Abstract
Accumulating evidence shows a progressive decline in the efficacy of coronavirus disease 2019 (COVID‐19) (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) messenger RNA (mRNA) vaccines such as Pfizer‐BioNTech (mRNA BNT161b2) and Moderna (mRNA‐1273) in preventing breakthrough infections due to diminishing humoral immunity over time. Thus, this review characterizes the kinetics of anti‐SARS‐CoV‐2 antibodies after the second dose of a primary cycle of COVID‐19 mRNA vaccination. A systematic search of the literature was performed and a total of 18 articles (N = 15 980 participants) were identified and reviewed. The percent difference of means of reported antibody titers was then calculated to determine the decline in humoral response after the peak levels postvaccination. Findings revealed that the peak humoral response was reached at 21–28 days after the second dose, after which serum levels progressively diminished at 4–6‐month postvaccination. Additionally, results showed that regardless of age, sex, serostatus, and presence of comorbidities, longitudinal data reporting antibody measurement exhibited a decline of both anti‐receptor binding domain immunoglobulin G (IgG) and anti‐spike IgG, ranging from 94% to 95% at 90–180 days and 55%–85% at 140–160 days, respectively, after the peak antibody response. This suggests that the rate of antibody decline may be independent of patient‐related factors and peak antibody titers but mainly a function of time and antibody class/molecular target. Hence, this study highlights the necessity of more efficient vaccination strategies to provide booster administration in attenuating the effects of waning immunity, especially in the appearance of new variants of concerns.
Keywords: COVID‐19, Moderna, mRNA 1273, mRNA BNT162b2, Pfizer‐BioNTech, vaccines
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic claimed millions of lives worldwide and remains an unprecedented challenge to global public health. 1 , 2 Despite the increasing availability of therapeutics against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, mass vaccination is the mainstay for limiting new infections, reinfections, breakthrough infections, and unwanted sequelae from COVID‐19. 3 , 4 , 5 , 6 Although nationwide immunization programs have already been implemented in most countries, the mass production, allocation, and accessibility to COVID‐19 vaccines remain a hurdle, especially in low‐income countries where administration of primary and adjunctive doses could be challenging. 7
Among different types of COVID‐19 vaccines, the utilization of messenger RNA (mRNA)‐based vaccines is unprecedented. 8 Despite the efficacy of mRNA vaccines in reducing the risk of developing severe COVID‐19 illness, ample evidence showed the progressive decline in their efficacy for preventing SARS‐CoV‐2 infections. 9 Such vulnerability is correlated to the significant decline in anti‐SARS‐CoV‐2 antibodies (Abs) over time. 10 , 11 , 12 This waning humoral response could lead to breakthrough infections, thus highlighting the potential role of serologic testing to assess vaccine immunogenicity and protective efficacy. Although the role of T cells and B memory cells from vaccine‐induced immunity has yet to be fully elucidated, multiple studies showed that Ab levels directly correlate with the risk of vaccine reinfection and breakthrough, with low levels of anti‐SARS‐CoV‐2 immunoglobulin G (IgG) in patients with infections correlating with high viral load and duration of viral shedding. 3 , 4 In addition, the emergence of the new SARS‐CoV‐2 Omicron variant (B.1.1.529), with potential for high immune escape, highlights the necessity for vaccine boosters and high Ab titers for an improved immunoprotection. 13 Thus, to better understand the rate of decay of anti‐SARS‐CoV‐2 Abs through time, this rapid systematic review was designed to analyze humoral response within a 6‐month period among recipients of the Pfizer‐BioNTech or Moderna COVID‐19 mRNA‐based vaccines to provide further evidence on the necessity of boosting vulnerable populations.
2. METHODS
2.1. Search strategy and eligibility criteria
A systematic literature search was conducted to identify studies reporting the decline of the humoral response of individuals post‐mRNA vaccination. As shown in Figure 1, a comprehensive search was carried out in PubMed, Cochrane CENTRAL, Google Scholar, Science Direct, medRxiv, and Research Square for articles published from January 2021 to November 30, 2021. The search keywords used included “SARS‐CoV‐2,” “COVID‐19,” “declining humoral response,” “post‐vaccination,” “mRNA vaccine” “Pfizer‐BioNTech,” “Moderna,” “mRNA‐1273,” and “mRNA BNT162b2” which resulted in 51 journal articles.
Figure 1.

Screening and appraisal of journal articles for inclusion in the systematic review
For the inclusion criteria, articles reporting the following data were considered: (1) all participants who received a primary vaccination cycle with two complete doses of Pfizer‐BioNTech (mRNA BNT162b2) or Moderna (mRNA‐1273), (2) with at least a record of 10 patients and above (3) individual IgG or IgA, total Ig, or neutralizing anti‐SARS‐CoV‐2 antibody titers, (4) quantitative or semi‐quantitative antibody tests, (6) articles reporting humoral response 4–8‐month postvaccination, (7) articles available in the English language, and (8) randomized controlled, cohort, preprint or published papers and (9) providing complete and extractable data given the limited papers available for this novel disease and the mRNA vaccine.
The exclusion criteria were: (1) participants who received a single dose of mRNA vaccine only, (2) participants who received other types of vaccines such as Sinovac, AstraZeneca, Johnson & Johnson, Novavax, and Sanofi‐GSK, (3) articles reporting humoral response <4‐month postvaccination, and (4) anti‐SARS‐CoV‐2 immunoglobulin M (IgM) response and cell‐mediated immunity; the former because it is now increasingly clear that IgM plays a minor role against COVID‐19, as it has lower sensitivity (64%), specificity (99%), and accuracy (94%) compared to IgG (93%, 100%, and 98%, respectively). 14 IgM antibodies were also found to decline early (i.e., at Day 20 postvaccination) and have lower neutralizing potential. 15 The latter because we only focused on humoral immunity due to limited data and lack of standardization of available cell‐mediated immunity assays, hence imposing difficulty in data analysis.
2.2. Data extraction
Data were extracted independently from each article by two authors into a spreadsheet. A third author checked the extracted data for completeness and accuracy. Any disagreements were resolved by consensus among the authors.
Descriptive and outcome data were extracted from the included studies, including the type of vaccine, country of origin, sample size, age range or median age of the population, type of serologic test employed and its manufacturing company, analyzer used, immunoglobulin measured, and molecular target of immunoglobulin. In addition, serial or nonserial serologic measurement determination was done, to differentiate which study performed better patient follow‐up, thus reducing methodologic bias due to greater accountability for interindividual variability despite generally lower sample size for serial serologic sampling. Additional data were requested from the original study authors when necessary.
2.3. Data analysis
All data reported in this study were in reference to the peak humoral response after a primary vaccination cycle with two doses. However, due to significant heterogeneity in the assays used to probe antibody titers, the antibody measurements reported in the articles were standardized using the percent difference of means. This shows the absolute value of the ratio of the difference between two groups, (groups A and B which pertain to antibody titer measurements on the peak and after the peak of humoral response) and their average, expressed as a percentage to enable standard comparison of these data regardless of their units of measurements and the diagnostic tools used for their quantification. It is computed using the formula below:
Group A—Antibody titer on the peak humoral response; Group B—Antibody titer after the peak humoral response
Therefore, values are reported as a percentual decrease from the peak. Time points were also standardized to represent the number of days after the second vaccine dose.
