Abstract
Broad‐spectrum antiviral agents targeting viral RNA‐dependent RNA polymerase (RdRp) are expected to be a key therapeutic strategy in the ongoing coronavirus disease 2019 (COVID‐19) pandemic and its future variants of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus that causes COVID‐19. Molnupiravir is a nucleoside analog that in vivo experiments have been reported to inhibit the replication of SARS‐CoV‐2, the virus that causes COVID‐19. Clinical trials of molnupiravir as a therapy for patients with mild‐to‐moderate COVID‐19 also suggest its significant therapeutic efficacy in comparison to placebo. Molnupiravir is lethally mutagenic against viral RNA, but its effect on host cell DNA is being questioned. Herein, the safety concerns of molnupiravir are discussed with recent findings from published reports and clinical trials. The unchanged efficacy of molnupiravir against mutated SARS‐CoV‐2 variants is also highlighted. With its administration via the oral route, molnupiravir is expected to turn the tide of the COVID‐19 pandemic.
Keywords: COVID‐19, EIDD‐2801, error catastrophe, mutagenesis, β‐d‐N4‐hydroxycytidine
1. INTRODUCTION
Since its first observation in humans in 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused the coronavirus disease 2019 (COVID‐19) pandemic, affecting the worldwide population. 1 Other coronavirus outbreaks, including severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012, did not have an infPection rate as high as that of the present COVID‐19 outbreak. 2 Scientists and clinicians immediately responded through drug repurposing and vaccine development as a strategy to control the pandemic. 3 , 4 While vaccine development is challenged by the emerging immune‐resistant strains of SARS‐CoV‐2 4 and high vaccine hesitancy, 5 , 6 the repurposing of drugs (among many other efforts) is expected to turn the tide of the pandemic. 7 For this purpose, broad‐spectrum antivirals are preferable because it could be used for new human‐infecting virus that would probably cause another outbreak in the future. 8
SARS‐CoV‐2 is a positive‐sense single‐stranded virus which requires RNA‐dependent RNA polymerase (RdRp) to replicate inside host cells. Therefore, many antiviral agents target this protein to attenuate the viral replication. 9 Remdesivir, an RdRp inhibitor, was the first approved drug for the treatment of SARS‐CoV‐2 infection. 10 For remdesivir to be sufficiently bioavailable to inhibit the virus, it must be administered intravenously. 11 This implies that remdesivir administration requires a medical setup, which is not convenient for nonhospitalized patients. This problem was soon addressed by the development of molnupiravir (MK‐4482 or EIDD‐2801), an orally bioavailable antiviral drug. 12 Moreover, molnupiravir can be synthesized using abundant cytidine, thus potentially contributing to a low‐cost production. 7 , 13
Not all RdRp inhibitors are effective. This is due to the fact that SARS‐CoV‐2 is one of the nidoviruses that have proofreading activity attributed to the acquisition of the 3′—5′ exoribonuclease (ExoN) domain. 14 , 15 Molnupiravir, along with two other nucleoside analogs (remdesivir and favipiravir), can escape the proofreading mechanism of SARS‐CoV‐2. 16 , 17 In addition to escaping the proofreading activity, it is preferable if it is difficult for the virus to become resistant against the antiviral agent. Herein, we discuss the mechanism of molnupiravir in attenuating viral replication via lethal mutagenesis and how it acts as a barrier to prevent antiviral resistance in SARS‐CoV‐2. Results from laboratory experiments and clinical trials regarding the safety, tolerability, and efficacy of molnupiravir are also discussed.
2. MOLNUPIRAVIR: A PRODRUG OF Β‐D‐N4‐HYDROXYCYTIDINE
To properly understand molnupiravir, the discovery of β‐d‐N4‐hydroxycytidine (NHC, also named as EIDD‐1931), a ribonucleoside analog, needs to be emphasized first. 18 Initially, the use of NHC as an antiviral agent was limited because of its oral bioavailability in nonhuman primates. 19 , 20 Later, a group of scientists from Emory University (USA) developed molnupiravir (also known as EIDD‐2801 or MK‐4482), which acts as a prodrug of NHC and allows a sufficiently high bioavailability of NHC. 12 Orally bioavailable antiviral agents are not limited by medical setup requirements unlike those administered intravenously; hence, they can be used for nonhospitalized patients. The ester group in molnupiravir is rapidly cleaved by host's esterase in the plasma, converting the molecule into NHC (Figure 1). This is the reason as to why, in the plasma, only free NHC substance was detected. 20 Upon its penetration into an infected cell, NHC intracellularly transforms into its active form, NHC‐5'‐triphosphate (NHC‐TP) (Figure 1). Researchers have proposed several patented chemical pathways to obtain molnupiravir. 21
Figure 1.
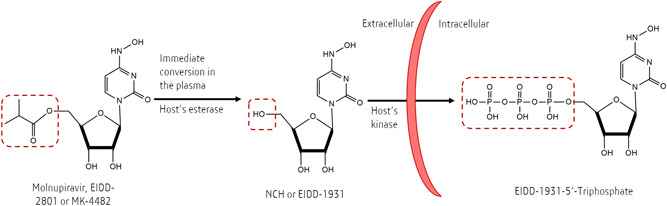
Conversion of molnupiravir into β‐d‐N4‐hydroxycytidine (NHC) and NHC‐triphosphate (the active form of molnupiravir), occurring in the extracellular and intracellular spaces, respectively. Highlighted in the red‐dashed boxes are focused functional groups changed during each conversion.
