Abstract
Outcomes of 109 hospitalized COVID‐19 patients who received at least one vaccine dose 14 or more days prior the disease onset were retrospectively compared to control cohort of 109 age, sex, and Charlson comorbidity index‐matched patients chosen among 2990 total hospitalized patients in our tertiary‐level institution in a period from January to June 2021. Among 109 vaccinated patients, 84 patients were partially and 25 fully vaccinated. Vaccinated patients experienced significantly lower 30 days mortality (30% vs. 49%; hazard ratio [HR]: 0.56 [0.37–0.85]; p = 0.008), less frequently required high flow oxygen therapy (17% vs. 34%; HR: 0.45 [0.26–0.76]; p = 0.005), and mechanical ventilation (8% vs. 18%; HR: 0.41 [0.20–0.88]; p = 0.027) in comparison to the matched cohort of unvaccinated patients. More favorable survival was observed in patients receiving vector in comparison to messenger RNA (mRNA) vaccine types in unadjusted analysis (30 days mortality 18% vs. 40%; HR: 0.45 [0.25–0.79]; p = 0.034). In the multivariable Cox regression analysis model both mRNA (HR: 0.59 [0.36–0.98]; p = 0.041) and vector vaccine types (HR: 0.30 [0.15–0.60]; p < 0.001) were associated with improved survival in comparison to unvaccinated patients, independently of age (HR: 1.03 [1.01–1.06]; p = 0.011), male sex (HR: 1.78 [1.14–2.76]; p = 0.010), severity of illness (HR: 2.06 [1.36–3.10]; p < 0.001) and functional status on admission (HR: 1.42 [1.07–1.85]; p = 0.013).
Keywords: Astra Zeneca, COVID‐19, Moderna, Pfizer, SARS‐CoV‐2, vaccination
Highlights
Vaccinated patients who required hospitalization were old and burdened with comorbidities.
Vaccinated in comparison to matched control patients experienced improved survival.
Favorable effects of vaccination were more pronounced in vector than mRNA vaccine recipients.
1. INTRODUCTION
COVID‐19, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has affected more than 230 million people worldwide and caused more than 4.8 million deaths till now. 1 It has largely influenced our way of life, health, social relationships, and economics at a global level. Up to 15%–20% of unvaccinated patients present with severe or critical COVID‐19 symptoms, develop respiratory insufficiency, and require hospitalization, 2 thus putting an enormous strain on the healthcare system.
Vaccination is an effective preventive measure for pandemics control, in addition to other epidemiological measures (wearing masks, distancing, disinfection). In most of the European Union, two messenger RNA (mRNA) (BNT162b2 and mRNA‐1273) and two vector COVID‐19 vaccines (ChAdOx1 nCoV‐19 AZD1222 and Ad26.COV2.S) are available. In Croatia vaccination started on January 27th, 2020 and the first available were mRNA vaccines for priority groups of population (older, long‐term facility residents, healthcare workers, care home workers, immunocompromised patients). From mid‐February 2021 there are also vector vaccines on disposition and mass vaccination of the general population started from April 2021 with all four available vaccines. All of them have proven their safety and efficacy, 3 , 4 , 5 , 6 not only in fully vaccinated then also in incomplete vaccinated population for BNT162b2 and ChAdOx1 nCoV‐19 vaccines. Notably, a great effect of a single dose against hospital admissions and mortality has been reported. 7
Due to the inability of vaccines to completely prevent the development of COVID‐19, patients exposed to COVID‐19 vaccines can still be hospitalized and encountered in clinical wards. Their clinical course is unknown. Thus, we aimed to investigate the clinical characteristics and disease course of these patients by analyzing a large cohort of real‐life hospitalized COVID‐19 patients from a tertiary institution.
