Abstract
Background
Most of the common risk factors for severe outcomes of coronavirus disease 2019 (COVID‐19) are correlated with poor oral health, tooth loss, and periodontitis. This has pointed to a possible relationship between oral and systemic health in COVID‐19 patients. Hence, this study aimed to assess the dental and periodontal status of hospitalized COVID‐19 patients and their associations with the incidence of adverse COVID‐19 outcomes.
Methods
We included 128 hospital patients aged between 20 and 97 years and with diagnoses of COVID‐19 in this prospective observational study. Dental and periodontal status was assessed using in‐hospital clinical examinations, including the Decayed, Missing, and Filled Teeth index, periodontal status, and tooth loss patterns (Eichner index). Associations between oral health measures, the severity of COVID‐19 symptoms, and hospitalization endpoints were tested using chi‐square test and incidence rate ratio (IRR) estimation using a generalized linear model with log‐Poisson regression. The regression models used a block‐wise selection of predictors for oral health‐related variables, comorbidities, and patients’ ages.
Results
Overall, poor oral health conditions were highly prevalent and associated with critical COVID‐19 symptoms, higher risk for admission in the intensive care unit (ICU), and death. Periodontitis was significantly associated with ICU admission (IRR = 1.44; 95% confidence interval [95%CI] = 1.07–1.95; P = 0.017), critical symptoms (IRR = 2.56; 95%CI = 1.44–4.55; P = 0.001), and risk of death (IRR = 2.05; 95%CI = 1.12‐3.76; P = 0.020) when adjusted for age and comorbidities. The Eichner index (classes B and C) was associated with ICU admission.
Conclusion
There was a positive association between deleterious oral health‐related conditions, especially periodontitis, and severe COVID‐19 outcomes in hospitalized COVID‐19 patients.
Keywords: COVID‐19, hospital medicine, oral health, periodontitis
1. INTRODUCTION
The new coronavirus infection by the severe acute respiratory syndrome‐2 virus (SARS‐CoV‐2) and the coronavirus disease 2019 (COVID‐19) pandemic dramatically affected human life worldwide and presented unprecedented challenges to health services, governments, and society. 1 In addition to efforts to minimize its socioeconomic impacts, control the dissemination of the infection, and develop effective preventive and treatment strategies, there was also a need to understand the epidemiological and pathophysiological aspects of the disease and related risk factors that predispose specific groups to severe disease manifestations and higher mortality risks.
The risk for severe disease and death increases with age and the presence of comorbidities such as diabetes, hypertension, and obesity. 1 The fact that most of the common risk factors for severe outcomes of COVID‐19 are correlated with poor oral health, tooth loss, and periodontitis pointed to a possible relationship between oral and systemic health in COVID‐19 patients. 2 , 3 , 4 , 5 A recent retrospective study based on oral examination records and panoramic X‐rays suggested a possible relationship between poor dental conditions and the severity and outcome of COVID‐19, as they observed higher hospitalization and death rates in patients with severe dental damage, including caries lesions, apical periodontitis, and cervical bone loss. 6
The presence of severe periodontitis was reported to be associated with COVID‐19, 7 and a higher risk for severe complications of COVID‐19 was also suggested, 2 , 3 , 4 , 5 , 8 especially in predisposed patients with hypertension. 9 A case‐control study assessed the periodontal conditions in posterior bitewings and panoramic radiographs from electronic medical records and outcomes of COVID‐19 from the same database. 10 After adjusting for potential confounders, periodontitis was associated with higher rates for all COVID‐19 complications, death, intensive care unit (ICU) admission, and the need for invasive ventilation. 10 In a cross‐sectional clinical study, Gupta et al. found that hospital admission, the requirement for assisted ventilation, and COVID‐19 pneumonia rose with a concomitant increase in the severity of periodontitis (but without adjustment for confounders). 11 Furthermore, the detection of SARS‐CoV‐2 in samples of periodontal tissues 12 and dental biofilm 13 suggest a role of periodontal and oral environment in the contamination and infectivity patterns of COVID‐19, 14 affecting strategies to avoid the spread, prevention of adverse outcomes, and potential therapeutic approaches for COVID‐19.
Nevertheless, there is scarce evidence derived from clinical data on the relationship between harmful oral conditions and the likelihood of aggravated COVID‐19 symptoms. Therefore, this study aimed to assess the oral health conditions in COVID‐19 patients and determine the association between oral health and disease outcomes, including the incidence of severe/critical symptoms, ICU admission. The study hypothesis was that the prevalence and severity of caries, periodontitis, and tooth loss are associated with severe COVID‐19 symptoms and poor outcomes in hospitalized patients.
2. MATERIALS AND METHODS
2.1. Study location, design, and sample
This short‐term prospective study was conducted in Goiania, State of Goiás, a city located in central Brazil with nearly 1.5 million inhabitants. Brazil has one of the highest death tolls due to COVID‐19 globally, and the incidence of the disease, hospitalization, and mortality in all the states in Brazil achieved high rates during the entire pandemic period. 15
This study was designed according to the STROBE guidelines for cohort studies (see in online Journal of Periodontology). 16 A convenience sample of COVID‐19 patients was selected from two reference hospitals for COVID‐19 patients (Clinical Hospital of the Federal University of Goiás, HC/UFG; and the COVID‐19 State‐run Hospital, HCamp). Participants were recruited between August 2020 and March 2021, when the two hospitals admitted 1,964 COVID‐19 patients. The university and the hospital ethical research committees approved the study (CAEE 38088920.9.0000.5083 / 38088920.9.3001.5078 / 38088920.9.3002.5082), ensuring anonymity and confidentiality of patient data. All participants or responsible relatives provided informed written consent to participate in the study. This study followed the Declaration of Helsinki's principles.
Eligible participants were hospitalized, infected COVID‐19 patients, with a minimum age of 18 years. Due to the limited access to restricted areas, assessment of potentially eligible patients occurred once per week during scheduled visits. The number of assessed patients in each visit varied according to the current hospital census rates throughout the data collection period. Inclusion criteria included a diagnosis of COVID‐19 confirmed by reverse‐transcription polymerase chain reaction (qRT‐PCR) and the presence of at least one typical COVID‐19 symptom. 17 Exclusion criteria included the impossibility of performing intraoral examinations and periodontal assessments, sepsis, infections with multiresistant bacteria, missing dates of qRT‐PCR examinations, missing information regarding the study endpoints, and missing data on the selected outcomes.
