Abstract
Background
Respiratory syncytial virus (RSV), human metapneumovirus (hMPV), and influenza are respiratory pathogens leading to hospitalization in adults. Our understanding of the disease burden is limited to data from single-center or 1-season studies in elderly patients. The HARTI study allows comparison of risk factors for progression to severe disease and medical resources utilization (MRU) during and post-hospitalization in adults diagnosed with influenza, RSV, or hMPV.
Methods
This was a prospective global study in adults hospitalized with acute respiratory tract infection (40 centers, 12 countries). Participants with influenza, RSV, or hMPV were enrolled in a substudy and followed for up to 3 months postdischarge.
Results
Overall, 366 influenza, 238 RSV, and 100 hMPV-infected participants enrolled in the substudy. RSV participants were older and had greater frequency of risk factors and longer duration of symptoms before hospitalization than influenza participants. The RSV and hMPV groups received more bronchodilators, corticosteroids, and oxygen supplementation. No significant differences in intensive care unit admissions or complications were observed. Readmission occurred in 20%–33% of patients within 3 months postdischarge, with the highest rates for RSV and hMPV. In-hospital death occurred in 2.5% of RSV, 1.6% of influenza, and 2% of hMPV participants. In multivariate analyses, length of stay was independently associated with country, renal disease, and increased age; probability of receiving supplemental oxygen was associated with pathogen (hMPV > RSV > influenza), abnormal chest x-ray, and increased age.
Conclusions
Although influenza is more frequent, the HARTI study demonstrates greater frequency of underlying risk factors and MRU for RSV and hMPV vs influenza in hospitalized adults, indicating a need for effective interventions.
Keywords: global prospective study, Influenza, RSV, hMPV, medical resource utilization
Lower respiratory tract infections (LRTIs) are a leading cause of mortality and morbidity worldwide, resulting in almost 2.38 million deaths in 2016, making LRTI the sixth leading cause of mortality for all ages [1]. In a systematic analysis of the global burden of disease in adults in 2010, about 500000 deaths annually were linked to influenza and 250000 to respiratory syncytial virus (RSV) infections [2]. In a prospective study among hospitalized adults aged ≥50 years, mortality was comparable between human metapneumovirus (hMPV) and RSV-infected patients [3].
Influenza is a well-recognized respiratory pathogen, with annual epidemics affecting 5%–20% of the global unvaccinated population in all age groups, resulting in 3–5 million cases of severe illness and 250000 to 500000 deaths [2, 4, 5]. The rates of serious influenza illness and death are highest among persons aged <2 years or ≥65 years and those who are immunocompromised or have medical conditions such as asthma, chronic obstructive pulmonary disease (COPD), cardiovascular disease, or diabetes [6–9].
There is increasing yet incomplete evidence of the burden associated with RSV and hMPV infection in adults. A prospective study in the United States revealed that 3%–7% of healthy elderly adults (≥65 years of age) and 4%–10% of high-risk adults (those with chronic heart or lung disease) suffer from RSV infection annually, with an estimated 177000 hospitalizations and 14000 deaths attributable to RSV each year in adults aged ≥65 years [6]. The burden of RSV infection in older adults may be underestimated due to lack of routine testing, low awareness among providers, and lack of detectability when it results in an exacerbation of an underlying chronic cardiac or pulmonary condition [10]. Similar to influenza, the risk of mortality is markedly increased among the elderly who are hospitalized with RSV [11]. First recognized in 2001, hMPV has been identified as an important cause of acute respiratory tract infections (ARTIs), affecting all age groups, with severe infections in children aged <5 years, the elderly, and patients with cardiac, pulmonary, or immunocompromising disease [12–15]. A prospective study during 4 consecutive winter seasons showed an annual incidence of hMPV infection of 2.2%–10.5% in outpatient adults, with 51% of patients developing symptomatic respiratory illness. During the same period, hMPV was associated with 8.5% of adult hospitalizations due to acute cardiopulmonary illness [15]. Like influenza, RSV and hMPV infections in adults are associated with acute exacerbation of asthma and COPD [9, 16, 17].
Many studies to date involve only older adults and provide data from single centers, most commonly in the United States and Europe [18]. The HARTI study aimed to further characterize adults throughout the age spectrum who were hospitalized with RSV, hMPV, and influenza. Risk factors and medical resources utilization (MRU) during hospitalization and up to 3 months post–hospital discharge were compared between RSV, hMPV, and influenza patients.
METHODS
Study Design
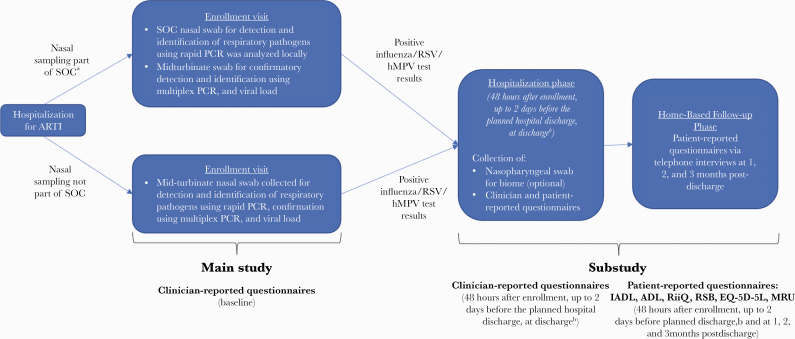
This was a prospective cohort study in adult patients (≥18 years old) hospitalized with ARTI during the influenza/RSV/hMPV season at 40 centers across 12 countries (Australia, Argentina, Brazil, Canada, France, Germany, Japan, Malaysia, Mexico, Republic of Korea, South Africa, United States), over 2 consecutive epidemic seasons (2017–2019). The clinical diagnosis of ARTI and decision to hospitalize the patient were made according to local standard-of-care (SOC) practices. The enrollment period was guided based on the local epidemic wave progression. The study included 2 parts: a main study and a substudy (Figure 1). Participants were consented and enrolled in the main study within 24 hours after admission. Viral testing was done as part of SOC or by study-specific molecular diagnostic testing, if not performed as SOC. All tests (whether SOC or study-specific) used reverse transcription polymerase chain reaction–based technology. Participants with influenza (up to a cap of the first 380 consecutive participants), RSV, or hMPV infection were invited to enroll in the substudy, which comprised a hospitalization phase with 3 visits (enrollment, 48 hours after enrollment, and within ~2 days before planned discharge) and a follow-up phase with telephone interviews at 1, 2, and 3 months postdischarge. This report describes results from the substudy (refer to Supplementary Table 1 for the main study population).