2. METHODS
We have retrospectively analyzed hospitalized COVID‐19 patients treated in our tertiary‐level institution (University hospital Dubrava) in the period from January 2021 to June 2021. Among 2990 patients, we have identified a total of 109 patients who received at least one dose of anti‐SARS‐CoV‐2 vaccine ≥14 days before hospitalization and compared them to 1:1 age, sex, and Charlson comorbidity index (CCI) matched cohort of hospitalized but unvaccinated patients. Matching procedure was done without COVID‐19 severity on admission as a criterion with the aim of assessing the potential difference in severity between vaccinated and unvaccinated patients, however proportion of severe or critical disease was well balanced between the two groups (there was no significant difference in the proportion of patients with severe or critical disease on presentation). CCI was chosen for quantification of comorbidity burden as a validated measure with prognostic implications in COVID‐19 patients due to the fact that the matching procedure could not be performed using a large number of comorbidities. Specific comorbidities were well balanced between the two groups after matching (no significant difference). The subgroup of patients exposed to single‐dose with illness onset <14 days after vaccination (75 patients) was excluded from analyses. Data on baseline characteristics and clinical course of patients are a part of the hospital registry project and were obtained through analysis of written and electronic medical records. Data on vaccination were collected from vaccination certificates or by plausible self‐report if they provided the number of vaccination doses, dates, and type of vaccine and were checked at the national immunization registry. All patients were tested COVID‐19 positive by polymerase chain reaction or antigen test. COVID‐19 severity was graded based on the World Health Organization recommendations as mild, moderate, severe, and critical. The study was approved by the Institutional Review Board.
The normality of the distribution of numerical variables was analyzed using the Shapiro–Wilk test. Numerical variables were presented as the median and interquartile range (IQR) and were compared between groups using the Kruskal–Wallis one‐way analysis of variance. Categorical variables were presented as frequencies and percentages and were compared between groups using the χ 2 test. Survival analyses were based on the Kaplan–Meier method. The main outcome of interest was survival and the need for mechanical ventilation and high flow oxygen therapy were assessed as additional outcomes of interest and were assessed in vaccinated and matched unvaccinated groups of patients. Survival was recorded from the start of hospitalization and included the postdischarge period for a median of 2 months postdischarge. Discharged patients were scheduled for control visits or contacted if not attending. Patients were censored if not obtaining an event of interest during the follow‐up period or lost to follow‐up. Survival was compared between groups using the log‐rank test for univariate and the Cox regression analysis for multivariable analysis. Variables that were considered clinically relevant for survival outcome (vaccine type, age, sex, COVID‐19 severity on admission, ECOG functional status on admission, CCI) were included in the multivariable model. Vaccination type was analyzed as a categorical variable with three levels (mRNA, vector, and nonvaccinated). Nonadjusted and adjusted hazard ratios (HRs) with 95% confidence intervals (CI) are provided for these parameters. Only one model adjusted synchronously for all included variables is presented. Adjustments for some of the factors also used for matching of the control group were deliberately implemented to control the analysis for potential small differences between vaccinated and unvaccinated patients as well as for heterogeneity of vaccinated group of patients due to the retrospective data set and obligation to ameliorate as much bias as possible. p < 0.05 were considered to be statistically significant. All analyses were performed using the MedCalc statistical software ver 20.010 (MedCalc Software Ltd.).
3. RESULTS
The median age of COVID‐19 patients hospitalized after vaccination was 82 years, IQR (73–86). There were 59/109 (54.1%) males. Median CCI was 5 points, IQR (4–7). Severe or critical COVID‐19 on admission was present in 100/109 (91.7%) patients. Patients' characteristics are shown in Table 1.
Table 1.
Patients' characteristics between vaccinated and age, sex, and Charlson comorbidity index‐matched control cohort of unvaccinated patients
| Matched controls (N = 109) | Vaccinated patients (N = 109) | p Value | |
