Abstract
The mitochondrial membrane potential (ΔΨ m) is a parameter often used to determine mitochondrial function; therefore, it can be used to determine the integrity and functionality of cells. A decrement of ΔΨ m is implicated in several inflammatory‐related pathologies, such phenomena can be related to COVID‐19 infection. The present work aimed to compare the ΔΨ m in leucocytes (human PBMCs; HPBMC) isolated from healthy control (HC) subjects, patients with COVID‐19 (C‐19), recovered subjects at 40 ± 13 (R1) and 335 ± 20 (R2) days after infection (dai). Obtained data showed that ΔΨ m decreased in HPBMC of subjects with C‐19, R1, and R2 compared with HC. When analyzing the ΔΨ m data by sex, in females, a significant decrease was observed in R1 and R2 groups versus HC. Regarding men, a significant decrease of ΔΨ m was observed in R1, with respect to HC, contrary to R2 group, who reestablished this parameter. Obtained results suggest that the loss of ΔΨ m could be related to the long‐COVID.
Keywords: leucocytes, mitochondrial membrane potential, response sex‐associated, SARS‐CoV‐2
Graphical Abstract
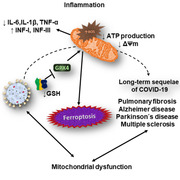
Patients recovered from COVID‐19 present loss of the ΔΨ m in HPBMC, leading to mitochondrial dysfunction, which is associated with the onset and progression of several chronic diseases such as pulmonary fibrosis, Alzheimer's, Parkinson's, and multiple sclerosis. Therefore, determining the ΔΨ m in patients recovered from SARS‐CoV‐2 could be an early sign of several diseases caused by post‐COVID‐19 sequelae.
Abbreviations
- C‐19
patients with COVID‐19
- COVID‐19
coronavirus disease 2019
- Ct
cycle threshold
- dai
days after infection
- DiOC6(3)
3,3′‐dihexyloxacarbocyanine iodide
- HC
group of healthy subjects or healthy controls
- HPBMC
human PBMCs
- R1
subjects recently recovered from COVID‐19 (40 ± 13 days after infection)
- R2
recovered subjects at 11 months post‐COVID‐19 (335 ± 20 days after infection)
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VTM
viral transport medium
- ΔΨ m
mitochondrial membrane potential
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is an enveloped RNA betacoronavirus, the outbreak of which occurred in December 2019 in Wuhan, China, which triggered the global pandemic. 1 , 2 , 3 SARS‐CoV‐2 causes the human infection known as coronavirus disease 2019 (COVID‐19). 3 , 4 Patients affected by this disease are often asymptomatic or exhibit mild symptoms (fever, cough, myalgia, and fatigue) and generally have a good prognosis. 5 , 6 , 7 However, a significant number of patients develop severe fatal consequences related to inflammatory processes largely promoted by exacerbated cytokine production, which involves major systemic disturbances. 2 , 8 , 9 These include iron dysregulation that manifests as hyperferritinemia associated with disease severity. Iron dysregulation induces the production of reactive oxygen species and promotes oxidative stress. The elevated inflammatory/oxidative state can lead to mitochondrial dysfunction leading to cell death. 2 , 10 , 11 , 12 , 13 Patients recovering from acute SARS‐CoV‐2 infection can develop long‐term sequelae, such as lung injury (pulmonary fibrosis) and other inflammatory affections, including neurodegenerative events. 14 , 15 , 16 Thus, mitochondrial integrity is essential to maintain an adequate immune response against SARS‐CoV‐2 infection. 9 , 17
As described above, mitochondrial integrity can determine differential vulnerability to SARS‐CoV‐2 infection, so some authors have proposed using mitochondrial function as a potential biomarker for developing severe COVID‐19. 2 , 17 , 18 , 19 An important indicator of mitochondrial integrity is mitochondrial membrane potential (ΔΨ m); this parameter can provide critical data related to the physiologic state of the cell and mitochondrial function of COVID‐19 patients. 20
Currently, there is information on the effects of SARS‐CoV‐2 on functional parameters of mitochondria in patients with active COVID‐19 12 , 13 , 14 , 15 , 16 , 17 , 18 ; however, there is no information on post‐COVID effects on mitochondrial functionality. Therefore, the present research aimed to evaluate ΔΨ m in human PBMCs (HPBMCs) in patients with COVID‐19 (C‐19), subjects recently recovered from COVID‐19 (R1) at 40 ± 13 days after infection (dai) and recovered subjects at 11 months postinfection (R2, at 335 ± 20 dai), as well as healthy control (HC) subjects.
