Abstract
Interleukin‐38 (IL‐38) has recently been considered as a cytokine with anti‐inflammatory properties in viral respiratory infections, particularly coronavirus disease 19 (COVID‐19), but the evidence has not been well elucidated. Therefore, a case‐control study was conducted to determine IL‐38 serum levels in 148 patients with COVID‐19 (45 moderate, 55 severe, and 48 critical) and 113 controls. Results demonstrated that IL‐38 levels did not show significant differences between patients and controls (68.7 [interquartile range: 62.7–75.6] vs. 67.7 [58.0–82.6] pg/ml; probability = 0.457). Similarly, patients stratified by disease severity, age group, gender, or chronic disease showed no significant differences between IL‐38 levels in each stratum. Whereas, overweight/obese patients had a significantly lower median of IL‐38 compared to normal‐weight patients. Further, IL‐38 showed significantly higher levels in the age group ≥50 years of patients with critical illness than in the age group <50 years. Female patients with severe disease also showed significantly elevated levels of IL‐38 compared to male patients. In conclusion, the study indicated that serum IL‐38 levels were not affected by COVID‐19 infection, but the distribution of patients according to disease severity, age, gender, and body mass index may better reveal the role of IL‐38 in disease pathogenesis.
Keywords: age, body mass index, COVID‐19, disease severity, gender, interleukin‐38
1. INTRODUCTION
With over 300 million reported cases and over 5.5 million deaths, the coronavirus disease 2019 (COVID‐19) pandemic has become one of the most serious health emergencies of the 21st century. It is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a single‐stranded enveloped RNA virus, first reported in Wuhan in December 2019. 1 The virus infects the human respiratory system and causes hyperactivity of the immune system, resulting in a high degree of inflammation, lung damage and other comorbidities. 2 A particular feature of this is increased levels of proinflammatory cytokines such as interleukin (IL)‐1, IL‐6, and tumor necrosis factor (TNF)‐α, particularly in patients with severe COVID‐19, a condition called cytokine storm or acute respiratory distress syndrome (ARDS). 3
Cytokines are low molecular weight signaling proteins produced by immune and nonimmune cells in response to various stimuli including pathogens such as viruses and bacteria to regulate innate and adaptive immune responses. 4 After infection with SARS‐CoV‐2, the host's immune system is primed leading to activation of inflammation‐related genes and production of cytokines from target cells. 5 Upregulated production of proinflammatory cytokines and the consequent recruitment of immune cells to the site of infection can lead to tissue damage, particularly in cases of ARDS, where the risk of death is increased. 6 Thus, investigation of the mechanisms by which the human body balances the activity of immune system (proinflammatory and anti‐inflammatory cytokines) to enable an appropriate response in viral clearance is a fundamental issue. 7
One group of cytokines that has been addressed for its role in immunopathogenesis of COVID‐19 and/or disease severity is the IL‐1 family of cytokines. Interestingly, this family includes cytokines with both proinflammatory (IL‐1α, IL‐1β, IL‐18, IL‐33, and IL‐36) and anti‐inflammatory (IL‐37 and IL‐38) effects, some of which are linked to damaging inflammation, while others mediate resistance to viral and bacterial infections. 8 In COVID‐19 infection, the IL‐1 cytokine family has been identified to play a pivotal role in the induction of cytokine storm due to dysregulated immune responses. 9 Further, the anti‐inflammatory cytokine IL‐37 showed reduced levels in serum of severely ill COVID‐19 patients. 10 A recent study also demonstrated for the first time that IL‐38 is an additional cytokine of this family that showed significantly elevated circulating levels in COVID‐19 patients. The study concluded that IL‐38 may exert a protective role against immune system hyperactivity, and may alleviate induced lung inflammation. 11 Besides, it has been indicated that dysregulated serum levels of IL‐38 are linked to immunopathogenesis of several inflammatory and immune‐mediated disorders, as well as lung‐related diseases. 12
Due to limited data on IL‐38 and COVID‐19, the current study examined serum levels of IL‐38 in patients with COVID‐19 and healthy controls to evaluate the potential role of this cytokine in pathogenesis of COVID‐19 infection. Further, disease severity (moderate, severe, and critical), as well as patient‐related parameters (age, gender, body mass index and chronic disease status) were taken into account in the evaluation.
