’s disease, while preservation of perivascular AQP4 abundance was observed in subjects remaining cognitively intact over the age of 85. The reduced perivascular AQP4 abundance was further associated with increasing amyloid-β and tau pathology, as well as with global measures of cognitive decline. In three recent genetics studies carried out in distinct human populations, single nucleotide polymorphisms in the human AQP4 gene were associated with variation in cognitive decline,69 amyloid burden and clinical status,70 and an association between sleep disruption and amyloid burden.71 A recent human transcriptomic study further demonstrated that in addition to the expression of AQP4, differences in the expression of genes whose products determine perivascular AQP4 localization (specifically genes encoding elements of the DAC, SNTA1, DTNA, DMD, DAG1) were associated with dementia status and temporal cortical tau pathology.72 These findings suggest that changes in AQP4 expression and localization may contribute to the development and progression of neurodegenerative diseases, including Alzheimer’s disease, in human populations. Understanding the emerging role of dynamic AQP4 subcellular relocalization provides a new framework to understand waste clearance in the healthy brain and opens up new treatment avenues to slow the progression of neurodegenerative diseases.
CNS oedema
Following a traumatic primary injury to the brain or spinal cord, a series of molecular cascades is triggered that results in further neuronal and glial cell death from inflammation, changes in brain energy metabolism and/or ischaemia/hypoxia, referred to as secondary damage.73,74 These molecular changes have architectural and functional consequences, including the development of oedema, the formation of glial scars and cavities, and neuronal cell loss.75 It is now clear that no single pathological feature can be explained in isolation in this complex process, which remains incompletely understood (Fig. 3). It is established that water flows into CNS tissue through AQP4, but the source of the water (whether the blood column, or the CSF/perivascular spaces) remains controversial.
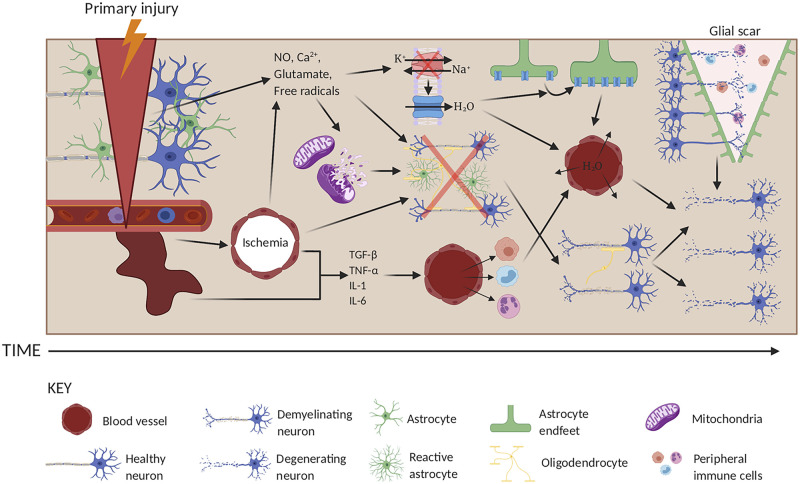
Figure 3.
The pathogenesis of traumatic injury in the CNS. In the primary injury phase, the brain or spinal cord is injured following external insult. This primary injury results in mechanical damage to neurons, astrocytes, oligodendrocytes and blood vessels. A series of secondary injury cascades then occurs that potentiates the primary injury. In the earlier post-injury stages, damaged blood vessels may haemorrhage, resulting in ischaemia and release of inflammatory cytokines (e.g. TGF-β, TNF-α, IL-1, and IL-6). These cytokines attract blood-borne inflammatory cells such as neutrophils, macrophages and leucocytes, which act both to clear up cellular debris, but also cause further damage to healthy cells by enhancing local inflammation, eventually leading to neuronal loss from inflammatory damage and through Wallerian degeneration following oligodendrocyte death and demyelination. Damaged neurons may secrete free radicals, nitric oxide (NO), glutamate, and Ca2+, which further potentiate cellular damage by causing mitochondrial dysfunction leading to the loss of ATP, and by causing localized excitotoxicity. Collectively, these two events result in the loss of Na+/K+-ATPase activity and the loss of oxygen tension in astrocytes, which results in cytotoxic oedema through increased water absorption through AQP4 (blue). This is followed by ionic dysregulation, eventually leading to swelling via vasogenic oedema and cavity formation limited by the formation of a glial scar, which obstructs neuronal regrowth and enhances cell damage. Created using www.biorender.com.
