Abstract
Background
We investigated the association of nutritional risk and inflammatory marker level with length of stay (LOS) in children and adolescents hospitalized for COVID‐19 infection in two pediatric teaching hospitals in a developing country.
Methods
This was a cross‐sectional analytical retrospective study performed in two pediatric hospitals. We included the data from all children and adolescents who were hospitalized with a SARS‐CoV‐2 infection between March and December 2020. Demographic, anthropometric, clinical, and laboratory data were extracted from electronic medical records. Nutritional risk was assessed according to the STRONGkids tool within 24 hours of admission and was categorized into two levels: ≥4 (high risk) and <4 (moderate or low risk). Means or medians were compared between nutritional risk groups using the t test and Mann–Whitney U test, respectively. The association of nutritional risk and inflammatory markers with LOS was estimated using the Kaplan–Meier method and log‐rank test. Cox proportional‐hazard and linear regression models were performed, and adjusted for sex, age, and respiratory symptoms.
Results
From a total of 73 patients, 20 (27.4%) had a STRONGkids score ≥4 at admission, which was associated with a longer LOS even after adjusting (β = 12.30; 1.74–22.9 95% CI; P = 0.023). The same association was observed between LOS and all laboratory markers except for D‐dimer.
Conclusion
Among children and adolescents with COVID‐19, a STRONGkids score ≥4 at admission, lower values of albumin, lymphocytes, and hemoglobin, and higher CRP values were associated with longer LOS.
Keywords: COVID‐19, hospitalized child, inflammation, length of stay, malnutrition, nutrition assessment, nutrition risk, pediatrics, SARS‐CoV‐2
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus has caused a global pandemic state with high morbidity and mortality. 1 The latest knowledge of this disease indicates that the virus causes a significant inflammatory response with organ dysfunction and, thus, a potentially lengthier hospitalization period, a need for mechanical ventilation, and an undernourished clinical condition. Sarcopenia and nutrition deterioration triggered by intense systemic inflammatory response have been described in adults. 2 , 3
Some studies have described a poorer outcome in undernourished or obese patients. 4 , 5 Nutrition risk scores applied earlier can be useful for the recognition of patients with lower nutrition reserves and poorer prognoses. 6 These patients need to receive effective nutrition therapy to avoid additional protein catabolism and the deterioration of their clinical condition. 2 , 7 The STRONGkids nutrition screening tool has been utilized frequently to evaluate nutrition risk upon hospital admission and, when combined with a complete anthropometric evaluation, can be useful to determine nutrition classification and a potential therapeutic response. 8
In coronavirus disease 2019 (COVID‐19), some laboratory test alterations may be related to prognosis. Lymphopenia and altered inflammatory markers, such as higher concentrations of ferritin, D‐dimer, and C‐reactive protein (CRP), as well as lower concentrations of serum albumin, may be associated with increased disease severity and poorer outcomes. 9 There are, however, no studies that have assessed the influence of nutrition status in the pediatric population on the outcome of the disease. In the context of the high prevalence of SARS‐CoV‐2 infection, currently mainly in developing countries, and the occurrence of severe cases in the pediatric population, we conducted a study to evaluate nutrition risk and inflammatory markers at admission and examine their association with outcome defined as length of stay (LOS) in children and adolescents hospitalized because of COVID‐19.
MATERIALS AND METHODS
This cross‐sectional analytical retrospective study assessed children and adolescents aged 1 month to 18 years who have been admitted to two tertiary care university pediatric hospitals because of COVID‐19 infection.
We included data from all children and adolescents who were hospitalized at the pediatric ward or the intensive care unit (ICU) with SARS‐CoV‐2 infection between March 2020 and December 2020. We excluded neonates (0–28 days). The data were extracted from patients' electronic medical records. The diagnosis of COVID‐19 was defined by a positive reverse transcriptase polymerase chain reaction test on nasopharyngeal swabs, serology, or antigen test, or by COVID‐19 exposure within the 4 weeks prior to the onset of symptoms, in accordance with the recommendations and definitions of the World Health Organization (WHO). 10 All patients were tested and had symptoms or signals compatible with the disease. The most frequent were fever and/or some respiratory discomfort.