2.2. Variables
Baseline data were collected after hospitalization under acceptable individual clinical conditions for the examination procedures. Sociodemographic data included age, sex, race, educational level, and monthly family income. Medical information was retrieved from medical records and included the following: body mass index (BMI); pre‐existing habits (smoking and alcohol intake); comorbidities, conditions, or diseases associated with a higher risk of COVID‐19 aggravation including diabetes, obesity (BMI ≥ 30), hypertension, asthma, and chronic obstructive pulmonary disease (COPD); pregnancy; cardiovascular diseases; liver diseases; cancer; osteoporosis; thyroid diseases; arthritis; and HIV or other sexually transmitted diseases.
The set of selected oral health variables were as follows:
1. Decayed, Missing, and Filled Teeth (DMFT) index; 18
2. The Eichner index is a tooth‐loss classification system based on the presence or absence of occlusal contact in the posterior area: Class A (four support zones posteriorly); Class B (one to three support zones posteriorly or the presence of occlusal contacts anteriorly); and Class C (no occlusal contact on the remaining teeth). Classes A, B, and C are further divided into subgroups according to the number of teeth lost and occlusal supporting zones; 19
3. Prosthodontic features: Use or need for a dental prosthesis, recording the type, extension, and arch;
4. Periodontal measures: Probing depth (PD), clinical attachment level (CAL), bleeding on probing;
5. Periodontal diagnosis: Patients were classified as healthy or with gingivitis 20 or periodontitis, 21 based on a classification of periodontal diseases and conditions. 22 Gingival health was defined as < 10% bleeding sites with PD ≤ 3 mm. Gingivitis was defined as ≥ 10% bleeding sites with PD ≤ 3 mm, and periodontitis was defined as a detectable interdental CAL at ≥ 2 nonadjacent teeth, or buccal or oral CAL ≥ 3 mm with pocketing ≥ 3 mm at ≥ 2 teeth when not ascribed to non‐periodontitis‐related causes.
The oral examinations assessing oral health‐related variables were performed within the first week after hospital admission for 63.3% and 84.7% within the first 2 weeks after hospital admission. Two dentists (CAC and NLC) performed an intraoral examination, including a periodontist (CAC) who performed the periodontal probing, helped by another assistant (ACSV), under external illumination in the patient care setting. The periodontal assessment was performed at six sites per tooth in all teeth. Inter‐ and intraexaminer reliability was assessed in a pilot analysis of PD and CAL measurements in 48‐hour duplicate assessments of five non‐COVID‐19 patients with Stage III generalized periodontitis, and acceptable agreement was achieved (intraclass correlation > 0.80).
All examinations and data collection in the hospital environment were conducted following the recommended infection prevention and control (IPC) practices for routine healthcare delivery during the COVID‐19 pandemic and recommended IPC practices when caring for a patient with SARS‐CoV‐2 infection. Patients and nursing teams were instructed to improve oral hygiene. Then, all patients discharged from the hospital were invited to receive dental and periodontal treatment at the university dental clinics. Data analysis also considered comorbidities that are well‐known risk factors for increase the risk of severe illness and mortality due to COVID‐19. The included comorbidities were (1) diabetes, (2) hypertension, and (3) obesity. Data were retrieved from medical charts registered at hospital admission based on objective measurements, medical history, and self‐reported data. Obesity was diagnosed based on the BMI ≥ 30.
The study dependent variables included COVID‐19 outcomes assessed at the end of the hospitalization period or clinical status achieved during hospitalization, follows:
1. Level of symptoms: the most severe status of symptoms during hospitalization, classified as mild, moderate, severe, or critical, according to the WHO; 23 , 24
2. Hospital admission and discharge criteria: duration (in days), and admission to an ICU;
3. Hospitalization endpoint: discharge or death;
4. Type of ventilation support: mechanical ventilator (invasive and noninvasive) or tracheostomy ventilator;
5. Clinical manifestations and symptoms.
2.3. Data analysis
Data analysis included descriptive statistics and the chi‐square test. The incidence rate ratio (IRR) was calculated to test the association between COVID‐19 outcomes and predictors (oral‐related variables, comorbidities, and age). A modified Poisson regression approach was used to calculate the IRR estimators (and their 95% confidence intervals [CIs]). Each predictor was associated with outcomes using a generalized linear model with a log‐Poisson regression model with robust error variances to provide unadjusted risk estimators. 25 Then, the IRRs were adjusted for comorbidities, oral health predictors, and patient age. For the multiple regression estimates, the independent variables were entered in the model in a forward block‐wise fashion that included comorbidities (diabetes, hypertension, and obesity) and oral health‐related predictor variables (DMFT index, Eichner index, and periodontitis). The patient's age was included as a potential confounder in all multivariate models, and the selection method of predictors were based on statistical significance (P < 0.05 for entry and P < 0.20 for removal). The significance level for statistical inferences was set at 0.05, and the Stata 13.0 software was used for data analysis.
3. RESULTS
During the 8‐month recruitment and data collection period, there were 43 research team visits to the hospital settings. A total of 394 currently hospitalized patients at the time of the visits were eligible to participate in the study. However, 95 patients were not available for the dental exam before the hospitalization endpoint and were excluded. The remaining 299 were assessed for eligibility, and 171 were excluded due to missing information about the specific date of the qRT‐PCR exam (n = 79), missing data on the COVID‐19 outcomes (n = 50), refusal to participate (n = 16), or impossibility of performing oral examinations (n = 26). Finally, 128 patients were assessed with complete baseline data and follow‐up data and included in the analysis (see Supporting Information Flowchart in online Journal of Periodontology ).