2.4. Scope and limitations
The demographic parameters used in this study were limited to age, sex, serostatus, and comorbidities such as hemodialysis or chronic kidney disease, immunologic disorders, metabolic derangements including heart disease, hypertension, and diabetes mellitus. It also includes a general section wherein no specific parameter was identified in determining the percent decrease in the humoral response. These factors were analyzed independently of each other.
Moreover, the authors utilized all available preprint and published journal articles on this topic written through November 30, 2021, which included vaccination time points from Days 0 to 201 of either Pfizer‐BioNTech (mRNA BNT162b2) or Moderna (mRNA‐1273) in demonstrating the decline of the humoral response. These vaccines were only discussed in this systematic review as they were the leading vaccine utilized worldwide and due to the wide range of available resources at the time of writing.
A quantitative meta‐analysis was not done because of the heterogeneity determined between studies, as well as between different immunoassays used in each study, their units, sensitivities/specificities, and antigenic targets, not appropriate to perform a reliable and sensible pooled analysis. Thus, percent (%) differences in titers between groups were calculated, as an attempt to standardize the antibody data and enable a combined evaluation of different studies.
Last, we did not evaluate cell‐mediated immunity and the kinetics of T cells after vaccination. The measurement of T‐cell responses against SARS‐CoV‐2 is complex and lacks standardization to allow for comparisons in the literature, but is a priority topic for future research. Nonetheless, Gilbert et al. 16 demonstrated that the majority (68.5%) of vaccine efficacy is mediated by neutralizing antibody responses, with the remaining 31.5% of vaccine efficacy may be attributed to cell‐mediated responses, other functional antibodies, and innate immunity. These findings are in agreement with studies demonstrating the relationship between neutralizing antibody titers and breakthrough infections. 3 , 4
3. RESULTS
Eighteen articles with a total population of N = 15 980 participants were selected in this systematic review in which 14 studies focused on Pfizer‐BioNTech (n = 13 364), 2 on Moderna (n = 234), and 2 included both Pfizer‐BioNTech and Moderna (n = 2382). These were further divided into four categories, humoral immunity post second dose vaccination influenced by age, sex, baseline serostatus, and presence of comorbidities. Thirteen articles were included in the general section, four under the age category, four under sex, four under serostatus, and three articles under comorbidities. For the sex category, a total of 3128 males and 6237 females were included in the analysis. Meanwhile, the comorbidities cohort was further grouped according to the reported condition. Three studies reported on hemodialysis or end‐stage renal disease, one study reported on heart disease and hypertension, one for diabetes mellitus, and one for immunologic disorders. It is also worth noting that articles under these categories were nonexclusive, as most studies included more than one factor in their analysis. With respect to the geographical distribution, seven studies were from Italy, three studies from the United States, three from Israel, two from Belgium, and one each from Japan, Estonia, and Austria. Of the reviewed articles that conducted serial study sampling, the largest sample size was from Italy (n = 787).
Tables 1, 2, 3, 4, 5 provide a summary of the following trends observed in this study. In general, there is a substantial decrease of Ab titers against SARS‐CoV‐2 following 6‐month post second dose mRNA vaccination (Figure 2); however, the amount and rate of decline of the Ab titers varied according to the factors analyzed. Participants exhibited up to 95% decline in Ab levels at least 120 days after the peak rise of the IgG titers post‐mRNA vaccination. In studies reporting longitudinal data on Ab measures, an observed decline from peak levels of anti‐RBD IgG ranged from 94% to 95% at 90–180 days post peak, while the anti‐spike IgG showed a decline that ranged from 55% to 85% at 140–160 days after the peak (Figure 2). With respect to the vaccine brand, Pfizer‐BioNTech displayed a 90%–94% decline of anti‐RBD IgG at 150–161 days following the peak and a decline of IgG anti‐spike protein ranging from 55% to 95% at 140–180 days after the peak. Meanwhile, Moderna exhibited a 69%–96% decrease in anti‐RBD IgG at 150–174 days from the peak, and a 45% decline in antis‐pike IgG 90 days posttiter peak.
Table 1.
Declining humoral response post second dose administration of SARS‐CoV‐2 mRNA vaccine
| Author | Vaccine | Country of origin | Sample size | Mean/median age | Immunoglobulin measurement | Serial/nonserial serologic measurement | Reported values | % Difference from peak |
|---|---|---|---|---|---|---|---|---|
| Campo et al 10 | Pfizer‐BioNTech | Italy | Healthcare workers: 274 | Median (range) | Diasorin, LIAISON, IgG, S1/S2 | Serial | Geometric means and 95% confidence Intervals | D28 |
| There is 9% decrease 21 days after the peak rise of IgG titer on Day 7 after full vaccinationD61There is 41% decrease 54 days after the peak rise of IgG titer on Day 7 after full vaccination | ||||||||
| Male: 99 | 46.1 years | D147 | ||||||
| Female: 175 | (23–69 years) | There is 55% decrease 140 days after the peak rise of IgG titer on Day 7 after full vaccination | ||||||
| Salvagno et al. 12 | Pfizer‐BioNTech | Italy | Healthcare workers: 787 | Mean age: 44 ± 12 years | Roche, Elecsys, total Ig, RBD | Serial | Medians and interquartile ranges | D90 |
| <65 years: 754 | There is 38% decrease 60 days after the peak rise of IgG titer on Day 30 after full vaccination | |||||||
| ≥65 years: 33 | D180 | |||||||
| Male: 268 | There is 55% decrease 150 days after the peak rise of IgG titer on Day 30 after full vaccination | |||||||
| Female: 519 | ||||||||
| Ponticelli et al. 17 | Pfizer‐BioNTech | Italy | Healthcare workers: 162 | Mean age: 42.5 ± 11.9 years | SNIBE, MAGLUMI, IgG, RBD | Serial | Medians and interquartile ranges | D180 |
| Male: 68 | ||||||||
| Female: 94 | There is 90% decrease 150 days after the peak rise of IgG titer on Day 30 after full vaccination | |||||||
| Salvagno et al. 18 | Pfizer‐BioNTech | Italy | Baseline SARS‐CoV‐2 | Median age: 42 years | Euroimmun, IgA, spike S1 subunit | Serial | Medians and interquartile ranges | D180 |
| Seronegative | ||||||||
| Healthcare workers: 97 | IQR: 31–52 years | There is 71% decrease 150 days after the peak rise of IgA titer on Day 30 after full vaccination | ||||||
| Kato et al. 19 | Pfizer‐BioNTech | Japan | Healthcare workers: 98 | Median age: 43 years | Tosoh, AIA‐CL, IgG, nucleocapsid/spike | Serial | Geometric means and 95% confidence intervals | D201 |
| Male: 24 | IQR: 38–49 years | |||||||
| Female: 74 | There is 93% decrease 180 days after the peak rise of IgG titer on Day 21 after full vaccination | |||||||
| Naaber et al. 20 | Pfizer‐BioNTech | Estonia | Laboratory employees: 122 | Median age: 34 years | Abbott, IgG, RBD | Serial | Medians and interquartile ranges | D42 |
| There is 48% decrease 35 days after the peak rise of IgG titer on Day 7 after full vaccination | ||||||||
| D84 | ||||||||
| There is 79% decrease 87 days after the peak rise of IgG titer on Day 7 after full vaccination | ||||||||
| Male: 21 | Range: 21–69 years | D180 | ||||||
| Female: 101 | There is 94% decrease 161 days after the peak rise of IgG titer on Day 7 after full vaccination | |||||||
| Laing et al. 21 | Pfizer‐BioNTech | USA | Healthcare workers: 187 | Mean age: 42.8 years | LakePharma, Multiplex microsphere‐based immunoassay, IgG, nucleocapsid/spike | Serial | Geometric meansand 95% confidence intervals | D180 |
| Male: 56 | IQR: 21–69 years | |||||||
| Female: 131 | There is 77% decrease 150 days after the peak rise of IgG titer on Day 30 after full vaccination | |||||||
| Israel et al. 22 | Pfizer‐BioNTech | Israel | 2653 | Mean age: 56.45 ± 15.87 years | Abbott, Alinity i, IgG, RBD | Nonserial | Medians and interquartile ranges | D30–59 |
| There is 48% decrease 30 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| D60–89 | ||||||||