2. MATERIAL AND METHODS
2.1. Selection of participants
Patients (adults) who attended the LANIIA‐Nayarit laboratory facilities (laboratory accredited by the Mexican Ministry of Health) for SARS‐CoV‐2 molecular detection, by RT‐qPCR, were invited to participate voluntarily in this study. All participants signed an informed consent form before sampling and clinical data collection. This study was carried out following the guidelines stated in the Declaration of Helsinki and was approved by the local bioethics commission (Comisión Estatal de Bioética del Estado de Nayarit‐ CEBN/01/21).
After the SARS‐CoV‐2 screening, patients were classified into 4 groups: (1) group of HC subjects: negative for SARS‐CoV‐2 and any symptoms or signs related to COVID‐19 were declared (n = 35); (2) group of patients with COVID‐19 (C‐19): subjects with SARS‐CoV‐2 active infection (qRT‐PCR positive) (n = 36); (3) group of subjects recently recovered from COVID‐19 (R1): individuals who were diagnosed as SARS‐CoV‐2 positive in our laboratory facilities, come back after several weeks of recovery (40 ± 13 dai), and resulted negative to SARS‐CoV‐2 molecular tests (n = 34); and (4) group of recovered subjects at 11 months post‐COVID‐19 (R2): individuals who were diagnosed as SARS‐CoV‐2 positive in our laboratory facilities, come back after 335 ± 20 dai (n = 18).
Patients who smoked or those with comorbidities (asthma, diabetes, hypertension, and neurodegenerative diseases) were excluded.
2.2. Sampling for SARS‐CoV‐2
For oropharynx/nasopharynx swab sampling, 2 flexible swabs were used per patient. After sampling, swabs were placed in 2.5 ml of sterile viral transport medium (VTM). Sterile VTM (pH 7.1) was prepared with HBSS supplemented with 4 mg/ml gentamicin sulfate (Sigma–Aldrich; Cat No. G1264), 50,000 U/50,000 μg penicillin/streptomycin (Sigma–Aldrich; Cat No. P4333), 0.4 mg/ml amphotericin B (Sigma–Aldrich; Cat No. A2942) and 5% BSA (Sigma–Aldrich; Cat No. A3311).
2.3. Molecular detection of SARS‐CoV‐2
RNA extraction was performed with the QIAamp RNA viral Mini Kit (Qiagen; Cat No/ID: 1020953 USA, Germantown). Samples were processed following the RT‐qPCR methodology indicated in the Berlin protocol with modifications (InDRE protocol), using the StarQ One‐Step RT‐qPCR kit (Qiagen; Cat No/ID: 210210, USA, Germantown). The genes to be amplified in each sample were the E gene (E_Sarbeco_F: ACAGGTACGTTAATAGTTAATAGCGT,E_Sarbeco_R:ATATTGCAGCAGCAGTACACGC‐ACACA, E_Sarbeco_P1: FAM‐CACTAGCCATCCTTACTTACTGCGCTTCG‐BBQ) and as a human control, the RNAseP gene (RNAseP F: AGATTTGGACCTGGAGCG, RNAseP R, GAGCGGCTGCTGCTCCACAAGT, RNAseP P1, FAM‐TTCTGACCTGAAGGCTGCGG‐BHQ1). 21 , 22 qRT‐PCR was performed with 5 μl (≈70 ng/μl) of RNA extracted in a total reaction of 25 μl. All samples were analyzed with an Applied Biosystems 7500 Fast Real‐Time PCR System, with the protocol: 50°C for 15 min, 95°C for 2 min and then 45 cycles of 95°C for 15 s and 60°C for 30 s. In all cases, amplification of RNAseP was used as an internal control. Samples with a cycle threshold (Ct) ≤ 38 were considered SARS‐CoV‐2 positive.
2.4. HPBMC isolation
Venous blood from volunteers was placed in EDTA tubes. Blood samples were diluted with phosphate buffer saline (PBS, pH 7.2) in a 1:1 ratio, deposited on a Ficoll‐Histopaque 1.077 g/ml column (1:2 ratio; Histopaque:blood), and centrifuged at 10,000 g for 25 min at room temperature. The cell ring was recovered and washed with PBS (pH 7.2) at 12,000 g for 7 min. The cell button was then resuspended in RPMI‐1640 supplemented with 10% FBS and 1% antibiotic (streptomycin/penicillin).