Demographic data (age and sex), anthropometric measurements (weight, height, body mass index for age [BMI/A], respiratory manifestations, and inflammatory markers concentrations [serum albumin level, CRP, ferritin, D‐dimer, hemoglobin, leukocyte, lymphocyte, and platelet counts]) were collected within 24 h of hospitalization.
Nutrition risk screening was performed by dietitians in real time within 24 h of hospital admission according to the STRONGkids tool. 11 This tool assesses the risk of malnutrition during hospitalization using the presence or absence of nutrition deficits, underlying disease, reduced food intake, or physiological/weight loss or insignificant gain in the last weeks or months, with a score ranging from 0 to 5 points. Risk classification according to STRONGkids is as follows: low risk (0 points), moderate risk (1–3 points), and high risk (4–5 points). In this study, we categorized the nutrition risk into two levels: STRONGkids ≥4 (high risk) and STRONGkids <4 (moderate or low risk).
Nutrition status on admission was defined by BMI/A z score (zBMI/A) using reference values from the WHO 12 : undernutrition (zBMI/A < −2), eutrophic (zBMI/A ≥ −2 and ≤+2), and overweight/obese (zBMI/A > +2).
Respiratory involvement was gauged in the case of an altered chest x‐ray (airspace opacities, signs of pneumonia, or ground‐glass opacities), altered computed tomography (ground‐glass opacities or consolidation), pulse oximetry <92%, or moderate‐to‐intense tachypnea for age on admission.
Hospital LOS was calculated according to the total number of days of hospitalization (pediatric ward and/or ICU).
Statistical analysis in this study was performed by categorizing the population into groups according to: serum albumin level >2.5 and ≤2.5 g/dl, absolute lymphocyte count >1500/ml and ≤1500/ml (lymphopenia), hemoglobin ≥9 and <9 g/dl (anemia), CRP ≥50 and <50 mg/L, leukocytes >4500/mm3 and <4500/mm3 (leukopenia), platelets ≥100,000 and <100,000/μl (thrombocytopenia), ferritin ≥200 and <200 ng/ml, and D‐dimer ≥250 and <250 ng/ml.
Normality was assessed by the Shapiro‐Wilk test. Data from continuous variables were reported as means with 95% CI when normally distributed, whereas medians and interquartile range (IQR) were used to describe variables without a normal distribution. The t test was used to compare means, and the comparison of medians was done using the Mann‐Whitney U test for comparison of demographic and laboratory data as well as LOS between the two groups STRONGkids ≥4 and STRONGkids <4. Categorical variables were reported as frequencies and analyzed by the Pearson chi‐square test. The Kaplan‐Meier time‐to‐event analysis was used to assess the influence of nutrition risk and inflammatory marker level on LOS, followed by the log‐rank test to verify any significant difference between the curves. To avoid the competing effect of mortality on the outcome (LOS), we performed a Kaplan‐Meier curve including only those patients who survived (we excluded patients who died in this analysis). If the Kaplan‐Meier curve showed good differentiation in assessing LOS, Cox proportional hazard models and linear regression models were created. The baseline model was adjusted for age and sex, in order to adjust for severity, we indicated specific respiratory symptoms (increased respiratory rate for age or the presence of dyspnea). Results were expressed as a hazard ratio or beta coefficient with 95% CI for Cox regression and linear models, respectively. All statistical analyses were conducted using the software Stata, version 15.1. A significance level of 5% was considered in all analyses.
This study was approved by the institution's Research and Ethics Committee, where the study was conducted. Informed consent was obtained from all participants or their legal guardians.