Figure 1.
Study design schema. aWhen nasal swab collected as part of SOC, midturbinate swab (collected from opposite nostril than used for SOC test). bIf a participant was hospitalized for a short period (ie, <72 hours) or transferred to another ward, an early discharge assessment was performed on the day of discharge. Abbreviations: ADL, activities of daily living; ARTI, acute respiratory tract infection; EQ-5D-5L, EuroQol 5 Dimensions 5 Levels; hMPV, human metapneumovirus; IADL, instrumental activities of daily living; MRU, medical resource utilization; PCR, polymerase chain reaction; RiiQ, Respiratory Intensity and Impact Questionnaire; RSB, Respiratory Symptoms Bother and Change in Health Status Questionnaire; RSV, respiratory syncytial virus; SOC, standard of care.
Data Collection
Clinical information and hospital MRU were collected through clinician-reported questionnaires (at enrollment, 48 hours after screening, and within 2 days before planned discharge) and through the MRU questionnaire during monthly phone interviews postdischarge. If the participant was discharged within 3 days of the enrollment visit, 1 hospital assessment was performed postbaseline (on the day of discharge). Vital signs and complications, including lower respiratory, cardiovascular, and other complications (Supplementary Figure 1), were collected during hospitalization. An MRU questionnaire that assessed hospital readmissions, medical consultations (general practitioner, internal medicine, pulmonologist, respiratory physiotherapy, or other), professional home care, and medications was administered at 1, 2, and 3 months postdischarge.
Statistical Analysis
Data collected from study questionnaires were summarized using descriptive statistics. The National Early Warning Score (NEWS), a tool that improves detection of clinical deterioration in adult patients, was calculated using vital signs [19, 20]. The NEWS score consists of 7 graded physiological measurements (respiratory rate, oxygen saturation, oxygen supplementation, temperature, blood pressure, heart rate, level of consciousness). Concomitant medications reported by the clinician during hospitalization were described by frequency distribution. Core risk factors for progression to severe disease (ie, hospitalization) and long-term sequelae (ie, slow recovery of lung function and functional capacity) were defined as age >65 years, chronic heart or renal disease, COPD, and asthma. Hospital length of stay (LOS) was summarized using Kaplan-Meier (KM) analysis and described categorically (by ≤3 days or >3 days of stay). Pairwise comparisons were performed between RSV and hMPV vs influenza participants, and any differences among the 3 respiratory pathogens were assessed. Multivariate regression analysis was applied on hospital LOS and probability of receiving supplemental oxygen during hospitalization. A multivariate accelerated failure time (AFT) model with log-logistic likelihood was applied to investigate which baseline covariates impact the observed LOS. The AFT model provides an alternative to the Cox proportional hazards (PH) model in the analysis of time to event data [21, 22]. Whereas the PH model assumes that the risk factors multiply the hazard of hospital discharge by some constant, the AFT model assumes that the risk factors have a multiplicative effect (acceleration factor [AF]) on the LOS. For example, a binary covariate (eg, risk group indicator) with an estimated AF of 1.49 would imply that patients at risk have an LOS 1.49 times longer than patients without risks and thus a 49% increase in LOS. To compare the results of the AFT model, a PH model was fit using the covariates included in the multivariate AFT model. More information on the advantages, model structure, and interpretation of the AFT model are provided in the Supplementary Data. P values without corrections for multiple comparison are reported in Supplementary Tables 2–5.
RESULTS
Disposition
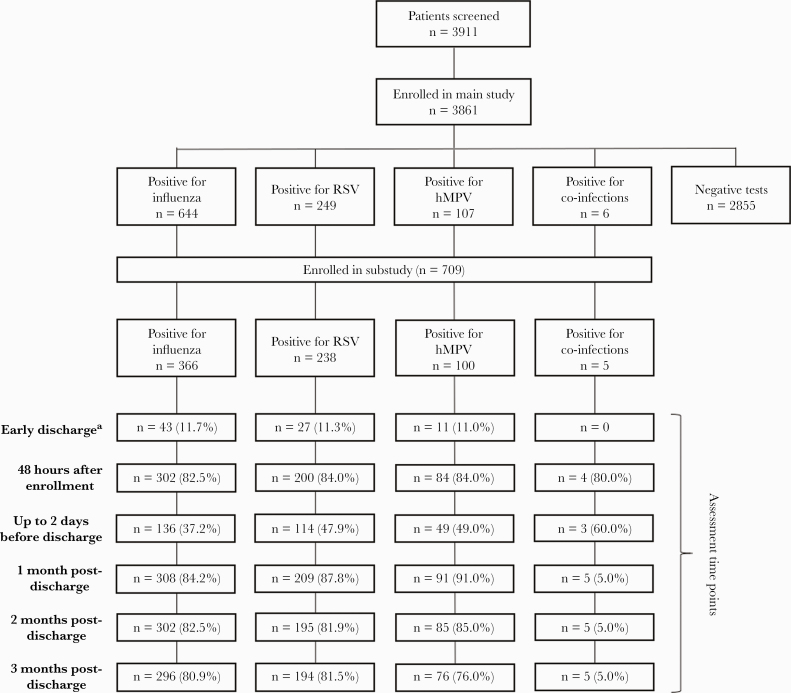
Overall, 3861 participants were consented and enrolled in the main study between March 2, 2017, and October 31, 2019. Of these 3861 participants, 26.1% (n=1006) had a positive test for influenza (16.7%), RSV (6.4%), hMPV (2.8%), or co-infection between any of the 3 viruses (0.2%). Among these 1006 main study participants with infections, 709 (18.1%) subsequently enrolled in the substudy, including 366 (9.5%) influenza-positive, 238 (6.2%) RSV-positive, and 100 (2.6%) hMPV-positive participants (and 5 [0.1%] participants with co-infections not described in this manuscript) (Figure 2).
Figure 2.