The main features of the participants are detailed in Table 1. Age ranged from 20 to 97 years (mean = 58.7; SD = 17.6), 53.1% were male, and most were from the lower socioeconomic stratum. There was a high prevalence of comorbidities, deleterious habits, and diseases. Table 2 shows the frequency of symptoms and hospitalization features regarding the overall duration of hospital stay, ICU admission, and time of the stay. A variety of symptoms were observed, and all patients had one or more concomitant symptoms. The symptomatic status ranged from mild to moderate in 73 patients (57.0%) and severe‐critical in 55 patients (43.0%). Thirty‐one patients (24.2%) died after a hospitalization time ranging from 1 to 61 days. Seventy‐eight (60.9%) patients were discharged due to remission of symptoms and COVID‐19 infection after hospitalization ranging from 2 to 49 days (mean = 13.2; SD = 11.0). Sixteen patients (12.5%) were discharged to another care facility, and 3 (2.3%) were self‐discharged from the hospital.
TABLE 1.
Baseline data of hospitalized patients with COVID‐19 (n = 128)
| Variables | Categories | n (%) | |
|---|---|---|---|
| Sociodemographic | Sex | Male | 68 (53.1) |
| Female | 60 (46.9) | ||
|
Age–mean (SD) = 58.7 (17.6)(3 missing data) |
≤ 30 years | 9 (7.2) | |
| >30 to 50 years | 28 (21.9) | ||
| >50 to 65 years | 41 (32.8) | ||
| ≥ 65 years | 47 (37.6) | ||
|
Race† (17 missing data) |
White | 22 (19.8) | |
| Brown | 81 (73.0) | ||
| Yellow | 6 (5.4) | ||
| Black | 2 (1.8) | ||
| Educational level (36 missing data) | Higher education | 18 (18.8) | |
| High school | 34 (35.4) | ||
| Elementary and middle school | 40 (41.7) | ||
| Monthly family income* (44 missing data) | > 5 BMW | 9 (10.7) | |
| > 3 and ≤ 5 BMW | 23 (27.4) | ||
| ≤ 3 BMW | 52 (61.9) | ||
| Hospital | University hospital | 76 (59.4) | |
| COVID‐19 state‐run hospital | 52 (40.6) | ||
| General health conditions | Hypertension | 65 (50.8) | |
| Obesity | 52 (40.6) | ||
| Diabetes | 39 (30.5) | ||
| COPD | 22 (17.2) | ||
| Asthma | 7 (5.5) | ||
| Alcohol intake (current and former) | 53 (41.4) | ||
| Smoking (current and former) | 50 (39.1) | ||
| Pregnancy (60 women) | 16 (26.7) | ||
| Cardiovascular diseases | 34 (26.6) | ||
| Liver diseases | 15 (11.7) | ||
| Cancer | 12 (9.4) | ||
| Osteoporosis | 10 (7.8) | ||
| Thyroid disease | 10 (7.8) | ||
| Arthritis | 7 (5.5) | ||
| HIV or other STD | 7 (5.5) | ||
| Date of the oral examination after hospital admission | ≤ 7 days | 81 (63.3) | |
| 7–14 days | 27 (21.1) | ||
| ≥ 15 days | 20 (15.6) | ||
Abbreviations: COPD, chronic obstructive pulmonary disease, HIV, human immunodeficiency virus, STD, sexually transmitted diseases.
*In Brazilian minimum wage (BMW).
†Race was self‐reported by study participants, and race categories (Black, White, Yellow, and Brown) were based on the medical records data, which were used as a reference for the racial categorization based on the 2008 Survey of Ethnic‐Racial Characteristics of the Population by the Brazilian Institute of Geography and Statistics (IBGE): https://www.ibge.gov.br/estatisticas/sociais/populacao/9372-caracteristicas-etnico-raciais-da-populacao.html?=&t=resultados.
TABLE 2.
Symptoms, hospitalization features, and outcomes of COVID‐19 patients (n = 128)
| Variables | Categories | n (%) |
|---|---|---|
| COVID‐19 related symptoms | Cough | 91 (71.1) |
| Fatigue | 87 (68.0) | |
| Dyspnea | 87 (68.0) | |
| Hyperoxia | 76 (59.4) | |
| Shortness of breath or difficulty breathing | 76 (59.4) | |
| Fever | 67 (52.3) | |
| Headache | 57 (44.5) | |
| Dry mouth | 54 (42.2) | |
| Congestion or runny nose | 54 (42.2) | |
| Anosmia | 52 (40.6) | |
| Diarrhea | 47 (36.7) | |
| Sore throat | 41 (32.0) | |
| Muscle pain | 41 (32.0) | |
| Nausea | 39 (30.5) | |
| Burning eyes | 36 (28.1) | |
| Ageusia | 35 (27.3) | |
| Vomiting | 34 (26.6) | |
| Metallic taste | 28 (21.9) | |
| Dysgeusia | 20 (15.6) | |
| Hypogeusia | 19 (14.8) | |
| Mumps | 10 (7.8) | |
| Conjunctivitis | 5 (3.9) | |
| Skin rashes | 3 (2.3) | |
| Admission and referral to ICU – n (%) | Yes | 69 (53.9) |
| No | 59 (46.1) | |
| Length of stay in ICU (in days) | Mean ± SD | 16.3 ± 13.9 |
| Median (IQR) | 12 (20) | |
| Length of stay in hospital (in days) | Mean ± SD | 15.5 ± 12.0 |
| Median (IQR) | 19 (15) | |
| Need for invasive ventilation in ICU | – | 45 (35.2) |
| Need for tracheostomy in ICU (n = 44) | – | 15 (11.7) |
| Level of symptoms – n (%) | Critical | 43 (33.6) |
| Severe | 12 (9.4) | |
| Moderate | 63 (49.2) | |
| Mild | 10 (7.8) | |
| Hospital endpoint – n (%) | Discharge | 78 (60.9) |
| Death | 31 (24.2) | |
| Discharge to another care facility | 16 (12.5) | |
| Self‐discharge | 3 (2.3) | |
| Mortality rates – n (%) | Admission to ICU (n = 69) | 31 (44.9) |
| Severe‐critical symptoms (n = 55) | 30 (54.5) | |
| Critical symptoms (n = 43) | 29 (67.4) |
Abbreviations: ICU, intensive care unit.
Dental and periodontal conditions are presented in Table 3. Overall characteristics showed a high prevalence of deleterious oral conditions. Near 22% were edentulous, median DMFT index was 18, 50.8% used some type of prosthesis, 56.8% were classified as Eichner index classes B and C, and 47.9% of dentate or partially dentate participants had periodontitis.
