Abstract
COVID‐19 vaccines provide high levels of protection against severe disease and hospitalization due to severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) infection. Vaccination may be less effective in preventing shedding of infectious viruses from otherwise immune patients. In this study, we describe breakthrough infections and shedding of infectious viruses in convalescent hamsters without significant replication in the lower respiratory tract following reinfection by Alpha and Delta variants despite high levels of circulating antibodies in sera. Using convalescent hamsters with long‐term immunity (up to 1 year) following infection by ancestral SARS‐CoV‐2, we can model aspects of recurring COVID‐19 in the context of preexisting immunity.
Keywords: COVID‐19, long‐term immunity, SARS‐CoV‐2, variants of concern
1. INTRODUCTION
The ongoing plague of COVID‐19 is perhaps the best‐documented pandemic in human history and the first major threat to global public health to emerge during the age of big data. Almost real‐time sequencing of clinical isolates and the availability of this information has led to a wealth of information about viral evolution. Variants of concern continue to emerge and are identified as severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) spreads and adapts to its new hosts. Although some beta coronaviruses such as MERS and SARS‐CoV‐1 were responsible for outbreaks of severe disease, then either faded away or contained, others have become endemic. As SARS‐CoV‐2 begins to contend with vaccination programs and naturally acquired immunity, infections and deaths continue to occur. Aged Syrian Hamsters recapitulate many aspects of COVID‐19 in humans 1 , 2 including upper respiratory tract (URT), lower respiratory tract (LRT) replication, pathology, and resistance to subsequent infection. 2 , 3
Starting in May–June of 2021, the Delta variant (B.1.617.2) emerged and quickly supplanted the Alpha variant (B.1.1.7) as the dominant SARS‐CoV‐2 lineage. This was most likely due to Delta being significantly more contagious compared to previous variants. 4 , 5 , 6 Vaccination has continued to be highly effective (80%–100%) in preventing hospitalization and illness due to COVID‐19. As time since vaccination increases, there is evidence that vaccine efficacy against SARS‐CoV‐2 infection wanes. 7 As infection can result in shedding by vaccinated or naturally immune persons, 8 this can provide a mechanism for continued viral transmission to susceptible populations or contribute to the maintenance of viral transmission cycles. Additionally, hundreds of millions have acquired immunity from natural infection, which can confound studies of vaccine efficacy as convalescent patients decline vaccination due to perceived immunity.
2. MATERIALS AND METHODS
Vero cells (ATCC CRL 1586) were obtained from the American Type Culture Collection and were maintained and passaged in DMEM medium (Gibco/Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (Hyclone). The following reagents were obtained through BEI Resources, NIAID, NIH: SARS‐CoV‐2, Isolate hCoV‐19/England/204820464/20200, NR‐54000 (Alpha) and Isolate, hCoV‐19/USA/PHC658/2021, NR‐55282 (Delta) and passaged once in Vero E6 cells before challenge. The identity of all viruses used in this study was confirmed by Sanger sequencing.
Convalescent male and female, 16–22 months old, Syrian hamsters (Mesocricetus auratus) were challenged with 104 PFU of either B.1.617.2 (n = 6) or B.1.1.7 (n = 7). were housed and challenged by the intranasal route with tissue homogenates collected on Days 4 and 7 postinfection as described previously 9 according to an animal protocol approved by the Food and Drug Administration IACUC. The hamsters were tested for neutralizing antibodies by a 50% plaque‐reduction neutralization assay 10 just before challenge. A summary of the hamsters used in this study is presented in Table S1.