2.5. HPBMC characterization, counting, and culture
HPBMC were characterized by size (forward side scatter) and granularity (forward scatter). Once the study population was established in the BD Accuri C6 software template, cell counting proceeded. Then, cell viability was determined through propidium iodide staining; a 95% viability in HPBMC was considered for cell culture. In brief, 1 × 106 HPBMC/ml resuspended in RPMI medium supplemented with FBS (10%) and streptomycin/penicillin (1%) were placed in each well at 37°C and 5% CO2 in 24‐well plates, for a period of 24 h.
2.6. Determination of mitochondrial membrane potential (ΔΨ m)
The determination of ΔΨ m was performed using a cationic cyanine dye, 3,3′‐dihexyloxacarbocyanine iodide (DiOC6(3) (Invitrogen™, Cat No. D273). 23 1 × 106 HPBMC/ml from each experimental group were centrifuged at 2000 rpm for 7 min, subsequently to the cell button, 500 μl of PBS and DiOC6(3) 20 nM was added and incubated at room temperature for 15 min. Finally, each sample was analyzed in the flow cytometer (10,000 events) with the 488 nm excitation laser (FL1).
2.7. Statistical analysis
Mean fluorescence intensity (MFI) of DiOC6(3) was obtained by processing the data with the FlowJo v10 software, whereas the statistical analysis was completed in Prism 6® software (GraphPad Software Inc.). MFI was represented as a bar graph with the means ± sem. For statistical analysis, the Kruskal–Wallis test and the Dunn's Multiple Comparison Test were used. The significance level was p < 0.05.
3. RESULTS AND DISCUSSION
In this work, mitochondrial membrane potential (ΔΨ m) was assessed for the first time in HPBMC from patients with COVID‐19 (C‐19), subjects recently recovered from infection (R1) at 40 ± 13 dai, and recovered subjects at 11 months post‐COVID‐19 (R2, at 335 ± 20 dai), as well as, HC subjects. A total of 105 subjects were studied, of whom 35 were HC, 36 with C‐19 active, 34 were R1, from the latter subjects 18 were analyzed again 11 months postinfection (R2 group). Of all patients, 67 were female and 38 were male. The ages of the subjects ranged from 18 to 74 years (Table 1).
TABLE 1.
Characteristics of the study subjects
| Subject | n (Total) | N (Female) | n (Male) | Mean age (range years) | qRT‐PCR result |
|---|---|---|---|---|---|
| HC | 35 | 22 | 13 | 40 (22–74) | − |
| C‐19 | 36 | 23 | 13 | 37 (18–65) | + |
| x̄ (CT) = 28 ± 5 | |||||
| R1 | 34 | 22 | 12 | 44 (24–71) | − |
| R2 | 18 | 11 | 7 | 42 (21–69) | − |
HC, healthy control group; C‐19, patients with COVID‐19 (active); R1, subjects recently recovered from COVID‐19 (40 ± 13 days after infection); R2, recovered subjects at 11 months post‐COVID‐19 (335 ± 20 days after infection).
The patients with C‐19 active indicated that they had not required hospitalization; however, 60% of the participants mentioned having had treatment prescribed by a physician with azithromycin (15%), ivermectin (10%), vitamins (8%), antipyretics (8%), anticoagulants (7%), corticosteroids (5%), anti‐inflammatory drugs (5%), and others (2%). All COVID‐19 participants were categorized as ambulatory patients (having a mild disease) since no one stated that they had been hospitalized, and no evidence of pneumonia or hypoxia (SpO2 ≥ 94%) was ever observed. 24 , 25
As for the 35 HC subjects, 22 were female and 13 were male, their ages ranged from 22 to 74 years, whereas of the 36 C‐19 patients, 23 were female and 13 were male, their ages ranged from 18 to 65 years. The mean time of active SARS‐CoV‐2 infection was 40 days, with recovered patients considered to be those who tested negative for SARS‐CoV‐2 in their last diagnostic test by RT‐qPCR; of the 34 R1 subjects (22 were women and 12 were men) 18 individuals agreed to participate after 11 months post‐infection with SARS‐CoV‐2 (11 women and 7 men) (Table 1).