Originally, molnupiravir was developed to tackle the increasing infection rate of alphaviruses in the United States. 12 Initially, molnupiravir was not specifically designed with broad‐spectrum antiviral activity. During the early stages of the COVID‐19 pandemic, this drug was tested in a clinical trial for seasonal influenza. 22 Only afterward, molnupiravir was observed to have broad‐spectrum antiviral activity even before the mechanism could not be explained. 20 Similar to other drug candidates, such as remdesivir, 23 molnupiravir has also been repurposed to treat COVID‐19. Following the acquisition of the exclusive right to develop molnupiravir by Merck, in collaboration with Ridgeback Biotherapeutics, molnupiravir entered clinical trials. 21
3. MOLNUPIRAVIR AND LETHAL MUTAGENESIS
SARS‐CoV‐2 is a positive‐sense single‐stranded RNA virus that requires RdRp, which functions as genome replication machinery for viral replication in human cells. 24 , 25 RdRp is less than 500 amino acid units in size and possesses three sub‐folded domains shaped like a human cupped right hand (consisting of thumbs, palm, and fingers). Binding sites of the RdRp are the most conserved and accessible region, suggesting RdRp targeting as an effective approach for the mechanism of action of an antiviral. 9 A repurposed and approved drug for COVID‐19 treatment, remdesivir, is known to have RdRp inhibition activity. 26
In case of coronaviruses (CoVs), immediate termination of RNA chain elongation is ineffective against the proofreading activities of the virus. 14 During the replication of CoVs, there are at least 16 nonstructural proteins (nsp1‐16) involved in the process. Of these, the nsp10/nsp14 complex performs 3′—5′ ExoN activity, which can evade nonspecific RNA degradation. 14 Moreover, nsp14 can hydrolyze the mismatched nucleotides at the 3′ end of a nascent RNA. 27 Remdesivir can escape such proofreading activity by allowing three more nucleotides before the termination of the RNA synthesis (delayed termination). 16 Escaping this proofreading activity improves the efficacy of the drug.
Molnupiravir can escape the proofreading activity because it relies on lethal mutagenesis mechanism in attenuating SARS‐CoV‐2 replication. Once inside the cells, NHC is phosphorylated to form the active ribonucleoside triphosphate (NHC‐TP). Viral RNA polymerase (nsp12) then incorporates NHC‐TP into the negative sense genomic RNA (‐gRNA) of SARS‐CoV‐2 as NHC‐monophosphate (NHC‐MP). 28 Following the incorporation, primers having NHC‐MP at their 3' end were efficiently extended, particularly at high ribonucleoside triphosphate (rNTP) concentrations. Moreover, like other mutagenic nucleotides, NHC‐TP likely exists in different tautomeric forms that affect base pairing. The hydroxylamine form acts like cytosine (C) and enables base pairing with guanine (G), whereas the oxime form (C = NOH) acts like uracil (U) and allows base pairing with adenine (A). 28 The NHC‐TP substrate exists predominantly in its hydroxylamine form and acts like CTP; however, when present as NHC‐MP in the template, both tautomeric forms seem to coexist and act like CTP or UTP, favoring incorporation of GTP and ATP, respectively. 28 Hence, there would be transition mutation of nucleotides (G to A or C to U) in nascent +gRNA. Since SARS‐CoV‐2 is a positive‐sense RNA virus, NHC‐TP can be incorporated in both −gRNA and +gRNA. 29 This alteration of nucleotide results in mutagenic transcription and translation products, which are known as lethal mutagenesis or error catastrophe. 30 An illustration of the mutagenesis mechanism of molnupiravir during SARS‐CoV‐2 replication is presented in Figure 2. Even though the inhibition of RNA synthesis is possible through the incorporation of G as the opposite of NHC‐MP, it can be easily overcome by the abundance of nucleoside triphosphate, leaving mutagenesis as the dominant mechanism of molnupiravir. 28
Figure 2.
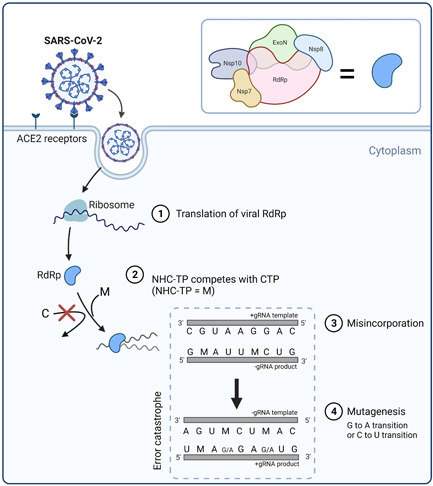
Lethal mutagenesis induction by molnupiravir during severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) replication. The RdRp complex has exoribonuclease (ExoN) and nsp10, which have proofreading activity and function. SARS‐CoV‐2 enters host cell via the ACE2 receptor and RdRp translation is initiated by the host cell ribosome (1). RdRp is used to replicate viral genomic RNA (gRNA), where competition between M and natural C occurs (3), resulting in the misincorporation of the nucleoside and consequently causing lethal mutagenesis (4). C—cytosine, CTP—C‐triphosphate, A—adenine, G—guanine, U—uracil, M—NHC‐5'‐monophosphate, ACE2—angiotensin‐converting enzyme 2, RdRp—RNA‐dependent RNA polymerase, and ExoN—exoribonuclease.