Flow diagram of participants at each study visit. Patients having a positive result for 2 or more respiratory pathogens between FLU, RSV, and hMPV are presented in the co-infections. All the percentages were calculated based on the total number of patients included for each pathogen. Missing patients at each study visit are not included in the diagram above. aIf a participant was hospitalized for a short period (ie, <72 hours) or transferred to another ward, an early discharge assessment was performed on the day of discharge. Abbreviations: hMPV, human metapneumovirus; FLU, influenza; RSV, respiratory syncytial virus.
Demographics and Baseline Clinical Characteristics
Almost half of the substudy participants were from the United States (49.9%) (Table 1). Overall, 44.3% of participants were between the ages of 18 and ≤64 years. The mean (SD) age of participants overall was 65.6 (16.2) years and was significantly higher among RSV participants (67.3 [16.5] years) compared with influenza (64.4 [16.1] years; P=.032). The majority of participants were female (57.0%), with a higher proportion among RSV compared with influenza cases (61.8% vs 52.7%, respectively; P=.035). A greater proportion of RSV participants had core risk factors compared with influenza (86.1% vs 75.4%, respectively; P=.002). Similar trends toward a higher percentage of female gender and more core risk factors with hMPV compared with influenza were noted, although differences were not statistically significant. Age (≥65 years) was the most common risk factor (influenza: 51.9%; RSV: 60.9%; hMPV: 57.0%) among all respiratory pathogen groups, followed by heart disease (influenza: 38.8%; RSV: 41.6%; hMPV: 33.0%) and COPD (influenza: 22.1%; RSV: 30.7%; hMPV: 32.0%).
Table 1.
Demographic and Baseline Clinical Characteristics by Respiratory Pathogen
| Influenza (n=366) | RSV (n=238) | hMPV (n=100) | Totala (n=709) | |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 64.4 (16.05) | 67.3 (16.52) | 65.9 (15.65) | 65.6 (16.19) |
| Median | 65.5 | 70.0 | 69.0 | 67.0 |
| (Range) | (18–99) | (18–98) | (24–93) | (18–99) |
| P value | .032b | .437c | .098d | |
| Female gender, No. (%) | 193 (52.7) | 147 (61.8) | 62 (62.0) | 404 (57.0) |
| P value | .035b | .124c | .051d | |
| Presence of core risk factor, No. (%)e | 276 (75.4) | 205 (86.1) | 85 (85.0) | 570 (80.4) |
| P value | .002b | .058c | .002d | |
| Age ≥65 y | 190 (51.9) | 145 (60.9) | 57 (57.0) | 395 (55.7) |
| P value | .036b | .429c | .089d | |
| Chronic disease–heart disease | 142 (38.8) | 99 (41.6) | 33 (33.0) | 276 (38.9) |
| P value | .548b | .345c | .334d | |
| Chronic disease–renal disease | 46 (12.6) | 39 (16.4) | 22 (22.0) | 109 (15.4) |
| P value | .231b | .027c | .055d | |
| Asthma | 47 (12.8) | 51 (21.4) | 20 (20.0) | 120 (16.9) |
| P value | .007b | .099c | .014d | |
| Respiratory tract morbidity–COPD | 81 (22.1) | 73 (30.7) | 32 (32.0) | 187 (26.4) |
| P value | .024b | .056c | .026 d | |
| Presence of other risk factor, No. (%)f | 57 (15.6) | 28 (11.8) | 11 (11.0) | 96 (13.5) |
| P value | .232b | .323c | .292d | |
| COPD severity, No. (%) | ||||
| No. | 81 | 73 | 32 | 187 |
| Mild | 20 (24.7) | 10 (13.7) | 6 (18.8) | 36 (19.3) |
| Moderate | 18 (22.2) | 20 (27.4) | 7 (21.9) | 46 (24.6) |
| Severe | 7 (8.6) | 17 (23.3) | 6 (18.8) | 30 (16.0) |
| Not reported | 36 (44.4) | 26 (35.6) | 13 (40.6) | 75 (40.1) |
| P value | .032b | .481c | .1776d | |
| Previous vaccinations, No. (%) | ||||
| Influenzag | 169 (46.2) | 143 (60.1) | 73 (73.0) | 388 (54.7) |
| P value | .001b | <.001c | <.001d | |
| Pneumococcal | 132 (36.2) | 93 (39.1) | 58 (58.0) | 286 (40.4) |
| Missing | 1 (0.3) | 0 | 0 | 1 (0.1) |
| P value | .508b | <.001c | <.001d | |
| Symptom length before hospitalization, d | ||||
| Mean (SD) | 4.5 (4.01) | 5.6 (7.27) | 5.1 (5.35) | 5.0 (5.50) |
| Median | 3.0 | 4.0 | 4.0 | 4.0 |
| Range | (0–31) | (0–92) | (0–35) | (0–92) |
| P value | .013b | .420c | .045d | |
| Reason for hospital admission | ||||
| Only ARTI | 165 (45.1) | 100 (42.0) | 51 (51.0) | 319 (45.0) |
| Only underlying medical conditions other than ARTI | 10 (2.7) | 6 (2.5) | 3 (3.0) | 19 (2.7) |
| Both ARTI and underlying medical conditions other than ARTI | 191 (52.2) | 132 (55.5) | 46 (46.0) | 371 (52.3) |
| P value | .733b | .548c | .636d | |
| Type of ARTI, No. (%) | ||||
| Asthma exacerbation | 19 (5.3) | 29 (12.5) | 10 (10.3) | 58 (8.4) |
| P value | .003b | .124c | .007d | |
| Bronchitis | 43 (12.1) | 26 (11.2) | 8 (8.2) | 78 (11.3) |
| P value | .849b | .380c | .571d | |
| COPD exacerbation | 52 (14.6) | 61 (26.3) | 26 (26.8) | 140 (20.3) |
| P value | .001 b | .008c | .001d | |
| Otherh | 148 (41.6) | 65 (28.0) | 22 (22.7) | 237 (34.3) |
| P value | .001 b | .001c | .001d | |
| Pneumonia | 138 (38.8) | 86 (37.1) | 42 (43.3) | 268 (38.8) |
| P value | .744 b | .489c | .572d | |
| Missing | 10 (2.7) | 6 (2.5) | 3 (3.0) | 19 (2.7) |
| Type of underlying medical condition other than ARTI, No. (%) | ||||
| Asthma or COPD | 48 (23.9) | 49 (35.5) | 18 (36.7) | 116 (29.7) |
| Congestive heart failure | 24 (11.9) | 21 (15.2) | 5 (10.2) | 50 (12.8) |
| Sepsis | 26 (12.9) | 13 (9.4) | 4 (8.2) | 43 (11.0) |
| Hypoxemia | 53 (26.4) | 39 (28.3) | 12 (24.5) | 105 (26.9) |
| Other | 51 (24.9) | 16 (11.6) | 10 (20.4) | 76 (19.5) |
| O2 supplement at screening visit | 186 (50.8) | 157 (66.0) | 64 (64.0) | 409 (57.7) |
| P value | <.001b | .026c | <.001d | |
| NEWS score at screening | ||||
| Mean (SD) | 3.99 (2.723) | 4.64 (2.677) | 4.12 (2.461) | 4.22 (2.682) |
| Median | 4.00 | 5.00 | 4.00 | 4.00 |
| Range | (0.0–13.0) | (0.0–12.0) | (0.0–11.0) | (0.0–13.0) |
| Missing | 35 (9.6) | 23 (9.7) | 15 (15.0) | 74 (10.4) |
| P value | .012b | .589c | .039d | |
Abbreviations: ANOVA, analysis of variance; ARTI, acute respiratory tract infection; COPD, chronic obstructive pulmonary disease; hMPV, human metapneumovirus; RSV, respiratory syncytial virus.