TABLE 3.
Frequency of dental and periodontal conditions of hospitalized COVID‐19 patients (n = 119)
| Variables | Categories | n (%) |
|---|---|---|
| DMFT index (four missing data) | ≤ 6 | 11 (8.9) |
| 7 – 14 | 37 (29.8) | |
| 15 – 21 | 25 (20.2) | |
| ≥ 22 | 51 (41.1) | |
| Number of teeth* | Present | 15.8 ± 11.3 |
| Missing | 12.7 ± 10.9 | |
| Filled | 4.2 ± 4.1 | |
| Decayed | 1.3 ± 2.1 | |
| Eichner index (three missing data) | A (A1+A2+A3) | 54 (43.2) |
| B (B1+B2+B3+B4) | 21 (16.8) | |
| C (C1+C2+C3) | 50 (40.0) | |
| Fully edentulous | – | 28 (21.9) |
| Use of prostheses (four missing data) | No | 61 (49.2) |
| Yes – maxillary | 26 (21.0) | |
| Yes – mandibular | 5 (4.0) | |
| Yes – both | 32 (25.8) | |
| Periodontal status (n = 96) | Healthy | 8 (8.3) |
| Gingivitis | 42 (43.8) | |
| Periodontitis | 46 (47.9) | |
| Highest probing depth (n = 96) | ≤ 3 mm | 43 (44.8) |
| ≥ 4 and ≤ 6 | 34 (35.4) | |
| ≥ 7 mm | 19 (19.8) |
* Mean ± standard deviation.
Table 4 shows that patients with periodontitis had higher rates of ICU admission (P = 0.008), and severe‐critical symptoms (P < 0.001). Poorer outcomes were also observed for patients with higher DMFT indexes and fewer occlusal pairs (P < 0.05). Periodontitis was also associated with higher rates of need for invasive ventilation (P = 0.008). Patients with hypertension had higher ICU admission rates, critical symptoms, and deaths (P < 0.05). No associations were found between poorer COVID‐19 outcomes and diabetes, cardiovascular disease, or obesity.
TABLE 4.
Relationship between oral health‐related conditions, comorbidities, and COVID‐19 outcomes
| ICU admission | Death | Level of symptoms* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Mild | Moderate | Severe | Critical | ||
| Oral health‐related conditions | |||||||||
| DMFT (n = 124) | Lower 50% | 26 (40.6) | 38 (59.4) | 9 (14.1) | 55 (85.9) | 8 (12.5) | 35 (54.7) | 6 (9.4) | 15 (23.4) |
| Higher 50% | 40 (66.7) | 20 (33.3) | 19 (31.7) | 41 (68.3) | 1 (1.7) | 28 (46.7) | 6 (10.0) | 25 (41.7) | |
| P‐value | 0.004 | 0.019 | 0.008 | ||||||
| Eichner index (n = 125) | A | 18 (33.3) | 36 (66.7) | 7 (13.0) | 47 (87.0) | 8 (14.8) | 30 (55.6) | 6 (11.1) | 10 (18.5) |
| B | 14 (66.7) | 7 (33.3) | 4 (19.0) | 17 (81.0) | 0 | 12 (57.1) | 0 | 9 (42.9) | |
| C | 35 (70.0) | 15 (30.0) | 18 (36.0) | 32 (64.0) | 1 (2.0) | 21 (42.0) | 6 (12.0) | 22 (44.0) | |
| P‐value | <0.001 | 0.019 | 0.001 | ||||||
| Periodontal status (n = 96) | Healthy | 1 (12.5) | 7 (87.5) | 0 | 8 (100) | 2 (25.0) | 6 (75.0) | 0 | 0 |
| Gingivitis | 18 (42.9) | 24 (57.1) | 6 (14.3) | 36 (85.7) | 5 (11.9) | 23 (54.8) | 8 (19.0) | 6 (14.3) | |
| Periodontitis | 30 (65.2) | 16 (34.8) | 14 (30.4) | 32 (69.6) | 1 (2.2) | 19 (41.3) | 2 (4.3) | 24 (52.2) | |
| P‐value | 0.008 | 0.056 | <0.001 | ||||||
| Comorbidities | |||||||||
| Diabetes (n = 128) | Yes | 19 (48.7) | 20 (51.3) | 10 (25.6) | 29 (74.4) | 4 (10.3) | 16 (41.0) | 6 (15.4) | 13 (33.3) |
| No | 50 (56.2) | 39 (43.8) | 21 (23.6) | 68 (76.4) | 6 (6.7) | 47 (52.8) | 6 (6.7) | 30 (33.7) | |
| P‐value | 0.436 | 0.804 | 0.824 | ||||||
| Hypertension (n = 128) | Yes | 42 (64.6) | 23 (35.4) | 23 (35.4) | 42 (64.6) | 1 (1.5) | 30 (46.2) | 5 (7.7) | 29 (44.6) |
| No | 27 (42.9) | 36 (57.1) | 8 (12.7) | 55 (87.3) | 9 (14.3) | 33 (52.4) | 7 (11.1) | 14 (22.2) | |
| P‐value | 0.014 | 0.003 | 0.003 | ||||||
| Cardiovascular disease (n = 128) | Yes | 17 (50.0) | 17 (50.0) | 9 (26.5) | 25 (73.5) | 2 (5.9) | 17 (50.0) | 3 (8.8) | 12 (35.3) |
| No | 52 (55.3) | 42 (44.7) | 22 (23.4) | 72 (76.6) | 8 (8.5) | 46 (48.9) | 9 (9.6) | 31 (33.0) | |
| P‐value | 0.594 | 0.721 | 0.751 | ||||||
| Obesity (n = 121) | Yes | 28 (53.8) | 24 (46.2) | 13 (25.0) | 39 (75.0) | 2 (3.8) | 26 (50.0) | 4 (7.7) | 20 (38.5) |
| No | 34 (49.3) | 35 (50.7) | 13 (18.8) | 56 (81.2) | 8 (16.6) | 35 (50.7) | 7 (10.1) | 19 (27.5) | |
| P‐value | 0.619 | 0.414 | 0.148 | ||||||
Note: Data are expressed as absolute frequency (%), and statistical significance is highlighted in bold.