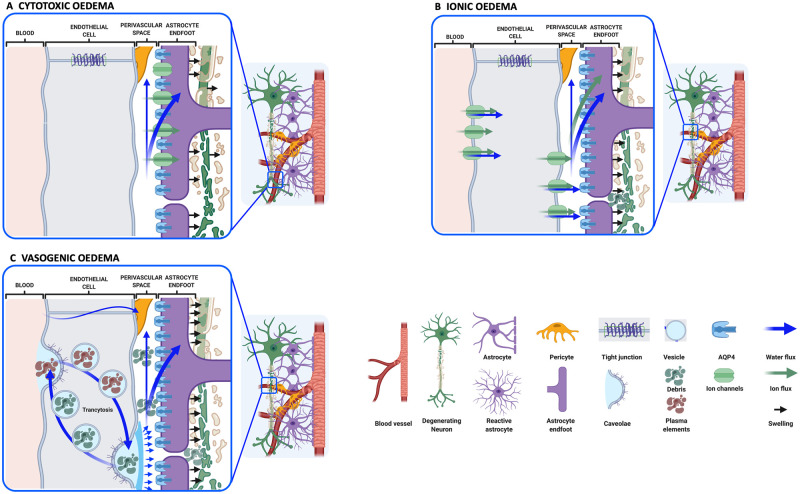
Oedema is a particular issue in the injured CNS because of the limited space (in the skull and spine) into which damaged tissue can swell. This is relevant following not only traumatic injury, but also in stroke and CNS tumours. In the last decade, the reclassification of oedema as cytotoxic, ionic or vasogenic (Fig. 4) based on observed changes in the brain has been widely adopted.76 Cytotoxic oedema (Fig. 4A) is defined as intracellular water accumulation without BBB disruption, usually as a consequence of the loss of oxygen tension. Morphologically, it is characterized by the swelling of astrocytes and the focal swelling of neuronal dendrites (known as beading).77,78 Ionic oedema (Fig. 4B) results from influx of water and sodium ions into the brain parenchyma prior to tight junction dysfunction, and is usually associated with cytotoxic oedema. Vasogenic oedema is a result of BBB dysfunction (Fig. 4C). The sources of water driving the formation of brain oedema remain a topic of debate.58,76,79,80 Methodological advances over the past decades, including two-photon microscopy and MRI, have led to new insights into the role of fluid in the perivascular spaces and the glymphatic system. In a recent study using a mouse ischaemic stroke model, the use of 22Na+ suggested that the CSF, not the blood, is the source of sodium ions.58 CSF was also identified as a major source of water driving AQP4-dependent oedema. Due to the incompressibility of CSF, enhanced influx of CSF into the parenchyma must be balanced either by enhanced secretion of CSF at the choroid plexus, enhanced drainage of CSF, or a change in the total volume of the ventricles and perivascular spaces. Temporarily limiting CSF secretion by targeting aquaporins or ion pumps in the choroid plexus membrane might therefore limit oedema formation in the short-term.
Figure 4.
Classification of CNS oedema. (A) Cytotoxic oedema is defined by astrocyte swelling (black arrows) followed by neuronal dendrite swelling. The net entry of water (blue arrows), most likely from the perivascular space, is caused by disruption of cellular ion homeostasis (green arrows) following hypoxic insult. (B) Ionic oedema is characterized by transcapillary sodium ion and anion fluxes associated with cellular uptake of ions from the perivascular CSF, and entry of water into the brain parenchyma. Astrocytes continue to be swollen (black arrows) by water from the perivascular space and the vascular compartment. Neuronal death produces cellular debris in the extracellular space (ECS). (C) Vasogenic oedema is a result of BBB dysfunction, possibly following ionic oedema. Increased transcytosis may contribute to the entry of plasma elements (brown), followed by water. Clearance of debris from the ECS produced by neuronal cell death may also occur by transcytosis (green). In some severe cases, the tight junctions between the endothelial cells are weakened leading to increased permeability of cerebral blood vessels to plasma components. Created using www.biorender.com.