Participants with co-infections (between influenza and/or RSV and/or hMPV; n=5) are not displayed in this table but are included in the Total column.
P value based on Student t test (age), Wilcoxon rank-sum test (symptom length, NEWS score), or chi-square test (categorical variables) for the pairwise comparison between RSV and influenza participants.
P value based on Student t test (age), Wilcoxon rank-sum test (symptom length, NEWS score), or chi-square test (categorical variables) for the pairwise comparison between hMPV and influenza participants.
P value based on 1-way ANOVA (age), Kruskal-Wallis rank-sum test (symptom length, NEWS score), or chi-square test (categorical variables) for differences between influenza, RSV and hMPV participants.
Core risk factors: age ≥65 years, chronic heart disease, COPD, chronic renal disease, asthma.
Other risk factors: behavior risk factor–alcoholism, behavior risk factor–other, behavior risk factor–smoking (≥20 cigarettes/d), chronic disease–HIV infection, chronic disease–liver disease, chronic disease–lung disease, chronic disease–other, congenital or acquired immunodeficiencies, diabetes, diagnosed atopy–hay fever, diagnosed atopy–other, neoplasia, neurological and/or neuropsychiatric condition, neuromuscular disorder–multiple sclerosis, neuromuscular disorder–myasthenia gravis, neuromuscular disorder–other, other, pregnancy, respiratory tract morbidity–cystic fibrosis, respiratory tract morbidity–other, respiratory tract morbidity–pulmonary hypertension, upper airway abnormality–other, upper airway abnormality–subglottic stenosis.
“Previous influenza vaccination” refers to vaccine receipt in the prior season.
Other types of ARTI (free-text) primarily consisted of viral influenza-like ARTI.
Reason for hospital admission was reported as both ARTI and other underlying medical conditions for 52.3% overall (RSV: 55.5%; influenza: 52.2%; hMPV: 46.0%). Underlying asthma or COPD was more common among RSV (35.5%) and hMPV (36.7%) participants compared with influenza (23.9%) participants. The mean (SD) length of symptoms before hospitalization was longer for RSV (5.6 [7.3] days) and hMPV (5.1 [5.4] days) participants compared with influenza (4.5 [4.0] days) participants, with a statistically significant difference between RSV and influenza (P=.013). Similarly, late presentation (symptoms >3 days before hospitalization) was more common among RSV (55.9%) and hMPV (53.0%) participants compared with influenza (49.5%) participants. The mean (SD) NEWS scale at screening was 4.2 (2.7) overall (RSV: 4.6 [2.7]; hMPV: 4.1 [2.5]; influenza: 4.0 [2.7]).
Medical Resource Utilization
MRU During Hospitalization
Hospital LOS varied by several factors including infecting virus, country, age, and core risk factors (Table 2). The median (interquartile range) LOS based on KM estimates was longer for RSV (6.0 [4–9] days) compared with influenza (5.0 [3–7] days) and hMPV (5.0 [4–9] days) participants (P=.0043), and a greater proportion of RSV (73.5%) and hMPV (75.0%) participants had an LOS >3 days compared with influenza (70.8%) participants. Overall, LOS was shortest in Mexico, the United States, and Australia (KM median, 3.0, 4.0, and 4.0 days, respectively) and longest in Japan and France (median, 10.0 and 8.0 days, respectively; P<.0001) (Supplementary Figure 2). Longer stays were reported for participants who received oxygen supplementation during hospitalization and in the 75+ years age group (P<.0001). Significantly longer LOS was also reported in participants with core risk factors (P=.0003) and high NEWS scale at enrollment (P<.0001).
Table 2.
