Abstract
To mitigate SARS‐CoV‐2 transmission, vaccines have been urgently approved. With their limited availability, it is critical to distribute the vaccines reasonably. We simulated the SARS‐CoV‐2 transmission for 365 days over four intervention periods: free transmission, structural mitigation, personal mitigation, and vaccination. Sensitivity analyses were performed to obtain robust results. We further evaluated two proposed vaccination allocations, including one‐dose‐high‐coverage and two‐doses‐low‐coverage, when the supply was low. 33.35% (infection rate, 2.68 in 10 million people) and 40.54% (2.36) of confirmed cases could be avoided as the nonpharmaceutical interventions (NPIs) adherence rate rose from 50% to 70%. As the vaccination coverage reached 60% and 80%, the total infections could be reduced by 32.72% and 41.19%, compared to the number without vaccination. When the durations of immunity were 90 and 120 days, the infection rates were 2.67 and 2.38. As the asymptomatic infection rate rose from 30% to 50%, the infection rate increased 0.92 (SD, 0.16) times. Conditioned on 70% adherence rate, with the same amount of limited available vaccines, the 20% and 40% vaccination coverage of one‐dose‐high‐coverage, the infection rates were 2.70 and 2.35; corresponding to the two‐doses‐low‐coverage with 10% and 20% vaccination coverage, the infection rates were 3.22 and 2.92. Our results indicated as the duration of immunity prolonged, the second wave of SARS‐CoV‐2 would be delayed and the scale would be declined. On average, the total infections in two‐doses‐low‐coverage was 1.48 times (SD, 0.24) as high as that in one‐dose‐high‐coverage. It is crucial to encourage people in order to improve vaccination coverage and establish immune barriers. Particularly when the supply is limited, a wiser strategy to prevent SARS‐CoV‐2 is equally distributing doses to the same number of individuals. Besides vaccination, NPIs are equally critical to the prevention of widespread of SARS‐CoV‐2.
Keywords: coronavirus disease 2019 (COVID‐19), nonpharmaceutical intervention (NPI), severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), stochastic dynamic model, vaccination
1. INTRODUCTION
The outbreak of COVID‐19 caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to an unprecedented public health and economic crisis worldwide since December 2019. 1 To mitigate the spread, a variety of nonpharmaceutical interventions (NPIs) have been implemented, including screening and isolation, travel restriction, remote schooling and work distancing. 2 , 3 , 4 Although these efforts are beneficial to control the spread in a short term, globally, as of March 4, 2022, there have been 440 807 756 confirmed cases of COVID‐19, including 5 978 096 deaths, reported by the World Health Organization (WHO). 5 Additionally, in many countries, relaxation of NPIs has led to a resurgence of the epidemic as herd immunity has not been reached thus far. 6 A long‐term solution, such as vaccines that protect from SARS‐CoV‐2 infection, remains urgently needed.
The competition for developing vaccines against SARS‐CoV‐2 started in early 2020 and more than 50 companies began development of the first vaccine. 7 At present, 14 vaccines have been approved for urgent use, including 3 nucleic acid vaccines (Curevac, Moderna, and BioNTech), 3 inactivated virus vaccines (Bharat, Sinovac, and Sinopharm), and 8 viral‐vectored vaccines (Clover Biopharmaceutical, Serum institute, Novavax, Sanofi, AstraZeneca, Janssen, Gamaleya, and CanSino). 8 As of March 5, 2022, a total of 10 704 043 684 vaccine doses have been administered globally. 5 The benefits of an effective vaccine for individuals and their communities have resulted in widespread demand, so it is critical that decision‐making on vaccine distribution is well‐motivated, particularly in the initial phases when vaccine availability is limited. 7 , 9 , 10 , 11
As the basis of regulatory approvals, the initial vaccine was released in early 2021, there are two main suggested approaches to vaccine prioritization: (i) directly vaccinate those at highest risk for severe outcomes and (ii) protect them indirectly by vaccinating those who do the most transmitting. 12 , 13 Model‐based investigations of the trade‐offs between these strategies have found that the optimal balance between direct and indirect protection depends on both vaccine efficacy and supply, recommending direct vaccination of older adults for low‐efficacy vaccines and for high‐efficacy but supply‐limited vaccines. 14 Rather than comparing prioritization strategies, others have compared hypothetical vaccines, showing that even those with lower efficacy for direct protection may be more valuable if they also provide better indirect protection by blocking transmission. 14 Prioritization of transmission‐blocking vaccines can also be dynamically updated on the basis of the current state of the epidemic, shifting prioritization to avoid decreasing marginal returns. 15 However, the strategies of prioritizing and optimizing doses complement are highly dependent on different vaccine efficacy (VE) and durability of immunity. An optimal resource allocation will largely reduce the transmission economically.
To evaluate the vaccine allocation strategies, we built an age and occupation stratified SEIRS (susceptible, exposed, infectious, recovered, and susceptible) model. Since age has been shown to be an important correlate of susceptibility, seroprevalence, severity, and mortality, this model includes an age‐dependent contact matrix, susceptibility to infection, and infection fatality rate (IFR), allowing us to estimate the cumulative incidence of SARS‐CoV‐2 infections by means of forward simulations of disease dynamics. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
2. METHODS
An individual‐based dynamic model, stratified by age and occupation, was built to simulate the transmission of SARS‐CoV‐2 based on the epidemical progression of susceptible‐exposed‐infectious‐removed‐susceptible (SEIRS) structure. This model includes NPIs aimed at mitigating the epidemic.