Using mutagenesis as the primary mechanism, where full‐length RNA can still be produced, ExoN proofreading would be easily circumvented by the molnupiravir. Although both favipiravir and molnupiravir perform mutagenesis against the viral genome, an in vitro study found the latter to be more efficacious. 31 In fact, the mutagenic activity of favipiravir was not observed in the aforementioned study and modest antiviral activity was only reached after the concentration was increased to 300 μM. 31 An investigation using thermal melting technique revealed that the stability of a complex between molnupiravir‐embedded RdRp and RNA strands was close to that of the non‐mutagenic one. 29 Favipiravir is weakly incorporated into the RNA primer (−gRNA), 32 making it more challenging to incorporate incorrect nucleotides into the growing RNA strand. A recent study revealed that RdRp backtracking, which can enhance proofreading activity, was stimulated by nsp13 during SARS‐CoV‐2 replication. 33 This indicates the importance of a strong binding affinity with RdRp to circumvent the proofreading activity of SARS‐CoV‐2, as observed in molnupiravir. 34
4. ANTI‐SARS‐COV‐2 ACTIVITY OF MOLNUPIRAVIR: IN VITRO, IN VIVO, AND EX VIVO STUDIES
Various in vitro and ex vivo studies (ex vivo study means a study using samples extracted from living organism, where the treatment carried out after the extraction) have been conducted to elucidate the effect of molnupiravir in suppressing SARS‐CoV‐2 replication. 35 , 36 The molnupiravir metabolite, NHC, reduced the SARS‐CoV‐2 titers in human airway epithelial cell cultures in a dose‐dependent manner with a half‐maximal inhibitory concentration (IC50) as low as 0.08 μM. 35 An ex vivo study using cryopreserved human lung tissues, which were heterogeneous in terms of interleukin (IL)‐6, IL‐8, and interferon (IFN)‐B1 expression corresponding to SARS‐CoV‐2 infection, revealed the effectiveness of NHC in inhibiting viral growth. 36 The efficacy of NHC was also attributed to its ability to circumvent the ExoN proofreading activity of MERS‐CoV. 15
When tested in a mouse model, NHC was effective against multiple CoVs through lethal mutagenesis but not against host cell RNA. 35 Furthermore, a mouse model implanted with human lung tissue was used in a study, which revealed significant inhibitory activity of molnupiravir against SARS‐CoV‐2 replication. 37 The transmission of SARS‐CoV‐2 was efficiently suppressed by molnupiravir confirmed by the absence of viral infection in nasal tissues and secretions of a ferret model with minimal clinical symptoms resembling the young‐adult human population. 38 In another study on the Syrian hamster model, which acts as a preclinical model of mild disease, similar efficient antiviral activity was observed. 39
Several studies have highlighted the advantages of molnupiravir among other anti‐SARS‐CoV‐2 drugs. 36 , 40 , 41 A meta‐analysis of in vivo studies on anti‐SARS‐CoV‐2 drugs suggested the efficacy of molnupiravir (along with remdesivir and amodiaquine) in reducing viral titers. 40 A comparative study using a cell‐based RdRp inhibition assay revealed that molnupiravir was the most potent among all the anti‐SARS‐CoV‐2 drugs studied (entecavir, favipiravir, penciclovir, remdesivir, ribavirin, and tenofovir). 39 In another report employing heterogeneous human lung tissue, molnupiravir was efficient in inhibiting SARS‐CoV‐2 replication compared to chloroquine and remdesivir that showed negligible effects. 36 It is worth mentioning that molnupiravir is one of two orally bioavailable anti‐SARS‐CoV‐2 drugs ready to enter mass production (the other one being the nirmatrelvir/ritonavir combination, or Paxlovid), which is an important aspect in controlling the pandemic. 40
5. MOLNUPIRAVIR EFFICACY: EVIDENCE FROM CLINICAL TRIALS
To accelerate clinical trials, unprecedented collaboration between the sponsor, contract research organization (CRO), and regulatory authorities is critical. 42 Molnupiravir efficacy, safety, virological outcome, and other outcomes have all been studied clinically, as shown in Table 1. Two Phase I clinical trials analyzing the efficacy of molnupiravir that were conducted in the United Kingdom have been completed, and the results have been published. 43 , 44 A randomized, double‐blind, placebo‐controlled study on 130 healthy volunteers investigated the pharmacokinetics of molnupiravir along with absorption inhibition by food intake. 44 The median time of maximum plasma NHC obtained was observed after 1 to 1.75 h with a geometric half‐life approaching 1 h. Slower elimination was observed in subjects with higher and increased doses, and accumulation did not appear after multiple dosing. Food intake was proven to be ineffective in reducing overall absorption, even though several reductions were observed in the absorption rate during the fed state. Adverse events occurred more frequently in the placebo group, with diarrhea being the most common adverse event. None of the subjects from the molnupiravir or placebo group showed indications of clinically significant abnormalities (based on laboratory results, vital sign monitoring, or electrocardiography). However, one subject receiving molnupiravir at a dosage of 800 mg at 2×/day had to discontinue the drug due to the development of a mild truncal maculopapular pruritic rash, which was correlated to the drug by the investigator. Overall, the investigation suggested that a dose of 50 to 800 mg for 5.5 days was safe and well‐tolerated. 44 Additionally, the same safety and tolerability were also observed in subjects receiving a single dose of molnupiravir (up to 1600 mg). 44 Another Phase I clinical trial with an open‐label, randomized controlled design and including COVID‐19 patients with a 5‐day disease duration (n = 18) also concluded that the molnupiravir dosage of 800 mg at 2×/day was safe and well‐tolerated. 43
Table 1.