In traumatic brain injury and spinal cord injury, where the BBB/BSCB can be damaged directly by the primary injury (i.e. cytotoxic and vasogenic oedema co-exist), the source of water and sodium ions is likely to be a mixture of CSF and blood, with the exact ratio depending on the extent of BBB damage. Further endothelial dysfunction, secondary to the primary insult, leads to vasogenic oedema (Fig. 4C). For many years, it was proposed that BBB breakdown is required to facilitate the entry of plasma proteins into the extracellular space. However, more recent work has shown that vasogenic oedema can occur without physical rupture of endothelial cells.81–83 Although the suppression of transcellular transport (transcytosis) at the BBB is an active process that maintains a functional barrier, increased transcytosis observed in injured capillary endothelial cells may contribute to plasma protein entry, exacerbating brain swelling.84 Transcytosis may also be involved in the elimination of some proteins from the perivascular space back into the blood stream. Relocalization of AQP4 to the perivascular astrocyte membrane facilitates cytotoxic oedema,7 and may also increase the rate at which ionic oedema develops, both by increasing astrocyte membrane water permeability and possibly by regulating the endfoot membrane localization of ion channels via direct interaction (e.g. with Kir4.1, TRPV4, SUR1-TRPM4).85–87
Current available therapies for the treatment of brain oedema are hypertonic mannitol or saline, steroids for tumour-induced brain swelling and, once the oedema becomes life-threatening, decompressive craniotomy.88 The reliability and validity of the results of high-dose mannitol trials in the treatment of traumatic brain injury have been questioned89; a Cochrane review concluded that insufficient evidence was available to recommend mannitol for the management of traumatic brain injury patients.90 Although hypertonic saline is used to treat brain oedema following ischaemic stroke,91 a Cochrane review similarly reported that conclusions could not be drawn about the efficacy and safety of hypertonic saline or other intracranial pressure‐lowering agents in the management of acute traumatic brain injury.92 While the use of steroids did not reduce oedema following stroke,93 some success was reported in reducing brain tumour-associated oedema with dexamethasone.94 However, the molecular pathogenesis of tumour-associated oedema is quite different from that of trauma or stroke-associated oedema, as it is primarily driven by neoangiogenesis of vessels under-expressing tight junction proteins within the tumour.95 A recent study suggested that loss of AQP4 assembly into OAPs may facilitate evasion of apoptosis and enhanced migration in glioma cells,96 but how this interacts with tumour-associated oedema or AQP4 localization remains unexplored.
Little is known about mechanisms controlling the resolution of brain oedema. Early experiments showed that increased AQP4 expression was associated with oedema resolution,77,97–101 and in a vasogenic oedema model, Aqp4−/− mice developed significantly increased intracranial pressure compared to wild-type mice, confirming a role for AQP4 in oedema resolution.102 Understanding this dynamic mechanism, including the role of the glymphatic system, will guide the development of new therapeutic approaches to treating oedema.
Neuromyelitis optica
Neuromyelitis optica (NMO) is a rare but severe demyelinating autoimmune inflammatory condition of the CNS, formerly classified as a type of multiple sclerosis that primarily affects the optic nerve and spinal cord.103 The majority of NMO patients have autoantibodies against AQP4 (termed NMO-IgG) detectable in their serum.104 The mechanisms by which NMO-IgG cause the pathophysiological features of NMO remain elusive,105 although administration of NMO-IgG leads to NMO-like pathology in rodents,106,107 providing strong evidence that NMO-IgG is causative. However, different NMO-IgGs can have large differences in their ability to activate complement upon AQP4 binding, with some epitopes more facilitative for IgG hexamerization, meaning that there is not a simple relationship between antibody titre and disease severity.108 There is also evidence to support the idea that NMO-IgG facilitates both complement-dependent and complement-independent astrocytopathy.109,110 Most NMO-IgGs preferentially bind the M23 isoform of AQP4, but this selectivity appears to depend on an OAP assembly-associated conformation of the extracellular loops of AQP4, rather than a difference between the M1 and M23 proteins per se.111,112 The effect of NMO-IgG on OAP size is unclear; one study reported an increase in average OAP size following NMO-IgG binding,113 another found no effect114 and a third reported a decrease in average OAP size.19 More recent work suggests that changes in the dynamics (rather than the average size of OAPs) may be altered by NMO-IgG, with potential consequences for glutamatergic synapse function.19 Similarly, whether NMO-IgG inhibits AQP4 water channel function is controversial113,114; an exquisitely tight seal between the extracellular domain of AQP4 and NMO-IgG would be required to inhibit water transport. The potential effects of NMO-IgG on AQP4-mediated glymphatic function remain unexplored.
Neuroinflammatory disorders
AQP4 may also have a role in CNS inflammation in a manner that is independent of autoantibody formation. AQP4 expression, either on peripheral immune cells, or on CNS astrocytes may regulate CNS immune cell migration and trafficking, or glial activation and cytokine production, respectively.115 One study using Aqp4−/− mice reported that the central neuroinflammatory response to CNS lipopolysaccharide (LPS) injection, including TNFα release, was reduced, suggesting a pro-inflammatory role for AQP4.116 In a more recent study, Aqp4 gene deletion altered astroglial cytokine release and exacerbated α-synuclein pathology in a rodent model of Parkinson’s disease.117 These studies suggest that AQP4 may function to regulate CNS cytokine signalling. Given the role of AQP4 in glymphatic clearance,37,56 one possible explanation for these findings is that AQP4-dependent glymphatic exchange contributes to the distribution and clearance of cytokines within the CNS. The impacts that physiological and pathological changes in AQP4 localization have on its inflammatory roles remain to be defined.