| There is 78% decrease 60 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| D90–119 | ||||||||
| There is 87% decrease 90 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| D120–149 | ||||||||
| There is 89% decrease 120 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| D150–179 | ||||||||
| There is 92% decrease 150 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| D180+ | ||||||||
| There is 95% decrease 180 days after the peak rise of total antibody titer on Days 0–29 after full vaccination | ||||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | 8395 | No data | Abbott, Quant II,IgG, RBD | Nonserial | Means and 95% confidence intervals | D30–59 |
| There is 42% decrease 30 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| D60–89 | ||||||||
| There is 78% decrease 60 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| D90–119 | ||||||||
| There is 87% decrease 90 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| D120–149 | ||||||||
| There is 89% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| D150–179 | ||||||||
| There is 92% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| D 180+ | ||||||||
| There is 95% decrease 180 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||||
| Salvagno et al. 24 | Pfizer‐BioNTech | Italy | 86 | Median age: 45 years | DiaSorin, LIAISON, IgG, spike trimeric | Serial | Medians and interquartile ranges | D180 |
| IQR: 31–53 years | New Industries Biomedical Engineering Co., LTD, MAGLUMI, IgG, RBD | There is 83% and 92% decrease 150 days afterthe peak rise of anti‐spike trimeric IgG and RBD IgG titers, respectively, on Day 30 after full vaccination | ||||||
| Khoury et al. 25 | Pfizer‐BioNTech | Israel | Healthcare workers: 100 | Mean age: | Abbot's © quant assay, IgG, RBD | Serial | Medians | D60 |
| and ranges | There is 56% decrease 30 days after the peak rise of total antibody titer on Day 30 after full vaccination | |||||||
| D90 | ||||||||
| Male: 55 | ||||||||
| Female: 45 | There is 88% decrease 60 days after the peak rise of total antibody titer on Day 30 after full vaccination | |||||||
| 40.7 ± 13.7 years | ||||||||
| D120 | ||||||||
| There is 94% decrease 90 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||||
| Ciabattini et al. 26 | Pfizer‐BioNTech | Italy | 145 | Mean age: 48.8 ± 14.3 years | In‐house ELISA, IgG, S1/S2 | Nonserial | Geometric means and 95% confidence Intervals | D97 |
| There is 65% decrease 90 days after the peak rise of anti‐spike IgG titer on Day 7 after full vaccination | ||||||||
| D167 | ||||||||
| There is 85% decrease 160 days after the peak rise of anti‐spike IgG titer on Day 7 after full vaccination | ||||||||
| Brisotto et al. 27 | Pfizer‐BioNTech & Moderna | Italy | 516 | Median age: 46.5 years | SNIBE, MAGLUMI, IgG, RBD | Serial | Medians and interquartile ranges | D20 |
| Male: 145 | IQR: 36–55 years | |||||||
| Female: 371 | There is 83% decrease 90 days after the peak rise of IgG titer on Day 30 after full vaccination |
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Effect of age in declining humoral response post second dose administration of SARS‐CoV‐2 mRNA vaccine
| Author | Vaccine | Country of origin | Sample size | Mean/median age | Immunoglobulin measurement | Serial/nonserial serologic measurement | % Difference from peak |
|---|---|---|---|---|---|---|---|
| Salvagno et al. 12 | Pfizer‐BioNTech | Italy | Healthcare workers: 787 | Mean age: 44 ± 12 years | Roche Elecsys, total Ig, RBD | Serial | D180 |
| <65 years | |||||||
| Male: There is 52% decrease 150 days after the peak rise of total antibody titer on day 30 after full vaccination | |||||||
| Female: There is 56% decrease 150 days after the peak rise of total antibody titer on day 30 after full vaccination | |||||||
| <65 years: 754 | ≥65 years | ||||||
| ≥65 years: 33 | Male: There is 56% decrease 150 days after the peak rise of total antibody titer on day 30 after full vaccination | ||||||
| Male: 268 | Female: There is 52% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Female: 519 | |||||||
| Salvagno et al. 24 | Pfizer‐BioNTech | Italy | 86 | Median age: 45 years | Diasorin, LIAISON, IgG, spike trimeric | Serial | D180 |
| Male: 41 | IQR: 31–53 years | <60 years: There is 83% and 81% decrease 150 days after the peak rise of anti‐spike trimeric IgG and RBD IgG titers, respectively, on day 30 after full vaccination | |||||
| Female: 45 | New Industries Biomedical Engineering Co., LTD, MAGLUMI, IgG, RBD | ≥60 years: There is 88% and 93% decrease 150 days after the peak rise of anti‐spike trimeric IgG and RBD IgG titers, respectively, on day 30 after full vaccination | |||||
| Doria‑Rose et al. 28 | Moderna | USA | Healthy adult participants: 33 | No data | Anti‐SARS‐CoV‐2, IgG, RBD | Serial | D180 |
| 18–55 years: There is 83% decrease 167 days after the peak rise of IgG titer on day 14 after full vaccination | |||||||
| 56–70 years: There is 96% decrease 174 days after the peak rise of IgG titer on day 7 after full vaccination | |||||||
| ≥71 years: There is 93% decrease 174 days after the peak rise of IgG titer on day 7 after full vaccination | |||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | 8395 | No data | Abbott, Quant II, IgG, RBD | Nonserial | D120–150 |
| <60 years old: There is 89% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| D150+ | |||||||
| <60 years old: There is 92% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| D120–150 | |||||||
| ≥60 years old: There is 87% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| Age in years <18: 92 | D150+ | ||||||
| 18–44: 2199 | ≥60 years old: There is 86% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||
| 45–59: 3016 | |||||||
| 60–74: 2515 | |||||||
| 75+ : 573 |
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Effect of sex in declining humoral response post second dose administration of SARS‐CoV‐2 mRNA vaccine
| Author | Vaccine | Country of origin | Sample size | Mean/median age | Immunoglobulin measurement | Serial/nonserial serologic measurement | % Difference from Peak |
|---|---|---|---|---|---|---|---|
| Salvagno et al. 12 | Pfizer‐BioNTech | Italy | Healthcare workers: 787 | Mean age: 44 ± 12 years | Roche Elecsys, total Ig, RBD | Serial | D180 |
| <65 years | |||||||
| Male: There is 52% decrease 150 days after the peak rise of total antibody titer on day 30 after full vaccination | |||||||
| Female: There is 56% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | |||||||
| <65 years: 754 | ≥65 years | ||||||
| ≥65 years: 33 | Male: There is 56% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Male: 268 | Female: There is 52% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Female: 519 | |||||||
| Salvagno et al. 18 | Pfizer‐BioNTech | Italy | Baseline SARS‐CoV‐2 seronegativehealthcare workers: 97 | Median age: 42 years | Euroimmun, IgA, spike S1 subunit | Serial | D180 |
| Males: 45 | IQR: 31–52 years | Males: There is 72% decrease 150 days after the peak rise ofIgA titer on Day 30 after full vaccination | |||||
| Females: 52 | Females: There is 71% decrease 150 days after the peak rise ofIgA titer on Day 30 after full vaccination | ||||||
| Salvagno et al. 24 | Pfizer‐BioNTech | Italy | 86 | Median age: 45 years | Diasorin, LIAISON, IgG, spike trimeric | Serial | D180 |
| Male: 41 | IQR: 31–53 years | Male: There is 84% and 92% decrease 150 days after the peak rise of anti‐spike trimeric IgG and RBD IgG titers, respectively, on Day 30 after full vaccination | |||||
| Female: 45 | New Industries Biomedical Engineering Co., LTD, MAGLUMI, IgG, RBD | Female: There is 83% and 93% decrease 150 days after the peak rise of anti‐spike trimeric IgG and RBD IgG titers, respectively, on Day 30 after full vaccination | |||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | 8395 | No data | Abbott, Quant II, IgG, RBD | Nonserial | D120–150 |
| Males: There is 86% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| Females: There is 88% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| D150+ | |||||||
| Males: 2774 | Males: There is 89% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||
| Females: 5621 | Females: There is 91% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination |
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Table 4.