Clinical studies evaluating molnupiravir therapeutic effects in COVID‐19 based on published articles
| Design (identified number) | Target participant | Participant size | Dosage | Results | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Efficacy | Safety | Virology | Others | |||||
| Randomized‐controlled (standard‐of‐care), open‐label, dose‐escalating. Phase I (NCT04746183) | Adult outpatients with SARS‐CoV‐2 infection confirmed by polymPCR within 5 days of symptom onset) | 18 participants (randomized 2:1 in groups of six participants to receive 300, 600, or 800 mg of molnupiravir orally twice daily for 5 daysor to receive a placebo) | Three dose cohorts (300, 600, and 800 mg molnupiravir) were studied sequentially | ‐ | Molnupiravir was found to be safe and well tolerated | ‐ | ‐ | 43 |
| Randomized, placebo‐controlled, double‐blind. Phase I (NCT04392219) | Subjects ranged in age from 19 to 60 years, with a mean body mass index of 24.4 to 25.4 kg/m2 | 130 participants (64 subjects received a single dose of molnupiravir/placebo, 55 subjects received a single dose of molnupiravir/placebo BID for 5.5 days, and 10 subjects received a single dose of 200 mg molnupiravir in the fed state followed by a single dose of 200 mg molnupiravir in the fasted state following a 14‐day washout period) | Single‐ascending‐ dose between 50 and 1600 mg molnupiravir or placebo | Molnupiravir was well tolerated at doses of 50 to 800 mg administered BID for 5.5 days and at single doses up to 1600 mg. In addition, molnupiravir is well absorbed when it is taken orally, and food intake has little effect on how well it is absorbed | Clinical laboratory, vital sign, and electrocardiogram data revealed no clinically significant findings or dose‐related trends | ‐ | This study found no clinically significant changes in hematological parameters | 44 |
| Multiple doses between 50 and 800 mg molnupiravir or placebo BID for 5.5 days | ||||||||
| Single dose of 200 mg molnupiravir in the fed state followed by a single dose of 200 mg molnupiravir in the fasted state | ||||||||
| Randomized, placebo‐controlled, multicenter, double‐blind. Phase IIa trial (NCT04405570) | Unvaccinated participants who had been diagnosed with SARS‐CoV‐2 and had symptoms lasting less than 7 days | 204 participants (202 receiving at least one dose of molnupiravir or placebo) | 200 mg molnupiravir or a placebo (1:1), followed by a 3:1 ratio of molnupiravir (400 or 800 mg) or a placebo, orally twice daily for 5 days | 800 mg molnupiravir versus placebo supports molnupiravir antiviral efficacy at the 800 mg dose | It was well tolerated, with no increase in treatment‐related or serious adverse events when compared to placebo‐treated participants | Molnupiravir decreased the isolation of infectious viruses, decreased the time required for viral RNA to be cleared, increased the proportion of participants who eliminated SARS‐CoV‐2 viral RNA | There are no safety signals or evidence of hematologic, renal, or hepatic toxicity at any doses | 45 |
| Randomized, placebo‐controlled, double‐blind. Phase II/III (NCT04575584) | Patients aged 18 years or older who require in‐hospital treatment for laboratory‐confirmed COVID‐19 infection with symptoms manifesting ten or fewer days prior to randomization | 304 participants (218 participants received at least one dose of molnupiravir, while 75 received a placebo) | 200, 400, or 800 mg molnupiravir (1:1:1:1), twice daily for 5 days | There is no indication of a treatment effect. | No safety concerns with molnupiravir were identified | Molnupiravir and placebo had no discernible effect on SARS‐CoV‐2 RNA viral load reduction from baseline | There is no evidence of toxicity to the blood, pancreas, or liver | 46 |
| Randomized, placebo‐controlled, double‐blind global. Phase II/III (NCT04575597) | Participants had mild or moderate laboratory‐confirmed COVID‐19 disease with onset of signs/symptoms up to (and including) 7 days before randomization | 302 participants to receive either molnupiravir 200 mg (n = 75), 400 mg (n = 77), or 800 mg (n = 76) or a placebo (n = 74) | Participants were randomly assigned to receive 200, 400, or 800 mg of molnupiravir or a placebo for 5 days and followed through Day 29 | The findings are insufficient to establish a meaningful metric of clinical efficacy. Regardless of treatment, the majority of participants experienced sustained resolution or improvement of COVID‐19 signs/symptoms by Day 29 and had a similar time to progression of COVID‐19 signs/symptoms through Day 29 | Molnupiravir was not associated with dose‐limiting adverse events and distinct safety signals | By Day 5, 5.6%, 20.6%, and 12.7% of participants in the 200‐, 400‐, and 800‐mg molnupiravir groups, respectively, had undetectable SARS‐CoV‐2 RNA levels, compared to 3.6% of placebo participants | Molnupiravir was not associated with clinically significant laboratory test result abnormalities, including hematologic parameters | 47 |
| Randomized, placebo‐controlled, double‐blind. Phase III (NCT04575597) | Nonhospitalized, unvaccinated adults with mild‐to‐moderate COVID‐19 infection confirmed in the laboratory and at least one risk factor for severe COVID‐19 illness and had symptom after 5 days | 1433 participants (716 were assigned to receive molnupiravir and 717 to receive placebo) | 800 mg molnupiravir or a placebo twice a day, for 5 days | Participants receiving molnupiravir had a lower risk of hospitalization or death after 29 days: 6.8% versus 9.7% in the placebo group | No safety concerns with molnupiravir were identified | Molnupiravir treatment was associated with a greater reduction in mean viral load from baseline than placebo treatment | ‐ | 48 |
Abbreviations: COVID‐19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
The efficacy of molnupiravir (800 mg 2×/day) to eliminate viral infection was found to be significant after 3 days of treatment (median) in a double‐blind, randomized, placebo‐controlled Phase IIa clinical trial (total, n = 202). 45 The patients included were unvaccinated outpatients with confirmed SARS‐CoV‐2 infection and symptom onset within 7 days. Patients who received 400 and 800 mg for the next 5 days had a significant reduction in infectious virus isolation compared with that in the placebo group. The duration required for viral RNA clearance was significantly shorter in the 800 mg group than in the placebo group. This clinical trial also suggested that molnupiravir was well‐tolerated, with adverse events (mostly headache, insomnia, and increased alanine aminotransferase) being observed in similar numbers across all the treatment groups. Interim results from a global‐scale Phase II/III clinical trial with identifier number NCT04575597 have been published in a peer‐reviewed journal. 47 , 48 In the Phase II component (n = 302), participants were nonhospitalized patients with a mild or moderate severity of COVID‐19 and 5‐day symptom onset before randomization. 47 The study concluded that treatment with molnupiravir essentially reduced hospitalization and/or death in comparison with the placebo group. 47 Interestingly, based on subgroup analyses, older participants (>60 years old) had a lower risk of hospitalization and/or death after receiving molnupiravir treatment than younger patients. 47 A Phase III clinical trial including 1433 nonhospitalized patients with mild‐to‐moderate COVID‐19 reported satisfactory results of molnupiravir treatment using a dosage of 800 mg at 2×/day for 5 days. 48 The number of hospitalization and/or death risks after 29 days of observation was significantly lower in the molnupiravir group than in the placebo group (p = 0.001). 48 Both studies confirmed the safety and tolerability of molnupiravir due to similar occurrence of adverse events in the molnupiravir or placebo group. 47 , 48
Merck, Sharp, and Dohme (MSD) first stated that the efficacy of molnupiravir in reducing hospitalization and/or death reached approximately 50%. 49 This number was obtained based on the interim results indicating that the risk reduction from 14.1% (in the placebo group) to 7.3% in the molnupiravir group was achieved. 48 It led to a great expectation from experts and policymakers on the drug, even a published article applauded the success. 50 However, the most recent update from the interim analysis of clinical trials revealed that the efficacy has decreased to 30%. 51 Moreover, a Phase II component of global Phase II/III clinical trials participated by 304 participants showed no clinical benefits from molnupiravir treatment on patients who have developed mild to moderate COVID‐19. 46 Currently published clinical trials have several limitations which predominantly derived from the insufficient number of participants. 43 , 45 , 47 Biased results could be obtained due to the uneven distribution of seropositive participants between the treated and placebo groups. 45 In addition, vaccinated individuals are not included in the clinical trials, whilst the more people have received vaccination. Taken altogether, these imply the importance of waiting for results from ongoing clinical trials.