2. MATERIALS AND METHODS
2.1. Patients and controls
A case‐control study was conducted on COVID‐19 patients admitted to COVID‐19 care units in Baghdad hospitals during September and December 2020 after obtaining approval of the Ethics Committee of the Iraqi Ministry of Health and Environment and written consent of the participants. Patients were studied 4−7 days after admission. A total number of 261 subjects were included: 148 COVID‐19 patients (mean age = 56.5 years; standard deviation = 14.3 years; range = 18–93 years; 60.1% males) and 113 controls (mean age = 37.8 years; standard deviation = 12.0 years; range = 18–57 years; 59.3% males). The RealLine SARS‐CoV‐2 kit (Bioron Diagnostics GmbH) was used to diagnose SARS‐CoV‐2 in nasopharyngeal swabs of patients. A chest tomography (CT) scan was also performed to confirm diagnosis. Included patients were those who showed a positive molecular test, had a CT scan indicating COVID‐19, and were over 18 years of age. Pregnant women were excluded. Patients were evaluated in terms of disease severity, and in this context, three categories were adopted; moderate (evident clinical and/or radiological observations of lower respiratory tract infection with ≥94% oxygen saturation), severe (<94% oxygen saturation and ≥30 breaths/minute respiratory rate) and critical (respiratory failure) illness. 13 Patients were also characterized for body mass index (BMI; normal‐weight and overweight/obese) and chronic disease (present and absent). Chronic disease was defined as cardiovascular disease (CVD), diabetes, or both. Patients were also profiled for the following laboratory parameters: hemoglobin (Hb), platelet count, white blood cell count (WBC), erythrocyte sedimentation rate (ESR), random blood glucose (RBG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total cholesterol, triglycerides, creatinine, blood urea nitrogen (BUN) and uric acid.
The control group included blood donors and health service personnel, who were healthy and did not suffer from respiratory and chronic diseases. They were seronegative for anti‐COVID‐19 IgG and IgM antibodies and C‐reactive protein, and their ESR was below 20 mm/h.
2.2. Immunoassay of IL‐38
Human IL‐38 enzyme‐linked immunosorbent assay (ELISA) kit was used to determine IL‐38 level in serum of participants (Cat. No E3276Hu Bioassay Technology Laboratory, China), and instructions of manufacturer were followed. Standard curve range of the kit was 0.5–200 pg/ml.
2.3. Statistical analysis
Categorical variables were expressed as number and percentage, and significant differences were assessed using Pearson chi‐square test. Continuous variables were subjected to two tests of normality; Kolmogorov‐Smirnov and Shapiro–Wilk tests. Normally distributed variables were given as mean and standard deviation (SD), and significant differences were assessed using one‐way analysis of variance (ANOVA) test. Skewed variables were presented through median and interquartile range (IQR), and significant differences were assessed using Mann–Whitney U (to compare two groups) or Kruskal–Wallis H (to compare more than two groups) test. Receiver operating characteristic (ROC) curve was applied to calculate area under the curve (AUC). All participants were distributed into two groups in relation to median levels of IL‐38 in controls; low and high production groups (< and ≥ median, respectively), and then logistic regression analysis was performed to calculate odds ratio (OR) and 95% confidence interval (CI). A probability (p) value ≤ 0.05 was taken statistically significant. GraphPad Prism version 8.0.0 and IBM SPSS Statistics 25.0 were used to perform statistical analysis.
3. RESULTS
3.1. Baseline characteristics of patients
The 148 patients enrolled in this study were categorized according to severity of COVID‐19. Forty‐five patients had moderate disease, while remaining patients experienced either severe or critical illness (55 and 48 patients, respectively). The latter two groups of patients had a significantly higher mean age compared to patients with moderate disease (58.1 ± 12.6 and 58.8 ± 14.9 vs. 52.2 ± 14.8 years, respectively; p = 0.048). In fact, more than 50% of moderate cases were classified under the age group < 50 years (53.3%), while 69.1% and 75% of severe and critical cases were above the age of 50 years, respectively. These differences were significant (p = 0.011). Distributions of gender (males and females), BMI (normal‐weight and overweight/obese) and chronic disease (present and absent) subgroups in the three disease severity groups showed no significant differences. With regard to chronic diseases (CVD, diabetes, or both), it is noteworthy that patients with moderate, severe, or critical disease shared an increased incidence of these diseases (53.3%, 63.6%, and 50.0%, respectively). Patients were also defined by the laboratory parameters listed in Table 1. Means of these parameters showed no significant differences between the three disease severity groups. WBC, ESR, and RBG were exceptions and positively correlated COVID‐19 severity. Their means were significantly higher in patients with severe or critical illness than in patients with moderate disease (p = 0.004, 0.001, and 0.006, respectively) (Table 1).
Table 1.