RESULTS
The study population consisted of 74 patients, with a median age of 94 months (IQR, 15.75–144 months); 40 patients (54%) were male. The distribution of STRONGkids scores categorized 71.6% of patients (n = 53) at <4 and 27% of patients (n = 20) at ≥4 (1 patient missing = 1.4%) (Figure 1). Anthropometric assessment was performed on 71 patients (3 patients missing = 4%) (Figure 1), of whom 15.5% (11 patients) were overweight/obese and 14% (10 patients) were undernourished. Forty‐eight patients (65%) had respiratory involvement, and seven died (9.6%). The high‐risk group (STRONGkids ≥4) had statistically significant lower values of serum albumin, lymphocytes, leukocytes, platelets, and hemoglobin, as well as higher CRP values. When we compared patients (in a simple analysis) with STRONGkids ≤4 and >4, significant differences were found in age (123 months vs 62 months; P = 0.017), LOS (11 days vs 5 days; P = 0.005), and pediatric ICU admission percentage (75% vs 28.3%; P = 0.000). Demographic, anthropometric, and clinical characteristics of all patients according to STRONGkids category are displayed in Table 1.
Figure 1.
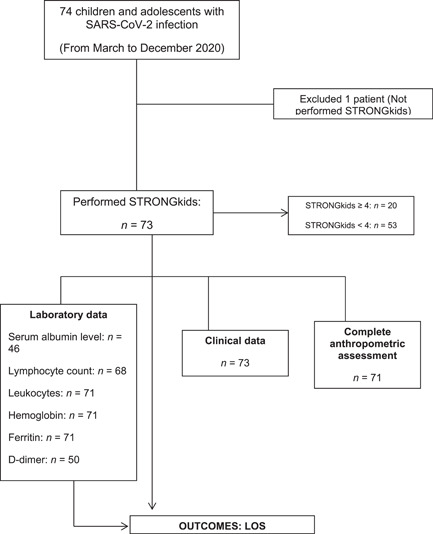
Study flowchart. CRP, C‐reactive protein; LOS, length of stay; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Table 1.
Characteristics of the study population according to STRONGkids
| All patients (n = 74) | STRONGkids (n = 73) | P value | ||
|---|---|---|---|---|
| ≥4 (n = 20) | <4 (n = 53) | |||
| Age, monthsa | 94 | 123 | 62 | 0.017 |
| Male %b | 54 | 60 | 50.9 | 0.489 |
| LOS, daysa | 6.5 | 11 | 5 | 0.005 |
| PICU admission, %b | 40.5 | 75 | 28.3 | 0.000 |
| zBMI/Ac | −0.28 | −0.33 | 0.09 | 0.419 |
| Deaths, %b | 9.6 | 20 | 5.7 | 0.084 |
| Respiratory symptoms, %b | 63.5 | 65 | 64 | 0.946 |
| Serum albumin level, g/dlc (n = 46) | 3.47 | 3.11 | 3.62 | 0.005 |
| Lymphocyte count/mla (n = 68) | 1965 | 1140 | 2550 | 0.000 |
| Leukocytes/mm3 a (n = 71) | 8530 | 6875 | 9510 | 0.027 |
| Platelets/μla (n = 71) | 242,000 | 115,500 | 285,000 | 0.004 |
| Hemoglobin, g/dlc (n = 71) | 10.9 | 9.8 | 11.4 | 0.009 |
| Ferritin, ng/mla (n = 41) | 341.8 | 570 | 319.8 | 0.440 |
| D‐dimer, ng/mla (n = 50) | 1243 | 1739 | 1125 | 0.480 |
| CRP, mg/La (n = 49) | 11.17 | 20.05 | 7.48 | 0.018 |
Abbreviations: CRP, C‐reactive protein; LOS, length of stay; PICU, pediatric intensive care unit; zBMI/A, body mass index for age z score.
Median.
Pearson chi‐square test.
Mean.
Nutrition risk at admission was significantly associated with hospital LOS in the survivors (n = 67) (performed analysis excluded patients who died). STRONGkids ≥4 was associated with a later discharge date (log‐rank test, P = 0.0107), as visualized in the Kaplan‐Meier curve represented in Figure 2. Lower values of serum albumin, lymphocytes, and hemoglobin, as well as higher values of CRP and D‐dimer, were also associated with longer LOS, as shown in Figure 3.
Figure 2.