6. ONGOING CLINICAL TRIALS
Ongoing clinical trials of molnupiravir are presented in Table 2. Among them, there is AGILE, a platform for a multicenter, Phase I/II randomized and controlled investigation on the rapid evaluation of COVID‐19 therapy candidates. In the first stage, the platform aimed to determine the optimal dose and safety of molnupiravir. 52 In Phase II/III multicenter clinical trial (NCT04575597), the effect of molnupiravir in nonhospitalized COVID‐19 patients was investigated. This study aimed to measure the efficacy of molnupiravir in treating COVID‐19 based on the percentage of hospitalized patients (24 h) and death (due to any cause). Calculation of the percentages of patients experiencing adverse events and treatment termination (due to adverse events) was also carried out in the trial (NCT04575597). A Phase III clinical trial including 1332 healthy volunteers living in close contact with someone infected with SARS‐CoV‐2 was conducted to test the hypothesis that molnupiravir could prevent infection (laboratory‐confirmed).
Table 2.
Ongoing clinical trials of molnupiravir obtained from ClinicalTrials.gov
| Identifier number (phase) | Title | Design | Participants | Location | Dosage |
|---|---|---|---|---|---|
| NCT04405739 (Phase II) | The safety of EIDD‐2801 and its effect on viral shedding of SARS‐CoV‐2 | Randomized, double‐blind, and placebo‐controlled | 96 adult patients with COVID‐19 7 days from symptom onset | United States | 2×/day for 5 days |
| NCT04746183 (Phase I/II) | AGILE: Seamless Phase I/IIa platform for the rapid evaluation of candidates for COVID‐19 treatment | Randomized, quadruple‐blind, and placebo‐ and SoC‐controlled | 600 adult patients with laboratory‐confirmed COVID‐19 | South Africa, United Kingdom | 2×/day for 5–6 days |
| NCT04575597 (Phase II/III) | A Phase II/III, randomized, placebo‐controlled, double‐blind clinical study to evaluate the efficacy, safety, and pharmacokinetics of MK‐4482 in nonhospitalized adults with COVID‐19 | Randomized, double‐blind, and placebo‐controlled | 1850 adult patients with COVID‐19 ≤5 days from symptom onset | Argentina, Brazil, Canada, Chile, Colombia, Egypt, France, Germany, Guatemala, Israel, Italy, Japan, Mexico, Philippines, Poland, Russian Federation, South Africa, Spain, Sweden, Taiwan, Ukraine, United Kingdom, United States | 200, 400, or 800 mg 2×/day for 5 days |
| NCT04939428 (Phase III) | A Phase III, multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of MK‐4482 for the prevention of COVID‐19 (laboratory‐confirmed SARS‐CoV‐2 infection with symptoms) in adults residing with a person with COVID‐19 | Randomized, triple‐blind, and placebo‐controlled | 1332 healthy volunteers who are living with a laboratory‐confirmed COVID‐19 patient | Argentina, Brazil, Colombia, Dominican Republic, France, Guatemala, Hungary, Japan, Malaysia, Mexico, Philippines, Romania, Russian Federation, South Africa, Spain, Thailand, Turkey, Ukraine, United States | 800 mg 2×/day for 5 days |
Abbreviations: COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
7. MOLNUPIRAVIR AND COMBINATION WITH OTHER DRUGS
Drug combination strategy aims to overcome the weakness of a drug or to enhance its efficacy through synergistic activity. Several combinations of molnupiravir with other antiviral agents have been reported. 53 , 54 , 55 , 56 An antiretroviral drug, nelfinavir, was found to exert synergistic activity against SARS‐CoV‐2 in Calu‐3 cells. 54 Synergism was also observed in the hamster model where molnupiravir was combined with another nucleoside analog, favipiravir. 53 , 56 The combination of IFN‐α and molnupiravir synergistically attenuated the replication of SARS‐CoV‐2 in Calu‐3 cells. 55 IFN‐α is capable of viral RNA degradation by inducing ribonuclease (RNase) transcription. 55
8. EMERGING SARS‐COV‐2 VARIANTS AND MOLNUPIRAVIR EFFECTIVENESS
Because of accumulated transition mutations, it is difficult for SARS‐CoV‐2 to develop resistance against molnupiravir. 15 In a recent study, both remdesivir and molnupiravir were found to have no statistically significant reduction in their efficacy against SARS‐CoV‐2 B.1.1.7 and B.1.351 variants. 57 Similar findings were also obtained in an in vivo study on hamster infection model in which replication of SARS‐CoV‐2 of B.1.1.7 and B.1.351 variants were efficiently inhibited by molnupiravir. 58 Intriguingly, the evolution of SARS‐CoV‐2 occurred in the presence of remdesivir in vitro, resulting in partial resistance to remdesivir. 59 Indeed, evidence of the global transmission of remdesivir‐resistant SARS‐CoV‐2 mutants is not currently present due to the viral fitness tradeoff following RdRp mutation. The study also showed that the SARS‐CoV‐2 RdRp mutation does not affect the efficacy of molnupiravir. 59 A recent study assessing the effectiveness of molnupiravir on Omicron variant found that 5 μM molnupiravir resulted in more than 900‐fold decrease in viral infectious titers assayed using TCID50 assay. 60 In addition, using cell lines the study also found a potent inhibition of viral replication when treated with molnupiravir. 60 These suggest that Omicron variant is highly sensitive to molnupiravir. However, preclinical and clinical trials are warrant to provide more solid data.