Baseline characteristics of COVID‐19 patients
| Characteristica | COVID‐19 cases; N = 148 | p‐value | |||
|---|---|---|---|---|---|
| Moderate; N = 45 | Severe; N = 55 | Critical; N = 48 | |||
| Age; years | 52.2 ± 14.8 | 58.1 ± 12.6 | 58.8 ± 14.9 | 0.048 | |
| Age group; years | <50 | 24 (53.3) | 17 (30.9) | 12 (25.0) | 0.011 |
| ≥50 | 21 (46.7) | 38 (69.1) | 36 (75.0) | ||
| Gender | Male | 29 (64.4) | 27 (49.1) | 33 (68.8) | 0.099 |
| Female | 16 (35.6) | 28 (50.9) | 15 (31.3) | ||
| BMI | Normal‐weight | 15 (33.3) | 16 (29.1) | 12 (25.0) | 0.676 |
| Overweight/obese | 30 (66.7) | 39 (70.9) | 36 (75.0) | ||
| Chronic disease | Present | 24 (53.3) | 35 (63.6) | 24 (50.0) | 0.344 |
| Absent | 21 (46.7) | 20 (36.4) | 24 (50.0) | ||
| Hemoglobin; g/dl | 11 ± 2.0 | 12.7 ± 2.2 | 12.6 ± 3.1 | 0.118 | |
| Platelets; × 109/L | 270.9 ± 100.5 | 306.5 ± 161.9 | 263.2 ± 127.4 | 0.220 | |
| WBC; ×109/L | 10.9 ± 3.3 | 11.1 ± 4.0 | 13.7 ± 6.1 | 0.004 | |
| ESR; mm/h | 37.9 ± 17.6 | 51.9 ± 23.8 | 76.5 ± 25.2 | 0.001 | |
| RBG; mg/dl | 179.4 ± 95.7 | 257.3 ± 140.1 | 219.6 ± 114.8 | 0.006 | |
| ALT; U/L | 49.8 ± 33.3 | 56.4 ± 60.1 | 67.0 ± 54.3 | 0.267 | |
| AST; U/L | 37.4 ± 12.3 | 40.3 ± 21.7 | 46.2 ± 28.6 | 0.147 | |
| ALP; IU/L | 86.5 ± 34.4 | 84.7 ± 53.8 | 94.2 ± 56.5 | 0.599 | |
| Cholesterol; mg/dl | 188.3 ± 50.2 | 199.5 ± 110.1 | 195.4 ± 125.7 | 0.861 | |
| Triglycerides; mg/dl | 202.8 ± 61.4 | 240.2 ± 129.5 | 232.3 ± 121.6 | 0.220 | |
| Creatinine; mg/dl | 1.0 ± 0.5 | 1.2 ± 0.7 | 1.1 ± 0.7 | 0.160 | |
| BUN; mg/dl | 57.4 ± 28.6 | 67.6 ± 47.4 | 54.7 ± 46.2 | 0.258 | |
| Uric acid; mg/dl | 5.9 ± 1.9 | 5.8 ± 2.4 | 5.3 ± 2.0 | 0.385 | |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; ESR, erythrocyte sedimentation rate; RBG, random blood glucose.
Values are given as mean ± standard deviation or a number followed by a percentage in parentheses; p, Pearson chi‐square or one‐way analysis of variance (ANOVA) test probability (significant p‐value is indicated in bold).
3.2. IL‐38 serum levels
Serum levels of IL‐38 showed no significant differences between COVID‐19 patients and controls (68.7 [IQR: 62.7–75.6] vs. 67.7 [58.0–82.6] pg/ml; p = 0.457). Similarly, patients stratified by disease severity, age group, gender, or chronic disease did not show significant differences between IL‐38 levels in each stratum. Whereas, overweight/obese patients were observed to have a significantly lower median of IL‐38 compared to normal‐weight patients (67.7 [61.6–74.2] vs. 72.6 [65.9–77.3] pg/ml; p = 0.011). Further, patients with moderate disease tended to have a higher median of IL‐38 than in patients with severe or critical disease, but the difference was not significant (69.7 [67.0–76.5] vs. 67.1 [60.6–74.0], and 68.0 [60.6–75.5] pg/ml, respectively; p = 0.078) (Figure 1).
Figure 1.
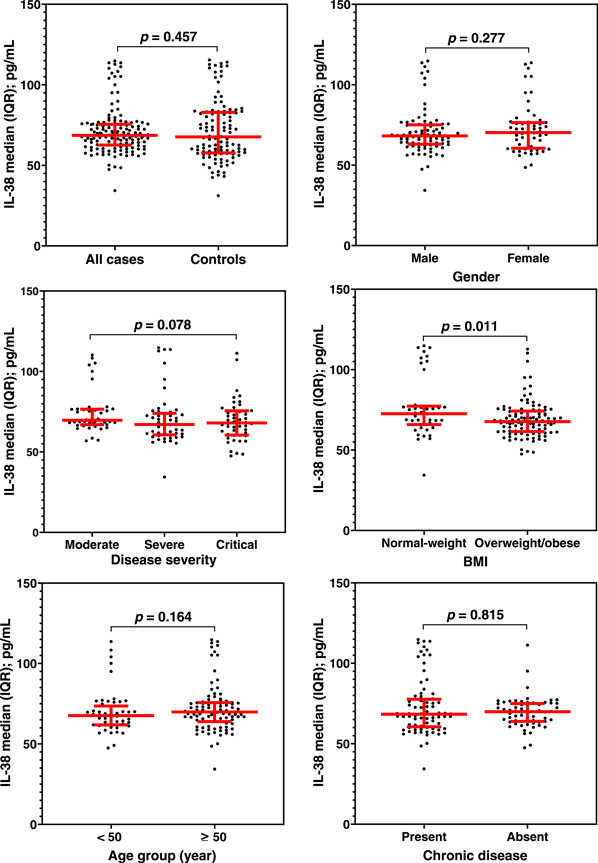
Scatter dot plots with median (horizontal red line) and interquartile range (vertical red line) of Interleukin‐38 (IL‐38) serum levels in all cases of COVID‐19 versus controls and in COVID‐19 cases stratified by disease severity, age group, gender, body mass index (BMI) and chronic disease. Significant differences between medians were assessed using Mann–Whitney U test. No significant differences were recorded except for the BMI category, in which normal‐weight patients showed a significantly higher level of IL‐38 than overweight/obese patients
To examine the concurrent effect of disease severity and patient‐related parameters on serum levels of IL‐38, each of the three disease severity groups (medium, severe, and critical) were classified into subgroups for each parameter (age: <50 and ≥50 years; gender; males and females; BMI: normal‐weight and over‐weight/obese and chronic disease: present and absent). IL‐38 tended to show higher levels in the age group ≥50 years of patients with severe or critical illness than in the age group <50 years (69.7 [59.4–74.0] vs. 63.5 [61.6–69.1] and 71.7 [61.9–77.4] vs. 64.4 [58.5–67.6] pg/ml, respectively), but the difference was only significant in critical cases (p = 0.015). Female patients with severe disease also showed significantly elevated levels of IL‐38 compared to male patients (71.7 [61.1–75.9] vs. 63.5 [60.5–69.6] pg/ml; p = 0.043). IL‐38 levels tended to be lower in overweight/obese patients than in normal‐weight patients in the three disease severity groups, but no significant differences were found. In the case of chronic disease, there were no significant differences between the IL‐38 levels in patients with and without chronic disease in the three disease severity groups (Figure 2).
