Conflict of interest
The authors declare that there is no conflict of interest.
Funding source
No funding has supported this work.
Dear Editor,
We report the first cases of Varicella‐zoster viral (VZV) infection after AZD1222 and BNT162b2 coronavirus disease 2019 (COVID‐19) mRNA vaccines in Greece. As the World Health Organization declared the COVID‐19 as a pandemic, the production of a safe and effective vaccine COVID‐19 became a global priority. 1 In December 2020, the European Medicines Agency first approved the BNT162b2 (Pfizer‐BioNTech COVID‐19 mRNA) vaccine and the AZD1222 (Oxford/AstraZeneca (University of Oxford, Oxford, UK) COVID‐19) vaccine. 2 There is a great variety of cutaneous reactions after COVID‐19 vaccination, 1 , 2 with only a few cases of Varicella‐zoster viral infection (VZV) reported. 3 , 4 , 5 , 6 , 7 , 8 , 9 Given the importance of widespread vaccination, recognition and understanding of these novel vaccines' adverse events are crucial. In this brief report, we present a case series of VZV infection after AZD1222 and BNT162b2 COVID‐19 mRNA COVID‐19 vaccination in Heraklion, Crete, Greece.
A retrospective case‐series study was performed at the Dermatology Department at the University Hospital of Heraklion in Heraklion, Crete, Greece from 1st of January 2021 until 15th of July 2021 regarding patients who attended Accident and Emergency (A&E) after developing herpes zoster (HZ) infection after COVID‐19 vaccination, to assess clinical features and timing of VZV infection after COVID‐19 vaccines.
From 1st of January 2021 until 15th of July 2021, 11 patients attended A&E Department at the University Hospital of Heraklion in Heraklion, Crete, Greece, who developed HZ viral (VZV) viral infection after COVID‐19 vaccination. There were six (6/11, 54.5%) females and five (5/11, 45.5%) males. The mean age of the patients was 67 years (SD ± 7.899). Eight patients developed VZV after the second dose of Pfizer vaccine, one patient developed VZV after the second dose of AstraZeneca vaccine (Fig. 1), and two patients developed VZV after the first dose of Pfizer vaccine. Both of these patients who developed VZV after the first dose of Pfizer vaccine had after three weeks the second dose of Pfizer vaccine with no further complications. The mean latency period till symptoms' onset was 7. Ninety‐one days (SD ± 4.86) and the mean latency period until vesicular eruptions onset was 11.09 days (SD ± 5.41). None of the patients was immunosuppressed and all of them received treatment with oral antiviral for seven days with good response.
Figure 1.
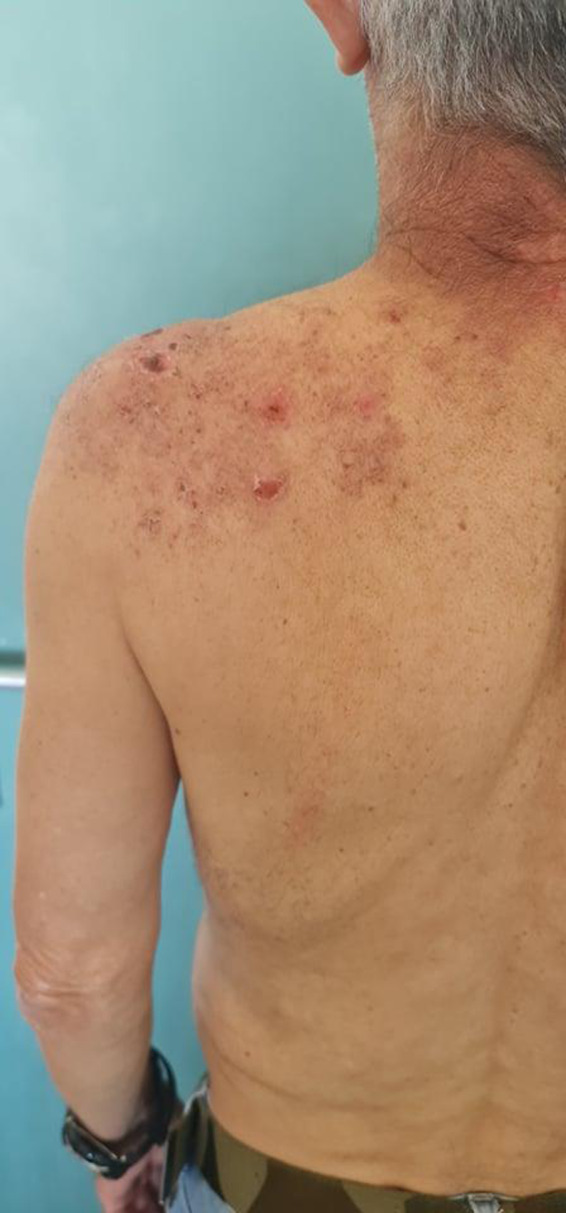
Vesicles, erosions and erythematous plaques in clusters on the left side of the upper back and arm on a male patient after AZD1222 vaccination, evocative of a herpes zoster viral (VZV) infection.
Here, we have reported a case series of VZV reactivation after AZD1222 and BNT162b2 COVID‐19 mRNA vaccines. In our case series, two patients developed VZV after the first dose of Pfizer vaccine and both were proceeded to the second dose of vaccine without any complications. Limitations of this study consist that this case series was from a single centre in Greece during a short period of time. In the literature, there are only few reports of VZV reactions after COVID‐19 vaccines. 4 , 5 , 6 , 7 , 8 , 9 , 10 In a study from Spain, VZV and herpes simplex virus (HSV) reactivations accounted for 13.8% of reactions. 1
The exact pathophysiology underlying cutaneous effects after AZD1222 and BNT162b2 COVID‐19 mRNA vaccines have still to be elucidated, and further prospective larger studies are needed. Nevertheless, even though VZV reactivation is rare, medical professionals should pay close attention to the possible adverse effects of the COVID‐19 vaccines.
Acknowledgement
The patients in this manuscript have given written informed consent to publication of their case details.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Català A, Muñoz‐Santos C, Galván‐Casas C et al. Cutaneous reactions after SARS‐CoV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol 2021. 10.1111/bjd.20639. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol 2021; 20: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 4. Lee C, Cotter D, Basa J, Greenberg HL. 20 Post‐COVID‐19 vaccine‐related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol 2021; 20: 1960–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aksu SB, Öztürk GZ. A rare case of shingles after COVID‐19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res 2021; 10: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tessas I. Kluger N Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35 (10):e620–e622. 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez‐Jiménez P, Chicharro P, Cabrera LM et al. Varicella‐zoster virus reactivation after SARS‐CoV‐2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep 2021; 12: 58–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of Varicella Zoster virus after vaccination for SARS‐CoV‐2. Vaccines (Basel) 2021; 9: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID‐19 vaccine. J Med Virol 2021; 93: 5231–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021; 35(9): e548–e551. 10.1111/jdv.17343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