9. CONCERNS OF MOLNUPIRAVIR GENOTOXICITY
Investigation of the effect of molnupiravir treatment on INF‐stimulated gene 15 (ISG15) transcripts suggested that the mutagenesis effect of molnupiravir was inefficient toward host DNA. 35 It had been argued that ribonucleotides are eliminated from eukaryotic cell DNA. 35 However, more recent study proved that NHC could cause DNA damage to the host. NHC, in its 2′‐deoxyribose form and concomitant to its conversion by ribonucleotide reductase, could act as one of DNA precursors. 31 The mutagenic activity of NHC (at low concentration) against mammalian cell DNA is based on the hypoxanthine phosphoribosyltransferase (HPRT) gene mutation assay. 31 These findings suggest the possibility of molnupiravir adversely affecting the host, especially by damaging the host DNA. 61 Consequently, pregnant women are not eligible for the treatment using molnupiravir, where a negative pregnancy test is required before the treatment. 62 Administration of molnupiravir to lactating mothers should be carried out with cautions as NHC was found to be transferrable via breast milk in vivo. 63 During the treatment, patients are strongly required to avoid pregnancy (female patients or female partner of male participants) even in the following 4 days posttreatment. 62
10. CONCLUSIONS
Molnupiravir is an orally bioavailable drug capable of inhibiting the replication of SARS‐CoV‐2 by inducing lethal mutagenesis and escaping viral proofreading activities. In vitro and in vivo studies have demonstrated potent antiviral activity of molnupiravir against SARS‐CoV‐2. Results from clinical trials confirmed good bioavailability, safety, and tolerability of the drug. The efficacy of molnupiravir was found to be significant in patients with mild or moderate COVID‐19. It could reduce the risk of hospital admission or death in nonhospitalized adults with mild‐to‐moderate COVID‐19. Nonetheless, the genotoxicity of molnupiravir is a matter of concern. Therefore, a careful assessment of clinical trials and long‐term safety evaluation of molnupiravir are warranted.
AUTHOR CONTRIBUTIONS
Conceptual: Sri Masyeni, Muhammad Iqhrammullah, Harapan Harapan. Design: Muhammad Iqhrammullah, Harapan Harapan. Supervision: Trina Tallei, Kuldeep Dhama, Harapan Harapan. Data collection/processing: Sri Masyeni, Muhammad Iqhrammullah, Andri Frediansyah. Analysis/interpretation: Sri Masyeni, Muhammad Iqhrammullah, Andri Frediansyah, Firzan Nainu, Trina Tallei, Talha Bin Emran, Youdiil Ophinni, Kuldeep Dhama, Harapan Harapan. Literature review: Sri Masyeni, Muhammad Iqhrammullah, Andri Frediansyah. Writing the article: Sri Masyeni, Muhammad Iqhrammullah, Andri Frediansyah, Harapan Harapan. Critical review: Sri Masyeni, Muhammad Iqhrammullah, Andri Frediansyah, Firzan Nainu, Trina Tallei, Talha Bin Emran, Youdiil Ophinni, Kuldeep Dhama, Harapan Harapan. All authors have read and approved the final draft.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
Harapan was supported by Lembaga Pengelola Dana Pendidikan (LPDP), managed by Indonesian Science Fund(ISF) (Grand No RISPRO/KI/B1/TKL/5/15448/2020).
Masyeni S, Iqhrammullah M, Frediansyah A, et al. Molnupiravir: a lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—a narrative review. J Med Virol. 2022;94:3006‐3016. 10.1002/jmv.27730
Contributor Information
Sri Masyeni, Email: masyeniputu@yahoo.com.
Harapan Harapan, Email: harapan@unsyiah.ac.id.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study. Data used in our study were presented in the main text.
