Abstract
The postantibiotic effects (PAEs) of seven antimycobacterial agents, tested at their respective peak concentrations in serum alone and in different combinations, against Mycobacterium tuberculosis ATCC 27294 were studied with a radiometric culture system in parallel with the viable count method. Rifampin gave the longest PAE (67.8 h) among the drugs used alone, and combinations of first-line drugs generally gave PAEs longer than 120 h. The data obtained might help provide a better understanding of the scientific basis of intermittently administered antituberculosis chemotherapy.
Postantibiotic effect (PAE) refers to the continued suppression of bacterial growth following limited exposure of organisms to an antimicrobial agent (1, 15, 21). A prolonged PAE may allow wider dosing intervals without the loss of therapeutic efficacy (3). For the treatment of tuberculosis, administration of drugs at wider intervals would reduce the costs and toxicities of drugs and facilitate directly observed antituberculosis chemotherapy, thus enhancing patient adherence (8) and leading to a favorable outcome. Earlier work on pulsed exposure to isoniazid, rifampin, and pyrazinamide for 6 to 96 h has provided hints about the suitability of intermittent administration of these drugs for the treatment of tuberculosis (4–7, 16). In an attempt to better understand the scientific basis of the efficacies of certain established intermittently administered antituberculosis regimens, as well as to gain pertinent knowledge about newer antimycobacterial agents, we embarked on a study that addresses the PAEs of various antituberculosis drugs in vitro.
The standard strain of Mycobacterium tuberculosis chosen for the study, strain H37Rv (ATCC 27294), was susceptible to all drugs tested. The MICs of all single drugs except pyrazinamide were determined by the broth macrodilution method (25), while susceptibility testing with pyrazinamide was done by the absolute concentration method (12). Altogether, seven antituberculosis drugs were assessed by using concentrations equivalent to their respective peak concentrations in serum (Cmax) in humans (17): amikacin (32 mg/liter), ethambutol (5 mg/liter), isoniazid (4 mg/liter), ofloxacin (8 mg/liter), rifampin (16 mg/liter), streptomycin (40 mg/liter), and pyrazinamide (60 mg/liter). Stock solutions of the drugs (all drugs except ofloxacin were purchased from Sigma, St. Louis, Mo.; ofloxacin was a gift from Daiichi, Tokyo, Japan) were prepared in appropriate solvents, stored at −70°C in 1.0-ml aliquots, and used within 6 months. For each experiment, aliquots of the stock solutions were thawed and subsequently diluted in Middlebrook 7H9 broth supplemented with 2% glycerol and 10% oleic acid-dextrose-catalase (Difco Laboratories).
To determine the PAE, a homogeneous suspension of cells whose turbidity matched that of a no. 1 McFarland standard was obtained from a 3-week-old culture and stored at −40°C in 1.0-ml aliquots. For each experiment, a single vial of cells was quickly thawed at 37°C and inoculated into 10 ml of BACTEC 12B medium supplemented with 2.5% Panta reconstituting fluid (PRF; Becton Dickinson, Towson, Md.). This suspension was incubated for 15 days at 37°C for use as the inoculum, which contained mycobacteria in the late logarithmic phase of growth. Prior to use, the seed vial was sonicated for 3 min in a Branson Ultrasonic water bath in order to minimize the number of bacterial aggregates. Nine milliliters of the various drugs at concentrations equivalent to 1.2 to 67 times the MICs of the different drugs on the basis of their Cmaxs, either alone or in different combinations, together with a drug-free control, were inoculated with 1.0 ml of the prepared seed (final inoculum, 2 × 106 to 7 × 106 CFU/ml). After 2 h of incubation, drug was removed by dilution 1:1,000 (1.0 ml in 9.0 ml and then 0.05 ml in 4.95 ml) into fresh prewarmed BACTEC 12B medium supplemented with 2.5% PRF. Drug controls containing similarly diluted unexposed organisms and drugs were included to monitor any residual antibiotic effects. Additional serially diluted controls were included in the combination experiments so that the control with the inoculum closest to that of the exposed culture could be selected for calculation of the PAE. All experiments were carried out thrice, in duplicate, on different days. Inoculated BACTEC vials were incubated and read daily (within 2 h) on a BACTEC 460 instrument until the BACTEC growth index (GI) reached 999. The numbers of viable organisms immediately before and after drug exposure and at daily intervals after the GI reading was taken were simultaneously determined by plating appropriate dilutions onto Middlebrook 7H11 agar slopes in screw-cap flasks. Growth was monitored for a maximum of 10 days. The agar slopes were incubated for 3 to 4 weeks, and the colonies were counted.