2.1. Model construction
In the model simulation, each healthy individual (susceptible) has a chance of being infected with SARS‐CoV‐2 under the force transmission rate depending on the number of daily contacts and the probability of SARS‐CoV‐2 being transmitted from an infected to uninfected contact. Once infected, the individual enters the exposed period. At the end of the exposure period, an individual will become infectious, either symptomatic or asymptomatic. Most infectious individuals recover but some will die (with IFR). We assumed that the recovered individual would be re‐infected after waned immunity, including natural immunity.
The population was grouped by age and occupation, and interactions between groups (or individuals) were simulated, taking into account the number of daily contacts. The global age structure was from the United Nations (2019), people aged 0–100 years were divided into eight groups (0–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, and over 65). The occupations (nonworker, student, worker, and others) and social contact patterns (home community, school, workplace, and other contacts) were then assigned according to the economic structure from the Chinese 2010 and 2020 census data.
Each individual was assigned a social contact parameter (location‐specific contact matrices, including home community, school, workplace, and other) by their occupation. This study assumed an individual has no age‐dependence in transmissibility, and the likelihood of viral exposure varied by individuals depending on the number of infectious people in their social network. We considered age‐stratified contact matrices from the BBC pandemic project in describing the average daily effective number of contacts that an individual has with others. 24 Age‐stratified IFRs were collected from a model‐based analysis under New York City during the 2020 spring pandemic wave. 25
2.2. Infection parameters
Each modeled individual was ascribed demographic characteristics (e.g., age and occupation) and epidemiological characteristics (e.g., exposed period, infectious period, symptomatic or asymptomatic status, recovery or death from infection). We incorporated asymptomatic infections into this model, although it remains unclear as to the asymptomatic rate and the extent asymptomatic patients contribute to viral transmission. 26 , 27 , 28 , 29 All the detailed information is presented in the supplementary materials.
Although existing studies have been focusing on the antibody responses to SARS‐CoV‐2, it is still vague in the natural and vaccine acquired immunity. 8 , 30 Therefore, we considered 90 and 120 days of acquired immunity priority before getting susceptible again in our simulation. 31 , 32 , 33 The transmission was dependent upon the period of exposure, period of infectiousness, and basic reproductive number (R0). 34 , 35 , 36 , 37 We also incorporated an asymptomatic rate denoting the probability of an infected case being asymptomatic and assumed a reduced rate of infection for asymptomatic cases. 24 , 26 , 35 , 36 , 38 After the infectious period, an individual has the chance to recover or die. 34 , 39
2.3. Interventions
In this model, we mimicked the transmission in four assumed periods. The first period is the free transmission period, during which no NPIs are implemented. The second period is the structural mitigation period, during which structural NPIs including isolation and quarantine are implemented. In this period, the infected individuals are isolated until they recover or die, and their close contacts are quarantined for 14 days. The accuracy rate of screening (sensitivity) was considered in this modeling. 40 , 41 The third period is the personal mitigation period, during which personal NPIs including social distancing, mask‐wearing, and hand washing are in place. The individuals begin to change their protection behavior depending on the government policy adherence rate. The assumed efficacy of mask‐wearing and hand washing is introduced into this model. 42 The final period is the vaccination period. In our study, we introduced VEs into our simulation to predict the effect of the vaccine, which is associated with the NPIs adherence rate. 8 Risk compensation was considered in our model. When the coverage rates reach the target value (≥50%), the adherence of personal NPIs becomes 50% lower among vaccinated people. In this situation, the theoretical immunity is elicited after all recommended doses of the vaccine are injected. We proposed two scenarios of vaccine‐allocation strategies (assumed two injections) based on the limited vaccine supply. Scenario 1 (one‐dose‐high‐coverage): distributing the two doses to two people, each person with one dose (lower VE); Scenario 2 (two‐doses‐low‐coverage): distributing the two doses to one person, each person with two doses (higher VE). Among these scenarios, we assumed time varied supplies, increased by 0.5% per day.
2.4. Simulation and sensitivity analysis
We simulated 100 million individual‐level transmission events by repeatedly generating contact distributions for a primary case and randomly generating infections among these contacts. This process was repeated a thousand times, and each simulation was to generate a set of epidemic trends including (1) daily newly confirmed cases and (2) daily total cumulative confirmed cases. Our primary sensitivity analyses were to the level of vaccination coverage (60% and 80%), the adherence rate of NPIs (50% and 70%), and the asymptomatic rate (30% and 50%). We further assumed the (natural and vaccinated) immunity waned after 90 and 120 days.
3. RESULTS
3.1. Main analysis
Overall, in ages, the distribution of infections was 1.06% (0–4 years old), 7.13% (5–14 years old), 19.14% (15–24 years old), 23.16% (25–34 years old), 19.82% (35–44 years old), 16.05% (45–54 years old), 9.13% (55–64 years old), and 4.50% (over 65 years old). In all, 53.64% of the dead were people over 35 years old. Most of the dead were students (16.09%) and workers (70.00%). In occupations, 12.88%, 17.71%, 67.28%, and 2.12% were accounted for by nonworker, students, workers and others (Figure 1).
Figure 1.