REFERENCES
- 1. Harrison AG, Lin T, Wang P. Mechanisms of SARS‐CoV‐2 transmission and pathogenesis. Trends Immunol. 2020;41:1100‐1115. 10.1016/j.it.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Zhou M, Nie X, et al. Epidemiological characteristics and transmission model of corona virus disease 2019 in China. J Infect. 2020;80:e25‐e27. 10.1016/j.jinf.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De P, Chakraborty I, Karna B, Mazumder N. Brief review on repurposed drugs and vaccines for possible treatment of COVID‐19. Eur J Pharmacol. 2021;898:173977. 10.1016/j.ejphar.2021.173977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Karmalawy AA, Soltane R, Abo Elmaaty A, et al. Coronavirus disease (COVID‐19) control between drug repurposing and vaccination: a comprehensive overview. Vaccines (Basel). 9, 2021:1317. 10.3390/vaccines9111317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID‐19 vaccine. Nat Med. 2021;27:225‐228. 10.1038/s41591-020-1124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosiello D, Anwar S, Yufika A, et al. Acceptance of COVID‐19 vaccination at different hypothetical efficacy and safety levels in ten countries in Asia, Africa, and South America. Narra J. 2021;1:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z, Yang L. Turning the tide: natural products and natural‐product‐inspired chemicals as potential counters to SARS‐CoV‐2 infection. Front Pharmacol. 2020;11:1013. 10.3389/fphar.2020.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ianevski A, Yao R, Fenstad MH, et al. Potential antiviral options against SARS‐CoV‐2 infection. Viruses. 2020;12:642. 10.3390/v12060642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elfiky AA. SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn. 2021;39:3204‐3212. 10.1080/07391102.2020.1761882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubin D, Chan‐Tack K, Farley J, Sherwat A. FDA approval of remdesivir—a step in the right direction. N Engl J Med. 2020;383:2598‐2600. 10.1056/NEJMp2032369 [DOI] [PubMed] [Google Scholar]
- 11. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324:1048‐1057. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Painter GR, Natchus MG, Cohen O, Holman W, Painter WP. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID‐19. Curr Opin Virol. 2021;50:17‐22. 10.1016/j.coviro.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snead DR, Gopalsamuthiram V, Williams C, Noble J, Jamison TF, Gupton BF. A concise route to MK‐4482 (EIDD‐2801) from cytidine: part 2. Synlett. 2020;32:326‐328. 10.1055/a-1275-2848 [DOI] [Google Scholar]
- 14. Bouvet M, Imbert I, Subissi L, Gluais L, Canard B, Decroly E. RNA 3'‐end mismatch excision by the severe acute respiratory syndrome coronavirus nonstructural protein nsp10/nsp14 exoribonuclease complex. Proc Natl Acad Sci U S A. 2012;109:9372‐9377. 10.1073/pnas.1201130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agostini ML, Pruijssers AJ, Chappell JD, et al. Small‐molecule antiviral beta‐d‐N (4)‐hydroxycytidine inhibits a proofreading‐intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93:e01348‐19. 10.1128/JVI.01348-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773‐4779. 10.1074/jbc.AC120.013056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID‐19: a systematic review of literature. Diabetes Metab Syndr. 2021;15:102329. 10.1016/j.dsx.2021.102329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stuyver LJ, Whitaker T, McBrayer TR, et al. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob Agents Chemother. 2003;47:244‐254. 10.1128/AAC.47.1.244-254.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon JJ, Toots M, Lee S, et al. Orally efficacious broad‐spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62:e00766‐18. 10.1128/AAC.00766-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toots M, Yoon JJ, Cox RM, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11:eaax5866 10.1126/scitranslmed.aax5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imran M, Kumar Arora M, Asdaq SMB, et al. Discovery, development, and patent trends on molnupiravir: a prospective oral treatment for COVID‐19. Molecules. 2021;26:19. 10.3390/molecules26195795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toots M, Plemper RK. Next‐generation direct‐acting influenza therapeutics. Transl Res. 2020;220:33‐42. 10.1016/j.trsl.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simonis A, Theobald SJ, Fätkenheuer G, Rybniker J, Malin JJ. A comparative analysis of remdesivir and other repurposed antivirals against SARS‐CoV‐2. EMBO Mol Med. 2021;13:e13105. 10.15252/emmm.202013105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia H, Gong P. A structure‐function diversity survey of the RNA‐dependent RNA polymerases from the positive‐strand RNA viruses. Front Microbiol. 2019;10:1945. 10.3389/fmicb.2019.01945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13:667‐673. 10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guy RK, DiPaola RS, Romanelli F, Dutch RE. Rapid repurposing of drugs for COVID‐19. Science. 2020;368:829‐830. 10.1126/science.abb9332 [DOI] [PubMed] [Google Scholar]
- 27. Malone B, Urakova N, Snijder EJ, Campbell EA. Structures and functions of coronavirus replication‐transcription complexes and their relevance for SARS‐CoV‐2 drug design. Nat Rev Mol Cell Biol. 2022;23:21‐39. 10.1038/s41580-021-00432-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon CJ, Tchesnokov EP, Schinazi RF, Götte M. Molnupiravir promotes SARS‐CoV‐2 mutagenesis via the RNA template. J Biol Chem. 2021;297:100770. 10.1016/j.jbc.2021.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir‐induced SARS‐CoV‐2 mutagenesis. Nat Struct Mol Biol. 2021;28:740‐746. 10.1038/s41594-021-00651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malone B, Campbell EA. Publisher correction: molnupiravir: coding for catastrophe. Nat Struct Mol Biol. 2021;28:955. 10.1038/s41594-021-00683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou S, Hill CS, Sarkar S, et al. Beta‐d‐N4‐hydroxycytidine inhibits SARS‐CoV‐2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415‐419. 10.1093/infdis/jiab247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naydenova K, Muir KW, Wu LF, et al. Structure of the SARS‐CoV‐2 RNA‐dependent RNA polymerase in the presence of favipiravir‐RTP. Proc Natl Acad Sci U S A. 2021;118:e2021946118. 10.1073/pnas.2021946118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malone B, Chen J, Wang Q, et al. Structural basis for backtracking by the SARS‐CoV‐2 replication‐transcription complex. Proc Natl Acad Sci U S A. 2021;118:e2102516118. 