Abstract
As statins decrease the progression of sepsis and its related mortality, this study aimed to evaluate the effect of atorvastatin on survival and symptom improvement in hospitalized patients with COVID‐19. This randomized controlled trial was performed on 156 hospitalized patients with COVID‐19 in Bojnourd city in 2021. Patients were randomly divided into comparison (standard therapy: hydroxychloroquine + Kaletra®) and intervention groups (atorvastatin 20 mg, SD, plus standard therapy). The main outcomes were the rate of symptom improvement, duration of hospitalization, need for intubation, and mortality rate. In this study, seven patients died, two patients (2.6%) in the comparison group and five (6.6%) in the intervention group. The mean hospitalization days (p = 0.001), the pulse rate (p = 0.004), and the frequency of hospitalization in the ICU ward (18.4% vs. 1.3%) were longer and greater in the intervention group. The remission probability in the comparison group was greater (p = 0.0001). The median hospitalization days in the intervention group was longer (p < 0.001) and remission in the comparison group occurred 1.71 times sooner (hazard ratio = 1.70, 95% confidence interval = 1.22–2.38, p = 0.002). Totally, adding atorvastatin to the standard regime in this study increased hospitalization days and imposed negative effects on symptom improvement in hospitalized patients with COVID‐19.
Keywords: atorvastatin, COVID‐19, pneumonia, treatment
1. INTRODUCTION
The novel coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first reported in Wuhan, China, in December 2019 and has rapidly become a pandemic. 1 It is primarily transmitted by respiratory droplets and close contact, 2 and its incubation period lasts 14–33 days. 3 Asymptomatic patients are able to spread this disease. 4 Infected patients who show mild to moderate symptoms may develop severe pneumonia and acute respiratory distress syndrome, septic shock, multiple organ failure, and even death. The most common sign and symptoms of this disease are fever (98%), dry cough (76%), myalgia or fatigue (44%), and dyspnea (55%), and the less common symptoms are sputum production (28%), headache (8%), hemoptysis (5%), and diarrhea (3%); and chest computerized tomography scans show pneumonia. 5 , 6 Laboratory markers include leukopenia (25%), lymphopenia (63%), thrombocytopenia (5%), and high lactate dehydrogenase (73%). 6
Cytokine storm is the manifestation of COVID‐19 severity which is accompanied by an increase in the plasma level of interleukin (IL)‐2, ‐4, ‐6, ‐7, and ‐10, granulocyte colony‐stimulating factor (GCSF), inducible protein 10 (IP‐10), monocyte chemoattractant protein 1 (MCP‐1), macrophage inflammatory protein 1 alpha (MIP1α), tumor necrosis factor α (TNF‐α) and interferon γ (IFN‐γ). Furthermore, a significant decrease in lymphocyte counts, especially CD8+ T cells, and an increase in neutrophil counts are seen. Thereafter, dynamic cytokine storms and neutrophil‐to‐lymphocyte ratio (NLR) are predictors of developing severe COVID‐19. 2
The pathogenesis of COVID‐19 is categorized into two main courses. The former is a replication cycle that is modulated by viral proteins and includes identification, fusion, entry, and replication of this virus. The latter is the infection progression phase with an inflammatory and immune response to the virus that causes tissue damage. Thus, both virus and host factors are important for the pathogenesis of this disease which is considered a promising target for COVID‐19 therapy. In this regard, probable effective drugs are classified into “target virus” and “target host” categories. 7
At the time of writing this article, just remdesivir has been approved by the Food and Drug Administration (FDA) for the treatment of COVID‐19 requiring hospitalization 8 and some drugs including tocilizumab, sotrovimab, propofol‐liuro 1%, bamlanivimab and etesevimab, casirivimab and imdevimab, baricitinib, COVID‐19 convalescent plasma, fresenius propoven 2%, and REGIOCIT have issued emergency use authorization declaration in certain conditions. 9
Furthermore, since the outbreak of COVID‐19, some old drugs are suggested to manage this disease in clinical trials based on the in vitro and in vivo studies. It seems drug repositioning is able to help in controlling this urgent outbreak faster and more economically. 7
A group of Iranian specialist physicians in treating COVID‐19 recommended antiviral medications to treat hospitalized patients with COVID‐19. At the time of designing this clinical trial study, the sixth edition of this recommendation was proposed. The recommended antiviral monotherapy regime was chloroquine (500 mg q12h on the first day followed by 250 mg q12h, orally) or hydroxychloroquine (HQ; 400 mg q12h on the first day followed by 200 mg q12h, orally) for 7–14 days based on the clinical progress. In combination therapy, lopinavir/ritonavir (400/100 mg q12h, orally) was administrated with chloroquine (500 mg SD, orally) or HQ (400 mg SD, orally) for the first day that was followed just with lopinavir/ritonavir (400/100 mg q12h, orally) for 7–14 days. In combination therapy with atazanavir/ritonavir, atazanavir/ritonavir (300/100 mg SD, orally) plus chloroquine (250 mg q12h, orally) or HQ (200 mg q12h, orally) were administrated from the first day for 7–14 days. 10