Morbidity and Medical Resource Utilization During Hospitalization and Postdischarge
| Influenza | RSV | hMPV | Totala | |
|---|---|---|---|---|
| MRU during hospitalization, No. | 366 | 238 | 100 | 709 |
| Highest clinical setting, No. (%) | ||||
| ICU | 28 (7.7) | 29 (12.2) | 8 (8.0) | 65 (9.2) |
| Inpatient ward | 338 (92.3) | 209 (87.8) | 92 (92.0) | 644 (90.8) |
| P value | .085b | 1.00c | .153d | |
| Mechanical ventilation, No. (%) | 9 (2.5) | 9 (3.8) | 5 (5.0) | 23 (3.2) |
| P value | .463b | .192c | .325d | |
| Concomitant medications | ||||
| Antibiotics | 243 (66.4) | 179 (75.2) | 74 (74.0) | 499 (70.4) |
| P value | .027b | .185c | .048d | |
| Antipyretics or antalgics | 226 (61.7) | 133 (55.9) | 62 (62.0) | 424 (59.8) |
| P value | .177b | 1.00c | .317 d | |
| Bronchodilators | 179 (48.9) | 175 (73.5) | 74 (74.0) | 432 (60.9) |
| P value | <.001 b | <.001c | <.001d | |
| Corticosteroids | 160 (43.7) | 150 (63.0) | 60 (60.0) | 375 (52.9) |
| P value | <.001b | .005c | <.001d | |
| Direct antivirals | 316 (86.3) | 34 (14.3) | 7 (7.0) | 362 (51.1) |
| P value | <.001b | <.001c | <.001d | |
| Supplemental oxygen | 193 (52.7) | 164 (68.9) | 67 (67.0) | 426 (60.1) |
| Mechanical | 6 (1.6) | 6 (2.5) | 4 (4.0) | 16 (2.3) |
| Nasal | 183 (50.0) | 154 (64.7) | 67 (67.0) | 406 (57.3) |
| Other | 19 (5.2) | 19 (8.0) | 1 (1.0) | 39 (5.5) |
| P value (supplemental oxygen) | <.001b | .015c | <.001d | |
| Complications during hospitalization, No. (%) | 145 (39.6) | 101 (42.4) | 45 (45.0) | 294 (41.5) |
| P value | .546b | .392c | .572d | |
| Lower respiratory complicationse,f | 72 (49.7) | 53 (52.5) | 22 (48.9) | 148 (50.3) |
| P value | .760b | 1.00c | .884d | |
| Cardiovascular complicationse,g | 35 (24.1) | 32 (31.7) | 11 (24.4) | 79 (26.9) |
| P value | .245b | 1.00c | .391d | |
| Bacterial superinfectione,h | 56 (38.6) | 37 (36.6) | 21 (46.7) | 115 (39.1) |
| P value | .855b | .431c | .509d | |
| Confusion, No. (%) | 22 (6.0) | 17 (7.1) | 3 (3.0) | 42 (5.9) |
| P value | .701b | .350c | .340d | |
| Deaths during hospitalization, No. (%) | 6 (1.6) | 6 (2.5) | 2 (2.0) | 14 (2.0) |
| MRU postdischarge, No. | 319 | 216 | 91 | 631 |
| Medical consultations, No. (%)i | 235 (73.7) | 175 (81.0) | 85 (93.4) | 499 (79.1) |
| P value | .062b | <.001c | <.001d | |
| Professional home care, No. (%)j | 71 (22.3) | 53 (24.5) | 26 (28.6) | 150 (23.8) |
| P value | .611b | .267c | .447d | |
| Hospital admission postdischarge, No. (%) | 65 (20.4) | 58 (26.9) | 30 (33.0) | 154 (24.4) |
| P value | .101b | .018c | .029d | |
| Institutional care postdischarge, No. (%) | 19 (6.0) | 25 (11.6) | 11 (12.1) | 55 (8.7) |
| P value | .031b | .080c | .038d | |
| Deaths during follow-up | 5 (1.4) | 5 (2.1) | 1 (1.0) | 11 (1.6) |
Abbreviations: hMPV, human metapneumovirus; ICU, intensive care unit; MRU, medical resource utilization; RSV, respiratory syncytial virus.
Participants with co-infections (between influenza and/or RSV and/or hMPV; n=5) are not displayed in this table but are included in the Total column.
P value based on chi-square test and Fisher exact test (mechanical ventilation) for the pairwise comparison between RSV and influenza participants.
P value based on chi-square test and Fisher exact test (mechanical ventilation) for the pairwise comparison between hMPV and influenza participants.
P value based on chi-square test and Fisher exact test (mechanical ventilation) for differences between influenza, RSV, and hMPV participants.
No. = participants who indicated “yes” for complications during hospitalization.
Lower respiratory complications included respiratory distress, arrest, and failure, empyema, other.
Cardiovascular complications included exacerbation of heart failure, atrial fibrillation, acute coronary events, acute cerebrovascular events.
Bacterial superinfection based on clinical judgment.
Medical consultations is set to “yes” if at least 1 of the following resources was used: general practitioner, internal medicine, pulmonologist, respiratory physiotherapy, or other.
Professional home care is set to “yes” if at least 1 of the following resources was used: general practitioner, nurse, respiratory physiotherapy, or other.
The most common concomitant medications reported were antibiotics and bronchodilators among RSV (75.2% and 73.5%, respectively) and hMPV (74.0% each) participants, and antivirals (86.3%) for influenza participants (Supplementary Figure 3). A greater proportion of RSV and hMPV participants received oxygen supplementation during hospitalization (68.9% and 67.0%, respectively) compared with influenza (52.7%) participants (P<.001).
Several trends, not statistically significant, were identified. Compared with influenza (39.6%) participants, a greater proportion of RSV and hMPV participants had complications during hospitalization (42.4% and 45.0%, respectively). Admission to the intensive care unit (ICU) and length of ICU stay were higher among RSV participants (12.2%; mean [SD], 9.1 [13.4] days) compared with influenza participants (7.7%; mean [SD], 8.0 [9.6] days). Twenty-three (3.2%) participants required mechanical ventilation, and 14 (2.0%) participants died during hospitalization (influenza: 1.6%; RSV: 2.5%; hMPV: 2.0%).
MRU Postdischarge
Overall, 24.4% of participants were readmitted to the hospital within 3 months postdischarge for any reason, with more readmissions among the RSV (26.6%) and hMPV (33.0%) groups compared with influenza (20.4%; P=.101 and P=.018 for RSV and hMPV, respectively, vs influenza). The majority of participants had at least 1 medical consultation postdischarge; most occurred in the RSV (81.0%) and hMPV (93.4%) groups compared with influenza (73.7%; P=.062 and P<.001 for RSV and hMPV, respectively, vs influenza). Bronchodilators were the main type of medication received postdischarge in over half of the RSV (51.4%) and hMPV (51.6%) participants, compared with 37.6% of influenza participants. A total of 103 (16.3%) participants overall reported using oxygen supplementation postdischarge, with a higher proportion observed among hMPV (20.9%) and RSV (19.4%) participants, compared with influenza (12.9%) participants. Among 104 participants with COPD who did not receive oxygen before hospitalization, 21.2% received oxygen supplementation postdischarge. Eleven participants died during follow-up (influenza: 1.4%; RSV: 2.1%; hMPV: 1.0%).