Effect of serostatus in declining humoral response post second dose administration of SARS‐CoV‐2 mRNA vaccine
| Author | Vaccine | Country of origin | Sample size | Mean/median age | Immunoglobulin measurement | Serial/nonserial serologic measurement | % Difference from peak |
|---|---|---|---|---|---|---|---|
| Campo et al. 10 | Pfizer‐BioNTech | Italy | Healthcare workers: 274 | Median (range) | Diasorin, LIAISON, IgG, S1/S2 | Serial | D147 |
| Male: 99 | 46.1 (23–69) years | Seronegative: There is 60% decrease in IgG titer 140 days after the peak rise of IgG titer on Day 7 after full vaccination | |||||
| Female: 175 | Seropositive: There is 68% decrease in IgG titer 140 days after the peak rise of IgG titer on Day 7 | ||||||
| Salvagno et al. 12 | Pfizer‐BioNTech | Italy | Healthcare workers: 787 | Mean age 44 ± 12 years | Roche Elecsys, total Ig, RBD | Serial | D180 |
| Baseline seronegative: 624 | Seronegative: There is 52% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Baseline seropositive: 163 | Seropositive: There is 74% decrease 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Bayart et al. 29 | Pfizer‐BioNTech | Belgium | Laboratory employees: 217 | Male | Abbott Architect, IgG, RBD | Serial | D159 |
| Mean age: 43 years | |||||||
| Range: 23–64 years | Seronegative: There is 90% decrease in IgG titer 152 days after the peak rise of IgG titer on day 7 after full vaccination | ||||||
| Female | Seropositive: There is 79% decrease in IgG titer 138 days after the peak rise of IgG titer on day 21 after full vaccination | ||||||
| Male: 61 | Mean age: 43 years | ||||||
| Female: 170 | Range: 23–66 years | ||||||
| Tré‐Hardy et al. 30 | Moderna | Belgium | Healthcare workers: 201 | Median age: 50.1 (46.9–52.4) years | LIAISON®, IgG,S1 and S2 subunit | Serial | D159 |
| Seronegative: There is 45% decrease 90 days after the peak rise of IgG titer on day 69 after full vaccination | |||||||
| Seropositive: IgG titer remains the same even 90 days after the peak rise on day 69 after full vaccination |
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Table 5.
Effect of comorbidities in declining humoral response post second dose administration of SARS‐CoV‐2 mRNA vaccine
| Author | Vaccine | Country of origin | Sample size | Mean/median age | Immunoglobulin Measurement | Serial/nonserial serologic measurement | % Difference from peak |
|---|---|---|---|---|---|---|---|
| Hemodialysis and kidney disease | |||||||
| Hsu et al. 31 | Pfizer‐BioNTech& Moderna | USA | Dialysis patients: 1866 | Mean age ± SD | ADVIA Centaur®, IgG, RBD | Nonserial | Pfizer‐BioNTech |
| D120 | |||||||
| There is 86% decrease in IgG titer 90 days after the peak rise of total antibody titer on Day 30 after full vaccination | |||||||
| D180 | |||||||
| There is 93.5% decrease in IgG titer 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | |||||||
| Moderna | |||||||
| D120 | |||||||
| There is no decrease in IgG titer 90 days after the peak rise of total antibody titer on day 30 after full vaccination | |||||||
| Male: 1084 | 63.9 ± 13.7 years | D180 | |||||
| Female: 782 | There is 69% decrease in IgG titer 150 days after the peak rise of total antibody titer on Day 30 after full vaccination | ||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | Without chronic kidney disease (CKD): 7715 | No data | Abbott, Quant II, IgG, RBD | Nonserial | With CKD |
| D120–150 | |||||||
| There is 87% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| D150+ | |||||||
| With chronic kidney disease (CKD): 680 | There is 72% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||
| Davidovic et al. 32 | Pfizer‐BioNTech | Austria | Hemodialysis patients: 41 | Mean ± SD | Diasorin, LIAISON, IgG, spike protein trimer | Serial | All |
| D180 | |||||||
| There is 92% decrease 136 days after the peak rise in IgG titers at Day 42 after full vaccination | |||||||
| Positive seroconversion | |||||||
| D180 | |||||||
| There is 91% decrease 136 days after the peak rise in IgG titers at Day 42 after full vaccination | |||||||
| No seroconversion | |||||||
| Positive seroconversion 6 months after vaccination: 27 | 67.3 ± 15.5 years | D180 | |||||
| No seroconversion 6 months after vaccination: 14 | There is 86% decrease 136 days after the peak rise in IgG titers at Day 42 after full vaccination | ||||||
| Heart disease and hypertension | |||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | Without heart disease: 7790 | No data | Abbott, Quant II, IgG, RBD | Nonserial | With heart disease |
| D120–150 | |||||||
| There is 91% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| D150+ | |||||||
| With heart disease: 605 | There is 79% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | ||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | Without hypertension: 5960 | No data | Abbott, Quant II, IgG, RBD | Nonserial | With hypertension |
| D120–150 | |||||||
| There is 86% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| With hypertension: 2435 | D150+ | ||||||
| There is 86% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| Diabetes mellitus | |||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | Without diabetes: 7342 | No data | Abbott, Quant II, IgG, RBD | Nonserial | With diabetes mellitus |
| D120–150 | |||||||