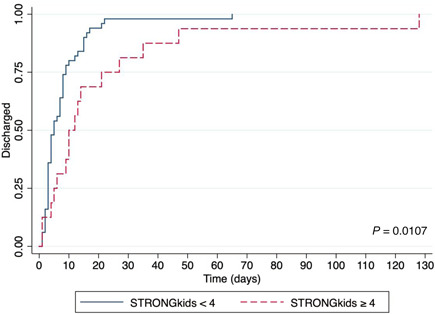
Kaplan‐Meier curves for length of stay (discharge) of children and adolescents with coronavirus disease 2019 according to nutrition risk at admission (STRONGkids ≥4 and STRONGkids <4)
Figure 3.
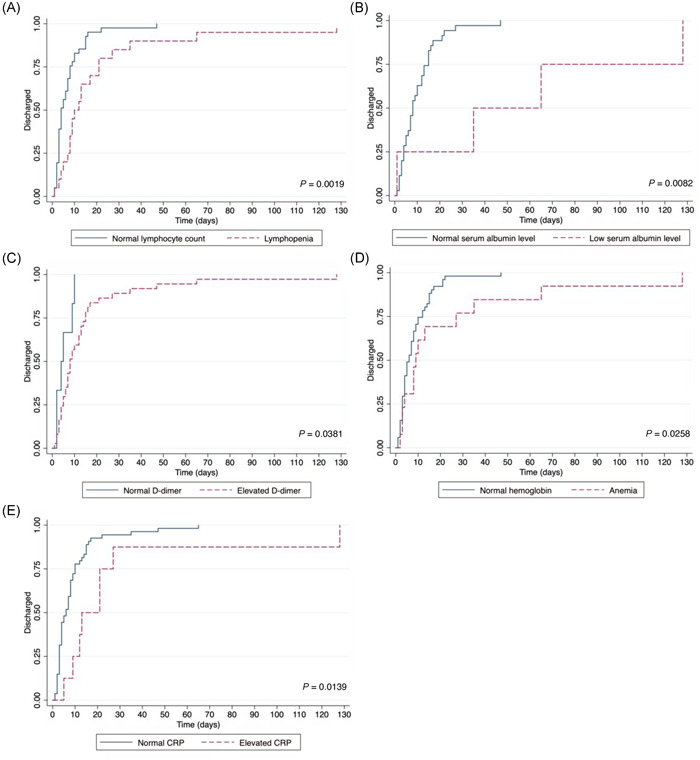
Kaplan‐Meier curves for length of stay (discharge) of children and adolescents with coronavirus disease 2019 according to (A) lymphocyte count, (B) serum albumin level, (C) D‐dimer, (D) hemoglobin, and (E) CRP. CRP, C‐reactive protein
There was no association of leukopenia (P = 0.933), thrombocytopenia (P = 0.314), or higher ferritin values (P = 0.539) with LOS. In the multivariate analysis, we observed that longer LOS remained statistically significant after adjustment for age, sex and respiratory symptoms, except for D‐dimer (Table 2).
Table 2.
Multiple linear regression: association of STRONGkids ≥4, serum albumin level, lymphocytes, hemoglobin, CRP, and D‐dimer with LOS in children and adolescents with COVID‐19
| Model | LOS | ||
|---|---|---|---|
| β | 95% CI | P value | |
| Model 1 (nutrition risk) | |||
| STRONGkids ≥4 | 12.30 | 1.74 to 22.9 | 0.023* |
| Age | 0.03 | −0.04 to 0.10 | 0.437 |
| Sex | 1.87 | −6.87 to 10.60 | 0.671 |
| Respiratory symptoms | −0.47 | −9.38 to 8.43 | 0.915 |
| Model 2 (serum albumin level) | |||
| Serum albumin level | 53.50 | 31.41 to 75.74 | 0.000* |
| Age | −0.48 | −0.15 to 0.05 | 0.333 |
| Sex | −3.81 | −15.75 to 8.13 | 0.521 |
| Respiratory symptoms | −2.50 | −14.34 to 9.34 | 0.671 |
| Model 3 (lymphocytes) | |||
| Lymphocytes | 12.98 | 2.27 to 23.70 | 0.018* |
| Age | 0.020 | −0.06 to 0.09 | 0.649 |
| Sex | 2.58 | −6.83 to 11.99 | 0.585 |
| Respiratory symptoms | −0.98 | −10.69 to 8.72 | 0.840 |
| Model 4 (CRP) | |||
| CRP | 19.37 | 5.43 to 33.30 | 0.007* |
| Age | 0.020 | −0.45 to 0.09 | 0.495 |
| Sex | 2.46 | −6.66 to 11.58 | 0.591 |
| Respiratory symptoms | −1.86 | −11.20 to 7.48 | 0.691 |
| Model 5 (hemoglobin) | |||
| Hemoglobin | 15.52 | 4.46 to 26.58 | 0.007* |
| Age | 0.040 | −0.03 to 0.10 | 0.258 |
| Sex | −0.14 | −9.09 to 8.82 | 0.976 |
| Respiratory symptoms | −1.58 | −10.63 to 7.47 | 0.728 |
| Model 6 (D‐dimer) | |||
| D‐dimer | 8.90 | −12.54 to 30.33 | 0.959 |
| Age | 0.03 | −0.08 to 0.14 | 0.604 |
| Sex | 2.91 | −11.07 to 16.88 | 0.676 |
| Respiratory symptoms | −0.38 | −15.16 to 14.39 | 0.728 |
Abbreviations: COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; LOS, length of stay.
Statistically significant.
DISCUSSION
The present study showed an association between higher nutrition risk at admission and hospital LOS among children and adolescents with COVID‐19 in two pediatric teaching hospitals. Time‐to‐discharge demonstrated by Kaplan‐Meier curves was longer in children who had a STRONGkids score ≥4. This confirms the importance of achieving an adequate nutrition status during hospitalization in these patients.