New horizons for drug discovery
IMD-0354/AER-270, TGN-020, acetazolamide, budesonide, furosemide, and various anti-epileptics have all been proposed to be AQP4 inhibitors on the basis of data primarily derived from the Xenopus laevis oocyte swelling assay.40 When retested in transport assays using primary astrocytes expressing endogenous AQP4, mammalian cell lines overexpressing exogenous AQP4 or recombinant AQP4 protein, many putative pore-blockers have been found to lack AQP4 inhibitory function.40,118,119 It therefore remains unclear, after several decades of effort, whether a specific AQP4 pore-blocking inhibitor can be developed, providing impetus to explore alternative strategies, such as targeting dynamic AQP4 subcellular relocalization.
Several lines of evidence over the last decade have also highlighted the diverse functions of aquaporins beyond water homeostasis.10 AQP4 has been proposed to associate with various ion channels in the astrocyte membrane, including the inwardly rectifying potassium channel Kir4.1,120 the mechanosensitive cation channel TRPV4,121 and the ABC protein/TRP channel complex SUR1-TRPM4.122 AQP4 and Kir4.1 are co-localized in astrocyte membranes,123 and co-immunoprecipitate from glial cells.120 This interaction was proposed to support potassium ion spatial buffering by astrocytes after neuronal activity,124 and cellular potassium ion reuptake is delayed in Aqp4−/− mice in an epilepsy model,125 although it still unclear whether there is a functional relationship between AQP4 and Kir4.1.126 However, there is some evidence that Kir4.1 limits the osmotic swelling of spinal cord astrocyte processes.127,128 In several cell types, TRP channel plasma membrane trafficking is dependent on the expression of an aquaporin protein.10 AQP4 and the SUR1-TRPM4 monovalent cation channel complex co-immunoprecipitated when overexpressed in COS-7 cells, preactivation of SUR1 with diazoxide increased astrocyte swelling in response to a calcium ionophore, and SUR1-TRPM4 was upregulated and TRPM4 knockout blocked astrocyte swelling in a mouse cerebellar cold injury model.122 Furthermore, inhibition of the SUR1-TRMP4 complex using glyburide reduces oedema formation in multiple rodent models of brain pathology.74 This work raises the intriguing possibility that as well as directly regulating astrocyte membrane water permeability, AQP4 facilitates membrane insertion of oedema-associated TRP channels. Based on the new molecular understanding of the role and mechanisms of dynamic AQP4 subcellular relocalization and protein–protein interactions in CNS oedema, novel anti-oedema therapies are likely to emerge. This is of the utmost importance because there are currently no pharmacological tools to prevent or reduce CNS oedema. Treatment therefore focuses on symptom management, which is only possible after the oedema has developed (and has caused secondary damage) and which uses interventions developed decades ago. These new possibilities for drug discovery offer new hope to the millions of people annually affected by CNS oedema and neurodegenerative diseases.7
The dependence of the field on static, in vitro models rather than dynamic, in vivo visualization may have contributed to both the glymphatic system and AQP4 subcellular relocalization remaining undiscovered for so long. Recapitulating the complex structure and function of the BBB in vitro is challenging. Rodent in vivo studies and slice cultures are anatomically more realistic, but are hampered by species differences in BBB function and by the isolation of tissue slices from the blood circulation, peripheral immune actors, and both CSF and intracranial pressure dynamics. New developments in 3D tissue engineering, organ-on-a-chip technologies, and induced pluripotent stem cell differentiation may help the field to begin to address some of these limitations.129–131 Future gains in our understanding of astroglial and AQP4 contributions to CNS physiology, and how their dysfunction contributes to the development of CNS disease, will likely depend on the combined use of emerging in vitro techniques, such as BBB/glymphatics-on-a-chip, to dissect specific physiological processes along with dynamic in vivo approaches that preserve the full anatomy and physiology of the glial–vascular unit.
Funding
We acknowledge grants from the Biotechnology and Biosciences Research Council (to R.M.B., A.C.C., and P.K. through BB/P025927/1), Aston University (to P.K. through a 50th Anniversary Prize Fellowship), the Swedish Research Council (to S.T.-H. through 2013–05945); the Crafoord Foundation (to S.T.-H. through 20140811 and 20180916) and the Magnus Bergvall Foundation (to S.T.-H. through 2015–01534).