The PAE calculated by the viable count method was the difference in time for the growth in the exposed culture (T) and the corresponding control (C) to increase by 1 log10 CFU/ml immediately after drug removal and is represented by the formula PAE = T − C (1, 3, 15, 21). The PAE quantitated by use of GI readings and the same formula given above was the difference in time for the exposed and control cultures to reach cumulative GI values of 100. This BACTEC T100 method was a modification of the method described by Inderlied et al. (13) for the susceptibility testing of Mycobacterium avium complex organisms and was used by Fuursted (9) and Zhanel et al. (26) for evaluation of the PAEs of drugs against M. avium complex organisms; it yielded a good correlation with the viable count method. The PAEs presented here are net PAEs corrected for residual antibiotic effects (data not shown). No notable decrease in the number of organisms was found following the 2-h exposure of strain H37Rv to the drugs used individually. However, serially diluted controls had to be run for experiments with drug combinations to avoid overestimation of the resultant PAEs because killing effects were detected after 2 h of exposure to some of the drug combinations.
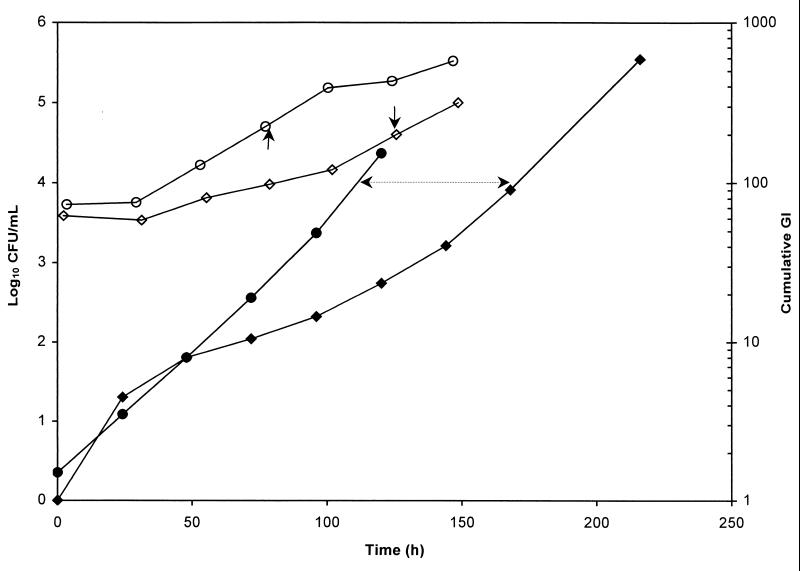
Growth curves for M. tuberculosis H37Rv were obtained as both the viable count (in CFU) versus time and the GI versus time. Figure 1 depicts a representative determination of the PAE of rifampin against H37Rv after 2 h of exposure to the drug at its Cmax. The mean time for H37Rv to increase 1 log10 CFU by the viable count method was 76.1 h, with a coefficient of variation (CV) of 17.9%. The mean time for the same organism to reach a cumulative GI of 100 was 113.9 h, with a CV of 7.8%. The PAEs of the drugs used alone against H37Rv, as determined by the viable count method and the BACTEC T100 method, in parallel, showed no significant difference (P > 0.05), with similarly high CVs (Table 1). The PAEs obtained by the BACTEC T100 method, at a constant volume without aliquots taken daily, for combinations of drugs as well as for individual drugs had improved CVs (Table 2). Rifampin gave the longest PAE (67.8 h), followed by streptomycin (32.2 h), isoniazid (18.1 h), amikacin (17.4), ofloxacin (6.2 h), pyrazinamide (1.9 h), and finally, ethambutol (1.8 h). The PAE induced by rifampin was significantly longer (P < 0.05) than the PAE produced by isoniazid, which was significantly longer than those produced by pyrazinamide (P < 0.02) and ethambutol (P < 0.001). Significant differences were also observed between the PAEs generated by amikacin and pyrazinamide (P < 0.01) and by amikacin and ethambutol (P < 0.001). The combinations isoniazid-rifampin-streptomycin-pyrazinamide, isoniazid-rifampin-streptomycin, isoniazid-rifampin, isoniazid-rifampin-ethambutol, isoniazid-rifampin-streptomycin-ethambutol, isoniazid-rifampin-pyrazinamide-ethambutol, isoiazid-rifampin-amikacin, and ofloxacin-rifampin-amikacin yielded PAEs in descending magnitude (Table 2); but none of these PAEs was significantly different (P > 0.05).
FIG. 1.