Hospital malnutrition is an important risk factor for increased morbidity, LOS, medical costs, and even mortality in children and adolescents, especially in those who are critically ill. 13 Malnutrition can cause severe immune dysfunction and predisposes patients to infections. Disabilities can appear as consequences of severe sarcopenia; it is one of the most important sequelae acquired during hospitalization, especially with intense inflammatory response. Studies with adult patients with COVID‐19 have shown increased muscle wasting caused by systemic inflammation, reduced physical activity, and inadequate nutrient intake in their studied population. 14 , 15 , 16 These patients displayed some signs of malnutrition, such as decreased serum albumin levels 17 , 18 and elevated concentrations of inflammatory markers 13 that cause metabolic changes, resulting in a negative nitrogen balance and worsening nutrition status. 19 Therefore, nutrition screening and support have been recommended for adults and children with COVID‐19. 20 , 21
Pediatric nutrition risk scores have been used to identify children and adolescents who are likely to develop further nutrition deterioration during hospitalization and the need for a strict monitoring of their nutrition clinical status to prevent poor outcomes. STRONGkids was incorporated by many hospitals in the world to evaluate nutrition risk in children and adolescents and may help determine the best plan of care for these patients. 22 , 23
Studies have shown a significant relationship of STRONGkids with current nutrition status 24 and outcome defined as LOS. Hulst et al. 11 showed that, in children with various clinical diagnoses, a lower STRONGkids risk score was associated with a significantly shorter LOS when compared with that of children with a moderate or higher risk score (median 2 days vs 3 days, respectively; P < 0.001). After adjusting for all clinical risk factors (age, underlying disease, and ethnicity), multivariate analysis demonstrated that the LOS difference between lower vs higher nutrition risk categories remained significant (P = 0.017).
Although the association between malnutrition and nutrition risk with unfavorable outcomes is well known, clinical evidence of this association in patients with COVID‐19 is limited, especially in children and adolescents. Mendes et al. 6 demonstrated this association in older patients with COVID‐19, whose highest nutrition risk scores were associated with prolonged LOS (by >3 days) in 173 survivors. In critically ill adults with COVID‐19, Zhao et al. 17 observed that 413 patients with higher nutrition risk scores had an increased risk of mortality and a longer stay in the hospital. In logistic regression models, an augmented nutrition risk score was associated with a risk of mortality increased by 1.23 times (adjusted odds ratio = 2.23; 95% CI, 1.10, 4.51; P = 0.026).