The results of this research, show that the ΔΨ m decreases significantly in subjects C‐19, R1, and R2, with respect to HC (Figure 1), suggesting that ΔΨ m in HPBMC is altered by acute SARS‐CoV‐2 infection and long‐COVID. These results align with previous reports by other authors, who indicate that SARS‐CoV‐2 causes mitochondrial dysfunction, since SARS‐CoV‐2 infection alters mitochondrial functionality, influencing its intracellular survival and/or evading host immunity. 4 , 17 , 26 , 27 , 28
FIGURE 1.
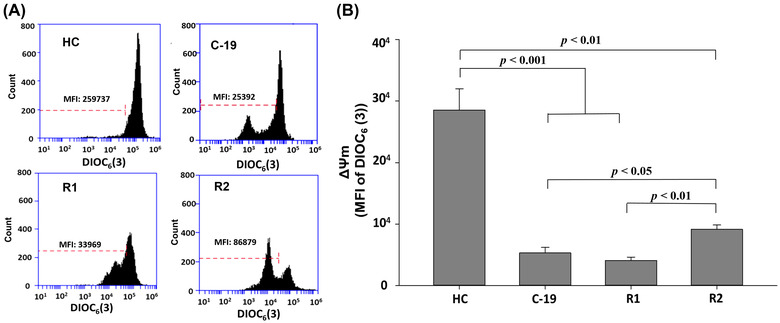
Mitochondrial membrane potential (MFI of DIOC6(3)) of HPBMC from healthy controls (HC) subjects, patients with COVID‐19 (C‐19), subjects recently recovered from COVID‐19 (R1) at 40 ± 13 days after infection (dai) and recovered subjects at 11 months post‐infection (R2, at 335 ± 20 dai). (A) Representative plots of ΔΨ m in HPBMC analyzed with software FlowJo. Each histogram shows DIOC6(3) MFI, which has affinity for stable ΔΨ m in cells. (B) MFI was represented as a bar graph with the means ± sem. Nonparametric Kruskal–Wallis and Dunn's multiple comparison tests were performed
For R2 group, data indicate that these subjects restore ΔΨ m as compared with the R1 group (Figure 1(B)); however, with respect to HC, the ΔΨ m is still significantly decreased (p < 0.05). These results suggest that the loss of ΔΨ m in subjects recovered 335 ± 20 days after SARS‐CoV‐2 infection can be associated with the so‐called long‐COVID, since of the 18 individuals who participated, 85% reported symptoms such as weariness, difficulty sleeping, fatigue, dyspnea, memory problems, anxiety, arthralgia, headache, dry cough, chest pain, myalgia, and anosmia (Figure 2).
FIGURE 2.
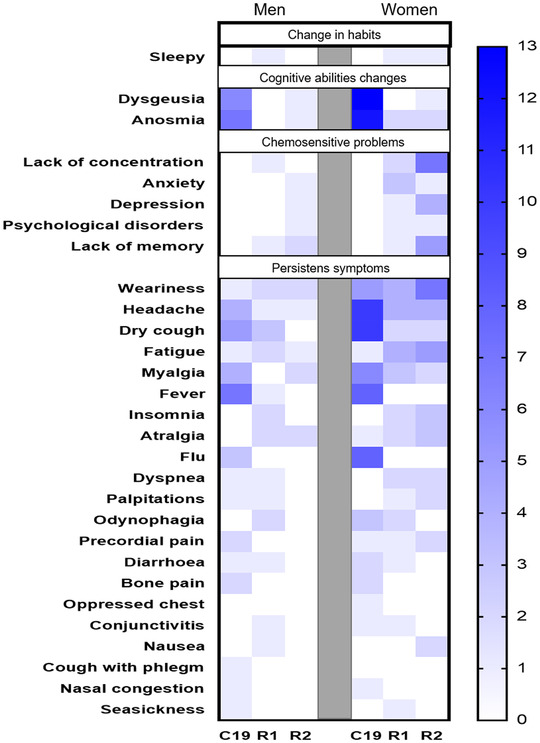
Symptomatology classification in male (left side) and female (right side) patients with COVID‐19 (C‐19), subjects recently recovered from COVID‐19 (R1) at 40 ± 13 days after infection (dai) and recovered subjects at 11 months post‐infection (R2, at 335 ± 20 dai). The intensity of blue color in each box indicates the frequency of individuals who presented each symptom
Furthermore, some authors have suggested that mitochondrial dysfunction is a predisposing factor for COVID‐19 severity. 2 , 17 , 18 , 19 However, the results of the present study suggest that the ΔΨ m could also be an early indicator of multiple COVID‐19‐associated diseases, since, in recovered patients (without comorbidities with a mean time of SARS‐CoV‐2 infection of 40 ± 13 dai) of COVID‐19 present loss of mitochondrial membrane potential, a phenomenon that persisted 11 months (335 ± 20 days) after COVID‐19 infection. In this regard, some authors have reported that patients who manage to survive the effects of acute SARS‐CoV‐2 infection may develop long‐term sequelae, such as lung injury (pulmonary fibrosis), neuronal injury (acute and chronic neuropathology), and neurodegenerative diseases (Alzheimer's, Parkinson's and multiple sclerosis), 14 , 15 , 16 , 29 , 30 which could be attributed to mitochondrial dysfunction associated to SARS‐CoV‐2 infection, since it has been suggested that mitochondrial dysfunction in conjunction with an abnormal innate immunity response plays a pivotal role in the onset and development of several chronic diseases. 25 , 26 , 27 , 30 , 31 , 32 , 33