Competing interests
The authors report no competing interests.
Abbreviations
- BBB
blood–brain barrier
- BSCB
blood–spinal cord barrier
- NMO
neuromyelitis optica
- OAPs
orthogonal arrays of particles
References
- 1.Rangel-Castilla L, Rangel-Castillo L, Gopinath S, Robertson CS.. Management of intracranial hypertension. Neurol Clin. 2008;26(2):521–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagelhus EA, Ottersen OP.. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mader S, Brimberg L.. Aquaporin-4 water channel in the brain and its implication for health and disease. Cells. 2019;8(2):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manley GT, Binder DK, Papadopoulos MC, Verkman AS.. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129(4):983–991. [DOI] [PubMed] [Google Scholar]
- 5.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC.. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758(8):1085–1093. [DOI] [PubMed] [Google Scholar]
- 6.Trillo-Contreras JL, Toledo-Aral JJ, Echevarría M, Villadiego J.. AQP1 and AQP4 contribution to cerebrospinal fluid homeostasis. Cells. 2019;8(2):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitchen P, Salman MM, Halsey AM, et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell. 2020;181(4):784–799.e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitchen P, Salman MM, Pickel SU, et al. Water channel pore size determines exclusion properties but not solute selectivity. Sci Rep. 2019;9(1):20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreida S, Törnroth-Horsefield S.. Structural insights into aquaporin selectivity and regulation. Curr Opin Struct Biol. 2015;33:126–134. [DOI] [PubMed] [Google Scholar]
- 10.Kitchen P, Day RE, Salman MM, Conner MT, Bill RM, Conner AC.. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim Biophys Acta. 2015;1850(12):2410–2421. [DOI] [PubMed] [Google Scholar]
- 11.Kitchen P, Conner MT, Bill RM, Conner AC.. Structural determinants of oligomerization of the aquaporin-4 channel. J Biol Chem. 2016;291(13):6858–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umenishi F, Verkman AS.. Isolation and functional analysis of alternative promoters in the human aquaporin-4 water channel gene. Genomics. 1998;50(3):373–377. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Pisani F, Nicchia GP, Svelto M, Frigeri A.. Evidences for a leaky scanning mechanism for the synthesis of the shorter M23 protein isoform of aquaporin-4: Implication in orthogonal array formation and neuromyelitis optica antibody interaction. J Biol Chem. 2010;285(7):4562–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman CS, Gorelick-Feldman DA, Davidson KGV, et al. Aquaporin-4 square array assembly: Opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci USA. 2003;100(23):13609–13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rash JE, Davidson KGV, Yasumura T, Furman CS.. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience. 2004;129(4):915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirt L, Fukuda AM, Ambadipudi K, et al. Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab. 2017;37(1):277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisjak M, Potokar M, Rituper B, Jorgacevski J, Zorec R.. AQP4e-based orthogonal arrays regulate rapid cell volume changes in astrocytes. J Neurosci. 2017;37(44):10748–10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Iwasaki Y, Yamamoto T, Konno H, Yoshimoto T, Suzuki J.. Disintegration of orthogonal arrays in perivascular astrocytic processes as an early event in acute global ischemia. Brain Res. 1984;300(1):141–145. [DOI] [PubMed] [Google Scholar]
- 19.Ciappelloni S, Bouchet D, Dubourdieu N, et al. Aquaporin-4 surface trafficking regulates astrocytic process motility and synaptic activity in health and autoimmune disease. Cell Rep. 2019;27(13):3860–3872.e3864. [DOI] [PubMed] [Google Scholar]
- 20.de Bellis M, Cibelli A, Mola MG, et al. Orthogonal arrays of particle assembly are essential for normal aquaporin‐4 expression level in the brain. Glia. 2021;69(2):473–488. [DOI] [PubMed] [Google Scholar]
- 21.Pisani F, Simone L, Mola MG, et al. Regulation of aquaporin-4 expression in the central nervous system investigated using M23-AQP4 null mouse. Glia. 2021;69(9):2235–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loughran G, Chou M-Y, Ivanov IP, et al. Evidence of efficient stop codon readthrough in four mammalian genes. Nucleic Acids Res. 2014;42(14):8928–8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bellis M, Pisani F, Mola MG, et al. Translational readthrough generates new astrocyte AQP4 isoforms that modulate supramolecular clustering, glial endfeet localization, and water transport. Glia. 2017;65(5):790–803. [DOI] [PubMed] [Google Scholar]
- 24.Rauch SM, Huen K, Miller MC, et al. Changes in brain β-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exper Neurol. 2011;70(12):1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoddevik EH, Khan FH, Rahmani S, Ottersen OP, Boldt HB, Amiry-Moghaddam M.. Amiry-Moghaddam M. Factors determining the density of AQP4 water channel molecules at the brain-blood interface. Brain Struct Funct. 2017;222(4):1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiry-Moghaddam M, Otsuka T, Hurn PD, et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100(4):2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belmaati Cherkaoui M, Vacca O, Izabelle C, et al. Dp71 contribution to the molecular scaffold anchoring aquaporine-4 channels in brain macroglial cells. Glia. 2021;69(4):954–970. [DOI] [PubMed] [Google Scholar]