Figure 2.
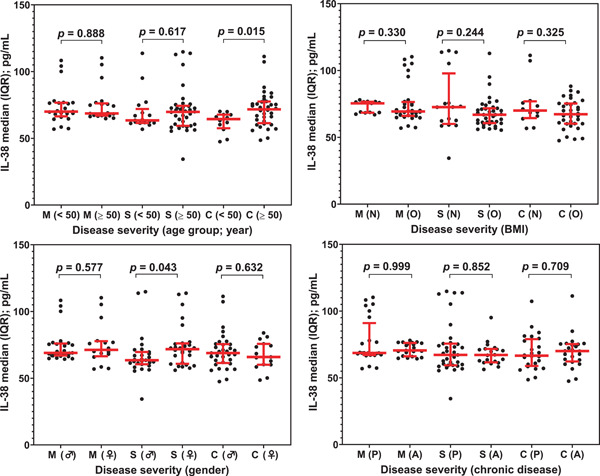
Scatter dot plots with median (horizontal red line) and interquartile range (vertical red line) of Interleukin‐38 (IL‐38) serum levels in COVID‐19 cases stratified by disease severity (M, moderate; S, severe, and C, critical) and age group (<50 and ≥50 years, gender (♂: male and ♀: female), BMI (body mass index; N: normal‐weight and O: overweight/obese) and chronic disease (P: present and A: absent). Significant differences between medians were assessed using Mann–Whitney U test. IL‐38 tended to show higher levels in the age group ≥50 years of patients with severe or critical illness than in the age group <50 years, but the difference was only significant in severe cases. Female patients with severe disease also showed significantly elevated levels of IL‐38 compared to male patients. IL‐38 levels tended to be lower in overweight/obese patients (O) than in normal‐weight patients in the three disease severity groups, but no significant differences were recorded. In the chronic disease condition, there were no significant differences between the levels of IL‐38 for patients with and without chronic disease in the three disease severity groups
3.3. ROC curve analysis
ROC curve analysis demonstrated that IL‐38 was a poor predictor of COVID‐19 as the estimated AUC was 0.522 (p = 0.546). A similar predicting power was indicated when the patients stratified by, age group, gender, or chronic disease. In the case of disease severity or BMI, a significant AUC was observed in moderate versus severe illness (AUC = 0.627; p = 0.030), and normal‐weight versus overweight/obese (AUC = 0.633; p = 0.012) (Figure 3).
Figure 3.