| There is 90% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| With diabetes: 1053 | D150+ | ||||||
| There is 84% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| Immunologic disorders | |||||||
| Kertes et al. 23 | Pfizer‐BioNTech | Israel | Without immunosuppressive disorder: 7411 | No data | Abbott, Quant II, IgG, RBD | Nonserial | With immunosuppressive disorder |
| D120–150 | |||||||
| There is 85% decrease 120 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
| With immunosuppressive disorder: 984 | D150+ | ||||||
| There is 73% decrease 150 days after the peak rise of total antibody titer on Days 7–29 after full vaccination | |||||||
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
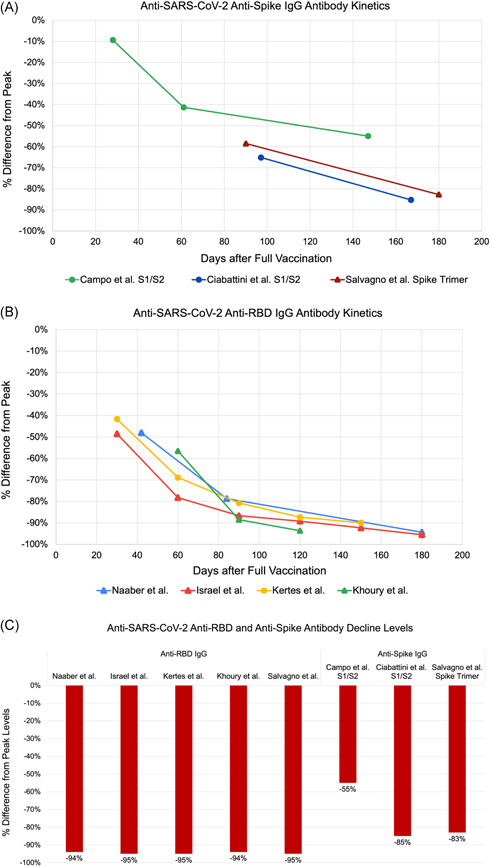
Kinetics of antibodies in studies reporting longitudinal data showing percentage decline from peak titer after vaccination for anti‐spike (A) and anti‐RBD (B) IgG antibodies. (C) Data summary of antibody percentage decline levels at 6‐month postvaccination. IgG, immunoglobulin G; RBD, receptor‐binding domain
A consistent decrease in humoral immunity postvaccination was found across all age groups ranging from 53% to 96% (Figure 3). Similarly, a consistent decline in Ab titers regardless of sex was also noted. Females reported a 91%–93% decrease in their anti‐RBD IgG levels, similar to males (ranging from 89% to 92%) (Figure 4). Anti‐spike IgG levels in females decreased by 83%, also close to the decline seen in males (84%) (Figure 4). Regardless of serostatus, a diminished humoral immunity post‐mRNA vaccination was evident. However, the Ab titers post second dose of mRNA vaccine displayed varying trends (Figure 5). One study showed a 90% decrease of titers in the seronegative group, which is higher than the decline observed in the seropositive group (79%) (Figure 5). This is in contrast with two studies, one showing a 52% and the other a 60% decline in their seronegative participants, which is lower than the observed decline in their seropositive participants (68% and 74%, respectively) (Figure 5). For comorbidities cohorts, there was a consistent decrease in the total antibody and IgG levels among those individuals with kidney disease and undergoing hemodialysis (93%), heart disease and hypertension (91%), diabetes mellitus (90%), and immunologic disorders (85%).
Figure 3.
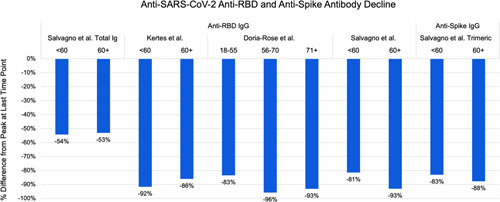
Comparison by age of the percentage decline of anti‐SARS‐CoV‐2 antibodies by immunoglobulin class and target from peak to last measured timepoint in each study. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Figure 4.

Comparison by sex of the percentage decline of anti‐SARS‐CoV‐2 antibodies by immunoglobulin class and target from peak to last measured timepoint in each study. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Figure 5.
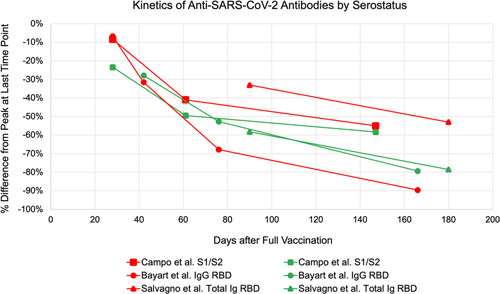
Comparison of antibody levels of seronegative and seropositive kinetics in studies reporting longitudinal data showing percentage decline from peak titer after vaccination. Red symbols indicate seronegative and green symbols indicate seropositive data
Although most of the studies focused on IgG titers, one study measured the levels of anti‐spike IgA with a decline up to 72% at 150 days after its peak, consistent with the rate of decay of other immunoglobulins. Conversely, a study from Italy reported the decline in total Ig reaching only up to 56% at 150‐day post peak, which is lower than the rate of decline measured in other studies.
4. DISCUSSION
4.1. Declining Ab levels post‐mRNA vaccination
In the midst of the COVID‐19 pandemic, the effectiveness and lasting protective effects of anti‐SARS‐CoV‐2 vaccines are topics of interest for the general population. Here, we highlight the trends of Ab titers post‐mRNA vaccination (Table 1 and Figure 2). Most studies reported Ab peak titers within 1–4‐week post second dose vaccination, followed by a steady decline up to 90% in anti‐SARS‐CoV‐2 Abs by 4–6‐month postvaccination. Consequently, as weeks of post‐mRNA vaccination increased, some studies reported an increase in the proportion of vaccine recipients whose Ab titers were below estimated protective thresholds. 22 , 24 Some studies noted that at 6‐month postvaccination, 16.1% of the study population had nonprotective levels of anti‐RBD IgG. 22 Similarly, other studies reported that at 6‐month postvaccination, 95% and 27% of the study population had anti‐RBD IgG and anti‐spike trimeric IgG levels below the protective threshold, respectively. 24 Although results are slightly dependent on the immunoassay used, the kinetics of Ab titer decline display similar properties. 24
Across all studies, the rate of decline was noted to be greater during the initial weeks after reaching peak levels compared to 4–6‐month post‐mRNA vaccination (Figure 2). While the Ab level needed for protection is not established yet, there is a correlation between seroimmunity and protection from infection as well as seroimmunity and the likelihood of transmission. 31 Therefore, this declining trend, with data demonstrating up to 95% decline in Ab levels depending on the antigenic target, supports the need for boosters and serologic monitoring moving forward to identify low responders and timely need for additional booster administration.