Multivariate Assessments
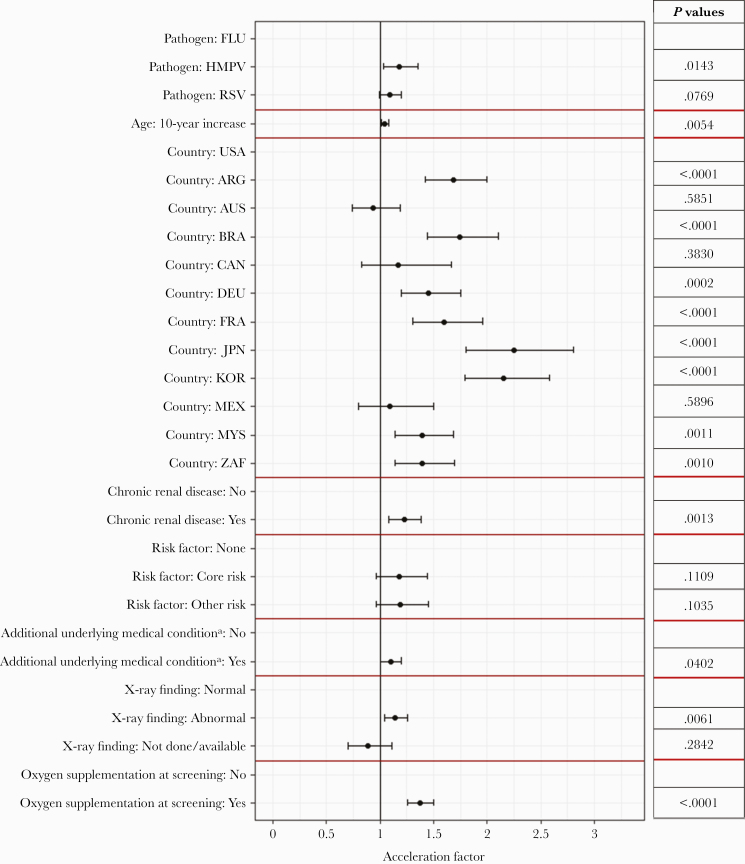
A multivariate AFT model showed that participants with chronic renal disease had a significant increase in LOS compared with those without renal impairment (AF, 1.22; 95% CI, 1.08–1.39; P=.0013), as well as oxygen supplementation at screening (AF, 1.37; 95% CI, 1.26–1.49; P<.0001). Hospital LOS increased for 10-year increase in age (AF, 1.05; 95% CI, 1.01–1.08; P=.0054). Compared with influenza participants, hMPV participants had a significant increase in LOS (AF, 1.18; 95% CI, 1.03–1.35; P=.0143), and RSV participants had a nonsignificant increase in LOS (AF, 1.09; 95% CI, 0.99–1.20; P=.0769) (Figure 3; Supplementary Table 3).
Figure 3.
Multivariate accelerated failure time model results for the length of hospital stay. Acceleration factors and 95% CIs of the variable included in the final model are presented. Rows without estimates are the reference category for that variable. aAdditional underlying medical condition on top of ARTI. Abbreviations: AF, acceleration factor; ARG, Argentina; ARTI, acute respiratory tract infection; AUS, Australia; BRA, Brazil; CAN, Canada; DEU, Germany; FRA, France; FLU, influenza; hMPV, human metapneumovirus; JPN, Japan; KOR, Korea; MEX, Mexico; MYS, Malaysia; RSV, respiratory syncytial virus; USA, United States of America; ZAF, South Africa.
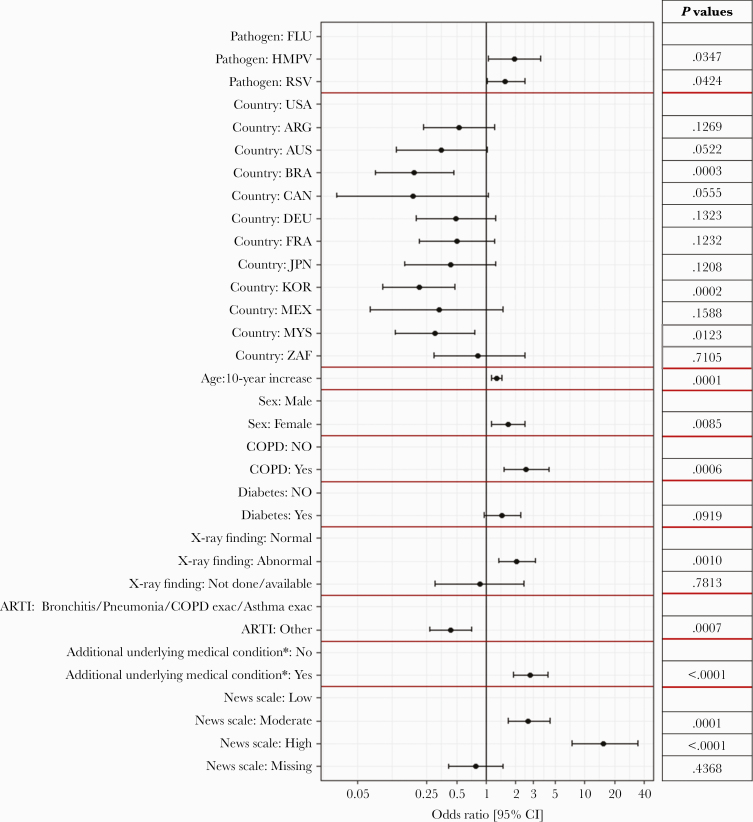
A multivariate logistic regression model showed that the probability of receiving oxygen supplementation during hospitalization was significantly higher for RSV (OR, 1.57; 95% CI, 1.02–2.45) and hMPV participants (OR, 1.93; 95% CI, 1.06–3.59) compared with influenza participants (P=.0424 and P=.0347, respectively). The probability was significantly lower compared with the United States in the following countries: Brazil (OR, 0.19; 95% CI, 0.07–0.47; P=.0003), Korea (OR, 0.21; 95% CI, 0.09–0.48; P=.0002) and Malaysia (OR, 0.31; 95% CI, 0.12–0.77; P=.0123). The probability of receiving oxygen supplementation was significantly higher for every 10-year increase in age (OR, 1.28; 95% CI, 1.13–1.46; P=.0001), for participants with COPD compared with no COPD (OR, 2.54; 95% CI, 1.51–4.37; P=.0006), and for participants with abnormal x-ray findings compared with normal x-ray findings (OR, 2.05; 95% CI, 1.34–3.14; P=.0010) (Figure 4; Supplementary Table 5).