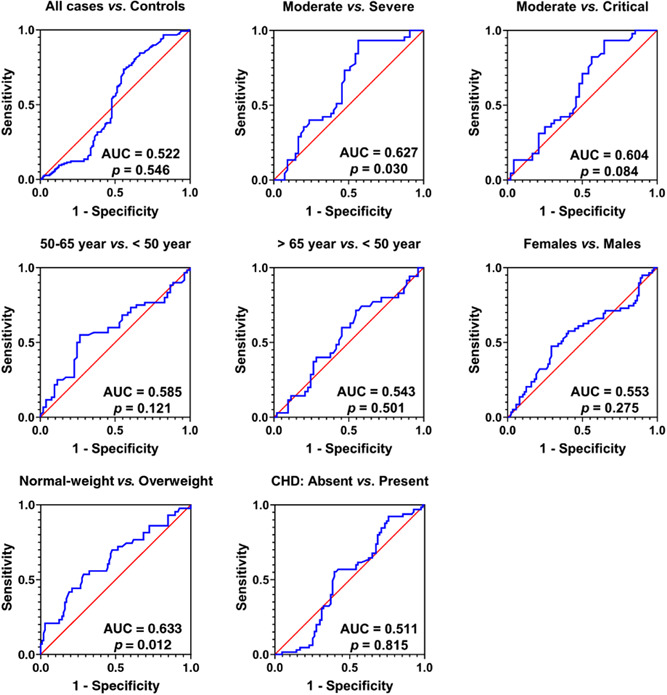
Receiver operating characteristic curve analysis of Interleukin‐38 (IL‐38) serum levels in all cases of COVID‐19 versus controls and in COVID‐19 cases stratified by disease severity, age group, gender, body mass index, and chronic disease (CHD). Area under the curve (AUC) and probability (p) are indicated. A significant AUC was observed in moderate versus severe illness (AUC = 0.627; p = 0.030), and normal‐weight versus overweight/obese (AUC = 0.633; p = 0.012)
3.4. Logistic regression analysis
Based on median levels of IL‐38 in controls, all subjects were classified as low and high production groups (<67.7 and ≥67.7 pg/ml, respectively), and then logistic regression analysis was performed to calculate OR and 95% CI. Although the analysis did not reveal a significant association between IL‐38 and the risk of developing COVID‐19, two findings can be considered important. First, about 50% of patients with severe disease were classified in the low production category (52.7%), while frequency of this category was much lower among patients with moderate disease (28.9%). This difference was significant and associated with an OR of 2.75 (95% CI = 1.20–6.27; p = 0.025). Second, the low production category was also more common in overweight/obese patients than in normal‐weight patients, and the difference was significant (49.5 vs. 30.2; OR = 2.26; 95% CI = 1.07–4.78; p = 0.044) (Table 2).
Table 2.
Low and high production groups of IL‐38 in COVID‐19 patients (all and subgroups) and controls
| Group | IL‐38 production group | OR (95% CI) | Reciprocal OR (95% CI) | p‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | |||||||
| N | % | N | % | |||||
| All | Controls | 56 | 49.6 | 57 | 50.4 | Reference | ||
| Patients | 65 | 43.9 | 83 | 56.1 | 0.80 (0.49–1.30) | 1.25 (0.77–2.05) | 0.383 | |
| Severity | Moderate | 13 | 28.9 | 32 | 71.1 | Reference | ||
| Severe | 29 | 52.7 | 26 | 47.3 | 2.75 (1.20–6.27) | 0.36 (0.16–0.83) | 0.025 | |
| Critical | 23 | 47.9 | 25 | 52.1 | 2.26 (0.97–5.29) | 0.44 (0.19–1.03) | 0.088 | |
| Age group; year | <50 | 27 | 50.9 | 26 | 49.1 | Reference | ||
| ≥50 | 38 | 40.0 | 57 | 60.0 | 0.64 (0.31–1.34) | 1.56 (0.74–3.26) | 0.228 | |
| Gender | Male | 43 | 48.3 | 46 | 51.7 | Reference | ||
| Female | 22 | 37.3 | 37 | 62.7 | 0.64 (0.33–1.26) | 1.56 (0.81–3.06) | 0.237 | |
| BMI | Normal‐weight | 13 | 30.2 | 30 | 69.8 | Reference | ||
| Overweight/obese | 52 | 49.5 | 53 | 50.5 | 2.26 (1.07–4.78) | 0.44 (0.21–0.93) | 0.044 | |
| Chronic disease | Present | 38 | 45.8 | 45 | 54.2 | Reference | ||
| Absent | 27 | 41.5 | 38 | 58.5 | 0.84 (0.44–1.61) | 1.19 (0.62–2.28) | 0.621 | |
Note: Low: <67.7 pg/ml; High: ≥67.7 pg/ml; p: Two‐tailed Fisher exact probability (significant p‐value is indicated in bold). Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
3.5. Spearman's rank order correlation analysis
Spearman's rank order correlation analysis was performed to calculate correlation coefficients (rs ) between IL‐38 and laboratory parameters in COVID‐19 patients. Four significant correlations were found. IL‐38 showed negative correlation with Hb (rs = −0.173; p = 0.035) and RBG (rs = −0.250; p = 0.002), while it was positively correlated with BUN (rs = 0.210; p = 0.011) and uric acid (rs = 0.177; p = 0.032) (Figure 4).
Figure 4.