In addition to cholesterol‐lowering ability, 3‐hydroxy‐3‐methyl‐glutaryl‐CoA (HMG‐CoA) reductase inhibitors (statins) are able to diminish inflammation and induce immunomodulatory, antioxidative, and anti‐atherosclerotic effects. 11 , 12 They inhibit liver production of C‐reactive protein by reducing interleukin‐6‐induced C‐reactive protein production in human hepatocytes. 13 Atorvastatin reduces C‐reactive protein‐induced chemokine secretion, ICAM‐1 upregulation and chemotaxis in adherent human monocytes. 14 Rosuvastatin reduces plasma concentrations of pro‐inflammatory cytokines TNF‐alpha and IFN‐gamma and decreases TNF‐alpha and IFN‐gamma production in stimulated T‐lymphocytes. It inhibits the Th‐1‐immune response. 15 Besides these pleiotropic effects, these drugs showed promising therapeutic effects against various infectious diseases. In a preclinical study, adding simvastatin, fluvastatin, or pravastatin increased antitubercular activity of rifampicin, isoniazid, and pyrazinamide. 16 In preclinical models, they showed favorable antiviral effects. For instance, atorvastatin restricts human immunodeficiency virus (HIV) replication in CD4+ T cells, 17 lovastatin inhibits respiratory syncytial virus (RSV) replication, and virus‐induced cell‐to‐cell fusion, 18 and simvastatin shows anti CMV effects. 19
Not only preclinical studies but also do clinical and retrospective studies prove honorable anti‐infective and antiviral effects of statins. They reduced the mortality rate in elderly patients with community‐acquired pneumonia and sepsis, 20 bacteriemia, 21 and viral pneumonia. 22
Due to the above‐mentioned subjects, questions have arisen whether statins are associated with the survival of patients with COVID‐19. In this regard, this study was designed to evaluate the therapeutic efficacy of atorvastatin in combination with national protocols on the survival of patients with COVID‐19.
2. PATIENTS AND METHODS
2.1. Study design
This single‐blind, randomized clinical trial was designed to evaluate the efficacy of atorvastatin in the treatment of patients with COVID‐19. Patients with severe COVID‐19 who were hospitalized from April 20 to May 20, 2021, in Imam Hasan Hospital Center, a referral hospital in Bojnurd, Iran were included. Ethical approval for this study was obtained from Research Ethics Committee in North Khorasan University of Medical Sciences (IR.NKUMS.REC.1399.035). Furthermore, the protocol of the study was registered at the Iranian Registry of clinical trials (IRCT20190831044653N5). Written informed consent was obtained from all patients or their first‐degree family members after receiving an explanation of the study. The sample size was determined through clinical significance. Based on the expert opinions, a 1‐day decrease in the length of stay in the hospital was considered significant. Therefore, the sample size was determined to consist of 50 patients for each group using the G‐power software with a confidence interval (CI) of 95% and a power of 80%. However, given the probability of a 25% drop in samples, at least 75 patients were assigned to each group.
2.2. Participants
COVID‐19 patients were diagnosed based on the WHO criteria and clinical symptoms/signs by an infectious diseases specialist. 10
Those who need hospitalization and did not meet the following exclusion criteria were included in this study. Exclusion criteria were including age <16 years old, need for hospitalization in an intensive care unit (ICU) at admission, a history of type 1 diabetes, ketoacidosis, uncompensated heart failure, severe renal failure (GFR < 30 ml/min), metabolic acidosis, severe respiratory failure need to intubation, and sensitivity to atorvastatin. Furthermore, pregnant and lactating women were not included.
2.3. Randomization, interventions, and follow‐up
Eligible patients were randomly divided into the intervention and comparison groups according to the permuted blocks of 4 in a randomization sequence (1:1 ratio). Patients in the intervention group received atorvastatin along with the national protocol medications, while in the comparison group, patients received only the national protocol medications. Allocation assignment was concealed from patients and statistical analyzers, but the clinicians and the nurse who were involved in managing and attending the patients in the ward were aware of the allocations and therapies. Atorvastatin (Sobhan, Iran) was administrated as 20 mg every day, orally. The national protocol 10 consisted of lopinavir/ritonavir (400/100 mg q12h, orally) with HQ (400 mg SD, orally) for the first day that was followed just with lopinavir/ritonavir (400/100 mg q12h, orally) for 7–14 days. Patients received drugs till they discharged from the hospital.