Our study is the first to describe nutrition characteristics in children and adolescents hospitalized with COVID‐19 and explore the relationship between nutrition risk and clinical outcomes. In addition, we demonstrated an association between altered inflammatory markers and LOS. Children and adolescents with lymphopenia, anemia, low serum albumin values, and higher CRP concentrations had longer LOS, which remained statistically significant after adjustment for age, sex and respiratory symptoms. Therefore, our data suggest that the use of STRONGkids in combination with inflammatory markers may help identify individuals at high risk of prolonged hospital LOS. Patients with COVID‐19 can have different levels of severity related to inflammatory status, so prognostic evaluation can be problematic because there is no cutoff value for the majority of the inflammatory markers. STRONGkids is a simple, easy‐to‐use score that can be applied at admission and can be useful to establish an index with inflammatory markers to estimate LOS. Zhao et al. 17 found similar results comparing nutrition risk and the inflammatory status of severe and critically ill adults. Critically ill patients had a significantly higher proportion of high nutrition risk scores, which were correlated with inflammatory and nutrition‐related markers.
Some authors have explored the prognostic value of inflammatory status in adults in various clinical settings, including cardiovascular diseases, infectious diseases, cancer, and, recently, COVID‐19. We found that an elevated nutrition risk, which was positively correlated with inflammatory markers, was associated with higher LOS. We think our data may encourage future researchers to create prognostic scores with inflammatory markers for children and adolescents as well. It is critical to have standardized tools to guide clinical decisions in the interest of improving outcomes, especially for the patients with a high nutrition risk.
This study has several limitations, including a relatively small sample size and a retrospective study design, with a possible loss of information related to chart analysis. Moreover, we could not evaluate the association of nutrition risk or nutrition status with other outcomes, including mortality and ventilator‐free days. Larger cohorts and a more intensive follow‐up of children and adolescents with COVID‐19 will be necessary to evaluate the degree to which the advantages of using nutrition scores for prognostic evaluation extend.
Despite the limitations, the study emphasizes the importance of a nutrition screening tool in children and adolescents with COVID‐19 and examines the possible negative impact of higher nutrition risk on the clinical outcomes of these patients.
In conclusion, the present study demonstrates that a higher nutrition risk score, as well as lower levels of serum albumin, lymphocytes, and hemoglobin, as well as higher CRP values at admission were associated with longer LOS in a cohort of children and adolescents with COVID‐19 admitted to two pediatric teaching hospitals in a developing country.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Werther Brunow de Carvalho contributed to the design of the research; Patrícia Zamberlan, Isadora Souza Rodriguez, Josiane de Carvalho Simas, Ariadne Beatriz Silvério, and Leila Costa Volpon equally contributed to the acquisition and analysis of the data; Karina Helena Canton Viani contributed to the analysis and interpretation of the data; Artur Figueiredo Delgado and Ana Paula de Carvalho Panzeri Carlotti contributed to the analysis and interpretation of the data and critically revised the manuscript; and Patrícia Zamberlan drafted the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank the teams (pediatric wards or the pediatric intensive care units) of the institutions involved and the university for their unrestricted support to the study.