- 28.Rao SB, Skauli N, Jovanovic N, et al. Orchestrating aquaporin-4 and connexin-43 expression in brain: Differential roles of alpha1- and beta1-syntrophin. Biochim Biophys Acta Biomembr. 2021;1863(8):183616. [DOI] [PubMed] [Google Scholar]
- 29.Simon MJ, Iliff JJ.. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862(3):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshio K, Watanabe H, Song Y, Verkman A, Manley GT.. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19(1):76–78. [DOI] [PubMed] [Google Scholar]
- 31.Smith AJ, Jin BJ, Ratelade J, Verkman AS.. Aggregation state determines the localization and function of M1- and M23-aquaporin-4 in astrocytes. J Cell Biol. 2014;204(4):559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicchia GP, Rossi A, Nudel U, Svelto M, Frigeri A.. Dystrophin-dependent and -independent AQP4 pools are expressed in the mouse brain. Glia. 2008;56(8):869–876. [DOI] [PubMed] [Google Scholar]
- 33.Nyblom M, Törnroth-Horsefield S.. Regulation of eukaryotic aquaporins. In: Soveral G, Nielsen S, Casini A, eds. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery. CRC Press; 2016. [Google Scholar]
- 34.Kwon T, Frøkiær J, Nielsen S.. Regulation of aquaporin-2 in the kidney: A molecular mechanism of body-water homeostasis. Kidney Res Clin Pract. 2013;32(3):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conner AC, Bill RM, Conner MT.. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol Membr Biol. 2013;30(1):1–12. [DOI] [PubMed] [Google Scholar]
- 36.Salman MM, Kitchen P, Woodroofe MN, et al. Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. Eur J Neurosci. 2017;46(9):2542–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchen P, Day RE, Taylor LH, et al. Identification and molecular mechanisms of the rapid tonicity-induced relocalization of the aquaporin 4 channel. J Biol Chem. 2015;290(27):16873–16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylvain NJ, Salman MM, Pushie MJ, et al. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim Biophys Acta Biomembr. 2021;1863(5):183573. [DOI] [PubMed] [Google Scholar]
- 39.Verkman AS, Anderson MO, Papadopoulos MC.. Aquaporins: Important but elusive drug targets. Nat Rev Drug Discov. 2014;13(4):259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abir-Awan M, Kitchen P, Salman MM, Conner MT, Conner AC, Bill RM.. Inhibitors of mammalian aquaporin water channels. Int J Mol Sci. 2019;20(7):1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen MK, Mestre H, Nedergaard M.. Fluid Transport in the Brain. Physiol Rev. Published online 5 May 2021. 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ringstad G, Vatnehol SAS, Eide PK.. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140(10):2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hablitz LM, Pla V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hablitz LM, Vinitsky HS, Sun Q, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5(2):eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison IF, Ismail O, Machhada A, et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143(8):2576–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundgaard I, Lu ML, Yang E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2017;37(6):2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zbesko JC, Nguyen TV, Yang T, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis. 2018;112:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener. 2015;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS.. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017;6:e27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mestre H, Hablitz LM, Xavier AL, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;7:e40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mestre H, Du T, Sweeney AM, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367(6483): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke. 2014;45(10):3092–3096. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, Ding F, Deng S, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37(11):2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren Z, Iliff JJ, Yang L, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33(6):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M, Iliff JJ, Liao Y, et al. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32(50):17948–17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis. 2016;93:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nedergaard M, Goldman SA.. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eide PK, Ringstad G.. MRI with intrathecal MRI gadolinium contrast medium administration: A possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015;4(11):2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eide PK, Ringstad G.. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2019;39(7):1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deike-Hofmann K, Reuter J, Haase R, et al. Glymphatic pathway of gadolinium-based contrast agents through the brain: Overlooked and misinterpreted. Invest Radiol. 2019;54(4):229–237. [DOI] [PubMed] [Google Scholar]
- 68.Zeppenfeld DM, Simon M, Haswell JD, et al. Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol. 2017;74(1):91–99. [DOI] [PubMed] [Google Scholar]