Regrowth curves of M. tuberculosis H37Rv following 2 h of exposure to rifampin at the rifampin Cmax. ○, viable count, control; ◊, viable count, exposed culture; ●, cumulative GI, control; ⧫, cumulative GI, exposed culture. Arrows indicate the difference in times, and the time interval between arrows denotes the PAE.
TABLE 1.
PAEs of antituberculosis drugs against H37Rv following 2-h exposures to the drugs at their respective Cmax
| Method | PAE (h [% CV])a
|
||||
|---|---|---|---|---|---|
| Isoniazid | Rifampin | Ethambutol | Amikacin | Ofloxacin | |
| VCb | 7.6 (88) | 42.9 (84) | 5.8 (133) | 22.3 (134) | 14.3 (144) |
| T100c | 12.5 (49) | 66.0 (115) | 5.5 (115) | 16.9 (109) | 6.0 (173) |
Values are means.
VC, viable count method.
T100, BACTEC T100 method.
TABLE 2.
PAEs against M. tuberculosis H37Rv after a 2-h exposure to single drugs and combinations of drugs, determined by the BACTEC T100 method
| Drug(s)a | MIC (mg/liter) | PAE (h)b | % CV |
|---|---|---|---|
| Isoniazid | 0.06 | 18.1 (5.7–31.2) | 46 |
| Rifampin | 0.5 | 67.8 (11.9–134.9) | 70 |
| Streptomycin | 1.0 | 32.2 (0.7–60.2) | 93 |
| Pyrazinamide | ≤50c | 1.9 (0.0–5.2) | 153 |
| Ethambutol | 2.0 | 1.8 (0.0–5.6) | 139 |
| Amikacin | 1.0 | 17.4 (13.0–19.9) | 22 |
| Ofloxacin | 0.25 | 6.2 (0.0–12.4) | 142 |
| HR | 159.8 (114.4–220.0) | ||
| HRS | 160.6 (97.0–275.1) | ||
| HRM | 155.3 (89.7–215.6) | ||
| HRSZ | 167.9 (72.6–286.9) | ||
| HRSM | 135.5 (90.3–202.8) | ||
| HRZM | 125.9 (90.0–176.1) | ||
| HRA | 97.2 (94.1–100.3) | ||
| ORA | 95.0 (94.3–95.8) |
Abbreviations used for combinations: H, isoniazid; R, rifampin; S, streptomycin; Z, pyrazinamide; M, ethambutol; A, amikacin; O, ofloxacin.
Values are means (ranges).
The breakpoint concentration used to determine susceptibility to pyrazinamide by the absolute concentration method.
In our previous study of the PAEs of amikacin and ofloxacin against the rapidly growing organism Mycobacterium fortuitum, we used the classical viable count method, which yielded informative results (19). However, when we applied the same method to the study of M. tuberculosis, it was soon realized that the performance of the viable count method can be compromised by several problems, including, in particular, the propensity for clumping, labor-intensive procedures, and the significant biohazard for the laboratory researchers. Measurement of CO2 generation with the BACTEC instrument for evaluation of PAEs against M. avium complex organisms was evaluated and was found to be an appropriate means of determination of the PAEs (9, 26). The cumulative GI of M. tuberculosis in growth plotted semilogarithmically as a function of time resembled the standard sigmoid growth curve obtained by the viable count method except for the absence of an initial lag phase (Fig. 1). Measurement of growth with the Bactec 460 system indicates an increase in bacterial cell numbers as well as metabolism, irrespective of the degree of clumping and filamentation. As a result, the PAEs of some antituberculosis drugs such as rifampin and isoniazid measured by this method might appear to be slightly extended compared with those obtained by the viable count method (Table 1). Owing to the greater sensitivity of the BACTEC instrument, the efficiency was enhanced and the biohazard was reduced. Although the PAEs obtained by both methods varied markedly, they were not significantly different and the CV was found to improve slightly when both the control and the drug-exposed cultures were maintained at constant volume when the BACTEC method was used (Table 2), indicating that the variation would likely come from clumping, which can hardly be controlled.
