CONFLICTS OF INTEREST
The authors declare no conflict of interest.
To the Editor,
1.
In November 2019, the social media app WeChat, the platform used within China, picked up chatter between physicians about a cluster of pneumonia cases reported as an illness in patients of unknown cause. This was followed by a report to the media on December 31, 2019, by the Wuhan Municipal Health Commissioner about a cluster of pneumonia cases in Wuhan, leading to the possibility of a new coronavirus outbreak. Following this report, the World Health Organization (WHO) came into action and announced that the causative agent is a novel coronavirus and issued the initial guidelines. By January 22, 2020, it was confirmed that this novel coronavirus could transmit from human to human. On February 11, the WHO announced the disease caused by the novel coronavirus would be named COVID‐19, and due to the widespread global reports of the disease by March 11, the WHO announced COVID‐19 as a pandemic. 1 The causative agent of the COVID‐19 is the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) virus that has spread from Wuhan, China, to the rest of the world and has been an ongoing pandemic ever since. 2 Approximately 468 million people have been infected with the SARS‐CoV‐2 and its variant of concerns (VOCs) andand its variants so far, and more than 6.0 million deaths have occurred. Human Coronavirus are classified into four major genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Of these, only the Alpha and Beta‐CoVs are known to infect humans. In contrast, the Gamma and Delta‐CoVs predominantly infect birds. 3 SARS‐CoV‐2 shares about 89% sequence identity with the other human coronaviruses. 4 The phylogenetic analysis has revealed that the SARS‐COV‐2 shares 89%–96% homology with the Chinese bat coronavirus (Bat‐SL‐CoV RaTG13, ZC45, and ZXC21), suggesting potential bat origin that was potentially transmitted to humans 2 , 5 , 6 , 7
Although the SARS‐CoV‐2 encodes an RNA proofreading exoribonuclease (nsp14‐exon), it has accumulated multiple random mutations over the viral genome during global transmission and led to different variants across the globe. 8 , 9 The first SARS‐CoV2variant of SARS‐CoV‐2, carrying the mutation at the D614G of Spike protein, emerged throughout the globe and became prevalent, suggesting a fitness advantage. 10 Later on, various variants were identified, including Alpha variant (B.1.1.7; United Kingdom), Beta (B.1.351; South Africa), Gamma (P1; Japan/Brazil), and Delta variants (B.1.617.2; India). As Delta was more transmissible than the other variants, it has evolved into a dominant variant globally over the past year (2021), potentially increasing the number of infections and deaths globally. 11 Recently the emergence of the novel variant C.1.2 and Omicron in South Africa have raised concerns as they are shown to potentially escape antibody responses induced by the available vaccines and/or virus‐induced immune responses following natural infection. Additionally, this variant is more transmissible than the Alpha and Delta variants. 12 These variants contain multiple mutations in the spike protein, raising concern about the degree of protection by the currently available vaccines and monoclonal antibody therapeutics, designed against the spike protein of the SARS‐CoV‐2 Wuhan strain or D614G strain circulating during the early phases of a pandemic. Coronaviruses are known to induce innate and virus‐specific adaptive immune responses to the infection. 13 , 14 These responses are reasoned to provide at least some degree of protection against reinfection. However, protection against the seasonal coronaviruses is limited and diminished with time, concurrent with declining neutralizing antibody titers. 15 A similar trend is also observed in specimens from SARS‐CoV‐2 infected individuals. They show 50% and 100% seroconversion rates on days 7 and 14 postsymptom onset, with ~90% seroconversion by day 10 postsymptom onset that gradually decreases over time. A significant percentage of individuals demonstrate a low or reduced level of neutralizing antibodies after 6 months of infection or vaccination. 16 , 17 The only effective protection currently available is the US Food and Drug Administration (FDA)‐approved direct‐acting small‐molecules antiviral against the SARS‐CoV‐2, which have been approved or in the advance clinical stage. These drugs do not target the highly mutated spike but rather conserved domain of main viral protease (Mpro or 3CL protease) or RNA‐dependent RNA polymerase (RdRp). However, recently FDA‐approved drugs like remdesivir, molnupiravir, and PAXLOVID remain sensitive toward the Omicron and other VOCs. 18 The mutation in the spike protein and other proteins of the SARS‐CoV‐2 that occurs as part of this adaptation process is reasoned to increase the fitness of the virus. Although the variants have been shown to have reduced capacity for antibody‐mediated neutralization, 19 the adjacent arm of the adaptive immunity, T‐cell response, is minimally affected and protecting against these variants. Similarly, a mutation in the regulatory region of N such as the Orf9b and Orf6 genes of the SARS‐CoV‐2 Alpha variant led to the higher expression at both subgenomic RNA and protein levels. Since these proteins are innate immune response antagonists, they delay the host's initial innate immune response and increase viral fitness. 20 Similar mutations are observed in the Delta and Omicron N/Orf9b regulatory regions. Omicron has been classified as having an extremely high number of mutations that are likely to influence higher transmission and thus pose a very high global risk. This strain has now been linked to rapid transmission/infections worldwide (Figure 1). The high number of mutations in the spike protein of Omicron variant as led to the suggestion that these mutations may have changed the biology of the virus as the variant replicate less efficiently in lung epithelial and lung organoid as compared to infection by other previous variants which showed a higher viral load in human nasal airway epithelial cells. 