- 69.Burfeind KG, Murchison CF, Westaway SK, et al. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer's disease. Alzheimers Dement (N Y). 2017;3(3):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chandra A, Farrell C, Wilson H, et al. ; Alzheimer's Disease Neuroimaging Initiative. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer's disease spectrum. Neurobiol Aging. 2021;97:1–9. [DOI] [PubMed] [Google Scholar]
- 71.Rainey-Smith SR, Mazzucchelli GN, Villemagne VL, et al. ; AIBL Research Group. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl Psychiatry. 2018;8(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon MJ, Wang MX, Murchison CF, et al. Transcriptional network analysis of human astrocytic endfoot genes reveals region-specific associations with dementia status and tau pathology. Sci Rep. 2018;8(1):12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pavlova V, Filipova E, Uzunova K, Kalinov K, Vekov T.. Pioglitazone therapy and fractures: Systematic review and meta-analysis. Endocr Metab Immune. 2018;18(5):502–507. [DOI] [PubMed] [Google Scholar]
- 74.Bordone MP, Salman MM, Titus HE, et al. The energetic brain - a review from students to students. J Neurochem. 2019;151(2):139–165. [DOI] [PubMed] [Google Scholar]
- 75.Halsey AM, Conner AC, Bill RM, Logan A, Ahmed Z.. Aquaporins and their regulation after spinal cord injury. Cells. 2018;7(10):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V.. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6(3):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Badaut J, Ashwal S, Obenaus A.. Aquaporins in cerebrovascular disease: A target for treatment of brain edema? Cerebrovasc Dis. 2011;31(6):521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Risher WC, Andrew RD, Kirov SA.. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57(2):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stokum JA, Gerzanich V, Simard JM.. Molecular pathophysiology of cerebral edema. J Cerebral Blood Flow Metab. 2016;36(3):513–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith AJ, Verkman AS.. CrossTalk opposing view: Going against the flow: Interstitial solute transport in brain is diffusive and aquaporin‐4 independent. J Physiol. 2019;597(17):4421–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haley MJ, Lawrence CB.. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab. 2017;37(2):456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Badaut J, Ajao DO, Sorensen DW, Fukuda AM, Pellerin L.. Caveolin expression changes in the neurovascular unit after juvenile traumatic brain injury: Signs of blood-brain barrier healing? Neuroscience. 2015;285:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayloo S, Gu C.. Transcytosis at the blood–brain barrier. Current Opin Neurobiol. 2019;57:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.King ZA, Sheth KN, Kimberly WT, Simard JM.. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: Evidence to date. Drug Des Devel Ther. 2018;12:2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tosun C, Kurland DB, Mehta R, et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke. 2013;44(12):3522–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rakers C, Schmid M, Petzold GC.. TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri‐infarct depolarizations in a stroke model. Glia. 2017;65(9):1550–1561. [DOI] [PubMed] [Google Scholar]
- 88.Jha RM, Kochanek PM, Simard JM.. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019;145(Pt B):230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts I, Smith R, Evans S.. Doubts over head injury studies. BMJ. 2007;334(7590):392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wakai A, McCabe A, Roberts I, Schierhout G.. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013;(8):CD001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogden AT, Mayer SA, Connolly ES Jr.. Hyperosmolar agents in neurosurgical practice: The evolving role of hypertonic saline. Neurosurgery. 2005;57(2):207–215. Discussion 207–215. [DOI] [PubMed] [Google Scholar]
- 92.Chen H, Song Z, Dennis JA.. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2020;1:CD010904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qizilbash N, Lewington SL, Lopez-Arrieta JM.. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev. 2002;(2):CD000064. [DOI] [PubMed] [Google Scholar]
- 94.Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL.. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: A randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44(4):675–680. [DOI] [PubMed] [Google Scholar]
- 95.Papadopoulos MC, Saadoun S, Binder DK, Manley GT, Krishna S, Verkman AS.. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129(4):1011–1020. [DOI] [PubMed] [Google Scholar]
- 96.Simone L, Pisani F, Mola MG, et al. AQP4 aggregation state is a determinant for glioma cell fate. Cancer Res. 2019;79(9):2182–2194. [DOI] [PubMed] [Google Scholar]
- 97.Badaut J, Ashwal S, Adami A, et al. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab. 2011;31(3):819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukuda AM, Adami A, Pop V, et al. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J Cereb Blood Flow Metab. 2013;33(10):1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fukuda AM, Pop V, Spagnoli D, Ashwal S, Obenaus A, Badaut J.. Delayed increase of astrocytic aquaporin 4 after juvenile traumatic brain injury: Possible role in edema resolution? Neuroscience. 2012;222:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meng S, Qiao M, Lin L, Del Bigio MR, Tomanek B, Tuor UI.. Correspondence of AQP4 expression and hypoxic-ischaemic brain oedema monitored by magnetic resonance imaging in the immature and juvenile rat. Eur J Neurosci. 2004;19(8):2261–2269. [DOI] [PubMed] [Google Scholar]