|---|---|---|---|
| Age (years), median and IQR | 82 (73−86) | 82 (73–86) | 0.929 |
| Sex | 1.000 | ||
| Male | 59 (54.1%) | 59 (54.1%) | |
| Female | 50 (45.9%) | 50 (45.9%) | |
| CCI, median and IQR | 5 (4−7) | 5 (4–7) | 0.699 |
| COVID‐19 severity | 0.719 | ||
| Mild | 6 (5.5%) | 10 (9.2%) | |
| Moderate | 3 (2.8%) | 2 (1.8%) | |
| Severe | 84 (77.1%) | 83 (76.1%) | |
| Critical | 16 (14.7%) | 14 (12.8%) | |
| ECOG status, median and IQR | 3 (2−4) | 3 (2−4) | 0.347 |
| Duration of symptoms, median and IQR | 5 (2−9) | 6 (1−10) | 0.498 |
| Arterial hypertension | 92 (84.4%) | 85 (78%) | 0.225 |
| Diabetes mellitus | 36 (33%) | 34 (31.2%) | 0.772 |
| Hyperlipoproteinemia | 33 (30.3%) | 31 (28.4%) | 0.766 |
| Obesity | 38 (34.9%) | 29 (26.6%) | 0.186 |
| Atrial fibrillation | 34 (31.2%) | 27 (24.8%) | 0.291 |
| Chronic kidney disease | 24 (22%) | 22 (20.2%) | 0.740 |
| Active malignancy | 10 (9.2%) | 5 (4.6%) | 0.181 |
| Dementia | 31 (28.4%) | 23 (21.1%) | 0.209 |
| CRP (mg/L), median and IQR | 100.7 (49.8−145.5) | 91.7 (43.7−156.8) | 0.880 |
| Ferritin (µg/L), median and IQR | 676 (364−1128) | 685 (375−1310) | 0.385 |
| d‐dimers (mg/L FEU), median and IQR | 1.4 (0.65−4.36) | 1.4 (0.69−3.29) | 0.911 |
| WBC (×109/L), median and IQR | 8.9 (6.1−11.8) | 8.1 (5.65−11.85) | 0.910 |
| Absolute neutrophils (×109/L), median and IQR | 7.02 (4.9−10.8) | 7.0 (5.0−9.5) | 0.697 |
| Absolute lymphocytes (×109/L), median and IQR | 0.79 (0.48−1.11) | 0.8 (0.59−1.13) | 0.713 |
| Hemoglobin (g/L), median and IQR | 121 (109−137) | 128 (113−140) | 0.146 |
| Platelets (×109/L), median and IQR | 210 (162−274) | 222 (169−305) | 0.300 |
| IL‐6 (pg/ml), median and IQR | 36.4 (13−60.32) | 57.5 (27.75−99.87) | 0.220 |
| Procalcitonin (ng/ml), median and IQR | 0.3 (0.14−1.02) | 0.3 (0.12−1.18) | 0.856 |
Abbreviations: CCI, Charlson comorbidity index; CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; IL, interleukin; IQR, interquartile range; WBC, white blood cells.
A total of 109 patients who received at least one vaccine dose comprised 3.6% of the total 2990 hospitalized patients during the study period. Among them, 84/109 (77%) patients received single‐dose and 25/109 (23%) were fully vaccinated, representing 2.8% and 0.8% of all hospitalized patients, respectively. A total of 60/109 (55%) of vaccinated patients received mRNA vaccine (51 BNT162b2 and 9 mRNA‐1273) and 49/109 (45%) received vector vaccine (ChAdOx1‐S/nCoV‐19). In comparison to other hospitalized patients, vaccinated patients were significantly older (median 82 vs. 72 years; p < 0.001) and had higher CCI (median 5 vs. 4; p < 0.001). Patients who were hospitalized after full vaccination in comparison to partially vaccinated were predominantly vaccinated with mRNA vaccines (92% vs. 44%; p < 0.001), were older (median 84 vs. 80 years; p = 0.015), more likely to have chronic kidney disease (40% vs. 17%; p = 0.013) and dementia (52% vs. 21%; p = 0.003).
We further compared clinical outcomes of the cohort of vaccinated patients with the age, sex, and CCI‐matched control cohort of 109 patients. Baseline patient characteristics were well balanced between two groups as shown in Table 1 with similar distribution of severe or critical COVID‐19 and different comorbidities. Vaccinated patients required lower rates of high flow oxygen therapy (17% vs. 34%; HR: 0.45, 95% CI: (0.26–0.76); p = 0.005) and mechanical ventilation (8% vs. 18%; HR: 0.41, 95% CI: (0.20–0.88); p = 0.027) and experienced significantly lower 30 days mortality in comparison to matched cohort of unvaccinated patients (30% vs. 49%; HR: 0.56, 95% CI: (0.37–0.85); p = 0.008) as shown in Figure 1A. More favorable survival was observed in vector in comparison to mRNA vaccinated patients (30 days mortality 18% vs. 40%; HR: 0.45, 95% CI: (0.25–0.79); p = 0.034) as shown in Figure 1B. However, patients hospitalized after receiving mRNA in comparison to vector vaccines were more likely to be male (67% vs. 43%; p = 0.012) and had higher CCI (median 6 vs. 4; p = 0.027) which are known negative prognostic factors. There was insignificant statistical trend for longer time from the first dose to the start of symptoms in patients receiving mRNA in comparison to vector vaccines (median 40 vs. 33 days; p = 0.085). Other clinically meaningful variables that were used for further adjustments showed significant univariate associations with survival: age (HR: 1.05, 95% CI: [1.03–1.08]; p < 0.001), male sex (HR: 1.65, 95% CI: [1.07–2.55]; p = 0.023), COVID‐19 severity (HR: 2.22, 95% CI: [1.49–3.30]; p < 0.001), CCI (HR: 1.17, 95% CI: [1.07–1.29]; p < 0.001), ECOG status on admission (HR: 1.63, 95% CI: [1.29–2.07]; p < 0.001).