Other supportive care such as fluid therapy, treatment of electrolyte disorders, and antibiotic therapy was considered according to the hospital protocols. The duration of the study was from the hospitalization of studied patients until the time of discharge from the hospital or death.
Patient demographic data, baseline diseases, symptoms at the time of disease presentation, vital signs, and laboratory data at the time of hospital admission were recorded. Patients were daily monitored in terms of changes in the vital signs, hemodynamic parameters, oxygenation status, laboratory data, and treatment strategies. The need for supplemental oxygen therapy and also invasive or noninvasive respiratory supports were evaluated regularly.
Based on the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines for the management of dyslipidemias, 23 emergency termination conditions during the study included an increase in liver enzyme levels (defined as more than three times the upper limit of normal) and new clinically diagnosed myopathy, as identified by treating clinicians.
2.4. Outcome measures
The primary outcome was the length of stay in the hospital from admission to discharge through survival analysis. Therefore, all eligible patients had been followed till they get better and were discharged from the hospital. Those with uncompleted data were considered as the right‐censored. Also, the secondary outcomes in this trial were the need for hospitalization in the ICU during the study and paraclinical findings.
2.5. Statistical analysis
After discharge of all patients from the hospital, the time until complete remission of symptoms and recovery was assessed. Patients who had incomplete information or who died at the time of discharge from the hospital were considered right‐censored. Cox proportional‐hazards regression models were used to estimate the relationship between the administration of atorvastatin and remission. In cox regression, the proportional hazard (PH) assumption is very important. This assumption means that the relative hazard is constant over time for the study groups. Before modeling, PH assumption for all predictor variables was checked through the Schoenfeld residuals analysis test. Then, each of the predictors was examined separately using cox regression. Variables that were significantly associated with remission in the univariate cox regression model were entered into the multivariate cox regression model. The results were reported as hazard ratio (HR) with a 95% CI. Kaplan–Meier analysis was applied to plot and estimate remission probabilities. Comparison of demographic and clinical characteristics of the studied groups was performed using appropriate analytical tests. All analyzes were done by SPSS version 24 software.
3. RESULTS
The flow diagram of participants in this clinical trial is shown in Figure 1. Initially, 191 patients with severe COVID‐19 were included in the study using the inclusion criteria. However, 26 patients who were later found not eligible and 9 patients who were not willing to participate were excluded. Finally, the sample consisted of 156 patients with severe COVID‐19 who were randomly assigned to two equal groups (intervention and comparison groups). The mean length of hospital stay was 6.40 ± 6.25 days for 154 patients with COVID‐19 who met the inclusion criteria. The mean age of the patients was 51.80 ± 17.40 years and 50.6% of the patients were male. The demographic and clinical characteristics of the study groups at baseline are shown in Table 1. Furthermore, the available baseline values of some laboratory tests of some patients are included in Table 1. The results showed that there was no significant difference between the two groups in terms of age, sex, respiratory rate, blood pressure, body temperature, cough, dyspnea, and diarrhea (p > 0.05). It seems two groups were not statistically different in platelets count, hemoglobin, plasma creatinine, aspartate transaminase, alanine transaminase, alkaline phosphatase, total bilirubin, direct bilirubin, and lactate dehydrogenase level (p > 0.05). Unfortunately, the data about CPK at the baseline was missed.
Figure 1.
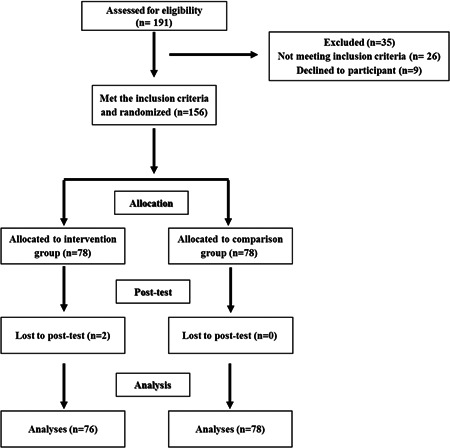
Flow diagram of the present study
Table 1.