- 101.Tourdias T, Dragonu I, Fushimi Y, et al. Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: A combined MRI-histological study. Neuroimage. 2009;47(2):659–666. [DOI] [PubMed] [Google Scholar]
- 102.Papadopoulos MC, Manley GT, Krishna S, Verkman AS.. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–1293. [DOI] [PubMed] [Google Scholar]
- 103.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG.. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999;53(5):1107–1114. [DOI] [PubMed] [Google Scholar]
- 104.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR.. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang X, Ransom BR, Ma JF.. The role of AQP4 in neuromyelitis optica: More answers, more questions. J Neuroimmunol. 2016;298:63–70. [DOI] [PubMed] [Google Scholar]
- 106.Kinoshita M, Nakatsuji Y, Kimura T, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386(4):623–627. [DOI] [PubMed] [Google Scholar]
- 107.Saini H, Rifkin R, Gorelik M, et al. Passively transferred human NMO-IgG exacerbates demyelination in mouse experimental autoimmune encephalomyelitis. BMC Neurol. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soltys J, Liu Y, Ritchie A, et al. Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J Clin Invest. 2019;129(5):2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nishiyama S, Misu T, Nuriya M, et al. Complement-dependent and -independent aquaporin 4-antibody-mediated cytotoxicity in human astrocytes: Pathogenetic implications in neuromyelitis optica. Biochem Biophys Rep. 2016;7:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yick LW, Ma OK, Ng RC, Kwan JS, Chan KH.. Aquaporin-4 autoantibodies from neuromyelitis optica spectrum disorder patients induce complement-independent immunopathologies in Mice. Front Immunol. 2018;9:1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS.. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286(18):16516–16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tuller F, Holzer H, Schanda K, et al. Characterization of the binding pattern of human aquaporin-4 autoantibodies in patients with neuromyelitis optica spectrum disorders. J Neuroinflammation. 2016;13(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012;109(4):1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS.. Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly. Glia. 2012;60(12):2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meli R, Pirozzi C, Pelagalli A.. New perspectives on the potential role of Aquaporins (AQPs) in the physiology of inflammation. Front Physiol. 2018;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS.. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011;25(5):1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xue X, Zhang W, Zhu J, et al. Aquaporin-4 deficiency reduces TGF-beta1 in mouse midbrains and exacerbates pathology in experimental Parkinson's disease. J Cell Mol Med. 2019;23(4):2568–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang B, Zhang H, Verkman AS.. Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides. Bioorg Med Chem. 2008;16(15):7489–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verkman AS, Smith AJ, Phuan PW, Tradtrantip L, Anderson MO.. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin Ther Targets. 2017;21(12):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Connors NC, Kofuji P.. Potassium channel Kir4.1 macromolecular complex in retinal glial cells. Glia. 2006;53(2):124–131. [DOI] [PubMed] [Google Scholar]
- 121.Benfenati V, Caprini M, Dovizio M, et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci USA. 2011;108(6):2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stokum JA, Kwon MS, Woo SK, et al. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia. 2018;66(1):108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagelhus EA, Mathiisen TM, Ottersen OP.. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129(4):905–913. [DOI] [PubMed] [Google Scholar]
- 124.Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP.. A novel role of vasopressin in the brain: Modulation of activity-dependent water flux in the neocortex. J Neurosci. 2001;21(9):3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT.. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53(6):631–636. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Verkman AS.. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol Cell Neurosci. 2008;37(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dibaj P, Kaiser M, Hirrlinger J, Kirchhoff F, Neusch C.. Kir4.1 channels regulate swelling of astroglial processes in experimental spinal cord edema. J Neurochem. 2007;103(6):2620–2628. [DOI] [PubMed] [Google Scholar]
- 128.Hanani M, Spray DC.. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci. 2020;21(9):485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahn SI, Sei YJ, Park HJ, et al. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat Commun. 2020;11(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salman MM, Marsh G, Kusters I, et al. Design and validation of a human brain endothelial microvessel-on-a-chip open microfluidic model enabling advanced optical imaging. Front Bioeng Biotechnol. 2020;8:573775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wevers NR, Kasi DG, Gray T, et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS. 2018;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]