Figure 1.
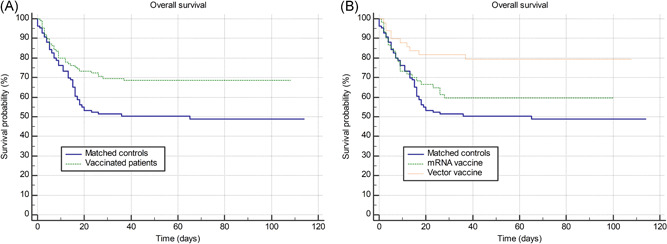
(A) Overall survival of vaccinated patients versus matched age, sex, and Charlson comorbidity index‐matched controls. (B) Same comparison with vaccinated patients was further stratified by mRNA and vector vaccine types. mRNA. messenger RNA
The multivariable Cox regression analysis revealed significantly improved survival for patients vaccinated with both vaccine types in comparison to unvaccinated patients in the model adjusted for clinically meaningful variables: mRNA vaccine type (HR: 0.59, 95% CI: [0.36–0.98]; p = 0.041), vector vaccine type (HR: 0.30, 95% CI: [0.15–0.60]; p < 0.001), age (HR: 1.03, 95% CI: [1.01–1.06]; p = 0.011), male sex (HR: 1.78, 95% CI: [1.14–2.76]; p = 0.010), COVID‐19 severity (HR: 2.06, 95% CI: [1.36–3.10]; p < 0.001), ECOG status on admission (HR: 1.42, 95% CI: [1.07–1.85]; p = 0.013), and CCI (HR: 1.07, 95% CI: [0.96–1.21]; p = 0.211). After additionally adjusting multivariable analysis for number of vaccine doses and calendar month of admission to the hospital, differences between vaccinated and unvaccinated patients remained significant for both vaccine types, whereas number of vaccine doses and calendar month of admission did not significantly affect the outcome.
4. DISCUSSION
To the best of our knowledge, our work is first to investigate clinical outcomes of hospitalized COVID‐19 patients who received prior vaccination in comparison to a comparable matched‐pair cohort of unvaccinated patients. Our results show the significant impact of vaccination on clinical outcomes. The majority of vaccinated patients who required hospitalization due to COVID‐19 were old with a high comorbidity burden thus being unable to develop a proper immune response to vaccination. Due to the fact that our hospital is a tertiary‐level institution, with up to 35% of hospitalized patients requiring intensive or critical care measures in total capacity, the majority of patients had severe or critical COVID‐19 on admission (91.7%). In comparison to the age, sex, and CCI‐matched control cohort of patients of comparable baseline characteristics who received no immunization before COVID‐19 infection, vaccinated patients had a tendency for lower high flow oxygen therapy and mechanical ventilation requirement and experienced improved survival. It is important to notice that despite having severe or critical COVID‐19 on admission in a similar proportion, vaccinated patients that required hospitalization in comparison to matched unvaccinated patients could expect limited disease progression with less further respiratory deterioration. The survival benefit was persisting for both mRNA and vector vaccine types in comparison to unvaccinated patients after adjusting for age, sex, COVID‐19 severity, CCI, and functional status on admission. However, it seems that favorable effects of vaccination regarding survival could be more pronounced in patients receiving vector vaccine in comparison to mRNA vaccine types, but this finding must be interpreted in the context of different populations vaccinated with those two types of vaccines and different time frames of vaccination (as discussed in continuation). It should be noted that a small subset of patients required hospitalization despite full vaccination status. These patients were of significantly older age and more burdened with comorbidities which indirectly implies that fully vaccinated people with a lower burden of age and comorbidities had not even been hospitalized.
Higher age and comorbidities are known to be a risk factor for poor outcomes, regardless of vaccination status 8 , 9 , 10 and high mortality rates in our study are typical for severe/critical COVID‐19 course in very old patient population burdened with comorbidities (median age 82 years, median CCI 5 points). Due to the availability of exclusively mRNA vaccines by mid‐February in Croatia and priority of vaccination among the older population, especially residents at long‐term care facilities, selected patients with unfavorable prognostic characteristics received mRNA vaccine type during the study period. Also due to recommendations of various professional societies, most immunocompromised patients were vaccinated with mRNA vaccines. These patients might have developed weaker immunogenic responses despite being fully vaccinated. However, this phenomenon may also be due to decreased neutralizing antibody titers which are shown for the BNT162b2 vaccine even for immunocompetent adults. 11 , 12 , 13 There was also a nearly significant difference for a longer time from the first dose to the start of symptoms presents in the mRNA in comparison to vector vaccines which might add to the observed difference. However, our study was not designed to properly assess potential mechanisms leading to differences between different vaccine types. Although mostly incomplete, vaccination provides protection from respiratory deterioration and death from COVID‐19. These phenomena might be more pronounced in patients receiving vector than mRNA vaccine types but were present for both vaccine types.