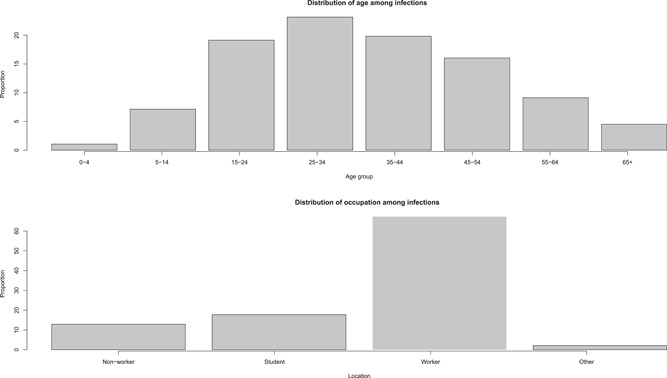
Distribution infections among age and occupation. The top panel shows the distribution of infections among eight age groups (0–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, and over 65 years old), and the bottom panel shows the distribution infections among four occupation groups (nonworker, student, worker, and other).
On average, the infection rate (in 100 million people) reduced from 2.68 (95% confidence interval [CI], 2.25–3.11) to 2.36 (95% CI, 1.94–2.78) as the NPIs adherence rate rose from 50% to 70% (Figure 2). 33.35% (95% CI, 22.22–43.78) and 40.54% (95% CI, 27.75–52.25) confirmed cases can be avoided if 50% and 70% of people followed the instruction.
Figure 2.
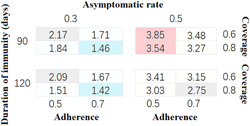
The proportion of total infected cases among the population. The infection rate of SARS‐CoV‐2 among 16 (24) different hypotheses. The primary sensitivity analyses were on the level of vaccination coverage (60% and 80%), the adherence of NPIs (50% and 70%), the asymptomatic rate (30% and 50%), and the assumed (natural and vaccinated) waned immunity after 90 and 120 days.
As the vaccination coverage reached 60% and 80%, the number of infections can be reduced by about 32.72% (95% CI, 22.03–43.41) and 41.19% (95% CI, 30.32–52.06), compared to the number without vaccination. The average IFRs were 0.68% (95% CI, 0.66–0.69) and 0.58% (95% CI, 0.57–0.58) when the vaccination coverages were 60% and 80%. When the durations of immunity were 90 and 120 days, the infection rates were 2.67 (95% CI, 2.20–3.14) and 2.38 (95% CI, 1.99–2.77).
Furthermore, our model suggested that with the increase in the asymptomatic infection rate, the prevention and control of SARS‐CoV‐2 was becoming more and more unfavorable. As the asymptomatic infection rate rose from 30% to 50%, the infection rate increased from 1.73 (95% CI, 1.59–1.87) to 3.31 (95% CI, 3.14–3.47), which was 0.92 (SD, 0.16) times higher. However, no matter how high the asymptomatic rate was, higher NPIs adherence and higher vaccination coverage can always prevent more SARS‐CoV‐2 infections (Figure 2).
According to our simulation, relying on a 30% asymptomatic rate, 70% NPIs adherence rate, 80% vaccination coverage, and 180 days of immunity, the infection rate remained at 1.42% (total confirmed cases, 142 243; 95% CI, 134 598–152 471), which reduced the infections most.
3.2. Vaccine distribution scenarios
The epidemiological impacts of the different dosing scenarios on mitigating the SARS‐CoV‐2 spread, when the vaccine supply was limited, are shown in Figure 3.
Figure 3.
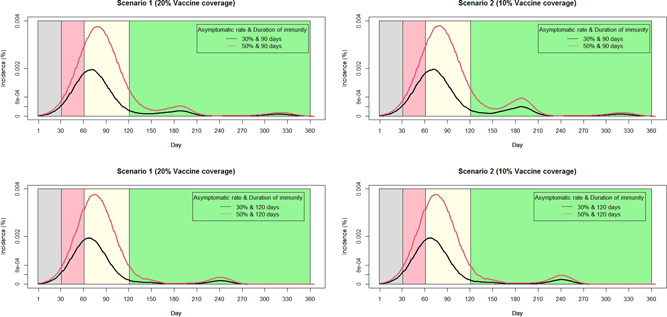
Daily SARS‐CoV‐2 incidence rate among the population. Focusing on 70% NPIs adherence rate and considering the same amounts of limited vaccine supplies. The daily incidence rates with different asymptomatic rates and durations of immunity are represented as black and red curves, respectively. The panels in the left and right columns represent different scenarios.
Assuming the adherence rate of NPIs was 70%, supposing the same amount of vaccines were available, under scenario 2 (completely vaccinated), relying on an immunity duration of 90 days, the IFR and infection rate were 0.74 (SD, 0.01%) and 3.38% (SD, 0.83%), whereas the values were 0.72% (SD, 0.01%) and 2.76% (SD, 0.90%) when immunity waning after 120 days. Considering scenario 1 (partially vaccinated), when the duration of immunity was 90 days, the IFR and infection rate were 0.74% (SD, 0.01%) and 2.69% (SD, 1.16%), whereas the values were 0.72% (SD, 0.01%) and 2.37% (SD, 1.12%) when immunity waning after 120 days (Table 1).
Table 1.