This trend was also observed in both Pfizer‐BioNTech and Moderna mRNA vaccines, and regardless of the vaccine brand used, the absolute Ab titers of those with older age, seronegative status, or presence of major comorbidities were consistently lower at each time point, findings consistent with previous studies. 33 Finally, with respect to the percentual decline in Ab titers 6‐month post second dose mRNA vaccination, there is a consistent rate of declining humoral response regardless of age, sex, serostatus, and comorbidities. Possible effects of the aforementioned factors on declining humoral response post second dose mRNA vaccination are summarized in Tables 2, 3, 4, 5 and discussed in subsequent sections.
4.2. Contributing factors to declining humoral immunity following complete mRNA vaccination
4.2.1. Age
Both humoral and cellular immune responses are impaired as individuals age, ultimately leading to poorer vaccine response. 34 Studies looking into the relationship of age and humoral response after undergoing Pfizer‐BioNTech vaccination noted an inverse relationship on the antibody titers mounted. 12 , 35 The studies included in Table 2 highlighted a similar trend. Regardless of the age group, there was a decline of titers starting 1 month after the second dose of vaccination (Figure 3). The mounted antibody titers are initially higher in the age group <60 years, but the rate of decline was consistent across all age groups. These all suggest the need for a booster vaccine dose for all age groups, but in low resource settings, prioritization should be given to older age groups who mount weaker humoral responses and overall lower antibody titers.
4.2.2. Sex
Ab titers of females were higher even after a humoral decline in all studies (Table 3); however, the percentage decline from the peak was similar for both sexes (Figure 4). 12 , 23 Although it is important to note that overall Ab titers may be influenced by the larger female population cohort (6238 females vs. 3128 males) involved in the included studies, this might be attributed to the sex‐based difference in humoral immunity which affects vaccine responses. Previous studies indicated that females have a greater humoral response attributed to hormonal differences and X chromosomes leading to biallelic expression of certain genes. 36 Several studies support this given the higher Ab titers mounted by females after vaccination against SARS‐CoV‐2. 35 , 37 Further, studies on the morbidity/mortality relationship due to SARS‐CoV‐2 infection between sexes found that males have three times higher probability of requiring intensive care unit admission and higher odds of mortality. 38 While these studies point to the increased risk and mortality among males, the overall percentage decline of Ab titers identified post‐second dose vaccination indicates the need for boosters regardless of sex.
4.2.3. Serostatus
Infection with SARS‐CoV‐2 elicits a humoral immune response via production of immunoglobulins targeting the spike, nucleocapsid, as well as other viral proteins. 39 This translates in detectable Ab titers before SARS‐CoV‐2 mRNA vaccine administration among previously infected individuals, and significantly higher Ab levels after the first dose compared to those with no history of prior infection. 39 However, Ab titers measured after the second vaccine dose report varying trends (Figure 5). Some studies reported that seropositive groups continue to have increased Ab levels compared to seronegative groups, while others reported that there is no significant difference between the two groups. 40 , 41 , 42 , 43 , 44 , 45 These disparities in trend are conveyed in the rate of decline of Ab titers at 5–6 months after full immunization compared to peak levels. Among the four journal articles included in this study, two reported that seropositive groups had a larger percentage decline in Ab titers from peak compared with seronegative groups. 10 , 12 This is possibly due to higher peak values making Ab catabolism more pronounced in seropositive groups. 12 Another possible explanation is due to timing, as seropositive groups reach peak Ab titers right after the first dose. Studies have observed that a second vaccine dose in seropositive groups does not significantly boost antibody titers higher, therefore these groups start their decline from peak much earlier than seronegative groups, who reach peak levels only after the second dose. 39 , 41 Conversely, two studies reported that seronegative groups had a larger percentage decline. 29 , 30 This led to a recommendation to prioritize seronegative groups for booster administration over seropositive in cases of limited booster supply. 30 However, given the heterogeneity of these findings, as well as a poor understanding of the immunity arising from the combination of native infection supplemented by vaccinated, booster administration should likely be recommended to everyone regardless of serostatus.
4.2.4. Comorbidities
Several studies established that elderly patients (age >60 years) with stage‐specific comorbidities along with other factors such as sex and serostatus elicit a comparably diminished humoral response postvaccination. 33 , 46 Moreover, the robustness of initial Ab response postvaccination is reported to predict the rapidity of Ab titer decline. 31 Those with higher maximum Ab response within 2 months from full vaccination were less likely to become seronegative or develop SARS‐CoV‐2 infection. 31 As such, it is important to follow the course of Ab titers several weeks after full vaccination among those with stage‐specific comorbidities to predict better when best to give additional booster vaccine doses and ensure appropriate protection.
In this systematic review, all three studies involving populations with comorbidities reported a consistent decrease in Ab titers from peak values at 4 months or later post second dose SARS‐CoV‐2 mRNA vaccination. 23 , 31 , 32 Interestingly, studies reported that the percentage decline of Ab titer for those with chronic kidney disease, heart disease, diabetes mellitus, and immunosuppressive disorders was greater at 120 days after peak levels (28–50 days after the first dose) compared to at 150 days after. 23 These findings, however, may be attributed to the study design, as it was a nonserial study where the sample size at Day 120 was less than at Day 150, which could have skewed the results. This particular study was also limited by the lack of control groups, preventing us from directly comparing the percent decline of those with comorbidities versus healthy populations, although the absolute values of Ab levels were significantly lower in those with comorbidities compared to the total sample population. 23 Regardless of this limitation, when taken together with other studies in this systematic review, those with comorbidities exhibited a similar trend in percent decline with the general population and other cohorts.
5. CONCLUSION
In this systematic review, we characterized the substantial decline in Abs at approximately 6 months after COVID‐19 mRNA vaccinations. To date, several studies identified a significant correlation between the time‐dependent waning of Ab levels and an increased risk for breakthrough infections. 47 , 48 In addition to the rise of SARS‐CoV‐2 variants of concern (VoC), like the Delta and more recently the Omicron variant, studies also suggest a correlation between cold weather and an increase in COVID‐19 cases. 49 This is consistent with the established association between the circulation of respiratory viruses and climatic factors, displaying a peak incidence in the winter months. 49 As such, the waning immunity, new VoC such as Omicron, uncontrolled community transmission of the Delta variant, and winter seasons could mark the culmination of major waves of COVID‐19, which could in part be abated by booster doses. Indeed, boosters are likely to provide cross‐reactive protection against SARS‐CoV‐2 infection, including VoC. 50
Individuals in the older age group and with preexisting comorbidities have lower levels of Abs compared to other individuals. 33 Hence, even with the same rate of decline in humoral immunity post second dose mRNA vaccination as observed in our study, their titers are consistently lower, and may in many cases be below adequate thresholds for protection. Thus, it is prudent to prioritize the elderly and those with comorbidities for booster administration in the coming months.
As evidence of waning Ab levels accumulates, several countries have expanded their vaccine coverage to include giving off booster doses to all adults after completion of primary vaccination. 51 , 52 As such, the rate of confirmed infection and the rate of severe illness were lower by a factor of 11.3 and 19.5, respectively, in the group that received the booster vaccine. 53 A secondary analysis had also shown that the rate of confirmed infection at least 12 days after booster administration was lower than the rate after 4–6 days by a factor of 5.4. 53 Further research is needed to understand the kinetics of antibody responses after vaccine boosters and to determine if a similar rate of decline is seen following adjunctive doses as observed with initial vaccination regimen.