*Chi‐squared for trend.
Regression analysis revealed no association between male sex and the risk of adverse outcomes, including ICU admission (IRR = 1.29; 95%CI = 0.93 – 1.80; P = 0.132), critical symptoms (IRR = 1.27; 95%CI = 0.71 – 2.27; P = 0.422), and death (IRR = 0.94; 95%CI = 0.51 – 1.74; P = 0.847). No significant effect of other socioeconomic variables was found.
Regression models in Table 5 reveal that increased age was independently and positively associated with poorer outcomes, including ICU admission, the critical level of symptoms (P < 0.001), and death (P < 0.001), when adjusted for other covariates in the models. Overall, patients with hypertension, periodontitis, and Eichner index, were at higher risk for poorer outcomes. When the predictors were adjusted for age and hypertension as confounders, periodontitis was significantly associated with ICU admission (IRR = 1.44; 95%CI = 1.07 – 1.95; P = 0.017), the incidence of critical symptoms (IRR = 2.56; 95%CI = 1.44 – 4.55; P = 0.001), and risk of death (IRR = 2.05 ; 95%CI = 1.12 – 3.76; P = 0.020). Eichner index (classes B and C) was positively associated with ICU admission risk, probably as a confounder of patient age. When the Eichner index was removed from Model 3 (including periodontitis as a predictor), admission to the ICU was positively associated with age (IRR = 1.02; 95%CI = 1.01 – 1.03; P < 0.001).
TABLE 5.
Risk estimation of the association between COVID‐19 outcomes and selected independent variables
| Model 1* | Model 2** | Model 3*** | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Predictors | IRR (95% CI) | P‐value | IRR (95% CI) | P‐value | IRR (95% CI) | P‐value | |
| ICU admission | Age (in years) | 1.02 (1.01 – 1.03) | <0.001 | 1.01 (1.00 – 1.02) | 0.131 | 1.01 (1.00 – 1.02) | 0.061 | |
| Block 1 | Hypertension | 1.50 (1.05 – 2.15) | 0.027 | 1.16 (0.82 – 1.64) | 0.393 | |||
| Diabetes | 0.82 (0.57 – 1.21) | 0.322 | ||||||
| Obesity | 1.09 (0.77 – 1.53) | 0.638 | ||||||
| Block 2 | Periodontitis | 1.43 (1.06 – 1.95) | 0.021 | 1.44 (1.07 – 1.95) | 0.017 | 1.44 (1.07 – 1.94) | 0.017 | |
| DMFT index | 1.03 (0.72 – 1.47) | 0.869 | ||||||
| Eichner index (A versus BC) | 2.09 (1.30 – 3.36) | 0.002 | 1.61 (1.01 – 2.56) | 0.043 | 1.64 (1.03 – 2.60) | 0.038 | ||
| Critical symptoms | Age (in years) | 1.03 (1.01 – 1.04) | <0.001 | 1.02 (1.00 – 1.04) | 0.070 | 1.03 (1.02 – 1.05) | <0.001 | |
| Block 1 | Hypertension | 2.54 (1.28 – 5.03) | 0.007 | 1.57 (0.78 – 3.17) | 0.210 | |||
| Diabetes | 0.86 (0.47 – 1.59) | 0.635 | ||||||
| Obesity | 1.33 (0.74 – 2.40) | 0.348 | ||||||
| Block 2 | Periodontitis | 2.26 (1.29 – 3.99) | 0.005 | 2.56 (1.44 – 4.55) | 0.001 | 2.52 (1.39 – 4.55) | 0.002 | |
| DMFT index | 1.05 (0.56 – 1.98) | 0.868 | ||||||
| Eichner index (A versus BC) | 2.74 (1.21 – 6.19) | 0.015 | 1.51 (0.71 – 3.23) | 0.284 | ||||
| Death | Age (in years) | 1.04 (1.02 – 1.06) | <0.001 | 1.04 (1.01 – 1.06) | 0.003 | 1.04 (1.02 – 1.06) | <0.001 | |
| Block 1 | Hypertension | 2.86 (1.28 – 6.42) | 0.011 | 1.74 (0.71 – 4.26) | 0.224 | |||
| Diabetes | 0.95 (0.48 – 1.93) | 0.888 | ||||||
| Obesity | 1.30 (0.67 – 2.51) | 0.437 | ||||||
| Block 2 | Periodontitis | 1.77 (0.92 – 3.39) | 0.088 | 2.05 (1.12 – 3.76) | 0.020 | 2.02 (1.08 – 3.77) | 0.028 | |
| DMFT index | 1.34 (0.62 – 2.89) | 0.453 | ||||||
| Eichner index (A versus BC) | 2.12 (0.86 – 5.25) | 0.104 | 1.05 (0.47 – 2.35) | 0.897 | ||||
Note: Data are expressed as incidence rate ratio (IRR) (95% confidence intervals) and P‐values. Statistically significant associations (P < 0.05) are highlighted in bold
* Associations between COVID‐19 outcome and predictors within each block.
** Associations between COVID‐19 outcome, age, and significant predictors (P < 0.20) in Model 1.
*** Only statistically significant predictors included in Model 2, controlled by age, using block‐wise criteria.
Then, patients were categorized as presenting with periodontitis and concurrent comorbidity, and those groups were tested for the occurrence of negative COVID‐19 outcomes (Figure 1). The combined presence of periodontitis and hypertension were associated with a higher risk for severe‐critical symptoms (71.4%; P = 0.004), ICU admission (81.0%; P = 0.005), and death (47.6%; P = 0.006). No greater risks for negative outcomes were observed when the patient presented with periodontitis and concurrent obesity/diabetes.
FIGURE 1.