Infection rate of SARS‐CoV‐2 among different scenarios
| Scenario 1 (partially vaccinated) | ||||
|---|---|---|---|---|
| Asymptomatic rate | Days | Vaccine coverage | ||
| 20% | 40% | |||
| Duration of immunity | 30% | 90 | 1.97 | 1.43 |
| 120 | 1.72 | 1.13 | ||
| 50% | 90 | 3.78 | 3.56 | |
| 120 | 3.34 | 3.29 | ||
| Scenario 2 (completely vaccinated) | ||||
|---|---|---|---|---|
| Asymptomatic rate | Days | Vaccine coverage | ||
| 10% | 20% | |||
| Duration of immunity | 30% | 90 | 2.84 | 2.50 |
| 120 | 2.30 | 1.71 | ||
| 50% | 90 | 4.19 | 3.97 | |
| 120 | 3.53 | 3.48 | ||
Figure 3 indicated as the duration of immunity prolonged, the second peak of SARS‐CoV‐2 will be delayed and the scale will be declined. On average, the total infections in scenario 2 was 1.48 times (SD, 0.24) higher than that in scenario 1.
Considering the same amount of supplies, and 20% and 40% vaccination coverage of scenario 1, the total number of infections were 270 256 (95% CI, 171 631–368 870) and 235 254 (95% CI, 113 440–357 060); corresponding to the scenario 2 with 10% and 20% vaccination coverage, the number of infections were 321 535 (95% CI, 241 342–401 658) and 291 523 (95% CI, 193 086–389 914).
4. DISCUSSION
In this modeling, the findings showed that the vaccines and NPIs substantially contributed to the SARS‐CoV‐2 transmission control. With higher vaccination coverage and NPIs adherence rate, more infections can be avoided. Compared to no vaccination, the number of infections can be reduced by 40% or 26% if the vaccination coverage reached 80% or 60%). Furthermore, when the adherence rate increases from 50% to 70%, 28% of infected cases can be further saved. To acquire the theoretical vaccine immunity, all recommended vaccine doses should be injected. However, when the vaccine (assumed more than two injections) supply was limited, the partially vaccinated strategy was superior to the completely vaccinated since it helped to reduce the infections by 67.57%. Finally, the transmission of SARS‐CoV‐2 was more difficult to control as the asymptomatic infection rate increased.
Vaccines, which can induce and establish the immune response against SARS‐CoV‐2, are crucial to the prevention and mitigation of morbidity and mortality cases by infection. 8 , 30 Multiple candidate vaccines, including nucleic acid vaccines (Moderna—mRNA‐1273; BioNTech—BNT162b1 and BNT162b2), inactivated virus vaccines (Sinovac—CoronaVac; Sinopharm—BBIBP‐CorV and Vero cells), live attenuated vaccines, protein or peptide subunit vaccines, and viral‐vectored vaccines (AstraZeneca—AZD1222; Janssen—Ad26. COV2.S; Gamaleya—rAd5 and rAd26; CanSino—Ad5CoV), are being developed and tested. 43 Each type has advantages and disadvantages and vaccine manufacturers have published articles to present the findings of their Phase III trials, each involving tens of thousands of participants, and commenced in various geographical locations. 7 , 9 , 10 , 11 These results have shown the VEs are over 90% with all recommended doses completed. Even after a partial dose, the VE can still reach 70%. In addition, the antibody responses from vaccination enhance the protection in preventing the disease progression. 7 , 9 , 10 , 11
SARS‐CoV‐2 variants are circulating globally and quickly became dominant in countries, such as the United Kingdom (WHO label, Alpha; Pango lineage B.1.1.7), South Africa (Beta; B.1.351), Brazil (Gamma; P.1), India (Delta and Kappa, B.1.617.2 and B.1.617.1), America (Iota, B.1.526), Peru (Lambda; C.37), and multiple countries (Omicron; B.1.1.529). 44 The reason for the strong infectivity of SARS‐CoV‐2 variants is that it can escape the neutralizing antibodies produced by the immune system, and the more lethal variants could substantially decrease the net benefit of vaccination. 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 Therefore, the current issues on whether a third dose of enhanced vaccine is needed and when to vaccinate it has also become a topic of public concern. 53 , 54 , 55 Moreover, how to cope with the evolution of the SARS‐CoV‐2 and develop a vaccine with a "broad‐spectrum effect" in avoiding virus escape is an important problem that researchers need to urgently solve. 56 , 57 The variants reduced the VE to varying degrees, but the vaccine is still protective. 58 , 59
Whichever vaccine appears, rational allocation of resources is very important. The current vaccination modeling research is mainly focusing on two directions. First, to minimize the deaths, Bubar et al. 12 recommended older adults enjoy a vaccine priority due to its higher fatality rate. Second, to mitigate the spread, Yang et al. 13 suggested several essential workers could be prioritized for vaccination to maintain essential services in the early phase of a vaccination program due to its higher contacts. In our model, we propose an interesting idea that when the vaccines are lacking in the early stage, to maximize the coverage, the total number of doses to be vaccinated should be equally distributed to the same amount of people even if it reduces the VE so that individuals can quickly establish an immune barrier.