Participant characteristics at baseline
| Variables | Standard | Intervention | p |
|---|---|---|---|
| Age,a median (IQR), year | 56 (36–64) | 46 (37–65) | 0.90 |
| Sex (male),b No (%) | 40 (51.9) | 38 (50) | 0.81 |
| Respiratory rate (the number of breaths per minute),a median (IQR) | 18 (17–19) | 18 (17–19) | 0.60 |
| Blood pressure (mmHg),c mean + SD | 124.56 (27.92) | 126.76 (17.61) | 0.56 |
| Pulse rate (beats per minute),a median (IQR) | 85 (80–89) | 90 (83–109) | 0.004 |
| Body temperature (°C),a median (IQR) | 37 (37–37.15) | 37.05 (37–38) | 0.10 |
| Cough,b No (%) | 49 (64.5) | 59 (77.6) | 0.07 |
| Myalgia,b No (%) | 44 (57.9) | 15 (19.7) | 0.001 |
| Dyspnea,b No (%) | 66 (85.7) | 62 (81.6) | 0.49 |
| Diarrhea,b No (%) | 4 (5.3) | 6 (7.9) | 0.51 |
| White blood cell (×103/µl),a median (IQR) | 5.7 (4.3–7.4) | 6.4 (5–9.8) | 0.04 |
| Hemoglobin (g/dl),a median (IQR) | 13.7 (12.5–14.7) | 13.5 (12.3–14.6) | 0.86 |
| Platelet (×103/µl),a median (IQR) | 172 (132–234) | 174 (142–219) | 0.80 |
| Plasma creatinine (mg/dl),a median (IQR) | 1.10 (0.90–1.30) | 1.10 (1–1.30) | 0.22 |
| Aspartate transaminase (U/L),a median (IQR) | 36.5 (21.25–50.75) | 28 (20–40.50) | 0.30 |
| Alanine transaminase (U/L),a median (IQR) | 22 (17.25–54.50) | 25 (18.5–40) | 0.83 |
| Alkaline phosphatase (IU/L),a median (IQR) | 188.5 (152.75–246) | 186 (130.50–206.50) | 0.58 |
| Total bilirubin (mg/dl),a median (IQR) | 0.86 (0.77–1.12) | 0.90 (0.70–1.15) | 0.73 |
| Direct bilirubin (mg/dl),a median (IQR) | 0.20 (0.115–0.325) | 0.20 (0.20–0.30) | 0.87 |
| Lactate dehydrogenase (U/L),a median (IQR) | 497 (384.5–603) | 491 (376.75–574.25) | 0.60 |
Note: The significant level is p < 0.05.
Abbreviation: IQR, interquartile range.
Mann–Whitney U‐test.
Chi‐squared tests.
Student's t‐test.
Follow‐up of the studied patients started from the time of admission and the beginning of the study and continued until the time of discharge from the hospital or death. During this period, a total of 7 patients with COVID‐19 died, 2 patients (2.6%) in the standard treatment group and 5 patients (6.6%) in the standard treatment group + atorvastatin) while other 147 patients recovered and were discharged from the hospital. The patients receiving atorvastatin had a higher mean length of hospital stay (7.72 days vs. 5.06 days, p = 0.001) and pulse rate (94.26 per minute vs. 87.87 per minute, p = 0.004) compared to the patients who did not receive atorvastatin. Also, the frequency of ICU hospitalization was higher in the patients receiving atorvastatin (18.4%) compared to the patients who did not receive atorvastatin (1.3%; Table 2).
Table 2.
Clinical characteristics of the study groups
| Variables | Standard | Intervention | p |
|---|---|---|---|
| Length of stay in the hospital,a median (IQR), day | 4 (3–6) | 6.5 (4–9) | 0.001 |
| Discharged alive,b No (%) | 76 (97.4) | 71 (93.4) | 0.27 |
| ICU admission,b No (%) | 1 (1.3) | 14 (18.4) | 0.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Mann–Whitney U‐test.
Fisher's exact test. The significant level is p < 0.05.
Figure 2 shows the Kaplan–Meier plot for the atorvastatin and atorvastatin‐free groups. The patients receiving standard treatment (97.4%) were more likely to recover than those receiving atorvastatin (93.4%), which is a statistically significant difference (p = 0.0001). Table 3 shows the mean and median length of hospital stay of the patients. The median length of hospital stay in the intervention and control groups was 7 and 4 days, respectively. In other words, 50% of the patients in the intervention group after 7 days and in the control group after 4 days from the start of the study have been discharged from the hospital.
Figure 2.
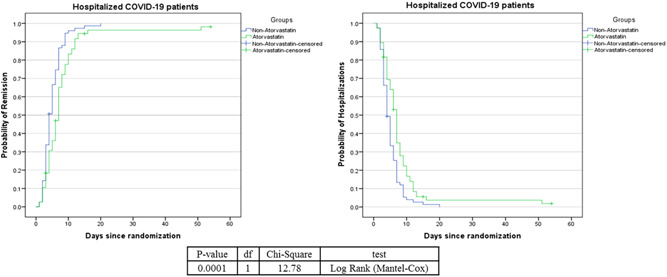
Kaplan–Meier plot comparing the survival time (in day) between the atorvastatin and non‐atorvastatin groups
Table 3.