When the antituberculosis drugs were used in different combinations, the PAEs obtained ranged from 95.0 to 167.9 h (Table 2). Combinations that are commonly used clinically, such as isoniazid-rifampin-streptomycin-pyrazinamide, isoniazid-rifampin-ethambutol-pyrazinamide, isoniazid-rifampin-streptomycin, isoniazid-rifampin-ethambutol, and even isoniazid-rifampin, could produce extended PAEs of over 120 h (Table 2) compared to those of single drugs. Amikacin and ofloxacin used in combination with rifampin gave PAEs of over 95 h (PAE for isoniazid-rifampin-amikacin, 97.2 h; PAE for ofloxacin-rifampin-amikacin, 95.0 h). The mechanisms by which PAEs occur have not been fully understood but are probably related to the time for recovery from the sublethal damage (structural or metabolic alterations) induced by the antimicrobial agent (10). It appears that the PAEs of rifampin and isoniazid contribute predominantly to the PAEs of combinations. The major mode of action of isoniazid against mycobacteria is the inhibition of mycolic acid synthesis, which results in the cells being more fragile (23), while the mode of action of rifampin is the inhibition of RNA transcription (11); and substances believed to increase the permeability of the mycobacterial cell wall, such as Tween 80, caused a significant increase in susceptibility to rifampin (20). Therefore, the synergistic activity of the isoniazid-rifampin combination against M. tuberculosisis is probably due to alteration of the integrity of the mycobacterial cell wall by isoniazid, which allows enhanced penetration of rifampin across the cell wall. In general, the enhancement of PAEs with drug combinations is primarily dependent on the ability of each individual drug to induce a PAE. The synergistic prolongation of PAE displayed by the isoniazid-rifampin combination is so extensive that any enhancement of the PAE that would result from the addition of other drugs or other combinations in the presence of the isoniazid-rifampin combination would not be noticeable. We considered a PAE of less than 24 h to be insignificant for antituberculous drugs since most of the chemotherapeutic regimens for tuberculosis are given daily or on alternate days. In the present study rifampin and streptomycin each produced a significant PAE against M. tuberculosis, while isoniazid and amikacin each induced a detectable PAE. Rifampin in combination with isoniazid produced a synergistic prolongation of the PAE that was over one generation (a mean of 23 h in the present study) longer than the sum of the PAEs for the individual drugs (Table 2). On the addition of streptomycin, pyrazanamide, or ethambutol to the isoniazid-rifampin combination, little or no extension (indifference) of the PAE was observed. It appeared that antagonism existed when amikacin was added to the rifampin-isoniazid combination, which resulted in a comparatively shorter PAE, but the difference was not statistically significant. As the mechanisms of the PAEs for the different drugs are still unclear, it would be difficult to speculate about the processes underlying the variations in PAEs when drugs are tested in different combinations. More in-depth studies with the drugs, as well as studies of their pharmacokinetics and pharmacodynamics, would be required to elucidate the underlying mechanisms.
The PAE of a drug against M. tuberculosis might be a putative pharmacodynamic parameter that would be of help in the design of an optimal dosing schedule for an antimicrobial agent (22). The results from our present study of PAEs (Table 2) might provide some additional insight into the scientific basis of the efficacies of commonly used first-line intermittent antituberculosis regimens, which generally comprise isoniazid-rifampin-streptomycin-pyrazinamide or isoniazid-rifampin-ethambutol-pyrazinamide given daily or thrice weekly initially, followed by isoniazid-rifampin given daily, twice weekly, or three times weekly (2, 14, 18). Our present findings on the PAEs of amikacin and ofloxacin might help to support the possibly rational use of ofloxacin daily and amikacin three times to five times per week (24). The lack of an impressive PAE for pyrazinamide in the present study is at variance with earlier findings (6), but this is likely accountable by the fact that pyrazinamide exerts its effect only at an acidic pH (pH 5.5), while in our drug combination experiments we used a medium with a neutral pH. Furthermore, the long serum half-life of pyrazinamide (9 to 11 h) (17) and the lack of obvious antagonism when pyrazinamide is used together with rifampin and isoniazid (Table 2) can possibly still enable the use of the drug on either a daily or a thrice-weekly basis. The current recommendation is that ethambutol may be given intermittently only when rifampin is included in the regimen, based on the results of a clinical trial (14). This seems to agree with the observed negligible PAE when ethambutol was used alone. However, as our findings are preliminary and limited in nature, much further evaluation and unraveling of the contribution of the knowledge of PAEs to providing an understanding and improving the practical aspects of antituberculosis chemotherapy are still definitely required.
Acknowledgments
This study was supported by earmarked grant CUHK 391/95M of the Research Grant Council, Hong Kong.