Figure 4.
Multivariate logistic regression model results for the probability of receiving supplemental oxygen during hospitalization. Odds ratios and 95% CIs of the variable included in final model are presented. Rows without estimates are the reference category for that variable. aAdditional underlying medical condition on top of ARTI. Abbreviations: ARG, Argentina; ARTI, acute respiratory tract infection; AUS, Australia; BRA, Brazil; CAN, Canada; DEU, Germany; FRA, France; FLU, influenza; hMPV, human metapneumovirus; JPN, Japan; KOR, Korea; MEX, Mexico; MYS, Malaysia; NEWS, National Early Warning Score; RSV, respiratory syncytial virus; USA, United States of America; ZAF, South Africa.
The results of the Cox proportional hazards model are presented in the Supplementary Data. Overall, conclusions were similar between the Cox proportional hazards model and the multivariate AFT model.
DISCUSSION
HARTI provides global prospective data on risk factors and MRU associated with RSV, hMPV, and influenza during hospitalization and after hospital discharge in adults. This study sheds light on the burden of these respiratory pathogens, regardless of age, as 44.3% patients were <65 years of age. RSV, hMPV, and influenza were diagnosed in 26% of ARTI hospitalizations screened. While influenza remains the most recognized viral respiratory pathogen diagnosed in adults, the results of the HARTI study confirmed the significant MRU associated with RSV and expanded the existing literature regarding MRU associated with hMPV infection. Although many similarities exist for the populations affected and clinical syndromes resulting from infection with influenza and other respiratory viruses, there is now growing evidence that suggests that subtle differences exist that may help target at-risk populations for interventions and support a better understanding of disease pathogenesis. In this study of hospitalized adults, RSV patients were older and had a greater frequency of underlying risk factors, as well as higher NEWS scale at screening, compared with influenza patients. Similar trends were noted for hMPV, though they did not reach statistical significance. Whether due to age or underlying conditions, the outcomes for RSV tended to be worse, with higher needs for supplemental oxygen, in-hospital complications, longer LOS, higher ICU use, and longer length of ICU stay. RSV and hMPV patients also showed greater MRU postdischarge, including hospital readmissions and medical consultations, than influenza patients. Long-term disease sequelae and postdischarge MRU were considerable, including all-cause 3-month readmission (24% overall) and new start of home-based therapy in 21% of COPD patients.
Other authors have noted similar findings when comparing RSV and influenza hospitalizations [10, 23, 24]. Tseng et al. (2020) reported high levels of MRU for RSV patients during hospitalization, with 21.4% of patients requiring ventilation support and 17.9% admitted to the ICU, as well as hospital readmissions within the first 30 days postdischarge exceeding 15% and substantial health care services utilization postdischarge [25]. Akerson et al. (2019) found that RSV infection was associated with greater odds of LOS ≥7 days and ICU admission in adjusted analyses compared with adults hospitalized with influenza [26]. Falsey et al. (1995) showed that RSV patients were more likely to receive therapy for bronchospasm and had a higher death rate than influenza patients [27]. In Bruyndonckx et al. (2020), the odds of having unresolved symptoms after 28 days and illness deterioration were significantly associated with age in RSV patients but not in influenza patients [16]. Furthermore, Sieling et al. (2021) found that RSV and hMPV were both associated with a longer median LOS (4.4 and 4.8 days, respectively) compared with influenza (3.9 days), with higher crude mortality [24]. In the current study, hMPV infections presented similar trends toward higher MRU compared with influenza. Although data are limited, other reports describe similar findings. In a recent study from France, hMPV patients were more frequently aged >65 years and presented with more acute heart failure during hospitalization compared with influenza patients [28].
In the current study, both RSV and hMPV participants had a longer duration of symptoms before hospitalization than participants with influenza. Others have made similar observations, and in a previous study [3], the median length of symptoms before hospital admission was the longest for RSV (6.5 days) in comparison to influenza and hMPV (both 5.0 days). The differences in time to presentation may reflect higher rates of systemic symptoms for influenza patients, leading them to seek health care sooner. The prolonged time to presentation is a significant challenge for testing and implementing early antiviral treatments for influenza, RSV, and hMPV.
Increased age, presence of chronic renal disease, and oxygen supplementation at screening were associated with increased LOS in the multivariate regression model. Similar to other publications [29], country/region influence on LOS was found; LOS was shortest in the United States, Australia, Canada, and Mexico and was longer in all other participating countries, with the longest LOS for Japan and Korea.
After adjusting for possible confounders, the probability of receiving oxygen supplementation during hospitalization was significantly higher for RSV and hMPV compared with influenza and for those with increased age, presence of COPD, and abnormal chest x-ray findings. Country-specific influence on probability of receiving oxygen was observed, with lower probability for Brazil, Korea, and Malaysia, compared with the United States. This trend could be related to the limited availability of medical oxygen in developing countries and different prescription practices. Medical oxygen is more readily available and less expensive in the United States and Europe, as has been highlighted by the recent COVID-19 pandemic [30, 31]. The burden of disease in many countries is still largely unexplored and deserves further study.
Strengths and Limitations
This was a prospective global study with a large number of participants, allowing for the assessment of disease severity and MRU during hospitalization and up to 3 months postdischarge. The study included 44.3% adults aged 18–≤64 years, whereas many previous studies have been conducted in elderly populations. HARTI was a prospective study conducted in 12 countries in both hemispheres over 2 full consecutive epidemic seasons, while other studies, particularly those assessing hMPV, have been limited to 1 country and are often retrospective. Some limitations should be noted: Despite the global design of HARTI, a large proportion of participants were enrolled from the United States (49.9%), limiting conclusions from countries with smaller sample sizes. However, the baseline characteristics of participants enrolled from other countries were similar to those of the United States. Most (88.4%) participants were enrolled on weekdays by protocol and may have differed from participants admitted to the hospital on weekends or holidays. The decision to discharge and provide oxygen supplementation followed the site’s SOC, whereas in the multivariate analysis, the data were pooled at the country level. Additionally, no source data verification cross-check of the clinician-reported questionnaires with the patient’s medical file was performed.