Mean and median of hospital stay (day) in the study groups
| Group | Mean | Standard error | 95% confidence interval | Median | Standard error | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Standard | 5.1 | 0.35 | 4.41 | 5.8 | 4 | 0.34 | 3.32 | 4.67 |
| Intervention | 8.31 | 1.12 | 6.1 | 10.52 | 7 | 0.38 | 6.24 | 7.75 |
| total | 6.62 | 0.58 | 5.51 | 7.79 | 5 | 0.4 | 4.26 | 5.79 |
Before performing the cox models (PH cox models), the PH assumption was checked and confirmed for all the studied variables. The crude and adjusted HRs for the relationship between the studied variables and survival time is shown in Table 4. Variables that were significant in the univariate/crude model were entered into the multivariate cox regression model to adjust or control them. In univariate analysis, it was observed that only there was a statistically significant relationship between age and groups with improvement, in such a way that with increasing age of the patients, the improvement decreases by 1% and also the improvement in the control group (standard treatment) occurred 1.71 times faster than intervention group (standard treatment + atorvastatin) (HR = 1.71, 95% CI = 1.23–2.38, p = 0.002). According to the results of the study, male patients recovered 1.14 times faster than female patients, but it was not statistically significant (HR = 1.14, 95% CI = 0.82–1.57, p = 0.430). Also, no statistically significant relationship was observed between other studied variables with improvement (p > 0.05). Variables that had a statistically significant relationship with improvement in the crude Cox regression model were used for multivariate modeling. As can be seen in the adjusted Cox regression model for age, there was still a significant relationship between the two groups with improvement, in which the HR of recovery in the control group (standard treatment) was higher than the intervention group (standard treatment + atorvastatin) by 1.70 times. In other words, the time of recovery in the control group was shorter than the time of recovery in the intervention group (HR = 1.70, 95% CI = 1.22–2.38, p = 0.002).
Table 4.
Data analysis using the crude and adjusted Cox regression model
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI for HR | p | HR | 95% CI for HR | p |
| Age, year | 0.99 | 0.98–1 | 0.048 | 0.99 | 0.981–1.00 | 0.039 |
| Sex, male | 1.14 | 0.82–1.57 | 0.43 | |||
| Respiratory rate in the first day, the number of breaths per minute | 0.919 | 0.83–1.01 | 0.087 | |||
| BP in the first day, mmHg | 0.996 | 0.99–1.003 | 0.27 | |||
| Pulse rate in the first day, beats per minute | 0.995 | 0.98–1.006 | 0.39 | |||
| Body temperature in the first day, °C | 1.014 | 0.94–1.092 | 0.71 | |||
| No cough | 1.06 | 0.73–1.53 | 0.74 | |||
| Myalgia | 1.17 | 0.84–1.64 | 0.35 | |||
| No dyspnea | 1.1 | 0.7–1.7 | 0.67 | |||
| Diarrhea | 1.07 | 0.56–2.04 | 0.83 | |||
| Standard therapy | 1.71 | 1.23–2.38 | 0.002 | 1.7 | 1.22–2.38 | 0.002 |
Abbreviations: BP, blood pressure; CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
For patients with COVID‐19 admitted to the hospital, giving atorvastatin 20 mg/day accompanied with the standard therapy resulted in a significant increase in the length of stay at the hospital, pulse rate, and the frequency of ICU admission compared with the standard group. The median days of stay at the hospital in the intervention group were longer than the standard group. Furthermore, remission in the intervention group occurred later than the standard group.
Our findings are consistent with this study that emphasized the deleterious effects of statins on COVID‐19 clinical outcomes including prolonged hospital stay (≥7 days) and/or need for invasive mechanical ventilation although this study didn't show any association between statin use and mortality. 24 Furthermore, the results from the CORONADO study verify our data that reported routine use of statins increased COVID‐19 related mortality in inpatients with type 2 diabetes. 25 Peymani et al. 26 in a retrospective cohort study in Iranian COVID‐19 patients (75 subjects) reported statin use did not decrease the risk of morbidity and death. But not significantly, statin use reduced the chance of being subjected to mechanical ventilation and normalizing computed tomography (CT) scan results. Three different types of used statins were atorvastatin (94.7%), rosuvastatin (2.7%), and simvastatin (2.7%). In these subjects, 14% of patients had been using statins for over 5 years, 22% for 1–5 years, and the rest (64%) for less than 1 year before hospital admission. Furthermore, the meantime of statin use during the stay at the hospital was 7.37 ± 4.84 (SD) days. 26 A randomized clinical trial showed administration of atorvastatin 20 mg once daily to adults with COVID‐19 who were hospitalized in the ICU for 30 days irrespective of hospital discharge status had no significant reduction in the composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all‐cause mortality. The authors relate this ineffectiveness to the time of study and explicate that the effect of atorvastatin on thrombosis or mortality may be apparent beyond the 30 days follow‐up; therefore, they are assessing this hypothesis in an ongoing 90 days study. Furthermore, designing a study with a more potent statin regimen or evaluation in a larger population of COVID‐19 patients was suggested. They mentioned that statin beneficial effects may be seen before the inflammatory response phase in early COVID‐19 and maybe for these very sick patients who are admitted to the ICU, it is just too late. 27