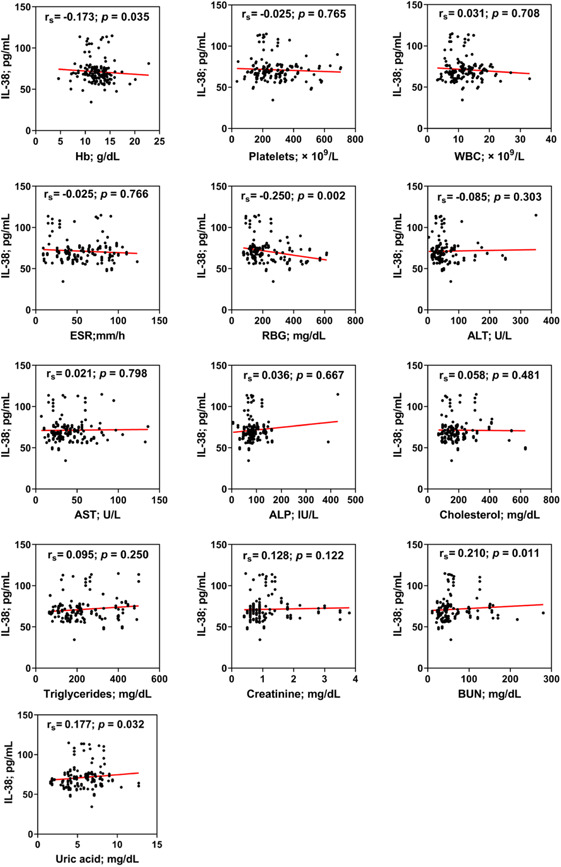
Spearman's rank order correlation coefficient (rs ) between Interleukin‐38 (IL‐38) and hemoglobin (Hb), platelets, white blood cell count (WBC), erythrocyte sedimentation rate (ESR), random blood glucose (RBG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total cholesterol, triglycerides, serum creatinine, blood urea nitrogen (BUN), and uric acid in COVID‐19 patients. Four significant correlations were found. IL‐38 showed negative correlation with Hb (rs = −0.173; p = 0.035) and random blood glucose (rs = −0.250; p = 0.002), while it was positively correlated with BUN (rs = 0.210; p = 0.011) and uric acid (rs = 0.177; p = 0.032). p: Two‐tailed Fisher's exact probability
4. DISCUSSION
A previous study has demonstrated that serum levels of IL‐38 increased in patients with SARS‐CoV‐2 infection and these levels were declined after recovery from the infection. This increase was more pronounced in nonsevere cases than in severe cases. Accordingly, the significance of IL‐38 in predicting the prognosis of SARS‐CoV‐2 infection has been proposed. 11 The results of the current study were not in favor of these findings and instead showed that serum levels of IL‐38 were not affected by COVID‐19 as no significant differences were found between patients and controls. Similarly, IL‐38 levels were not affected by disease severity, gender, age, or chronic disease status in COVID‐19 patients. Whereas, BMI might have an effect as it was observed that overweight/obese patients had significantly lower levels of IL‐38 compared to normal‐weight patients. Logistic regression analysis also added that patients with severe disease or those who were overweight/obese in the low production group of IL‐38 were more likely to have COVID‐19 than the high production group in patients with moderate disease or normal‐weight, respectively. In addition, ROC curve analysis indicated that IL‐38 could be used to distinguish between moderate and severe cases or normal‐weight and overweight/obese cases, although the AUC values revealed poor predictability (0.627 and 0.633, respectively). This may indicate that the association of IL‐38 with COVID‐19 is complicated by the influence of some factors; for instance, disease severity and BMI. Simultaneous examination of both factors revealed a tendency for IL‐38 to lower levels in overweight/obese patients compared to normal‐weight patients whether the disease was moderate, severe, or critical, but the differences were not statistically significant. This observation was extended to examine the role of disease severity and age in determining IL‐38 levels in COVID‐19 patients. In patients with critical illness, IL‐38 levels significantly increased in the age group ≥50 years compared to the age group <50 years, and a similar observation was made in severely ill patients but the difference was not significant. Further, female patients with severe illness showed a significantly increased levels of IL‐38 compared to male patients. These results suggest that disease severity and age, gender, or BMI may have concurrent effects on IL‐38 levels in COVID‐19 patients. In fact, most studies have linked COVID‐19 severity to age, gender, and BMI, with a significant proportion of critical cases over 50 years of age, particularly males and overweight/obese. 14 , 15 Besides, the cytokine profile in healthy and diseased populations, including COVID‐19 patients, is influenced by age, gender, and body mass index. 16 , 17
COVID‐19 infection is known to cause dysregulated production of cytokines with a hallmark of elevated levels of proinflammatory cytokines associated with suppressed antiviral immunity, and this positively correlates with disease severity and increased morbidity and mortality. 18 Elevated levels of proinflammatory cytokines, such as IL‐1, IL‐6, and TNF‐α, trigger an intense inflammatory response by recruiting immune cells such as macrophages to the alveoli, which in turn recruit more inflammatory cells such as monocytes that produce greater amounts of cytokines, thus increasing the inflammatory response, particularly in patients with severe COVID‐19. 19 The effects of proinflammatory cytokines can be counterbalanced by anti‐inflammatory cytokines which may reduce the inflammatory response through suppression of proinflammatory cytokines. In COVID‐19 patients, although there is no apparent imbalance in proinflammatory and anti‐inflammatory mechanisms, 20 decreased serum levels of IL‐6, IL‐17, IL‐22, CXCL10, IL‐1β, IFN‐γ, and TNF‐α have been reported in mice treated with IL‐38, while genes involved in regulatory T cell functions, such as Foxp3, showed increased expression. 