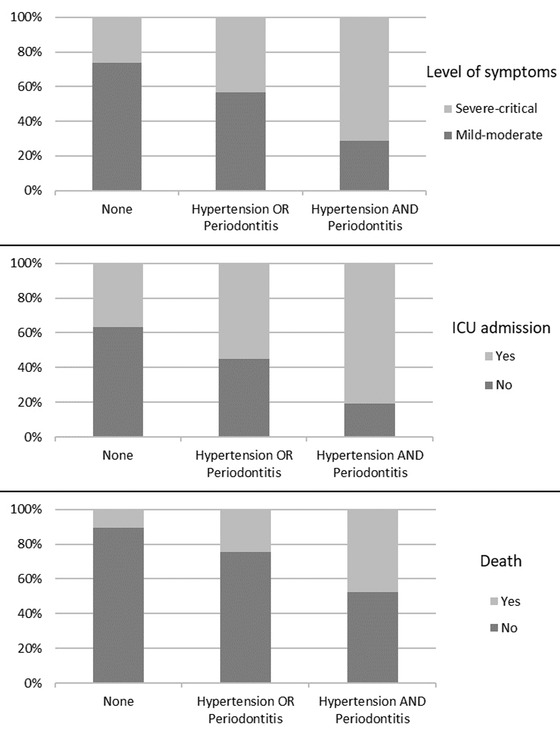
Effects of the combined occurrence of hypertension and periodontitis. Higher incidence rates of severe‐critical symptoms (top panel), intensive care unit admission (middle panel), and death (bottom panel) were observed for patients with hypertension and periodontitis (chi‐squared test, P < 0.01)
4. DISCUSSION
This clinical study reported outcomes of COVID‐19 in hospitalized patients and explored the association between patient outcomes and oral health conditions. Our findings suggest that poor dental and periodontal status are associated with aggravated COVID‐19 and a higher risk for severe‐critical symptoms, ICU admission, and death. Nevertheless, such relevant oral health‐related variables, especially the presence of periodontitis, must be considered in the context of a patient's age and comorbidities, as a relevant confounding variable assessed in this study and may influence both the occurrence of poorer COVID‐19 outcomes and poorer oral health conditions. Even after adjusting for age and hypertension, periodontitis was an important predictor for all severe outcomes of COVID‐19.
A hypothesis for the potential contributory role of periodontitis to aggravate the severity of COVID‐19 was proposed by Lloyd‐Jones et al. (2021), assuming an oral‐vascular‐pulmonary route of SARS‐CoV‐2 infection. 14 The authors suggested that the intraoral SARS‐CoV‐2 can translocate the virus from saliva to the gingival sulcus/periodontal pocket, evading the oral mucosal immune response and promoting the subsequent, direct vascular delivery to the pulmonary vessels. 14 Disease aggravation may be related to inducing the expression of angiotensin‐converting enzyme 2, a receptor for SARS‐CoV‐2, and the consequent production of inflammatory cytokines in the lower respiratory tract and primary pulmonary vasculopathy. 14 , 26 , 27 It was also suggested that the systemic inflammatory breakdown observed in severe COVID‐19 patients and severe periodontitis could aggravate SARS‐CoV‐2 infection. 2 , 4 , 26 , 28 Therefore, our findings reinforce the findings of Marouf et al. (2021), 10 Gupta et al. (2022), 11 and Anand et al. (2021), 7 and provide clinical information concerning the concurrent periodontal status in hospitalized patients, eliminating the likelihood of misdiagnosis of periodontitis in previously treated patients with a healthy reduced periodontium and offering robust evidence of the association of periodontitis and COVID‐19, even after adjustment for significant comorbidities.
Nevertheless, our findings may not be regarded as direct etiological evidence. First, because dental and periodontal diseases, as well as partial and total edentulism, are more prevalent in socially and economically disadvantaged populations, 29 , 30 which may be more likely to be infected by the SARS‐CoV‐2 virus due to higher exposure to the virus spread in the familiar, social, and work environments and are less likely to adopt appropriate preventive measures and to receive proper and timely medical care. 31 Second, there is sound evidence that the incidences of caries, periodontitis, and tooth loss increase with age 32 , 33 and are also highly prevalent in participants with severe comorbidities and deteriorated general health conditions. 34 , 35 Since the risk for aggravated symptoms and mortality rates are higher in COVID‐19 patients with advanced age 36 and participants with risk factors such as obesity, 37 these confounders should be considered in regression models. It is interesting that, in the present study, while the caries index lost significance when adjusting for the other covariates, the magnitude of the association between periodontitis and COVID‐19 outcomes increased when the effect of hypertension was adjusted. This highlights that the importance of the relationship between oral health and COVID‐19 be considered within the context of a common risk factor approach, as the key determinants of health to address risk factors that are common to many chronic conditions within the context of the broader socio‐environmental background for health promotion. 38
We found a significant independent association between hypertension and negative COVID‐19 outcomes in the bivariate regression models. However, well‐known risk factors associated with COVID‐19 outcomes, such as diabetes, obesity, and cardiovascular diseases, were not found to be significant risk factors, and some possible explanations may be considered. First, the diagnoses of the comorbidities included in this study were primarily based on self‐reported information obtained within the stressful context of the pandemic patient care and were not objectively measured by specific exams or previous medical reports. Therefore, there are concerns about the reliability of data related to comorbidities because the prevalence of obesity and diabetes based on self‐reporting was not identical to objective measurements. 39 , 40 , 41 Second, our study may have a sampling bias because hospitalized COVID‐19 patients represent only a small fraction of all participants infected by SARS‐CoV‐2. Considering that the overall prevalence of diabetes and obesity may be higher in patients who develop severe symptoms that require hospitalization, the risk estimates for hospitalized patients may differ from population‐based risk estimates and would affect the estimation of the actual role of such comorbidities in this study. For example, the last National Health Survey in Brazil estimated prevalence of diabetes and cardiovascular disease of 7.7% and 5.3%, respectively, 42 while higher prevalence of comorbidities (30.5% for diabetes, 26.6% for cardiovascular disease, and 40.6% for obesity) were found in our study sample. Finally, most of the initial studies on risk factors for poor COVID‐19 outcomes focused on systemic diseases and did not include oral diseases or conditions as potentially detrimental factors in infected patients. 43 , 44 Therefore, it is plausible to consider that the potential role of periodontitis may have been overlooked in previous studies, and there is substantial evidence to suggest the impact of poor oral conditions on aggravated symptoms and death in COVID‐19, 7 , 11 especially considering the well‐documented impact of periodontitis on the incidence of chronic diseases. 45