All kinds of NPIs have been introduced in mitigating the transmission of SARS‐CoV‐2. The major NPIs, including isolation and quarantine, social distancing, mask, and hand washing, are recommended by WHO. 60 Isolating confirmed cases stops the offspring generating and effectively blocks the transmission of SARS‐CoV‐2, and the contact tracing helps to minimize the potential transmission from second cases. 61 However, this NPI is limited substantially by delays from testing of index cases to the tracing of their contacts, because secondary cases might have been transmitting for a number of days in the community during the time that contact tracing is taking place. In addition, the delays in isolating confirmed cases from the infected date to the symptom onset or hospitalization is another issue, not to mention the sensitivity of the screening test. Moreover, asymptomatic cases do not know they are carriers and will not attend hospitals to conduct self‐screening. Thus, with the higher asymptomatic rate of SARS‐CoV‐2, it is not surprising that this NPI alone could not contain the SARS‐CoV‐2 pandemic. 62
Since the transmission routes, including droplet and contact transmission, of SARS‐CoV‐2, have been identified, several personal health behaviors, such as social distancing, mask‐wearing, and hand washing, have been encouraged by WHO to avoid transmission. These NPIs have been proven effective against SARS‐CoV‐2 in our modeling, as shown in other previous research. 63 By changing their personal behaviors, such as reducing social distancing, mask‐wearing, and handwashing, people can lower the risk of potential SARS‐CoV‐2 infections or reduce the probability of spread to susceptible individuals. 64 However, the effectiveness of these NPIs can be limited by low adherence. For example, due to the government policy, people who are living in China have a higher NPIs adherence rate and it achieves a lower total infection even with a larger population. 65 , 66 , 67 For those countries with moderate or lower adherence rates, the governments should advocate the importance of these protective measures in preventing the SARS‐CoV‐2 transmission. In addition, maintaining a high level of mask utilization is also necessary and masks should be replaced frequently for a permanent protective effect. 64 Proper use and disposal of masks is also essential to avoid increasing risk of transmission. 68 , 69
Our study also has several limitations. First, in this modeling, we did not consider the time‐varied antibody responses and viral load dynamics. 70 Due to its RNA virus structure, SARS‐CoV‐2 is continuously mutating. A much clear genomics‐informed response and serology data should be further adopted for simulation. After adding the distribution of virus load and the immune waning into the model, we can much better predict the future epidemic trend and make a response early. Second, the risk compensation after vaccination should be considered. 71 In another ongoing study, we have found that among the people who have been vaccinated, they have begun neither to use protections nor adhere to NPIs. In other words, we know that vaccines can protect against death after infection, but whether they can reduce the spread of SARS‐CoV‐2 seems to be an interesting research direction. Finally, the seasonal dependent transmission patterns should be introduced into the model. Based on little published data, the impact of unknown factors such as temperature or humidity changes could not be assessed in our model. 72 , 73 , 74 However, two similar human coronaviruses, HCoV‐OC43, and HCoV‐HKU1, cause annual wintertime outbreaks of respiratory illness in temperate regions, suggesting that climate and host behaviors may facilitate transmission as is true for influenza. 75 , 76 , 77 , 78 , 79 , 80
In conclusion, although the world has taken many different NPIs and vaccines to control and prevent the SARS‐CoV‐2 epidemic, the epidemic of SARS‐CoV‐2 has led to the current global large‐scale spread and there is no sign of complete control. 81 , 82 , 83 This study investigates the effectiveness of vaccination and NPI strategies in various situations. It is crucial to encourage people to vaccinate in order to improve vaccination coverage and establish immune barriers. Particularly, when the vaccine supply is limited, an optimal strategy to prevent SARS‐CoV‐2 transmission is equally distributing doses to the same number of individuals. Besides vaccination, NPIs are equally critical to the prevention of widespread of SARS‐CoV‐2.
AUTHOR CONTRIBUTIONS
Huachun Zou and Yuelong Shu conceived the idea and protocol. Yi‐Fan Lin built the model, collected data, finalized the analysis, interpreted the findings, and wrote the manuscript. Yuwei Li conducted the visualization. All authors have contributed to the interpretation of data, and study findings and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank all members for carefully reading and commenting on the manuscript. We thank staff members at disease control institutions, hospitals, and health administrations across China where outbreaks occurred for field investigation, administration, and data collection. We thank all open source websites for sharing data. This study was supported by the GuangDong Basic and Applied Basic Research Foundation (2021A1212110938), the China Postdoctoral Science Foundation (2021M693595), the Natural Science Foundation of China Excellent Young Scientists Fund (82022064), Natural Science Foundation of China International/Regional Research Collaboration Project (72061137001), Natural Science Foundation of China Young Scientist Fund (81703278), the National Science and Technology Major Project of China (2018ZX10721102), the Sanming Project of Medicine in Shenzhen (SZSM201811071), the High‐Level Project of Medicine in Longhua, Shenzhen (HLPM201907020105), the National Key Research and Development Program of China (2020YFC0840900), the Shenzhen Science and Technology Innovation Commission Basic Research Program (JCYJ20190807155409373), Special Support Plan for High‐Level Talents of Guangdong Province (2019TQ05Y230) and the Fundamental Research Funds for the Central Universities (58000‐31620005). All funding parties did not have any role in the design of the study or in the explanation of the data.
Lin Y‐F, Li Y, Duan Q, et al. Vaccination strategy for preventing the spread of SARS‐CoV‐2 in the limited supply condition:a mathematical modeling study. J Med Virol. 2022;94:3722‐3730. 10.1002/jmv.27783
Yi‐Fan Lin contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding open accessed websites.