CONCLUSIONS
RSV and hMPV participants had a greater frequency of underlying risk factors, including age >65 years, and substantial MRU during hospitalization and postdischarge compared with influenza participants. RSV participants had the longest length of symptoms before hospitalization, longest median LOS, highest proportion of ICU admission, and a significantly higher probability of receiving oxygen supplementation during hospitalization. Significant differences in LOS were observed by country. The results of the HARTI study expand upon existing literature and highlight the significant MRU associated with RSV and hMPV in hospitalized adults, indicating a need for effective interventions and informing future vaccine and antiviral research agendas.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
HARTI Study Group. Ting Soo Chow, Xavier Duval, Thomas Harrer, Nobuhisa Ishikawa, Odile Launay, Jacob Lee, Analia Mykietiuk, Mozar Neto, Marina Okoshi, Jan Rupp, Dimitar Sajkov, Desmond Samuel, Masaharu Shinkai, Heidi Siebert, Selim Suner, Seong-Heon Wie, IQVIA Real World Solutions (Rupali Naik, Sara Waugh).
Patient consent. Patient written consent was obtained for this study, and the study was approved by local ethical committees.
Financial support. This work was supported by Janssen Pharmaceutica NV.
Potential conflicts of interest. S. House has received consulting fees from Janssen. A. Falsey has received research grants from Pfizer, Mark Sharp and Dohme, Janssen, and BioFire and is on the Data Safety and Monitoring Board for Novavax. J. Witek and S. Keim are J&J stockholders. Y. Vandenijck, X. Ren, D. Kang, S. Kiem, and G. Ispas are employees of Janssen Pharmaceutica NV. E. Walsh has received grants from Merck, Janssen, and Pfizer and was a paid member of the Data Safety and Monitoring Board for GSK. P. Peeters: no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Troeger C, Forouzanfar M, Rao PC, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2016; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widmer K, Zhu Y, Williams JV, et al. . Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 2012; 206:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falloon J, Yu J, Esser MT, et al. . An adjuvanted, postfusion f protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG, Webster RG, Hay A.. Textbook of Influenza. Blackwell Science Ltd; 1998. [Google Scholar]
- 6.Falsey AR, Hennessey PA, Formica MA, et al. . Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Shay DK, Broder K, et al. ; Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 8.Lee N, Lui GC, Wong KT, et al. . High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 2013; 57:1069–77. [DOI] [PubMed] [Google Scholar]
- 9.Walsh EE, Falsey AR.. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets 2012; 12:98–102. [DOI] [PubMed] [Google Scholar]
- 10.Branche AR. Why making a diagnosis of respiratory syncytial virus should matter to clinicians. Clin Infect Dis 2019; 69:204–6. [DOI] [PubMed] [Google Scholar]
- 11.Colosia AD, Yang J, Hillson E, et al. . The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS One 2017; 12:e0182321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas LEM, Thijsen SFT, van Elden L, Heemstra KA.. Human metapneumovirus in adults. Viruses 2013; 5:87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev 2006; 19:546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Hoogen BG, Osterhaus DM, Fouchier RA.. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J 2004; 23:S25–32. [DOI] [PubMed] [Google Scholar]
- 15.Walsh EE, Peterson DR, Falsey AR.. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 2008; 168:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruyndonckx R, Coenen S, Butler C, et al. ; GRACE project group. Respiratory syncytial virus and influenza virus infection in adult primary care patients: association of age with prevalence, diagnostic features and illness course. Int J Infect Dis 2020; 95:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt H, Das A, Nam H, et al. . Epidemiology and outcomes of hospitalized adults with respiratory syncytial virus: a 6-year retrospective study. Influenza Other Respir Viruses 2019; 13:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirolos A, Christides A, Xian S, et al. . A landscape review of the published research output relating to respiratory syncytial virus (RSV) in North & Central America and Europe between 2011-2015. J Glob Health 2019; 9:010425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royal College of Physicians. National Early Warning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party. RCP; 2012. [Google Scholar]
- 20.National Health Service. National Early Warning Score (NEWS). 2017. Available at: https://www.england.nhs.uk/ourwork/clinical-policy/sepsis/nationalearlywarningscore/. Accessed 11 August 2021.
- 21.Kalbfleisch J, Prentice R.. The Statistical Analysis of Failure Time Data. 2nd ed. Wiley; 2002. [Google Scholar]
- 22.Keiding N, Andersen PK, Klein JP.. The role of frailty models and accelerated failure time models in describing heterogeneity due to omitted covariates. Stat Med 1997; 16:215–24. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Han X, Bai L, Zhang J.. Clinical characteristics and outcomes in adult patients hospitalized with influenza, respiratory syncytial virus and human metapneumovirus infections. Expert Rev Anti Infect Ther 2021; 19:787–96. [DOI] [PubMed] [Google Scholar]
- 24.Sieling WD, Goldman CR, Oberhardt M, et al. . Comparative incidence and burden of respiratory viruses associated with hospitalization in adults in New York City. Influenza Other Respir Viruses 2021; 15:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng HF, Sy LS, Ackerson B, et al. . Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis 2020; 222:1298–310. [DOI] [PubMed] [Google Scholar]
- 26.Ackerson B, Tseng HF, Sy LS, et al. . Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis 2019; 69:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falsey AR, Cunningham CK, Barker WH, et al. . Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 1995; 172:389–94. [DOI] [PubMed] [Google Scholar]
- 28.Loubet P, Mathieu P, Lenzi N, et al. Characteristics of human metapneumovirus infection in adults hospitalized for community-acquired influenza-like illness in France, 2012-2018: a retrospective observational study. Clin Microbiol Infect 2021; 27:127.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milenkovic M, Russo CA, Elixhauser A.. Hospital stays for influenza, 2004. HCUP Statistical Brief #16. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 30.Duke T, Graham SM, Cherian MN, et al. ; Union Oxygen Systems Working Group. Oxygen is an essential medicine: a call for international action. Int J Tuberc Lung Dis 2010; 14:1362–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins K, Isah A.. Covid-19 has turned the spotlight on the uneven provision of oxygen—a stark health inequity. The BMJ Opinion. 11 December 2020. Available at: https://blogs.bmj.com/bmj/2020/12/11/covid-19-has-turned-the-spotlight-on-the-uneven-provision-of-oxygen-a-stark-health-inequity/. Accessed 13 January 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.