Because periodontal disease and the DMFT index are closely related to tooth loss, the Eichner index was used in our study to assess the extent of teeth loss and lack of occlusal support. The findings suggested that advanced tooth loss patterns may be associated with poorer COVID‐19 outcomes. This positive association may be similar to other major chronic diseases and conditions such as stroke, 46 indirectly influenced by common risk factors such as advanced age 38 and increased likelihood of poor nutrition and modified eating behaviors. 47 Local factors associated with tooth loss also predispose individuals to robust inflammatory and immune responses, especially when the primary cause of tooth loss is periodontal disease. 48
Findings from this study emphasize the potential relationship between oral health and the pathophysiology of COVID‐19 and reinforce the role of the dentist and the dental team in the management of COVID‐19 patients and in confronting the pandemic. Our findings suggest that the dentist may play a role in identifying and educating individuals who are more susceptible to severe COVID‐19 outcomes based on their oral health status, alerting patients about their potential risks and the influence of oral diseases on general health. Furthermore, community oral health strategies should be encouraged to disseminate the importance of the daily oral hygiene measures, oral healthcare, and management of oral diseases, especially for older and vulnerable participants, to reduce the colonization of viruses and bacteria in the mouth, and consequently, the risk of related respiratory tract diseases. 49
This study has clear limitations related to data collection. The restricted access to the COVID‐19 areas in the hospital environment and the inherent risk of contamination were major factors hampering patient recruitment and examination. The missing data were mainly due to the difficulty of performing oral examinations and interviews of frail patients or those with severe illness receiving intensive medical care in the ICU setting and incomplete personal and medical data from the patients’ records. Another limitation was the time that the oral examination occurred during hospitalization. The oral examinations should be performed preferably within the first 2 weeks after hospital admission. However, this period may vary depending on the patient's medical condition and availability according to the dental team's schedule. Therefore, to minimize the influence of the current disease state and hospitalization on the development of gingival inflammation, when oral hygiene measures are often neglected, the diagnosis of the periodontal status was dichotomized as healthy/gingivitis or periodontitis. Patients were diagnosed with periodontitis only when the detectable CAL was ≥ 2, assuming that the progression of periodontal disease and associated clinical attachment loss may not develop during the hospitalization period. 50
Concerning the sampling methods and data analysis, the nonrandomized selection of participants may be subjected to bias because some patients with mild and moderate symptoms did not remain hospitalized for more extended periods sufficient to be assessed in the scheduled visits by the research team. Therefore, the magnitude of the associations in this study may be underestimated. Moreover, the relatively small sample size may lead to inaccuracy of the risk estimates; further studies with larger samples size are needed to support the findings of this preliminary observational study. Interventional studies may also be helpful to assess the effect of the control of deleterious oral conditions on the risk of severe COVID‐19 outcomes.
5. CONCLUSION
Within the limitations of this study, we observed a high prevalence of deleterious oral conditions in hospitalized COVID‐19 patients. Among the deleterious oral conditions assessed, periodontitis was associated with a higher occurrence of critical COVID‐19 symptoms and the need for intensive medical care and death, even when adjusted for age and presence of comorbidities.
Further studies with larger sample sizes and more robust designs are required to confirm the role of oral health‐related conditions as contributory risk factors for the aggravation of the disease. Nevertheless, our findings suggest that regular dental/periodontal check‐ups, preventive and treatment measures, and effective self‐oral care appear to positively affect the prevention and minimization of systemic complications in hospitalized patients and should be encouraged within the COVID‐19 pandemic context.
CONFLICT OF INTEREST
The authors declare no conflicts of interest concerning this article's research, authorship, and/or publication.
AUTHOR CONTRIBUTIONS
Camila Alves Costa, Ana Carolina Serafim Vilela, Cláudio Rodrigues Leles, and Nádia Lago Costa contributed to conception and design, acquisition, analysis and interpretation of data, and drafted and critically revised the manuscript. Suzane Aparecida Oliveira, Tiago Dias Gomes, and Alex Alves Costa Andrade contributed to acquisition and analysis of data, and drafted the manuscript. Nádia Lago Costa also contributed to acquisition of funding.
ETHICS STATEMENT
The university and the hospital ethical research committees approved the study (CAEE 38088920.9.0000.5083/38088920.9.3001.5078/38088920.9.3002.5082).