REFERENCES
- 1. Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133(9):1015‐1024. 10.1097/cm9.0000000000000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou H, Shu Y, Feng T. How Shenzhen, China avoided widespread community transmission: a potential model for successful prevention and control of COVID‐19. Infect Dis Poverty. 2020;9(1):89. 10.1186/s40249-020-00714-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou KA. Sample of Shenzhen in the War Against COVID‐19. 2020. https://newschinacomcn/2020-04/20/content_75953969htm
- 4. Network . Ten Questions SN on Anti‐COVID‐9 in Shenzhen. Why Shenzhen Could Detect Human‐to‐Human Transmission of COVID‐19 at its Early State. 2020. https://wwwsznewscom/news/content/-04/04/content_23031583htm
- 5. Dashboard WCC . WHO Coronavirus (COVID‐19) Dashboard With Vaccination Data. 2021. https://covid19whoint/
- 6. Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS‐CoV‐2: a systematic review and meta‐analysis. Lancet Glob Health, 9(5):e598‐e609. 10.1016/s2214-109x(21)00026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham BS. Rapid COVID‐19 vaccine development. Science. 2020;368(6494):945‐946. 10.1126/science.abb8923 [DOI] [PubMed] [Google Scholar]
- 8. Poland GA, Ovsyannikova IG, Kennedy RB. SARS‐CoV‐2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595‐1606. 10.1016/s0140-6736(20)32137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB. SARS‐CoV‐2 vaccine development: current status. Mayo Clin Proc. 2020;95(10):2172‐2188. 10.1016/j.mayocp.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur SP, Gupta V. COVID‐19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. 10.1016/j.virusres.2020.198114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amanat F, Krammer F. SARS‐CoV‐2 Vaccines: status report. Immunity. 2020;52(4):583‐589. 10.1016/j.immuni.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bubar KM, Reinholt K, Kissler SM, et al. Model‐informed COVID‐19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6532):916‐921. 10.1126/science.abe6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Zheng W, Shi H, et al. Who should be prioritized for COVID‐19 vaccination in China? A descriptive study. BMC Med. 2021;19(1):45. 10.1186/s12916-021-01923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckner JH, Chowell G, Springborn MR. Dynamic prioritization of COVID‐19 vaccines when social distancing is limited for essential workers. 2020. 10.1101/2020.09.22.20199174/JmedRxiv [DOI] [PMC free article] [PubMed]
- 15. Sandmann FG, Davies NG, group CftMMoIDC‐w , Vassall A, Edmunds WJ, Jit M. The potential health and economic value of SARS‐CoV‐2 vaccination alongside physical distancing in the UK: transmission model‐based future scenario analysis and economic evaluation. 2020. 10.1101/2020.09.24.20200857/JmedRxiv [DOI] [PMC free article] [PubMed]
- 16. Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS‐CoV‐2 in households, schools, and the community. J Infect Dis. 2021;223(3):362‐369. 10.1093/infdis/jiaa691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. Age‐dependent effects in the transmission and control of COVID‐19 epidemics. Nature Med. 2020;26(8):1205‐1211. 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Litvinova M, Liang Y, et al. Changes in contact patterns shape the dynamics of the COVID‐19 outbreak in China. Science. 2020;368(6498):1481‐1486. 10.1126/science.abb8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mueller AL, McNamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people? Aging. 2020;12(10):9959‐9981. 10.18632/aging.103344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of COVID‐19. Eur Respir J. 2020;55(5). 10.1183/13993003.01112-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levin AT, Hanage WP, Owusu‐Boaitey N, Cochran KB, Walsh SP, Meyerowitz‐Katz G. Assessing the age specificity of infection fatality rates for COVID‐19: systematic review, meta‐analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123‐1138. 10.1007/s10654-020-00698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS‐CoV‐2 in France. Science. 2020;369(6500):208‐211. 10.1126/science.abc3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Westmeier J, Paniskaki K, Karaköse Z, et al. Impaired cytotoxic CD8(+) T cell response in elderly COVID‐19 patients. mBio. 2020;11(5). 10.1128/mBio.02243-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klepac P, Kucharski AJ, Conlan AJ, et al. Contacts in context: large‐scale setting‐specific social mixing matrices from the BBC Pandemic project. J medRxiv . 2020. 10.1101/2020.02.16.20023754/ [DOI]