21 Omicron vairant has a unprecedented higher mutation rate, specifically with more than 55 mutations compared to the Wuhan strain. It has more than 30 mutations in the spike protein, but it still binds to the ACE2 receptor at a rate that is 44% higher in affinity than the wild‐type virus. 22 One of the most striking effects observed in the Omicron is the reduced processing of the S glycoprotein into S1/S2, which has significantly reduced syncytia formation during the cytopathic effect of infection. This property appears to lead to a drastic reduction in cell‐to‐cell transmission of this variant. 23 Similarly, Omicron variant prefers to use cathepsin‐dependent endosomal fusion strategy for cell‐surface fusion for viral entry. 21 Similarly, the poor processing of S also results in the reduced incorporation of the spike protein during the derivation of pseudovirus particles. 24 This Omicron pseudovirus exhibits a temperature‐sensitive decay at a rate similar to those noted for the D614G and Delta pseudoviruses at room temperature and 37°C. Still, it displays a comparatively faster decay rate at 0°C. As the SARS‐COV‐2 infects the cell types present in the nasal passage and the lower respiratory tract at a cooler temperature and <37°C, respectively, this temperature‐dependent S regulation may have implications for pathogenesis. 24 Although the Omicron variant is less virulent with a high transmission rate, it still presents a considerable risk to people with comorbidities, compromised immune response, and older people.
Figure 1.
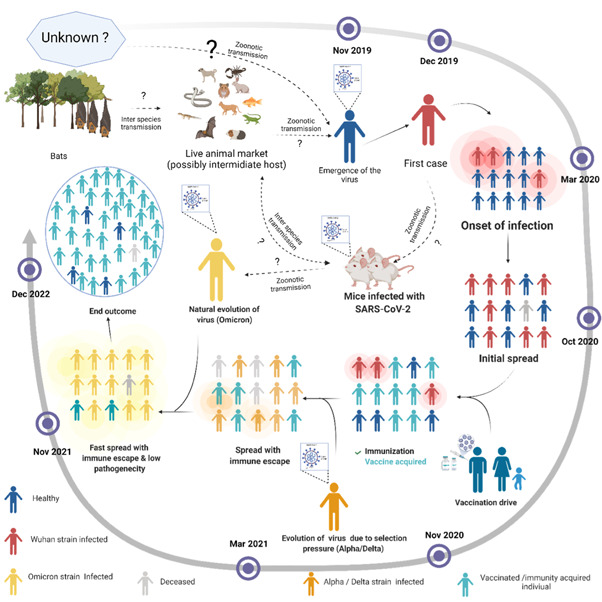
Timeline for the emergence of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and its evolution and possible end outcome. The SARS‐CoV‐2 genome is closely related to the bat coronavirus. It crossed the interspecies barrier and was likely transmitted to animals in the live animal market in Wuhan, China. The live‐animal market subsequently transmitted the pathogen to humans through direct contact with the virus. Later, the virus acquired the ability of human‐to‐human transmission through droplets, and local and long‐distance travel by infected individuals led to a pandemic shortly thereafter. Chronic infection, colossal transmission, and vaccination, mounted a selection pressure on SARS‐CoV2 resulting in continuous viral evolution. This evolution continued until the virus reached maximum transmissibility, immune evasion, and low pathogenicity. Later in the evolution, the virus may act as an attenuated natural vaccine and protect most of the infected individuals, which may potentially lead to an end of the pandemic. The solid black arrow represents the confirmed transfer, whereas the dotted black arrow shows the possibility of viral transfer
The emergence of the Omicron variant with a significantly higher number of mutations than the other SARS‐CoV‐2 variants during what appears as a single burst has been the subject of considerable concern to scientists. Along these lines, it is essential to note that virus origin is still debated; we do not understand fully how this variant will behave in the long run. Based on phylogenetic analyses, it was found that it diverged from the B.1.1 lineage, most likely, around mid‐2020 and appeared quite unusual, and its evolution has been a subject of considerable debate. Three reasons have been so far forwarded for the evolution, (1) It might have acquired the mutations during the chronic infection of an immunocompromised patient, (2) may have circulated and evolved in a hidden population of Africa, or (3) It might have been transmitted to rodents and then retransmitted back to humans acquiring this high number of mutations during this process. As the Omicron variant virus has shown minimal similarity in mutations with other variants that have been isolated from several other clinically chronically infected patients, the theory of Omicron originating from a single chronically infected patient is less likely. As the vaccination drive in Africa is not at the level compared to that in other countries, the second and third theories might be a more likely scenario. A recent study by Wei et al. has shown that the Omicron SARS‐CoV‐2 variant had a more substantial positive selection than normally observed viruses isolated from humans, and thus the potential of change in host species has been entertained (host‐jumping). This finding supports this view that the mutation observed in the Omicron spike protein is similar to that ofSARS‐CoV‐2 variant experimentally adapted to the murine host. 25 It is thus reasoned that the virus jumped from humans to mice, then back into human, and in the process, it acquired rapid mutations due to host adaptation by the virus. This suggests the Omicron origin as the interspecies evolutionary jump. The reduced pathogenicity and severity of Omicron, high transmissibility, and its inter‐species evolution have made it an excellent candidate for formulating an attenuated natural vaccine. Maybe it will act as a vaccine dose for the unvaccinated and a booster dose for vaccinated people. This pandemic has caused a global health crisis and a socioeconomic emergency. The emergence of the Omicron variant might help in reducing the burden and end the pandemic. However, the effectiveness and durability of the immunity in the individual mounting a weaker immune response is a matter of concern. Whether elicited by natural infection or vaccination, it is crucial to maintain high levels of neutralizing antibodies against all circulating variants to minimize viral transmission and promote protection in the upper respiratory tract. 26 The knowledge of the serological status of the individual or population is crucial for its immunity. The Omicron variant emergence and the fact that natural infection leads to an immune response against the spike and nucleocapsid protein of the SARS‐CoV‐2 27 might show us a ray of hope to end this pandemic (Figure 1) sooner.