Our results were in contrast with Davoodi et al.'s study 28 that conducted a similar study on COVID‐19 inpatients and evaluated the impact of adding atorvastatin to standard therapy, lopinavir/ritonavir, on the duration of hospitalization. However, the dose of atorvastatin (40 mg daily) and the number of enrolled patients (n = 20 in each group) were different in our study. They prescribed atorvastatin concomitantly with Kaletra® just for 5 days and the duration of follow‐up and the other therapies were not mentioned, while in our study patients received standard regimes for 7–14 days and atorvastatin till they were discharged from the hospital that was not more than 14 days based on Tables 2 and 3, so intervention group received atorvastatin exactly with Kaletra® in the whole of the study. Furthermore, in the standard regime, none of Davoodi et al. study's 28 patients received HQ that was included in our study. HQ is the substrate of CYP2D6 and CYP3A4, and ritonavir, a component of Kaletra®, is a potent CYP3A inhibitor, so can elevate the plasma concentration of HQ and potentiates HQ's side effects including cardiovascular, gastrointestinal, nervous system and dermatological problems, neuromuscular, skeletal, ophthalmic, and otic disorders. 7
A cohort study showed statin use in residents diagnosed with COVID‐19 was accompanied by less severe symptoms and improvement of clinical outcomes. 29 Based on another retrospective study by Daniels et al., 30 the use of statins one month before hospitalization for COVID‐19 was associated with a lower risk of severe COVID‐19, and a faster time to disease recovery. Gupta et al. 31 reported that antecedent statin use in patients hospitalized with COVID‐19 is associated with lower inpatient mortality. These studies did not mention the types of used statins. Another study showed pretreatment with simvastatin reduces LPS‐induced human lung injury. Simvastatin induces anti‐inflammatory in the pulmonary and systemic compartment, reduces neutrophilia, myeloperoxidase, tumor necrosis factor‐alpha, matrix metalloproteinase 7, 8, and 9 in the bronchoalveolar lavage fluid and C‐reactive protein in plasma. 32
Furthermore, these controversial differences and conflicting results about statins in COVID‐19 clinical outcomes may be results of their effects on angiotensin‐converting enzyme (ACE) 2 expression, and paying attention to drug–disease interactions may explain the negative impacts of statins in COVID therapy as described in the following.
The renin‐angiotensin (Ang) system cascade is divided into ACE/Ang II type 1 receptor (AT1)/AngII and ACE2/Ang(1–7) receptor Mas (Mas)/Ang(1‐7) axes. The vasoconstrictor and proliferative actions of the renin Ang system are mediated by ACE and AngII, while ACE2 opposes the ACE action. It converts AngI to Ang(1–9) and AngII to Ang(1–7) causing vasodilator and anti‐inflammatory properties. 33 Recently, ACE2 which is expressed in the respiratory airways, intestine, kidney, heart, and pancreas has been proposed as SARS‐CoV‐2 virus entering receptor. 34 On the other side, ACE2 overexpression is associated with reduced severity of acute respiratory distress syndrome. 35 Therefore, ACE2 overexpression on one hand increases the chance of SARS‐CoV‐2 virus‐cell entrance and on the other hand can diminish the severity of COVID‐19, two opposite outcomes!
Statins by fitting into the active site of the 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A (HMG‐CoA) reductase enzyme inhibit its action competitively and block the l‐mevalonate pathway. 36 By blocking this pathway, they inhibit cholesterol synthesis and induce immune‐modulatory effects. 37 Furthermore, statins promote tissue‐specific upregulation of ACE2, 38 the used receptor by the SARS‐CoV‐2 virus for cell entrance. 34
Guiding statin effects in the way of immunomodulatory and the way that the overexpression of ACE2 results in reduced severity of acute respiratory distress syndrome instead of promoting SARS‐CoV‐2 virus‐cell entrance is important and finding it could answer why there are controversial differences about statins effects in COVID‐19 clinical outcomes.
Based on this aforementioned information, the Bikdeli et al. 27 conclusion, and some related studies, 29 , 30 , 31 it seems the start of statin therapy at the early phase of COVID‐19, before need to hospitalization or start of the inflammatory process, could be effective and need to be evaluated in future clinical trials.
Beyond many clinical benefits, the adverse effects of statins are not rare. Muscle adverse effects are the most recognized contrary effects of statins including myositis, myalgia, and rhabdomyolysis secondary to severe muscle damages; many other organ failures have been reported in the context of statins rhabdomyolysis. CYP3A has a fundamental role in statins metabolism, so any competition in this pathway affects statins serum concentration and leads to drug interactions. 39 Both lopinavir and ritonavir are potent CYP3A inhibitors and their concurrent administration with statins raises statin concentrations and the risk of toxicity. 40 It is a predictable drug interaction and based on references atorvastatin 20 mg/day 41 was selected herein, however, it was better we evaluated enrolled patients for rhabdomyolysis, an outcome that was missed in this study, and its measuring was just limited to symptomatic patients during the study, while Davoodi et al. 28 excluded patients with myositis and liver injury form their study.