- 25. Yang W, Kandula S, Huynh M, et al. Estimating the infection‐fatality risk of SARS‐CoV‐2 in New York City during the spring 2020 pandemic wave: a model‐based analysis. Lancet Infect Dis. 2021;21(2):203‐212. 10.1016/s1473-3099(20)30769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect Dis. 2020;94:154‐155. 10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS‐CoV‐2 in Singapore: a modelling study. Lancet Infect Dis. 2020;20(6):678‐688. 10.1016/s1473-3099(20)30162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson MA, Quandelacy TM, Kada S, et al. SARS‐CoV‐2 transmission from people without COVID‐19 symptoms. JAMA Network Open. 2021;4(1):e2035057. 10.1001/jamanetworkopen.2020.35057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID‐19 outbreaks. Proc Natl Acad Sci USA. 2020;117(30):17513‐17515. 10.1073/pnas.2008373117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra SK, Tripathi T. One year update on the COVID‐19 pandemic: Where are we now? Acta Trop. 2021;214:105778. 10.1016/j.actatropica.2020.105778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Capuano R, Bisecco A, Conte M, et al. Six‐month humoral response to mRNA SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Multi Scler Relat Disord. 2022;60:103724. 10.1016/j.msard.2022.103724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evans JP, Zeng C, Carlin C, et al. Neutralizing antibody responses elicited by SARS‐CoV‐2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022: eabn8057. 10.1126/scitranslmed.abn8057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID‐19. Science. 2022;375(6585):1122‐1127. 10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- 34. Lin Y‐F, Duan Q, Zhou Y, et al. Spread and impact of COVID‐19 in China: a systematic review and synthesis of predictions from transmission‐dynamic models. Front Med. 2020;7:321. 10.3389/fmed.2020.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019‐nCoV) infections among travellers from Wuhan, China, 20‐28 January 2020. Euro Surveill. 2020;25(5). 10.2807/1560-7917.Es.2020.25.5.2000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You C, Deng Y, Hu W, et al. Estimation of the time‐varying reproduction number of COVID‐19 outbreak in China. 2020. 10.1101/2020.02.08.20021253/JmedRxiv [DOI] [PMC free article] [PubMed]
- 37. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689‐697. 10.1016/s0140-6736(20)30260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He D, Zhao S, Lin Q, et al. The relative transmissibility of asymptomatic COVID‐19 infections among close contacts. Int J Infect Dis. 2020;94:145‐147. 10.1016/j.ijid.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen M, Peng Z, Xiao Y, Zhang L. Modelling the epidemic trend of the 2019 novel coronavirus outbreak in China. Innovation. 2020;1(3):100048 10.1101/2020.01.23.916726/JbioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing for coronavirus disease 2019 (COVID‐19) in China: s report of 1014 vases. Radiology. 2020;296(2):E32‐E40. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zitek T. The appropriate use of testing for COVID‐19. West J Emerg Med. 2020;21(3):470‐472. 10.5811/westjem.2020.4.47370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smereka J, Ruetzler K, Szarpak L, Filipiak KJ, Jaguszewski M. Role of mask/respirator protection against SARS‐CoV‐2. Anesth Analg. 2020;131(1):e33‐e34. 10.1213/ane.0000000000004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. 10.1016/s0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang J, Marziano V, Deng X, et al. Despite vaccination, China needs non‐pharmaceutical interventions to prevent widespread outbreaks of COVID‐19 in 2021. Nat Hum Behav. 2021;5:1009‐1020. 10.1038/s41562-021-01155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farinholt T, Doddapaneni H, Qin X, et al. Transmission event of SARS‐CoV‐2 Delta variant reveals multiple vaccine breakthrough infections. 2021. 10.1101/2021.06.28.21258780/JmedRxiv [DOI] [PMC free article] [PubMed]
- 46. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596:276‐280. 10.1038/s41586-021-03777-9 [DOI] [PubMed] [Google Scholar]
- 47. Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS‐CoV‐2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220‐4236. 10.1016/j.cell.2021.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS‐CoV‐2 B.1.617.2 (Delta) variant COVID‐19 outbreak associated with a gymnastics facility—Oklahoma, April‐May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(28):1004‐1007. 10.15585/mmwr.mm7028e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alizon S, Haim‐Boukobza S, Foulongne V, et al. Rapid spread of the SARS‐CoV‐2 Delta variant in some French regions, June. Eurosurveillance. 2021;26(28), 10.2807/1560-7917.Es.2021.26.28.2100573 [DOI] [PMC free article] [PubMed]
- 50. Ito K, Piantham C, Nishiura H. Predicted dominance of variant Delta of SARS‐CoV‐2 before Tokyo Olympic Games, Japan, July 2021. Eurosurveillance. 2021;26(27). 10.2807/1560-7917.Es.2021.26.27.2100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hetemäki I, Kääriäinen S, Alho P, et al. An outbreak caused by the SARS‐CoV‐2 Delta variant (B.1.617.2) in a secondary care hospital in Finland, May 2021. Eurosurveillance. 2021;26(30). 10.2807/1560-7917.Es.2021.26.30.2100636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Connor BA, Couto‐Rodriguez M, Barrows JE, et al. Monoclonal antibody therapy in a vaccine breakthrough SARS‐CoV‐2 hospitalized delta (B.1.617.2) variant case. Int J Infect Dis. 2021;110:232‐234. 10.1016/j.ijid.2021.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta‐Variant. 2021. https://investorsbiontechde/news-releases/news-release-details/pfizer-and-biontech-provide-update-booster-program-light-delta.
- 54.Joint CDC and FDA Statement on Vaccine Boosters. https://wwwfdagov/news-events/press-announcements/joint-cdc-and-fda-statement-vaccine-boosters. 2021.