In conclusion, our findings show that the levels of protective Abs significantly and consistently decline 6–8 months after the second dose of anti‐SARS‐CoV‐2 mRNA vaccines regardless of age, sex, baseline serostatus, and comorbidities. This supports that the rate of Ab decline is mostly independent of patient‐related factors and peak Ab titer achieved after the second mRNA dose, but instead mainly a function of time and Ab class/molecular target, with some variability arising from the sensitivity of the immunoassay. 54 Given this information, it may then be possible to predict the decline in Ab levels over time to an insufficient titer, based only on the patient's peak Ab titer (~4 weeks after the second dose for seronegative patients), and thus identify in advance the optimal time when a booster vaccine dose should be indicated without the need for serial serologic monitoring. Such a strategy should be investigated for those who are likely to be low responders or at high risk of breakthrough infection. 55 A similar strategy could be used following booster vaccine doses upon evaluation of Ab kinetics. Alternatively, research should explore the use of age, sex, serostatus, and comorbidity to gauge the most suitable time to measure the antibody titer to assess the need for a booster dose based on the data presented in our report. Given the significant decline of Abs observed in our systematic review, as well as studies showing a correlation between Ab titers and breakthrough infections, it is of utmost importance to provide equal opportunities in obtaining the vaccines, create more efficient vaccination strategies, and emphasize the necessity of providing booster shots in attenuating the effects of waning immunity, especially in the appearance of new VoC, in addition to the forthcoming winter season.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All the authors cited in the manuscript had substantial contributions to the concept and design, the execution of the work, or the analysis and interpretation of data; drafting or revising the manuscript, and have read and approved the final version of the paper. Kin Israel Notarte: conceptualization, visualization, methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. Israel Guerrero‐Arguero: conceptualization, formal analysis, data curation, writing—review and editing. Jacqueline Veronica Velasco: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. Abbygail Therese Ver: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. Maria Helena Santos de Oliveira: formal analysis, data curation, writing—review and editing. Jesus Alfonso Catahay: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. Md. Siddiqur Rahman Khan: Writing—review and editing. Adriel Pastrana: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. Grzegorz Juszczyk: Conceptualization, data interpretation, writing—review and editing. Jordi B. Torrelles: methodology, data interpretation, writing—review and editing. Giuseppe Lippi: conceptualization, data interpretation, writing —review and editing. Luis Martinez‐Sobrido: methodology, data interpretation, writing—review and editing. Brandon Michael Henry: conceptualization, visualization, validation, formal analysis, writing—review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27688
Notarte KI, Guerrero‐Arguero I, Velasco JV, et al. Characterization of the significant decline in humoral immune response six months post‐SARS‐CoV‐2 mRNA vaccination: a systematic review. J Med Virol. 2022;94:2939‐2961. 10.1002/jmv.27688
Kin Israel Notarte and Israel Guerrero‐Arguero are equal contributors.
DATA AVAILABILITY STATEMENT
The supporting data are available within the article.
REFERENCES
- 1. Albano PM, Notarte KI, Macaranas I, Maralit B. Cross‐contamination in molecular diagnostic laboratories in low‐ and middle‐income countries: a challenge to COVID‐19 testing. Philippine Journal of Pathology. 2020;5:7‐11. 10.21141/PJP.2020.09 [DOI] [Google Scholar]
- 2. Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang C, , bin Bernardini S . The COVID‐19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365‐388. 10.1080/10408363.2020.1783198 [DOI] [PubMed] [Google Scholar]
- 3. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough Infections in vaccinated healthcare workers. N Engl J Med. 2021;385(16):1474‐1484. 10.1056/nejmoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Henry BM, Plebani M. Anti‐sars‐cov‐2 antibodies testing in recipients of covid‐19 vaccination: why, when, and how? Diagnostics. 2021;11(6):1‐10. 10.3390/diagnostics11060941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lippi G, Henry BM, Plebani M. Optimizing effectiveness of COVID‐19 vaccination: will laboratory stewardship play a role? Clin Chem Lab Med. 2021;59(12):1885‐1888. 10.1515/cclm-2021-0972 [DOI] [PubMed] [Google Scholar]
- 6. Noor R. A Review on the effectivity of the current COVID‐19 drugs and vaccines: are they really working against the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) variants? Current Clinical Microbiology Reports. 2021;8(3):186‐193. 10.1007/s40588-021-00172-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asundi A, O'Leary C, Bhadelia N. Global COVID‐19 vaccine inequity: the scope, the impact, and the challenges. Cell Host and Microbe. 2021;29(7):1036‐1039. 10.1016/j.chom.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Q, Zeng J, Yan J. COVID‐19 mRNA vaccines. J Genet Genomics. 2021;48(2):107‐114. 10.1016/j.jgg.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID‐19 vaccine over 6 months. New England Journal of Medicine. Published online. 2021;385:1‐11. 10.1056/nejmoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campo F, Venuti A, Pimpinelli F, et al. Antibody persistence 6 months post‐vaccination with bnt162b2 among health care workers. Vaccines. 2021;9(10):1‐9. 10.3390/vaccines9101125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klompas M. Understanding breakthrough infections following mRNA SARS‐CoV‐2 vaccination. JAMA. Published online. 2021;326:1‐3. 10.1001/jama.2021.19063 [DOI] [PubMed] [Google Scholar]
- 12. Salvagno GL, Lippi G, Henry BM, Pighi L, de Nitto S. Total anti‐SARS‐CoV‐2 antibodies measured 6 months after Pfizer‐BioNTech COVID‐19 vaccination in healthcare workers. J Med Biochem. 2022;41:1‐5. 10.5937/jomb0-33999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS‐CoV‐2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021. 10.1101/2021.11.11.21266068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffman T, Nissen K, Krambrich J, et al. Evaluation of a COVID‐19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS‐CoV‐2. Infect Ecol Epidemiol. 2020;10(1):20030916. 10.1080/20008686.2020.1754538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, Wang J, Xu X, Liao G, Chen Y, Hu CH. Patterns of IgG and IgM antibody response in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):1269‐1274. 10.1080/22221751.2020.1773324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert PB, Montefiori DC, McDermott A. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy trial. Science. Published online. 2021;23:43‐50. 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponticelli D, Antonazzo IC, Caci G, et al. Dynamics of antibody response to BNT162b2 mRNACOVID‐19 vaccine after 6 months. J Travel Med. 2021;28(8):taab173. 10.1093/jtm/taab173 [DOI] [PubMed] [Google Scholar]
- 18. Salvagno GL, Henry BM, Pighi L, de Nitto S, Lippi G Six‐month decline of serum anti‐spike S1 subunit IgA in SARS‐CoV‐2 in seronegative healthcare workers after mRNA‐based COVID‐19 vaccination. Published online 2021. 10.21203/rs.3.rs-1019658/v1 [DOI]
- 19. Kato H, Miyakawa K, Ohtake N, et al. Vaccine‐induced humoral and cellular immunity against SARS‐CoV‐2 at 6 months post BNT162b2 vaccination 10.1101/2021.10.30.21265693 [DOI] [PMC free article] [PubMed]