Disease–drug interactions are the other important item in pharmacotherapy. Myalgia is one of the earliest clinical characteristics of COVID‐19, and an increase in liver enzymes may occur in this disease. 42 Both myalgia, the main symptom of myositis, and liver function tests elevation are not rare side effects of statins. 39 Thereafter, statin therapy in patients with COVID‐19 may increase the risk of these aforementioned side effects 40 and may explain the deteriorative effects of statins in COVID‐19.
A hospital‐based study in Iranian patients determined the prevalence of atorvastatin‐induced myalgia is 44.3% and discussed gender, age, atorvastatin dose, duration of atorvastatin usage, and presence of myotoxic disease are the main predictors of myalgia in the Iranian population. 41 In our study, there are no significant differences in age and gender of both control and intervention groups, but we do not have any information about the medical history of our patients and duration of atorvastatin usage before admission to this study. This is another limitation of our study, while patients with the use of statins, chloroquine, HQ, and lopinavir/ritonavir were not eligible in the Davoodi et al. study. 28
5. CONCLUSION
Among inpatients with CoVID‐19, atorvastatin along with the national protocol medications, compared with only the national protocol medications, significantly increased the length of stay at the hospital and the frequency of ICU admission. The findings do not support the use of atorvastatin for the treatment of inpatients with COVID‐19.
The effects of more potent doses of statins with tight control on the inclusion criteria and side effects, and evaluation in a more targeted population of patients with COVID‐19 may clarify, support, or reject this data in the future. It is recommended that critical care scores, such as SOFA or qSOFA scores, be added to the data to more accurately understand and assess the true situation between the two groups of patients. Furthermore, it might be beneficial to test and verify the effect of statins in outpatients who are in the earlier phase of the COVID‐19, to prevent the start of the inflammatory process, rather than trying to stop it.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Maryam Rameshrad and Majid Ghafoori: Substantial contributions to the conception and design of the work; Marzieh Pakzad and Morteza Behnamfar: Data curation; Hassan Saadati: Design of the work, data analysis, and interpretation; Majid Ghafoori, Mohammadreza Taghavi, Amir Azimian, and Mina Sadat Mohajerzadeh: Investigation; writing – review and editing; Maryam Rameshrad, Majid Ghafoori, Hassan Saadati, and Peiman Alesheikh: Writing – original draft. All authors have read and approved the manuscript and take full responsibility for its content.
ACKNOWLEDGMENTS
The authors thank the Research Vice‐Chancellors of North Khorasan University of Medical Sciences. This study was supported by the North Khorasan University of Medical Sciences (Grant no. 990004).
Ghafoori M, Saadati H, Taghavi M, et al. Survival of the hospitalized patients with COVID‐19 receiving atorvastatin: A randomized clinical trial. J Med Virol. 2022;94:3160‐3168. 10.1002/jmv.27710
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu B, Huang S, Yin L. The cytokine storm and COVID‐19. J Med Virol. 2021;93(1):250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bikbov B, Bikbov A. Maximum incubation period for COVID‐19 infection: do we need to rethink the 14‐day quarantine policy? Travel Med Infect Dis. 2021;40:101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382(10):970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019‐nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rameshrad M, Ghafoori M, Mohammadpour AH, Nayeri M, Hosseinzadeh H. A comprehensive review on drug repositioning against coronavirus disease 2019 (COVID19). Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1137‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beware of Fraudulent Coronavirus Tests, Vaccines and Treatments . 2021. https://www.fda.gov/consumers/consumer-updates/beware-fraudulent-coronavirus-tests-vaccines-and-treatments.