Limitations of our work are single‐center experience and retrospective study design. Small sample size precludes further meaningful analyses of subgroups of interests (separate evaluation of partially and fully vaccinated patients, additional stratification of vaccine types by the number of received doses, etc.). No significant differences in outcomes were present between partially and fully vaccinated patients but due to small numbers results are inconclusive. Nevertheless, the overall large cohort of patients hospitalized in our institution allows us to properly match the control cohort of patients regarding baseline patient and disease characteristics. However, matching was performed for obtaining comparably vaccinated vs unvaccinated groups and it does not apply for comparison of one vaccine type to another. Due to the small sample of vaccinated patients, we were unable to properly match mRNA vs vector vaccinated patients to obtain meaningful conclusions. Our results are representative of a tertiary referral center for COVID‐19 with the majority of severe and critical patients and for the specific context of low vaccination rates in the general population at the start of the vaccination program (ranging from <5% to 30% over the study period). They also represent the pandemic period of second and third disease waves that were dominated by alpha and beta SARS‐CoV‐2 strains and timeframe <6 months from vaccination, thus avoiding additional biases introduced by later occurring strains and waning effects of vaccination that are planned to be evaluated separately in the future studies when data become more mature. Our study provides important insights into the clinical course of dominantly partially vaccinated COVID‐19 patients who despite incomplete vaccination status were able to experience less respiratory deterioration and improved survival. This corroborates efforts to increase the extent of primary series application on the global level. Further studies on this topic are needed to understand what factors are important for differences in survival between different vaccine types.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the University Hospital Dubrava Review Board.
AUTHOR CONTRIBUTIONS
All authors fulfilled ICMJE authorship criteria and agree to be accountable for all aspects of the work. Nikolina Busic participated in the study design, data acquisition, data interpretation, drafting of the manuscript, revising it critically for important content, and approving the final version. Tomo Lucijanic participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Bruno Barsic participated in study design, data interpretation, critically revising the manuscript for important content, and approved the final version. Ivica Luksic participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Iva Busic participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Goran Kurdija participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Ljubo Barbic participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Sanja Kunstek participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Tea Jelic participated in data interpretation, critically revising the manuscript for important content, and approved the final version. Marko Lucijanic participated in the study design, data acquisition, statistical analysis, data interpretation, drafting of the manuscript, revising it critically for important content, and approved the final version.
ACKNOWLEDGMENTS
This paper is a part of the project “Registar hospitalno liječenih bolesnika u Respiracijskom centru KB Dubrava“/“Registry of hospitalized patients in University Hospital Dubrava Respiratory center.”
Busic N, Lucijanic T, Barsic B, et al. Vaccination provides protection from respiratory deterioration and death among hospitalized COVID‐19 patients: differences between vector and mRNA vaccines. J Med Virol. 2022;94:2849‐2854. 10.1002/jmv.27666
DATA AVAILABILITY STATEMENT
Data available on reasonable request.
REFERENCES
- 1. WHO . Coronavirus dashboard. Accessed: January 10, 2022. https://covid19.who.int/.
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer‐BioNTech and Oxford‐AstraZeneca vaccines on covid‐19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case‐control study. BMJ. 2021;373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scobie HM, Johnson AG, Suthar AB, et al. Monitoring incidence of COVID‐19 cases, hospitalizations, and deaths, by vaccination status—13 U.S. Jurisdictions, April 4‐July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1284‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muthukrishnan J, Vardhan V, Mangalesh S, et al. Vaccination status and COVID‐19 related mortality: a hospital‐based cross‐sectional study. Med J Armed Forces India. 2021;77(suppl 2):S278‐S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agrawal U, Katikireddi SV, McCowan C, et al. COVID‐19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV‐19 vaccinations in 2.57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med. 2021;9:1439‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS‐CoV‐2 infection in Qatar. N Engl J Med. 2021;385:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer‐BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID‐19 Hospitalizations among adults without immunocompromising conditions—United States, March‐August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on reasonable request.