10.1073/pnas.2102516118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y, Li P, Solanki K, et al. Viral polymerase binding and broad‐spectrum antiviral activity of molnupiravir against human seasonal coronaviruses. Virology. 2021;564:33‐38. 10.1016/j.virol.2021.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12:eabb5883 10.1126/scitranslmed.abb5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schaller MA, Sharma Y, Dupee Z, et al. Ex vivo SARS‐CoV‐2 infection of human lung reveals heterogeneous host defense and therapeutic responses. JCI Insight. 2021;6:e148003. 10.1172/jci.insight.148003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahl A, Gralinski LE, Johnson CE, et al. SARS‐CoV‐2 infection is effectively treated and prevented by EIDD‐2801. Nature. 2021;591:451‐457. 10.1038/s41586-021-03312-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK‐4482/EIDD‐2801 blocks SARS‐CoV‐2 transmission in ferrets. Nat Microbiol. 2021;6:11‐18. 10.1038/s41564-020-00835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenke K, Hansen F, Schwarz B, et al. Orally delivered MK‐4482 inhibits SARS‐CoV‐2 replication in the Syrian hamster model. Nat Commun. 2021;12:2295. 10.1038/s41467-021-22580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ebenezer O, Jordaan MA, Ogunsakin RE, Shapi M. Potential SARS‐COV preclinical (in vivo) compounds targeting COVID‐19 main protease: a meta‐analysis and molecular docking studies. Hippokratia. 2020;24:99‐106. [PMC free article] [PubMed] [Google Scholar]
- 41. Han B, Song Y, Li C, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy children and adolescents: a double‐blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;14:812‐819. 10.1016/S1473-3099(21)00319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holman W, Holman W, McIntosh S, et al. Accelerated first‐in‐human clinical trial of EIDD‐2801/MK‐4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS‐CoV‐2. Trials. 2021;22:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dose and safety of molnupiravir in patients with early SARS‐CoV‐2: a phase I, open‐label, dose‐escalating, randomized controlled study. J Antimicrob Chemother. 2021;76:3286‐3295. 10.1093/jac/dkab318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Painter WP, Holman W, Bush JA, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad‐spectrum oral antiviral agent with activity against SARS‐CoV‐2. Antimicrob Agents Chemother. 2021;65:e02428‐20. 10.1128/AAC.02428-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fischer WA, JJ Eron 2nd, Holman W Jr., et al. A phase 2a clinical trial of Molnupiravir in patients with COVID‐19 shows accelerated SARS‐CoV‐2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2021;14:eabl7430. 10.1126/scitranslmed.abl7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arribas JR, Bhagani S, Lobo SM. Randomized trial of molnupiravir or placebo in patients hospitalized with covid‐19. NEJM Evid. 2021;1(2). 10.1056/EVIDoa2100044 [DOI] [PubMed] [Google Scholar]
- 47. Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of covid‐19 in nonhospitalized adults. NEJM Evid. 2021;1 (2). 10.1056/EVIDoa2100043 [DOI] [PubMed] [Google Scholar]
- 48. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of covid‐19 in nonhospitalized patients. N Engl J Med. 2021;386:509‐520. 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA‐1273 COVID‐19 vaccines in preventing SARS‐CoV‐2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020‐March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495‐500. 10.15585/mmwr.mm7013e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahase E. Covid‐19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;375:n2422. 10.1136/bmj.n2422 [DOI] [PubMed] [Google Scholar]
- 51.Merck and ridgeback biotherapeutics provide update on results from MOVe‐OUT study of molnupiravir, an investigational oral antiviral medicine, in at risk adults with mild‐to‐moderate COVID‐19. Merck. Accessed January 17th, 2022.
- 52. Griffiths G, Fitzgerald R, Jaki T, et al. AGILE‐ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID‐19: a structured summary of a study protocol for a randomised platform trial. Trials. 2020;21:544. 10.1186/s13063-020-04473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eloy P, Le Grand R, Malvy D, Guedj J. Combined treatment of molnupiravir and favipiravir against SARS‐CoV‐2 infection: one + zero equals two? EBioMedicine. 2021;74:103663. 10.1016/j.ebiom.2021.103663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ianevski A, Yao R, Biza S, et al. Identification and tracking of antiviral drug combinations. Viruses. 2020;12:1178. 10.3390/v12101178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ianevski A, Yao R, Zusinaite E, et al. Synergistic interferon‐alpha‐based combinations for treatment of SARS‐CoV‐2 and other viral infections. Viruses. 2021;13:2489. 10.3390/v13122489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdelnabi R, Foo CS, Kaptein SJF, et al. The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS‐CoV‐2 hamster infection model. EBioMedicine. 2021;72:103595. 10.1016/j.ebiom.2021.103595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee J, Lee J, Kim HJ, Ko M, Jee Y, Kim S. TMPRSS2 and RNA‐dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID‐19 caused by SARS‐CoV‐2 variants (B.1.1.7 and B.1.351). Microbiol Spectr. 2021;9:e0047221. 10.1128/Spectrum.00472-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdelnabi R, Foo CS, De Jonghe S, Maes P, Weynand B, Neyts J. Molnupiravir inhibits replication of the emerging SARS‐CoV‐2 variants of concern in a hamster infection model. J Infect Dis. 2021;224:749‐753. 10.1093/infdis/jiab361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Szemiel AM, Merits A, Orton RJ, et al. In vitro selection of remdesivir resistance suggests evolutionary predictability of SARS‐CoV‐2. PLoS Pathog. 2021;17:e1009929. 10.1371/journal.ppat.1009929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li P, Wang Y, Lavrijsen M, et al. SARS‐CoV‐2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Schalkwyk JM. Buyer beware: molnupiravir may damage DNA. BMJ. 2021;375:n2663. 10.1136/bmj.n2663 [DOI] [PubMed] [Google Scholar]
- 62. Singh AK, Singh A, Singh R, Misra A. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID‐19. Diabetes Metab Syndr. 2022;16:102396. 10.1016/j.dsx.2022.102396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fact sheet for healthcare providers: emergency use authorization for molnupiravir. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study. Data used in our study were presented in the main text.