Zamberlan P, Carlotti APdCP, Viani KHC, et al. Increased nutrition risk at admission is associated with longer hospitalization in children and adolescents with COVID‐19. Nutr Clin Pract. 2022;37:393‐401. 10.1002/ncp.10846
REFERENCES
- 1. Giacomet V, Barcellini L, Stracuzzi M, et al. Gastrointestinal symptoms in severe COVID‐19 children. Pediatr Infect Dis J. 2020;39(10):e317‐e320. [DOI] [PubMed] [Google Scholar]
- 2. Headey D, Heidkamp R, Osendarp S, et al. Impacts of COVID‐19 on childhood malnutrition and nutrition‐related mortality. Lancet. 2020;396(10250):519‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toptas M, Yalcin M, Akkoc İ, et al. The relation between sarcopenia and mortality in patients at intensive care unit. BioMed Res Int. 2018;2018:5263208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallagher D, DeLegge M. Body composition (sarcopenia) in obese patients: implications for care in the intensive care unit. JPEN J Parenter Enteral Nutr. 2011;35(suppl 5):21S‐28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulkarni R, Rajput U, Dawre R, et al. Severe malnutrition and anemia are associated with severe COVID in infants. J Trop Pediatr. 2021;67(1):fmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendes A, Serratrice C, Herrmann FR, et al. Nutritional risk at hospital admission is associated with prolonged length of hospital stay in old patients with COVID‐19. Clin Nutr. Published online March 23, 2021. 10.1016/j.clnu.2021.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galmés S, Serra F, Palou A. Current state of evidence: influence of nutritional and nutrigenetic factors on immunity in the COVID‐19 pandemic framework. Nutrients. 2020;12(9):E2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bang YK, Park MK, Ju YS, Cho KY. Clinical significance of nutritional risk screening tool for hospitalised children with acute burn injuries: a cross‐sectional study. J Hum Nutr Diet. 2018;31(3):370‐378. [DOI] [PubMed] [Google Scholar]
- 9. Leulseged TW, Hassen IS, Ayele BT, et al. Laboratory biomarkers of COVID‐19 disease severity and outcome: findings from a developing country. PLoS One. 2021;16(3):e0246087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Multisystem inflammatory syndrome in children and adolescents with COVID‐19: scientific. Brief 2020. May 15, 2020. Accessed December 17, 2021. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 11. Hulst JM, Zwart H, Hop WC, Joosten KF. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr. 2010;29(1):106‐111. [DOI] [PubMed] [Google Scholar]
- 12. WHO Multicentre Growth Reference Study Group . WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76‐85. [DOI] [PubMed] [Google Scholar]
- 13. Delgado AF, Okay TS, Leone C, Nichols B, Del Negro GM, Vaz FA. Hospital malnutrition and inflammatory response in critically ill children and adolescents admitted to a tertiary intensive care unit. Clinics (Sao Paulo). 2008;63(3):357‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welch C, Greig C, Masud T, Wilson D, Jackson TA. COVID‐19 and acute sarcopenia. Aging Dis. 2020;11(6):1345‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang PY, Li Y, Wang Q. Sarcopenia: an underlying treatment target during the COVID‐19 pandemic. Nutrition. 2021;84:111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11(4):863‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao X, Li Y, Ge Y, et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID‐19 patients. JPEN J Parenter Enteral Nutr. 2021;45(1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rouget A, Vardon‐Bounes F, Lorber P, et al. Prevalence of malnutrition in coronavirus disease 19: the NUTRICOV study. Br J Nutr. 2021;126(9):1296‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leite HP, Rodrigues da Silva AV, de Oliveira Iglesias SB, Koch Nogueira PC. Serum albumin is an independent predictor of clinical outcomes in critically ill children. Pediatr Crit Care Med. 2016;17(2):e50‐e57. [DOI] [PubMed] [Google Scholar]
- 20. Thibault R, Seguin P, Tamion F, Pichard C, Singer P. Nutrition of the COVID‐19 patient in the intensive care unit (ICU): a practical guidance. Crit Care. 2020;24(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stachowska E, Folwarski M, Jamioł‐Milc D, Maciejewska D, Skonieczna‐Żydecka K. Nutritional support in coronavirus 2019 disease. Medicina (Kaunas). 2020;56(6):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dokal K, Asmar N, Shergill‐Bonner R, Mutalib M. Nutrition evaluation screening tool: an easy to use screening tool for hospitalised children. Pediatr Gastroenterol Hepatol Nutr. 2021;24(1):90‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santos CAD, Rosa COB, Franceschini SDCC, Firmino HH, Ribeiro AQ. Nutrition risk assessed by STRONGkids predicts longer hospital stay in a pediatric cohort: a survival analysis. Nutr Clin Pract. 2021;36(1):233‐240. [DOI] [PubMed] [Google Scholar]
- 24. Huysentruyt K, Alliet P, Muyshont L, et al. The STRONG (kids) nutritional screening tool in hospitalized children: a validation study. Nutrition. 2013;29(11‐12):1356‐1361. [DOI] [PubMed] [Google Scholar]