Regarding the ΔΨ m in HPBMC between males and females from the analyzed groups (HC, C‐19, R1, and R2). In general, the results indicate that female HC have higher ΔΨ m than male HC (Figure 3). In this sense, some authors report that between both sexes there are differences in mitochondrial function, in this way, compared with men, women present higher levels of enzymes (citrate synthase activity) ATP, and antioxidant compounds, 34 , 35 due to a higher mitochondrial activity, facts related to a longer life expectancy, 36 and a lower occurrence of diseases and aging. 37
FIGURE 3.
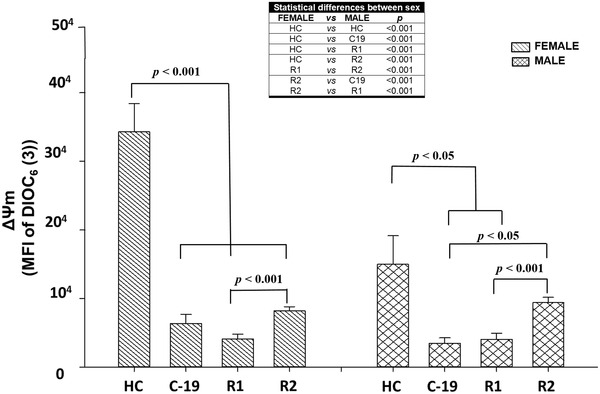
Mitochondrial membrane potential (MFI of DIOC6(3)) of HPBMC from healthy controls (HC), subjects, patients with COVID‐19 (C‐19), subjects recently recovered from COVID‐19 (R1) at 40 ± 13 days after infection (dai) and recovered subjects at 11 months postinfection (R2, at 335 ± 20 dai). MFI was represented as a bar graph with the means ± sem. The table shows the statistically significant differences between male and female. Nonparametric Kruskal–Wallis and Dunn's multiple comparison tests were performed
On the other hand, the obtained data indicate that both sexes decrease significantly ΔΨ m in C‐19 and R1 groups (Figure 3). Regarding males with C‐19 and R1, the ΔΨ m is significantly decreased with respect to HC males. However, in R2 males this parameter recovers significantly with respect to the C‐19 (p < 0.05) and R1 group, reaching similar values to HC males. Otherwise, in the C‐19, R1, and R2 groups of females, the ΔΨ m is significantly decreased with respect to HC female group. When comparing this parameter in R2 females with respect to R1 female, the ΔΨ m was significantly increased (p < 0.05); however, the ΔΨ m continues to be diminished (p < 0.05) with respect to HC females. Unlike men, R2 women did not restore their ΔΨ m with respect to the HC group, suggesting that this effect in women is caused by long‐COVID since all women (100%) reported presenting 5 symptoms on average (63% fatigue and difficulty sleeping, 45% fatigue, dyspnea, memory problems and anxiety, 36% headache, and arthralgia, 18% dry cough, chest pain and myalgia, 9% anosmia) after 335 ± 20 dai, contrary to men (58%) who reported having 2 symptoms on average (28% fatigue, memory problems, myalgia, and palpitations, whereas 14% fatigue, arthralgia, or headache) (Figure 2). This agrees with Ortona et al., 38 who indicate that women seem to be twice as likely to develop long‐COVID as men, due to hormonal factors are stronger in women than in men.