PATIENT CONSENT STATEMENT
All the participants were informed about this study's objectives, risks, and benefits, and those who agreed to participate signed the free informed consent form.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
This study was financially supported by the Foundation for Research Support in the State of Goiás (FAPEG), Brazil, Grant #CVD2020051000009. The funder had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Costa CA, Vilela ACS, Oliveira SA, et al. Poor oral health status and adverse COVID‐19 outcomes: a preliminary study in hospitalized patients. J Periodontol. 2022;00 1‐13. 10.1002/JPER.21-0624
REFERENCES
- 1. Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol. 2020;30:313‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrera D, Serrano J, Roldán S, Sanz M. Is the oral cavity relevant in SARS‐CoV‐2 pandemic?. Clin Oral Investig. 2020;24:2925‐2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID‐19. Oral Dis. 2021;27:768‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahni V, Gupta S. COVID‐19 & periodontitis: the cytokine connection. Med Hypotheses. 2020;144:109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vieira AR. Oral manifestations in coronavirus disease 2019 (COVID‐19). Oral Dis. 2021;27(3):770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sirin DA, Ozcelik F. The relationship between COVID‐19 and the dental damage stage determined by radiological examination. Oral Radiol. 2021;37:600‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anand PS, Jadhav P, Kamath KP, Kumar SR, Vijayalaxmi S, Anil S. A case‐control study on the association between periodontitis and coronavirus disease (COVID‐19). J Periodontol. 2021. 10.1002/jper.21-0272 [DOI] [PubMed] [Google Scholar]
- 8. Botros N, Iyer P, Ojcius DM. Is there an association between oral health and severity of COVID‐19 complications? Biomed J. 2020;43:325‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampson V, Kamona N, Sampson A. Could there be a link between oral hygiene and the severity of SARS‐CoV‐2 infections? Br Dent J. 2020;228:971‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID‐19 infection: a case‐control study. J Clin Periodontol. 2021;48:483‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta S, Mohindra R, Singla M, et al. The clinical association between periodontitis and COVID‐19. Clin Oral Investig. 2022;26(2):1361‐1374. 10.1007/s00784-021-04111-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matuck BF, Dolhnikoff M, Maia GVA, et al. Periodontal tissues are targets for Sars‐Cov‐2: a post‐mortem study. J Oral Microbiol. 2020;13:1848135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomes SC, Fachin S, Fonseca JG, et al. Dental biofilm of symptomatic COVID‐19 patients harbours SARS‐CoV‐2. J Clin Periodontol. 2021;48:880‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lloyd‐Jones G, Molayem S, Pontes CC, Chapple I. The COVID‐19 pathway: a proposed oral‐vascular‐pulmonary route of SARS‐CoV‐2 infection and the importance of oral healthcare measures. J Oral Med Dent Res. 2021;2:1‐25. [Google Scholar]
- 15. World Health Organization . WHO coronavirus (COVID‐19) dashboard. Situation by region, country, territory & area. Accessed February 3, 2022. https://covid19.who.int/table
- 16. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. [DOI] [PubMed] [Google Scholar]
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Oral Health Survey. Basic Methods, 5th edition. World Health Organization; 2013. [Google Scholar]
- 19. Eichner K. Renewed examination of the group classification of partially edentulous arches by Eichner and application advices for studies on morbidity statistics. (Article in German). Stomatol DDR. 1990;40:321‐325. [PubMed] [Google Scholar]
- 20. Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. J Clin Periodontol. 2018;45:S68‐S77. [DOI] [PubMed] [Google Scholar]
- 21. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. J Periodontol. 2018;89:S173‐S182. [DOI] [PubMed] [Google Scholar]
- 22. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions – introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45:S1‐S8. [DOI] [PubMed] [Google Scholar]
- 23. Xia L, Chen J, Friedemann T, et al. The course of mild and moderate COVID‐19 infections – the unexpected long‐lasting challenge. Open Forum Infect Dis. 2020;7:ofaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. General Office of Chinese National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020;133:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolkewitz M, Bruckner T, Schumacher M. Accurate variance estimation for prevalence ratios. Methods Inf Med. 2007;46:567‐571. [DOI] [PubMed] [Google Scholar]
- 26. Shamsoddin E. Is periodontitis associated with the severity of COVID‐19?. Evid Based Dent. 2021; 22: 66‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. Aspiration of periodontophatic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID‐19. J Oral Sci. 2020;63:1‐3. [DOI] [PubMed] [Google Scholar]
- 28. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chisini LA, Sarmento HR, Collares K, Horta BL, Demarco FF, Correa MB. Determinants of dental prosthetic treatment need: a birth cohort study. Community Dent Oral Epidemiol. 2021;49:394‐400. [DOI] [PubMed] [Google Scholar]
- 30. Ghassib IH, Batarseh FA, Wang HL, Borgnakke WS. Clustering by periodontitis‐associated factors – a novel application to NHANES data. J Periodontol. 2021;92:1136‐1150. [DOI] [PubMed] [Google Scholar]
- 31. Mishra V, Seyedzenouzi G, Almohtadi A, et al. Health inequalities during COVID‐19 and their effects on morbidity and mortality. J Healthc Leadersh. 2021;13:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GBD 2017 Oral Disorders Collaborators , Bernabe E, Marcenes W, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the Global Burden of Disease 2017 study. J Dent Res. 2020;99:362‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. López R, Smith PC, Göstemeyer G, Schwendicke F. Ageing, dental caries and periodontal diseases. J Clin Periodontol. 2017;44:S145‐S152. [DOI] [PubMed] [Google Scholar]
- 34. Felton DA. Edentulism and comorbid factors. J Prosthodont. 2009;18:88‐96. [DOI] [PubMed] [Google Scholar]
- 35. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. 2020;83:7‐13. [DOI] [PubMed] [Google Scholar]
- 36. Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID‐19 infection, severity, ICU admission and death: a meta‐analysis of 59 studies. BMJ Open; 11:e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pitones‐Rubio V, Chávez‐Cortez EG, Hurtado‐Camarena A, González‐Rascón A, Serafín‐Higuera N. Is periodontal disease a risk factor for severe COVID‐19 illness?. Med Hypotheses. 2020;144:109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28:399‐406. [DOI] [PubMed] [Google Scholar]
- 39. Flegal KM, Ogden CL, Fryar C, Afful J, Klein R, Huang DT. Comparisons of self‐reported and measured height and weight, BMI, and obesity prevalence from national surveys: 1999‐2016. Obesity (Silver Spring). 2019;27:1711‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onur I, Velamuri M. The gap between self‐reported and objective measures of disease status in India. PLoS One. 2018;13:e0202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie D, Wang J. Comparison of self‐reports and biomedical measurements on hypertension and diabetes among older adults in China. BMC Public Health. 2020;20:1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brazilian Institute of Geography and Statistics (IBGE) . National health survey: 2019: perception of health status, lifestyles, chronic diseases and oral health: Brazil and major regions. (Article in Portuguese). 2020. Accessed February 3, 2022. https://biblioteca.ibge.gov.br/visualizacao/livros/liv101764.pdf [Google Scholar]
- 43. Li J, Huang DQ, Zou B, et al. Epidemiology of COVID‐19: a systematic review and meta‐analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kapila YL. Oral health's inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 2021;87:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chang Y, Woo HG, Lee JS, Song TJ. Better oral hygiene is associated with lower risk of stroke. J Periodontol. 2021;92:87‐94. [DOI] [PubMed] [Google Scholar]
- 47. Kim JK, Baker LA, Davarian S, Crimmins E. Oral health problems and mortality. J Dent Sci. 2013;8(2):115‐120. 10.1016/j.jds.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomaa N, Glogauer M, Tenenbaum H, Siddiqi A, Quiñonez C. Social‐biological interactions in oral disease: a ‘Cells to Society’ view. PLoS One. 2016;11:e0146218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465‐1482. [DOI] [PubMed] [Google Scholar]
- 50. Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986;13:431‐445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