- 9. Drug and Biological Therapeutic Products . 2021. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs
- 10. Flowchart Diagnosis and Treatment of COVID 19 Disease at Outpatient and Inpatient Levels , 6th edition. 2020. https://medcare.behdasht.gov.ir/uploads/%D9%86%D8%B3%D8%AE%D9%87_%D8%B4%D8%B4%D9%85_%D9%81%D9%84%D9%88%DA%86%D8%A7%D8%B1%D8%AA.pdf
- 11. Kwak BR, Mulhaupt F, Mach F. Atherosclerosis: anti‐inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2(6):332‐338. [DOI] [PubMed] [Google Scholar]
- 12. Kim SW, Kang HJ, Jhon M, et al. Statins and inflammation: new therapeutic opportunities in psychiatry. Front Psychiatry. 2019;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer C, Gruber HJ, Landl EM, et al. Rosuvastatin reduces interleukin‐6‐induced expression of C‐reactive protein in human hepatocytes in a STAT3‐ and C/EBP‐dependent fashion. Int J Clin Pharmacol Ther. 2007;45(6):319‐327. [DOI] [PubMed] [Google Scholar]
- 14. Garjani A, Rezazadeh H, Andalib S, et al. Ambivalent effects of atorvastatin on angiogenesis, epidermal cell proliferation and tumorgenesis in animal models. Iran Biomed J. 2012;16(2):59‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Link A, Ayadhi T, Böhm M, Nickenig G. Rapid immunomodulation by rosuvastatin in patients with acute coronary syndrome. Eur Heart J. 2006;27(24):2945‐2955. [DOI] [PubMed] [Google Scholar]
- 16. Dutta NK, Bruiners N, Zimmerman MD, et al. Adjunctive host‐directed therapy with statins improves tuberculosis‐related outcomes in mice. J Infect Dis. 2020;221(7):1079‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS. 2016;30(2):171‐183. [DOI] [PubMed] [Google Scholar]
- 18. Gower TL, Graham BS. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob Agents Chemother. 2001;45(4):1231‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blanc M, Hsieh WY, Robertson KA, et al. Host defense against viral infection involves interferon mediated down‐regulation of sterol biosynthesis. PLOS Biol. 2011;9(3):e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sapey E, Patel JM, Greenwood H, et al. Simvastatin improves neutrophil function and clinical outcomes in pneumonia. A pilot randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(10):1282‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stamatogiannis N, Makris D, Zakynthinos E. Statins in bacteremia, sepsis and pneumonia: have we found the holy grail. Recent Pat Inflamm Allergy Drug Discov. 2009;3(3):167‐176. [DOI] [PubMed] [Google Scholar]
- 22. Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin‐converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018;31(4):419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41(1):111‐188. [DOI] [PubMed] [Google Scholar]
- 24. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID‐19 outcomes in hospitalized patients. PLOS One. 2021;16(9):e0256899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cariou B, Goronflot T, Rimbert A, et al. Routine use of statins and increased COVID‐19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2021;47(2):101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peymani P, Dehesh T, Aligolighasemabadi F, et al. Statins in patients with COVID‐19: a retrospective cohort study in Iranian COVID‐19 patients. Transl Med Commun. 2021;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bikdeli B, Talasaz AH, Sharif‐Kashani B, et al. Atorvastatin versus placebo in patients with covid‐19 in intensive care: randomized controlled trial. BMJ. 2022;376:e068407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davoodi L, Jafarpour H, Oladi Z, et al. Atorvastatin therapy in COVID‐19 adult inpatients: a double‐blind, randomized controlled trial. IJC Heart Vasc. 2021;36:100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Spiegeleer A, Bronselaer A, Teo JT, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21(7):909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daniels LB, Sitapati AM, Zhang J, et al. Relation of statin use prior to admission to severity and recovery among COVID‐19 inpatients. Am J Cardiol. 2020;136:149‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta A, Madhavan MV, Poterucha TJ. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID‐19. Res Sq. 2020:rs.3.rs‐56210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shyamsundar M, McKeown ST, O'Kane CM, et al. Simvastatin decreases lipopolysaccharide‐induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179(12):1107‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santos RA, Ferreira AJ, Simões ESAC. Recent advances in the angiotensin‐converting enzyme 2‐angiotensin(1‐7)‐Mas axis. Exp Physiol. 2008;93(5):519‐527. [DOI] [PubMed] [Google Scholar]
- 34. Zamorano Cuervo N, Grandvaux N. ACE2: evidence of role as entry receptor for SARS‐CoV‐2 and implications in comorbidities. Elife. 2020;9:e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wösten‐van Asperen RM, Bos AP, Bem RA, et al. Imbalance between pulmonary angiotensin‐converting enzyme and angiotensin‐converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med. 2013;14(9):e438‐e441. [DOI] [PubMed] [Google Scholar]
- 36. Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;33(11):1569‐82. [PubMed] [Google Scholar]
- 37. Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tikoo K, Patel G, Kumar S, et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93(3):343‐351. [DOI] [PubMed] [Google Scholar]
- 39. Golomb BA, Evans MA. Statin adverse effects. Am J Cardiovasc Drugs. 2008;8(6):373‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dashti‐Khavidaki S, Khalili H. Considerations for statin therapy in patients with COVID‐19. Pharmacotherapy. 2020;40(5):484‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dastan F, Salamzadeh J, Saffaei A, Nabavi Y, Abbasinazari M. Atorvastatin‐induced myalgia in Iranian patients: a hospital‐based study to determine the prevalence and associated risk factors. J Pharm Care. 2020;8:4. [Google Scholar]
- 42. Guan W‐j, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