On the other hand, some authors have compared SARS‐CoV‐2 infection between men and women, indicating a high mortality rate in older men; suggesting that women conduct a more effective viral clearance, perhaps since females show more robust innate interferon antiviral response, in addition to greater adaptive immunity towards viral antigens. 4 , 17 , 38 , 39 Besides, it has been reported that outbreaks of SARS and the Middle East respiratory syndrome have also shown a male predominance in disease susceptibility, a feature also observed in SARS‐CoV‐2. 40 , 41 , 42 In this regard, Channappanavar et al., 43 experimentally infected male and female mice with SARS‐CoV, observing that males were more susceptible than females; however, ovariectomy or estrogen receptor antagonists increased the mortality of females, concluding that estrogens could play a protective effect against coronavirus infection. 42 , 43 , 44 Similarly, other studies have reported that ovariectomy decreases mitochondrial oxidative phosphorylation and increases oxidative stress. 33 , 45 In this sense, the present study results suggest that the decrease of ΔΨ m in HPBMC from infected and recovered female patients to SARS‐CoV‐2 could be related to an estrogen alteration (lack of menstruation or ovulation); notwithstanding the above, further studies are still necessary to support this hypothesis.
4. CONCLUSION
Mitochondrial dysfunction is associated with the onset and development of several chronic diseases (neurodegenerative, cardiovascular, gastrointestinal, diabetes, and cancer). Nowadays, mitochondrial dysfunction is considered a predisposing factor for COVID‐19 severity. The present results indicate that COVID‐19 recovered subjects presented loss of ΔΨ m, even 11 months (335 ± 20 dai) after infection, suggesting that this effect is associated with the so‐called long‐COVID since 85% of the subjects reported persistent symptoms. It is therefore proposed that a sustained decrease of ΔΨ m could be a sign of susceptibility to the development of diseases associated with post‐COVID sequelae.
AUTHORSHIP
K. J. G. D. R. contributed with the development of clinical survey, coordination of sample processing, data analysis, data interpretation, and writing of the manuscript; A. B. B. T. worked on the application of clinical survey, sample processing, and data analysis. C. E. C. R. contributed with sample processing and comments on the final version of the paper; G. A. T. I. performed the sample processing; P. C. O. L. contributed with the conceptual advice and comments on the final version of the paper; D. A. G. P. contributed with the sample processing; A. Y. B. D. performed the collection and processing of sample; D. A. P. D. worked on the development and application of clinical survey and data analysis; R. G. B. G. performed the sample collection and sample processing; M. I. G. P. contributed with the design of the study and comments on the final version of the paper.
DISCLOSURES
The authors declare no competing or financial interests.
FUNDING
This work was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) project number 313590 “Caracterización y validación de propiedades inmunomoduladoras de una formulación mexicana de fucoidano, como potencial tratamiento alternativo para COVID‐19.”
ACKNOWLEDGMENTS
The authors acknowledge to CONACyT for the approved project, and to the Immunotoxicology Laboratory from Universidad Autónoma de Nayarit (Mexico).
Díaz‐Resendiz KJG, Benitez‐Trinidad AB, Covantes‐Rosales CE, et al. Loss of mitochondrial membrane potential (ΔΨm) in leucocytes as post‐COVID‐19 sequelae. J Leukoc Biol. 2022;112:23–29. 10.1002/JLB.3MA0322-279RRR
Summary Sentence: Loss of mitochondrial membrane potential in leucocytes of recovered COVID‐19 subjects.