3. RESULTS
To compare the ability of Alpha and Delta variants to cause breakthrough infections, convalescent hamsters with long‐term immunity (6–15 months) following inoculation in previous experiments were sorted based on age and challenged with either Alpha (n = 7) or Delta (n = 6) variants of SARS‐CoV‐2. All hamster sera had neutralizing antibodies (Figure 1A and 1E) against the Alpha variant with an approximately fourfold reduced average titer against the Delta variant. LRT infection was restricted in the B.1.1.7 challenge group, with both infectious virus loads and viral replication below the limits of detection in lungs harvested on Days 4 and 7 postinfection (Figure 1B,C). LRT infection was also prevented in the B.1.617.2 challenge group (Figure 1F,G), as viral loads and replication were below the limits of detection in lungs harvested on Days 4 and 7 postinfection. In the URT, viral replication was not detected in tracheae collected from the B.1.1.7 group, however, viral replication was observed in a single animal (WH055) among the B.1.617.2 challenged hamsters. Although viral challenge did not result in a productive infection of the lungs or trachea, breakthrough infections of the nasal turbinates were observed in 6/7 hamsters in the B.1.1.7 group and 4/6 in the B.1.617.2 group. Hamsters shed infectious virus in nasal wash samples from 1‐ to 2‐day postinfection (DPI) after B.1.1.7 challenge (Figure 1D) and 1–3 DPI after B.1.617.2 challenge (Figure 1H).
Figure 1.
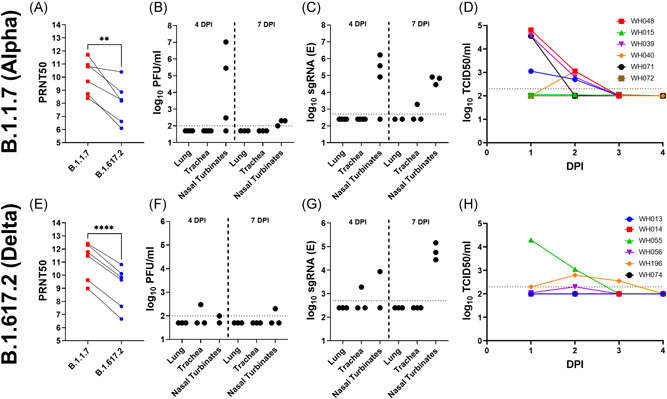
Comparison of viral replication in convalescent hamsters challenged with B.1.1.7 or B.1.617.2 strains of SARS‐CoV‐2. Immune, convalescent hamsters were tested for neutralizing antibodies (A and E) before challenge with 104 PFU B.1.1.7 (n = 7, top) or B.1.617.2 (n = 6, bottom) SARS‐CoV‐2. Viral loads (B and F), as well as viral subgenomic RNA (C and G), were tested in lung, trachea, and nasal turbinate samples collected 4 and 7 DPI. Shedding of infectious virus was assayed by TCID50 assay of nasal washes collected 1–4 DPI (D and H). DPI, days‐postinfection; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Neither B.1.1.7 nor B.1.617.2 challenge resulted in significant (>2%) weight loss for the convalescent hamsters in this study. Likewise, consolidation and overall pathology observed in lung sections were similar between B.1.1.7 and B.1.617.2 infected groups at 4 and 7 DPI (Figure 2B,C). Corresponding to the low levels of viral replication in lung homogenates, pathology in the lungs was mostly limited to bronchiole mucosal hyperplasia and mild (≤10%) consolidation, which was seen in all infected hamsters except WH074 (Figure 2D,E).
Figure 2.
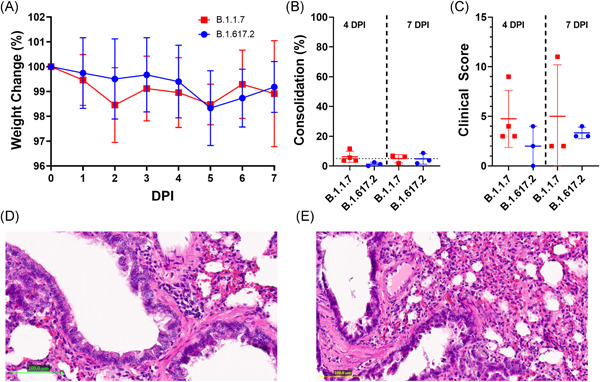
Morbidity and pathology of convalescent Syrian hamsters challenged with B.1.1.7 or B.1.617.2 strains of SARS‐CoV‐2. Following the challenge of convalescent animals, morbidity was assessed by daily weight measurements (A) as well as percent consolidation (B) and clinical score (C) of harvested lung tissues at 4‐ and 7‐day postinfection (DPI). The principal pathological sign in B.1.1.7 (D) and B.1.617.2 (E) challenged animals was bronchiole mucosal hyperplasia. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. CONCLUSIONS
Although uninfected convalescent animals were not available to be used in this study, serologically immune hamsters were vulnerable to breakthrough infection by both B.1.1.7 and B.1.617.2 clinical isolates. Despite the presence of neutralizing antibodies, hamsters showed evidence of URT infection and shedding of infectious viruses in nasal washes. However, the LRT was spared detectable viral replication and weight loss in infected animals was minimal in both groups. Although lung pathology was present in infected animals, it was not as severe as has been reported previously for SARS‐CoV‐2 infection 2 , 9 and could be due to the advanced age of the animals or to lingering damage from previous infection. Hamster WH074 had the highest PRNT50 values in serum and was clear of detectable viral replication in the LRT. Additionally, no pathology in lung tissues harvested 4 DPI was observed for WH074. Unfortunately, the sample size used in this study did not support correlation studies between neutralizing antibodies in the serum and viral replication or pathogenesis and a more detailed investigation awaits further studies.