21 Accordingly, it has been proposed that IL‐38 can ameliorate inflammation by inhibiting the production of certain proinflammatory cytokines such as IL‐17 and IL‐22. 22 Recently, IL‐37, an anti‐inflammatory cytokine functionally related to IL‐38, showed low levels in serum of severely ill COVID‐19 patients, and accordingly it has been suggested that low levels of IL‐37 are associated with disease risk. 10 Moreover, lower levels of IL‐38 were found in severely ill patients compared to patients with nonsevere disease. 11 In the current study, increased levels of IL‐38 were found in severe/critical patients over 50 years of age. At older ages, cytokine dysregulation was indicated with a progressive tendency toward a proinflammatory phenotype. 23 COVID‐19 is also associated with upregulated levels of proinflammatory cytokines. 3 Therefore, increased levels of IL‐38 in patients over 50 years of age may counteract these up‐regulations of proinflammatory cytokines. It has also been shown that serum IL‐38 levels exhibit an age‐related modality in healthy aging, while elevated IL‐38 levels were reached more rapidly in older patients with type 2 diabetes mellitus, and an increase in IL‐38 levels might have occurred to exert an anti‐inflammatory effects. 24
Besides age, IL‐38 levels were significantly increased in severely ill females compared to male patients. There is no direct evidence to support upregulated levels of IL‐38 in females compared to males. In Behçet's disease, a chronic multisystem autoimmune disease, serum levels of IL‐38 were higher in female patients with a positive pathergy test than in patients with a negative test and in patients with ocular involvement than in patients without ocular involvement. These differences were not found in male patients. 25 In multiple sclerosis and systemic sclerosis, IL‐38 levels showed no significant differences between male and female patients. 26 In the case of COVID‐19, IL‐10, an anti‐inflammatory cytokine, was demonstrated to exhibit a favorable female phenotype, and a more robust anti‐inflammatory response was observed in female patients compared to males. 27 Accordingly, gender difference in levels of anti‐inflammatory cytokines is an important issue in understanding why female COVID‐19 patients have a better prognosis and lower mortality than male patients, but the evidence has not been conclusive and further studies are warranted.
There was a tendency for IL‐38 levels to decrease in overweight/obese patients compared to normal weight patients regardless of disease severity. In fact, more than 50% of COVID‐19 patients were overweight/obese, and a positive association between disease severity and obesity has been reported in the literature. 14 The relationship between IL‐38 and obesity has not been well investigated, but two related studies deserve mention. In the first, a relative deficiency of IL‐38 has been reported in healthy, overweight Europeans and was associated with higher systemic inflammation in the elderly, and those with CVD and metabolic disease. 28 In the second, IL‐38 has been proposed as a potential target for the treatment of obesity‐induced adipose tissue inflammation. The study also indicated that IL‐38 could improve insulin resistance in mice with induced obesity. 29 Regarding this point, the current study documented that IL‐38 levels were negatively correlated with RBG (rs = −0.250; p = 0.002). This may indicate that the level of IL‐38 is affected by hyperglycemia, but more studies are needed to explore this issue.
The study was limited by the low sample size of patients in each of the disease severity groups. The statistically significant observations obtained should also be interpreted with caution as p‐values were not corrected to reduce the type I error rate caused by alpha inflation due to multiple comparisons.
In conclusion, the current study indicated that serum IL‐38 levels were not affected by COVID‐19 infection, but the distribution of patients according to disease severity, age, gender and BMI may better reveal the role of IL‐38 in the pathogenesis of COVID‐19.
AUTHOR CONTRIBUTIONS
All authors contributed equally to conceptualization, visualization, methodology, investigation, validation, and writing‐reviewing and editing.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
The authors sincerely appreciate the cooperation of the medical staff at the COVID‐19 care units in Baghdad hospitals.