REFERENCES
- 1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID‐19 pathogenesis. Mitochondrion. 2020;54:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS‐CoV‐2 hijacking of host mitochondria in COVID‐19 pathogenesis. Am J Physiol Cell Physiol. 2020;319:C258‐C267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fratta Pasini AM, Stranieri C, Cominacini L, Mozzini C. Potential role of antioxidant and anti‐inflammatory therapies to prevent severe SARS‐Cov‐2 complications. Antioxidants. 2021;10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nunn AV, Guy GW, Brysch W, et al. SARS‐CoV‐2 and mitochondrial health: implications of lifestyle and ageing. Immun Ageing. 2020;17:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 11. Phua J, Weng L, Ling L, et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respir Med. 2020;8:506‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alarcón‐Rodríguez J, Fernández‐Velilla M, Ureña‐Vacas A, et al. Manejo y seguimiento radiológico del paciente post‐COVID‐19. Radiología. 2021;63:258‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernando JEC. Seguimiento de los pacientes con secuelas no respiratorias de la COVID‐19. FMC Form Med Contin Aten Primaria. 2021;28:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang F, Kream RM, Stefano GB. Long‐term respiratory and neurological sequelae of COVID‐19. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e928996‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP. Mitochondria–in the crossfire of SARS‐CoV‐2 and immunity. Iscience. 2020:101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakahira K, Kyung SY, Rogers AJ, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ShenoyS . Coronavirus (Covid‐19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. JoVE. 2011;51:e2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasan MR, Mirza F, Al‐Hail H, et al. Detection of SARS‐CoV‐2 RNA by direct RT‐qPCR on nasopharyngeal specimens without extraction of viral RNA. PloS one. 2020;15:e0236564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortiz‐Lazareno PC, Bravo‐Cuellar A, Lerma‐Díaz JM, et al. Sensitization of U937 leukemia cells to doxorubicin by the MG132 proteasome inhibitor induces an increase in apoptosis by suppressing NF‐kappa B and mitochondrial membrane potential loss. Cancer Cell Int. 2014;14:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . COVID‐19 clinical management: living guidance. 2021. https://apps.who.int/iris/handle/10665/338882
- 25. Sánchez‐Ríos CP, Jiménez‐Cabrera OG, Barreto‐Rodríguez O, Téllez‐Navarrete NA. Enfermedad COVID‐19 en adultos jóvenes mexicanos hospitalizados. Neumol Cir Torax. 2021;80:105‐110. [Google Scholar]
- 26. De Las Heras N, Martín Giménez VM, Ferder L, Manucha W, Lahera V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID‐19: therapeutic effects of Vitamin D. Antioxidants. 2020;9:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi CS, Qi HY, Boularan C, et al. SARS‐coronavirus open reading frame‐9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol Res. 2014;193:3080‐3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anand SK, Tikoo SK. Viruses as modulators of mitochondrial functions. Adv Virol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papa SM, Brundin P, Fung VSC. Impact of the COVID‐19 pandemic on Parkinson's disease and movement disorders. Mov Disord. 2020;7:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calderón‐Garcidueñas L, Torres‐Jardón R, Franco‐Lira M, et al. Environmental nanoparticles, SARS‐CoV‐2 brain involvement, and potential acceleration of Alzheimer's and Parkinson's diseases in young urbanites exposed to air pollution. Int J Alzheimers Dis. 2020:1‐25. [DOI] [PubMed] [Google Scholar]
- 31. Wilkins H, Swerdlow R. Relationships between mitochondria and neuroinflammation: implications for Alzheimer's disease. Curr Top Med Chem. 2016;16:849‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Ortencio A, Navigante A. Disfunción mitocondrial y enfermedades cardiovasculares. Insufic Card. 2016;11:201‐214. [Google Scholar]
- 33. Rodríguez‐Violante M, Cervantes‐Arriaga A, Vargas‐Cañas S, Corona T. Papel de la función mitocondrial en las enfermedades neurodegenerativas. Arch Neurocienc. 2010;15:39‐46. [Google Scholar]
- 34. Cardinale DA, Larsen FJ, Schiffer TA, et al. Superior intrinsic mitochondrial respiration in women than in men. Front Physiol. 2018;9:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silaidos C, Pilatus U, Grewal R, et al. Sex‐associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol Sex Differ. 2018;9:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seifarth JE, Mcgowan CL, Milne KJ. Sex and life expectancy. Gend Med. 2012;9:390‐401. [DOI] [PubMed] [Google Scholar]
- 37. Tower J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch Biochem Biophys. 2015;576:17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ortona E, Buonsenso D, Carfi A, Malorni W. Long COVID: an estrogen‐associated autoimmune disease?. Cell Death Discov. 2021;7:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguere T. COVID‐19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do?. Am J Epidemiol. 2004;159:229‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinna G. Sex and COVID‐19: a protective role for reproductive steroids. Trends Endocrinol Metab. 2021;32:3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex‐based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costeira R, Lee KA, Murray B, et al. Estrogen and COVID‐19 symptoms: associations in women from the COVID symptom study. PLOS ONE. 2021;16:e0257051. 10.1371/journal.pone.0257051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaignard P, Savouroux S, Liere P, et al. Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology. 2015;156:2893‐2904. [DOI] [PubMed] [Google Scholar]