As COVID‐19 continues to spread and the nonimmune population diminishes, the pandemic may shift towards URT infections that are communicable between otherwise immune individuals. Our hamster model and epidemiological studies indicate that vaccination reduces the incidence of severe disease. Breakthrough infections and transmission to immune individuals still occur, and this is reflected in our experience with the hamster model discussed in this letter. As long as reservoirs of susceptible individuals persist and our countermeasures fail to prevent transmission, COVID‐19 will remain a health risk in the foreseeable future.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Charles B. Stauft: contributed conceptualization, formal analysis, investigation, methodology, visualization and had a role in writing the original draft as well as review and editing. Prabhuanand Selvaraj: contributed investigation as well as review and editing. Christopher Z. Lien: assisted with the investigation. Matthew F. Starost: assisted with the investigation, methodology (pathology), and review of the manuscript. Tony T. Wang: contributed conceptualization, formal analysis, investigation, methodology, project administration, as well as writing and editing of the manuscript.
Supporting information
table 1 caption: Vital statistics of male and female convalescent Syrian hamsters challenged with B.1.617.2 or B.1.1.7 SARS‐CoV‐2.
Stauft CB, Selvaraj P, Lien CZ, Starost MF, Wang TT. Long‐term immunity in convalescent Syrian hamsters provides protection against new‐variant SARS‐CoV‐2 infection of the lower but not upper respiratory tract. J Med Virol. 2022;94:2833–2836. 10.1111/jmv.27641
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID‐19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71:2428‐2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nuñez IA, Lien CZ, Selvaraj P, et al. SARS‐CoV‐2 B.1.1.7 Infection of Syrian Hamster does not cause more severe disease, and naturally acquired immunity confers protection. mSphere. 2021;6(3):e0050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selvaraj P, Lien CZ, Liu S, et al. SARS‐CoV‐2 infection induces protective immunity and limits transmission in Syrian hamsters. Life Sci Alliance. 2021;4(4):e202000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mikszewski A, Stabile L, Buonanno G, Morawska L. Increased close proximity airborne transmission of the SARS‐CoV‐2 Delta variant. Sci Total Environ. 2021;176:151499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen H, Vusirikala A, Flannagan J, et al. Household transmission of COVID‐19 cases associated with SARS‐CoV‐2 delta variant (B.1.617.2): national case‐control study. Lancet Reg Health Eur. 2021;12:100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS‐CoV‐2 is far higher compared to the ancestral SARS‐CoV‐2 virus. J Travel Med. 2021;28(7):taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS‐CoV‐2‐breakthrough infections to time‐from‐vaccine. Nat Commun. 2021;12(1):6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS‐CoV‐2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021;22:183‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stauft CB, Lien CZ, Selvaraj P, Liu S, Wang TT. The G614 pandemic SARS‐CoV‐2 variant is not more pathogenic than the original D614 form in adult Syrian hamsters. Virology. 2021;556:96‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stauft CB, Song Y, Gorbatsevych O, et al. Extensive genomic recoding by codon‐pair deoptimization selective for mammals is a flexible tool to generate attenuated vaccine candidates for dengue virus 2. Virology. 2019;537:237‐245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
table 1 caption: Vital statistics of male and female convalescent Syrian hamsters challenged with B.1.617.2 or B.1.1.7 SARS‐CoV‐2.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