REFERENCES
- 1.Bundtzen R W, Gerber A U, Cohn D L, Craig W A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981;3:28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Cohn D L, Catlin B J, Peterson K L, Judson F N, Sbarbaro J A. A 62-dose, 6-month therapy for pulmonary and extra-pulmonary tuberculosis. A twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med. 1990;112:407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 3.Craig W A, Ebert S C. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl. 1990;74:63–70. [PubMed] [Google Scholar]
- 4.Dickinson J M, Ellard G A, Mitchison D A. Suitability of isoniazid and ethambutol for intermittent administration in the treatment of tuberculosis. Tubercle. 1968;49:351–366. doi: 10.1016/s0041-3879(68)80016-9. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson J M, Mitchison D A. In vitro and in vivo studies to assess the suitability of antituberculous drugs for use in intermittent chemotherapy regimens. Tubercle Suppl. 1968;49:66–70. doi: 10.1016/s0041-3879(68)80051-0. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson J M, Mitchison D A. Observations in vitro on the suitability of pyrazinamide for intermittent chemotherapy of tuberculosis. Tubercle. 1970;51:389–396. doi: 10.1016/0041-3879(70)90004-8. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson J M, Mitchison D A. Suitability of rifampicin for intermittent administration in the treatment of tuberculosis. Tubercle. 1970;51:82–94. doi: 10.1016/0041-3879(70)90131-5. [DOI] [PubMed] [Google Scholar]
- 8.Fox W. Whither short course chemotherapy? Br J Dis Chest. 1981;75:331–357. doi: 10.1016/0007-0971(81)90022-x. [DOI] [PubMed] [Google Scholar]
- 9.Fuursted K. Evaluation of the post-antibiotic effect of six anti-mycobacterial agents against Mycobacterium avium by the Bactec radiometric method. J Antimicrob Chemother. 1997;40:33–38. doi: 10.1093/jac/40.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Fuursted K. Postantibiotic effect in vitro. APMIS. 1999;107(Suppl. 90):2. , 4–23. [PubMed] [Google Scholar]
- 11.Hartmann G, Honikel K O, Knussel F, Nuesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145:843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied C B, Nash K A. Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biologic fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 127–175. [Google Scholar]
- 13.Inderlied C B, Young L S, Yamada J K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987;31:1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher D, Chaulet P, Spinaci S, Harries A. Treatment of tuberculosis: guidelines for national programmes. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 15.McDonald P J, Craig W A, Kunin C M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977;135:217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Mitchison D A, Dickinson J M. Laboratory aspects of intermittent drug therapy. Postgrad Med J. 1971;47:737–741. doi: 10.1136/pgmj.47.553.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peloquin C A. Using therapeutic drug monitoring to dose the antimycobacterial drugs. Clin Chest Med. 1997;18:79–87. doi: 10.1016/s0272-5231(05)70357-9. [DOI] [PubMed] [Google Scholar]
- 18.Singapore Tuberculosis Service/British Medical Research Council. Five year follow up of a clinical trial of three 6-month regimens of chemotherapy given intermittently in the continuation phase in the treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1988;137:1147–1150. doi: 10.1164/ajrccm/137.5.1147. [DOI] [PubMed] [Google Scholar]
- 19.Tsui S Y, Yew W W, Li M S, Chan C Y, Cheng A F. Postantibiotic effects of amikacin and ofloxacin on Mycobacterium fortuitum. Antimicrob Agents Chemother. 1993;37:1001–1003. doi: 10.1128/aac.37.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Boxtel R M, Lambrecht R S, Collins M T. Effects of colonial morphology and Tween 80 on antimicrobial susceptibility of Mycobacterium paratuberculosis. Antimicrob Agents Chemother. 1990;34:2300–2303. doi: 10.1128/aac.34.12.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelman B S, Craig W A. Postantibiotic effects. J Antimicrob Chemother. 1985;15(Suppl. A):37–46. doi: 10.1093/jac/15.suppl_a.37. [DOI] [PubMed] [Google Scholar]
- 22.Vogelman B S, Gudmundsson, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 23.Winder F G. Mode of action of the antimycobacterial agents and associated aspects of the molecular biology of the mycobacteria. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. New York, N.Y: Academic Press, Inc.; 1982. pp. 353–438. [Google Scholar]
- 24.Yew W W, Chan C K, Chau C H, Tam C M, Leung C C, Wong P C, Lee J. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–751. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 25.Yew W W, Piddock L J, Li M S, Lyon D, Chan C Y, Cheng A F B. In-vitro activity of quinolones and macrolides against mycobacteria. J Antimicrob Chemother. 1994;34:343–351. doi: 10.1093/jac/34.3.343. [DOI] [PubMed] [Google Scholar]
- 26.Zhanel G G, Saunders M H, Wolfe J N, Hoban D J, Karlowsky J A, Kabani A M. Comparison of CO2 generation (Bactec) and viable-count methods to determine the postantibiotic effect of antimycobacterial agents against Mycobacterium avium complex. Antimicrob Agents Chemother. 1998;42:184–187. doi: 10.1128/aac.42.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]