- 20. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. 10.1016/j.lanepe.2021.100208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laing ED, Weiss CD, Samuels EC, et al. Durability of antibody responses and frequency of clinical and subclinical SARS‐CoV‐2 Running title: Antibody durability post‐BNT162b2 Mitre. 10.1101/2021.10.16.21265087 [DOI]
- 22. Israel A, Shenhar Y, Green I, et al. Large‐scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS‐CoV‐2 infection. medRxiv. 2021;Published online August 21, 10.1101/2021.08.19.21262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kertes J, Baruch Gez S, Saciuk Y, et al. Effectiveness of the mRNA BNT162b2 vaccine six months after vaccination: findings from a large Israeli HMO. medRxiv. Published online 2021;10:1. 10.1101/2021.09.01.21262957 [DOI] [Google Scholar]
- 24. Salvagno GL, Henry BM, Pighi L, de Nitto S, Lippi G, Gianfilippi G The pronounced decline of anti‐SARS‐CoV‐2 spike trimeric IgG and RBD IgG in baseline seronegative individuals 6 months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Published online 2021. doi: 10.21203/rs.3.rs-1063499/v1 [DOI] [PubMed]
- 25. Khoury J, Najjar‐Debbiny R, Hanna A, et al. COVID‐19 vaccine—long term immune decline and breakthrough infections. Vaccine. 2021;39(48):6984‐6989. 10.1016/j.vaccine.2021.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciabattini A, Pastore G, Fiorino F, et al. Evidence of SARS‐CoV‐2‐specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12:740708. 10.3389/fimmu.2021.740708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brisotto G, Muraro E, Montico M, et al. IgG antibodies against SARS‐CoV‐2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin Chim Acta. 2021;523:476‐482. 10.1016/j.cca.2021.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doria‐Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA‐1273 vaccine for Covid‐19. N Engl J Med. 2021;384(23):2259‐2261. 10.1056/nejmc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayart JL, Douxfils J, Gillot C, et al. Waning of igg, total and neutralizing antibodies 6 months post‐vaccination with bnt162b2 in healthcare workers. Vaccines. 2021;9(10):1092. 10.3390/vaccines9101092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tré‐Hardy M, Cupaiolo R, Wilmet A, et al. Six‐month interim analysis of ongoing immunogenicity surveillance of the mRNA‐1273 vaccine in healthcare workers: A third dose is expected. Journal of Infection. Published online. 2021;1:559‐564. 10.1016/j.jinf.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsu CM, Weiner DE, Manley HJ, et al. Seroresponse to SARS‐CoV‐2 vaccines among maintenance dialysis patients over six months. medRxiv. Published online 2021. 10.1101/2021.09.13.21263535 [DOI] [Google Scholar]
- 32. Davidovic T, Schimpf J, Abbassi‐Nik A, et al. Waning humoral response 6 months after SARS‐CoV‐2 vaccination with the mRNA‐BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021;100(6):1334‐1335. 10.1016/j.kint.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Notarte KI, Senanayake S, Macaranas I, et al. Effects of age, sex, serostatus and underlying comorbidities on humoral response post‐SARS‐CoV‐2 Pfizer‐BioNTech vaccination—a systematic review. medRxiv. Published online 2021;13, 10.1101/2021.10.10.21264825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10(3):330‐335. 10.1016/j.arr.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abu Jabal K, Ben‐Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 MRNA COVID‐19 vaccine: real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):403. 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fink AL, Klein SL. The evolution of greater humoral immunity in females than males: implications for vaccine efficacy. Current Opinion in Physiology. 2018;6:16‐20. 10.1016/j.cophys.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS‐CoV‐2 BNT162b2 vaccine. EClinicalMedicine. Published online. 2021;36:100928. 10.1016/j.eclinm.2021.100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):536. 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fraley E, LeMaster C, Geanes E, et al. Humoral immune responses during SARS‐CoV‐2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021;19(1):265. 10.1186/s12916-021-02055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93(7):4612‐4615. 10.1002/jmv.26982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebinger JE, Fert‐Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nature Med. 2021;27(6):981‐984. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kelsen SG, Braverman AS, Aksoy MO, et al. A Longitudinal Study of BNT162b2 Vaccine‐Induced Humoral Response and Reactogenicity in Health Care Workers with Prior COVID‐19 Disease. 10.1101/2021.03.18.21253845 [DOI] [PMC free article] [PubMed]
- 43. Modenese A, Paduano S, Bargellini A, et al. Neutralizing anti‐SARS‐CoV‐2 antibody titer and reported adverse effects, in a sample of italian nursing home personnel after two doses of the BNT162b2 vaccine administered four weeks apart. Vaccines. 2021;9(6):652. 10.3390/vaccines9060652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Padoan A, Dall'Olmo L, Rocca F, et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;519:60‐63. 10.1016/j.cca.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salvagno GL, Henry BM, di Piazza G, et al. Anti‐sars‐cov‐2 receptor‐binding domain total antibodies response in seropositive and seronegative healthcare workers undergoing covid‐19 mrna bnt162b2 vaccination. Diagnostics. 2021;11(5):832. 10.3390/diagnostics11050832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salmerón Ríos S, Mas Romero M, Cortés Zamora EB, et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID‐A study. J Am Geriatr Soc. 2021;69(6):1441‐1447. 10.1111/jgs.17153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS‐CoV‐2 breakthrough infections to time‐from‐vaccine; preliminary study. medRxiv. 2021;384:403. 10.1101/2021.07.29.21261317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike‐antibody waning after second dose of BNT162b2 or ChAdOx1. The Lancet. 2021;398(10298):385‐387. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bolano‐Ortiz TR, Camargo‐Caicedo Y, Puliafito SE, et al. Spread of SARS‐CoV‐2 through Latin America and the Caribbean region: a look from its economic conditions, climate and air pollution indicators. Environ Res. 2020;191:109938. 10.1016/j.envres.2020.109938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Corbett KS, Gagne M, Wagner DA, et al. Protection against SARS‐CoV‐2 beta variant in mRNA‐1273 vaccine–boosted nonhuman primates. Science. Published online. 2021;21. 10.1126/SCIENCE.ABL8912/SUPPL_FILE/SCIENCE.ABL8912_SM.PDF [DOI] [PubMed] [Google Scholar]
- 51. Mahase E. Covid‐19 booster vaccines: what we know and who's doing what. BMJ (Clinical research ed). 2021;374:n2082. 10.1136/bmj.n2082 [DOI] [PubMed] [Google Scholar]
- 52. Mbaeyi S, Oliver SE, Collins JP, et al. The advisory committee on immunization practices' interim recommendations for additional primary and booster doses of COVID‐19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545‐1552. 10.15585/mmwr.mm7044e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl J Med. 2021;385(15):1393‐1400. 10.1056/nejmoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Danese E, Montagnana M, Salvagno G, et al. Comparison of five commercial anti‐SARS‐CoV‐2 total antibodies and IgG immunoassays after vaccination with BNT162b2 mRNA. J Med Biochem. 2021;40(4):335‐340. 10.5937/JOMB0-31475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA‐1273 vaccines in U.S. veterans. New England Journal of Medicine. Published online. 2021;1:105‐115. 10.1056/NEJMoa2115463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supporting data are available within the article.