AUTHOR CONTRIBUTIONS
Siddappa N. Byrareddy conceived the idea, Anurag R. Mishra and Siddappa N. Byrareddy wrote first draft of the manuscript, Anurag R. Mishra created the figure with help of Siddappa N. Byrareddy, Debasis Nayak, and Siddappa N. Byrareddy, reviewed and edited the manuscript. All the authors reviewed and approved the final submission.
ACKNOWLEDGMENT
A. R. M. is supported by the Department of Science and Technology (DST, Government of India) INSPIRE graduate fellowship. This study was partially supported by the National Institute of Allergy and Infectious Diseases grants R01 AI129745, R01 AI113883, and NIDA DA052845 to S. N. B. Further, S. N. B. acknowledges independent research and development (IRAD) funding from the National Strategic Research Institute (NSRI) at the University of Nebraska.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JFW, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giovanetti M, Benedetti F, Campisi G, et al. Evolution patterns of SARS‐CoV‐2: snapshot on its genome variants. Biochem Biophys Res Commun. 2021;538:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019‐new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl). 2020;133(9):1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callaway E. The coronavirus is mutating—does it matter? Nature. 2020;585(7824):174‐177. [DOI] [PubMed] [Google Scholar]
- 9. Gribble J, Stevens LJ, Agostini ML, et al. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021;17(1):e1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182(4):812‐827e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kannan SR, Spratt AN, Cohen AR, et al. Evolutionary analysis of the Delta and Delta Plus variants of the SARS‐CoV‐2 viruses. J Autoimmun. 2021;124:102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannan SR, Spratt AN, Sharma K, Chand HS, Byrareddy SN, Singh K. Omicron SARS‐CoV‐2 variant: unique features and their impact on pre‐existing antibodies. J Autoimmun. 2022;126:102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS‐CoV‐2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torbati E, Krause KL, Ussher JE. The immune response to SARS‐CoV‐2 and variants of concern. Viruses. 2021;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS‐CoV‐2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240‐e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike‐antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS‐CoV‐2 Omicron and other variants of concern. Antiviral Res. 2022;198:105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS‐CoV‐2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331‐2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thorne LG, Bouhaddou M, Reuschl AK, et al. Evolution of enhanced innate immune evasion by the SARS‐CoV‐2 B.1.1.7 UK variant. bioRxiv. 2021. 10.1101/2021.06.06.446826 [DOI] [Google Scholar]
- 21. Pia L, Rowland‐Jones S. Omicron entry route. Nat Rev Immunol. 2022;22:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golcuk M, Yildiz A, Gur M. The Omicron variant increases the interactions of SARS‐CoV‐2 spike glycoprotein with ACE2. bioRxiv. 2021. 10.1101/2021.12.06.471377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willett BJ, Grove J, MacLean OA, et al. The hyper‐transmissible SARS‐CoV‐2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. medRxiv. 2022;397:2331 [Google Scholar]
- 24. Wang Q. Functional properties of the spike glycoprotein of the emerging SARS‐CoV‐2 variant B.1.1.529. bioRxiv. 2021. 10.1101/2021.12.27.474288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei C, Shan KJ, Wang W, Zhang S, Huan Q, Qian W. Evidence for a mouse origin of the SARS‐CoV‐2 Omicron variant. J Genet Genomics. 2021;48:1111‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corbett KS, Werner AP, Connell SO, et al. mRNA‐1273 protects against SARS‐CoV‐2 beta infection in nonhuman primates. Nat Immunol. 2021;22(10):1306‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piccoli L, Park YJ, Tortorici MA, et al. Mapping Neutralizing and Immunodominant Sites on the SARS‐CoV‐2 Spike Receptor‐Binding Domain by Structure‐Guided High‐Resolution Serology. Cell. 2020;183(4):1024‐1042e1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