Al‐bassam WW, Al‐Karaawi IA, Sharquie IK, Ad'hiah AH. Evaluation of interleukin‐38 levels in serum of patients with coronavirus disease 2019. J Med Virol. 2022;94:3642‐3652. 10.1002/jmv.27762
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mallah SI, Ghorab OK, Al‐Salmi S, et al. COVID‐19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. 2021;20(1):1‐36. 10.1186/s12941-021-00438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trougakos IP, Stamatelopoulos K, Terpos E, et al. Insights to SARS‐CoV‐2 life cycle, pathophysiology, and rationalized treatments that target COVID‐19 clinical complications. J Biomed Sci. 2021;28(1):1‐18. 10.1186/s12929-020-00703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J, Yang X, Li Y, Huang Jan, Jiang J, Su N. Specific cytokines in the inflammatory cytokine storm of patients with COVID‐19‐associated acute respiratory distress syndrome and extrapulmonary multiple‐organ dysfunction. Virol J. 2021;18(1):1‐12. 10.1186/s12985-021-01588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. 10.3390/ijms20236008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodríguez L. SARS‐CoV‐2 infection: the role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev. 2020;54:62‐75. 10.1016/j.cytogfr.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelaia C, Tinello C, Vatrella A, De Sarro G, Pelaia G. Lung under attack by COVID‐19‐induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 2020;14:1‐9. 10.1177/1753466620933508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye CH, Hsu WL, Peng GR, et al. Role of the immune microenvironment in SARS‐CoV‐2 Infection. Cell Transplant. 2021;30:1‐15. 10.1177/09636897211010632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dinarello CA. Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8‐27. 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mardi A, Meidaninikjeh S, Nikfarjam S, Majidi Zolbanin N, Jafari R. Interleukin‐1 in COVID‐19 Infection: immunopathogenesis and Possible Therapeutic Perspective. Viral Immunol. 2021;34(10):679‐688. 10.1089/vim.2021.0071 [DOI] [PubMed] [Google Scholar]
- 10. Ahmed AA, Ad'hiah AH. Interleukin‐37 is down‐regulated in serum of patients with severe coronavirus disease 2019 (COVID‐19). Cytokine. 2021;148:155702. 10.1016/j.cyto.2021.155702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao X, Chan PKS, Lui GCY, et al. Interleukin‐38 ameliorates poly(I:C) induced lung inflammation: therapeutic implications in respiratory viral infections. Cell Death Dis. 2021;12(1):1‐18. 10.1038/s41419-020-03283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esmaeilzadeh A, Bahmaie N, Nouri E, Hajkazemi MJ, Rafie MZ. Immunobiological properties and clinical applications of interleukin‐38 for immune‐mediated disorders: a systematic review study. Int J Mol Sci. 2021;22(22):12552. 10.3390/ijms222212552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019‐nCoV) Infection is Suspected: Interim Guidance 28 January. WHO; 2020. https://apps.who.int/iris/handle/10665/330893e
- 14. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity is associated with higher in‐hospital mortality in a cohort of patients with COVID‐19 in the Bronx, New York. Metabolism. 2020;108:154262. 10.1016/j.metabol.2020.154262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendren NS, De Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID‐19: results from the American Heart Association COVID‐19 Cardiovascular Disease Registry. Circulation. 2021;143:135‐144. 10.1161/CIRCULATIONAHA.120.051936 [DOI] [PubMed] [Google Scholar]
- 16. Karaba AH, Zhou W, Hsieh LL, et al. Differential cytokine signatures of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and influenza infection highlight key differences in pathobiology. Clin Infect Dis. 2021;74:254‐262. 10.1093/cid/ciab376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azizian M, Mahdipour E, Mirhafez SR, et al. Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann Clin Biochem. 2016;53(6):663‐668. 10.1177/0004563216629997 [DOI] [PubMed] [Google Scholar]
- 18. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID‐19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. 10.3389/fimmu.2020.01708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabaan AA, Al‐Ahmed SH, Muhammad J, et al. Role of inflammatory cytokines in covid‐19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436. 10.3390/vaccines9050436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Notz Q, Schmalzing M, Wedekink F, et al. Pro‐ and anti‐inflammatory responses in severe COVID‐19‐induced acute respiratory distress syndrome—an observational pilot study. Front Immunol. 2020;11:2631. 10.3389/fimmu.2020.581338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu WD, Huang AF. Role of interleukin‐38 in chronic inflammatory diseases: a comprehensive review. Front Immunol. 2018;9(JUN):1462. 10.3389/fimmu.2018.01462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie L, Huang Z, Li H, Liu X, Zheng S, Su W. IL‐38: A new player in inflammatory autoimmune disorders. Biomolecules. 2019;9(8):345. 10.3390/biom9080345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Denis Alexander H, Ross OA. Age and age‐related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurău F, Silvestrini A, Matacchione G, et al. Plasma levels of interleukin‐38 in healthy aging and in type 2 diabetes. Diabetes Res Clin Pract. 2021;171:108585. 10.1016/j.diabres.2020.108585 [DOI] [PubMed] [Google Scholar]
- 25. Zarrabi M, Gholijani N, Shenavandeh S, Aflaki E, Amirghofran Z. IL‐38 serum levels in patients with Behcet's disease and the relationship with clinical features. Eur Cytokine Netw. 2019;30(3):82‐87. 10.1684/ecn.2019.0430 [DOI] [PubMed] [Google Scholar]
- 26. Zarrabi M, Nazarinia M, Rahimi Jaberi A, Gholijani N, Amirghofran Z. Elevated IL‐38 serum levels in newly diagnosed multiple sclerosis and systemic sclerosis patients. Med Princ Pract. 2021;30(2):146‐153. 10.1159/000510915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi S, Ngwa C, Morales Scheihing DA, et al. Sex differences in the immune response to acute COVID‐19 respiratory tract infection. Biol Sex Differ. 2021;12(1):1‐10. 10.1186/s13293-021-00410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Graaf DM, Jaeger M, van den Munckhof ICL, et al. Reduced concentrations of the B cell cytokine interleukin 38 are associated with cardiovascular disease risk in overweight subjects. Eur J Immunol. 2021;51(3):662‐671. 10.1002/eji.201948390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu K, Sun J, Chen S, et al. Hydrodynamic delivery of IL‐38 gene alleviates obesity‐induced inflammation and insulin resistance. Biochem Biophys Res Commun. 2019;508(1):198‐202. 10.1016/j.bbrc.2018.11.114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