- 55. Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine‐induced thrombotic thrombocytopenia. N Engl J Med. 2021;385:720‐728. 10.1056/NEJMoa2107051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Starr TN, Czudnochowski N, Liu Z, et al. SARS‐CoV‐2 RBD antibodies that maximize breadth and resistance to escape. Nature. 2021;597:97‐102. 10.1038/s41586-021-03807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walls AC, Miranda MC, Pham MN, et al. Elicitation of broadly protective sarbecovirus immunity by receptor‐binding domain nanoparticle vaccines. BioRxiv. 2021. 10.1101/2021.03.15.435528/JbioRxiv [DOI] [PMC free article] [PubMed]
- 58. Chen X, Wang W, Chen X, et al. Prediction of long‐term kinetics of vaccine‐elicited neutralizing antibody and time‐varying vaccine‐specific efficacy against the SARS‐CoV‐2 Delta variant by clinical endpoint. BMC Med. 2022;20(1):36. 10.1186/s12916-022-02249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pormohammad A, Zarei M, Ghorbani S, et al. Effectiveness of COVID‐19 vaccines against Delta (B.1.617.2) variant: a systematic review and meta‐analysis of clinical studies. Vaccines. 2021;10(1). 10.3390/vaccines10010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organization . Coronavirus disease (COVID‐19) advice for the public. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. [PubMed]
- 61. Quilty BJ, Clifford S, Hellewell J, et al. Quarantine and testing strategies in contact tracing for SARS‐CoV‐2: a modelling study. Lancet Public Health. 2021;6(3):e175‐e183. 10.1016/s2468-2667(20)30308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lei H, Wu X, Wang X, et al. Different transmission dynamics of COVID‐19 and influenza suggest the relative efficiency of isolation/quarantine and social distancing against COVID‐19 in China. Clin Infect Dis. 2020;73:4305. 10.1093/cid/ciaa1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020;368(6491):638‐642. 10.1126/science.abb6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. World Health Organization . Advice on the use of masks in the community, during home care and in healthcare settings in the context of the novel coronavirus (2019‐nCoV) outbreak: interim guidance, 29 January 2020. 2020. https://apps.who.int/iris/handle/10665/330987
- 65. Fang LQ, Zhang HY, Zhao H, et al. Meteorological conditions and nonpharmaceutical interventions jointly determined local transmissibility of COVID‐19 in 41 Chinese cities: a retrospective observational study. Lancet Reg Health West Pac. 2020;2:100020. 10.1016/j.lanwpc.2020.100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Wang Z, Rader B, et al. Associations between changes in population mobility in response to the COVID‐19 pandemic and socioeconomic factors at the city level in China and country level worldwide: a retrospective, observational study. Lancet Digital Health. 2021;3(6):e349‐e359. 10.1016/s2589-7500(21)00059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xiao H, Dai X, Wagenaar BH, et al. The impact of the COVID‐19 pandemic on health services utilization in China: time‐series analyses for 2016‐2020. Lancet Reg Health West Pac. 2021;9:100122. 10.1016/j.lanwpc.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. World Health Organization . Advice on the use of masks in the context of COVID‐19. 2020. https://www.who.int/publications/i/item/advice-on-the-use-of-masks-the-community-during-home-care-and-in-health-care-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak.
- 69. Han Q, Lin Q, Jin S, You L. Coronavirus 2019‐nCoV: a brief perspective from the front line. J Infect. 2020;80(4):373‐377. 10.1016/j.jinf.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malik M, Kunze AC, Bahmer T, Herget‐Rosenthal S, Kunze T. SARS‐CoV‐2: viral loads of exhaled breath and oronasopharyngeal specimens in hospitalized patients with COVID‐19. Int J Infect Dis. 2021;110:105‐110. 10.1016/j.ijid.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Trogen B, Caplan A. Risk compensation and COVID‐19 vaccines. Ann Intern Med. 2021;174(6):858‐859. 10.7326/m20-8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chan KH, Peiris JS, Lam SY, Poon LL, Yuen KY, Seto WH. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. 10.1155/2011/734690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin K, Yee‐Tak Fong D, Zhu B, Karlberg J. Environmental factors on the SARS epidemic: air temperature, passage of time and multiplicative effect of hospital infection. Epidemiol Infect. 2006;134(2):223‐230. 10.1017/s0950268805005054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science. 2020;368(6493):860‐868. 10.1126/science.abb5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of Coronaviruses. Trends in Microbiol. 2016;24(6):490‐502. 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014‐2017. J Clin Virol. 2018;101:52‐56. 10.1016/j.jcv.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Neher RA, Dyrdak R, Druelle V, Hodcroft EB, Albert J. Potential impact of seasonal forcing on a SARS‐CoV‐2 pandemic. Swiss Med Wkly. 2020;150:w20224. 10.4414/smw.2020.20224 [DOI] [PubMed] [Google Scholar]
- 78. Shaman J, Pitzer V, Viboud C, Lipsitch M, Grenfell B. Absolute humidity and the seasonal onset of influenza in the continental US. PLoS Curr. Dec 18 2009:2:Rrn1138. 10.1371/currents.RRN1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shaman J, Goldstein E, Lipsitch M. Absolute humidity and pandemic versus epidemic influenza. Am J Epidemiol. 2011;173(2):127‐135. 10.1093/aje/kwq347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chattopadhyay I, Kiciman E, Elliott JW, Shaman JL, Rzhetsky A. Conjunction of factors triggering waves of seasonal influenza. eLife. 2018;7 . 10.7554/eLife.30756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. The Lancet M . COVID‐19 vaccines: the pandemic will not end overnight. Lancet Microbe. 2021;2(1):e1. 10.1016/s2666-5247(20)30226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Skegg D, Gluckman P, Boulton G, et al. Future scenarios for the COVID‐19 pandemic. Lancet. 2021;397(10276):777‐778. 10.1016/s0140-6736(21)00424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saad‐Roy CM, Wagner CE, Baker RE, et al. Immune life history, vaccination, and the dynamics of SARS‐CoV‐2 over the next 5 years. Science. 2020;370(6518):811‐818. 10.1126/science.abd7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding open accessed websites.


