Abstract
Sequences of the folP1, rpoB, and gyrA genes were analyzed for 88 isolates of Mycobacterium leprae from leprosy patients in Japan, Haiti, Indonesia, Pakistan, and the Philippines. Thirteen isolates (14.8%) showed representative mutations in more than two genes, suggesting the emergence of multidrug-resistant M. leprae.
Leprosy is a chronic infectious disease caused by Mycobacterium leprae and is still a major health problem in several countries of Asia, Latin America, and Africa. Global efforts to control leprosy by intensive chemotherapy have led to a significant decrease in the number of registered patients. However, the annual reports of new cases indicate more than 500,000 even after the introduction of multidrug therapy (MDT) by the World Health Organization (WHO) (19). Current recommended control measures for treating leprosy with MDT are designed to prevent the spread of drug-resistant M. leprae. However, drug resistance has been reported since 1964 for dapsone (12), 1976 for rifampin (5), and 1996 for ofloxacin (7).
To prevent the emergence and transmission of multidrug-resistant (MDR) leprosy and to identify and treat existing cases of MDR leprosy, it is necessary to establish rapid methods for detection of drug resistance in M. leprae. However, M. leprae has not been cultivated on artificial media; therefore, to identify drug susceptibility patterns, bacteria must be tested using Shepard's mouse footpad assay (14). This in vivo method requires at least 6 months and relatively large numbers of bacteria. Recently, there have been advances in the elucidation of molecular events responsible for drug resistance in mycobacteria (6, 11, 13).
In the present study, we analyzed the DNA sequences of particular regions of M. leprae folP1, rpoB, and gyrA, which are responsible for resistance to dapsone, rifampin, and fluoroquinolones, respectively. Several M. leprae isolates showed point mutations in the genes. These results suggest the emergence of MDR M. leprae.
Bacterial isolates and materials.
A total of 88 M. leprae isolates, obtained from patients receiving antileprosy chemotherapy, were used in this study. The treatments and status of the leprosy patients are listed in Table 1. The treatment history for the patient from whom Zensho-4 was isolated has been described in detail (10). All available details of the cases are listed; however, for some cases, the details are unclear because records were not available. The patients in Japan were individually taking original medical treatments, including monotherapy. The patients in countries other than Japan received the WHO treatment regimen for multibacillary leprosy, except for a Pakistani case. For patients whose condition was improved by medical treatment had a return of leprosy with an increasing bacillary index after 5 to 10 years, the word “relapse” is used in Table 1. In cases Haiti-1 and Peshawar-5, the condition did not improve, though the patients were treated continuously. M. leprae isolates were obtained from biopsy samples of patients with leprosy (multibacillary type) or from footpads of mice infected with M. leprae: Japan (21 cases: 15 biopsy samples and 6 footpads), Haiti (l biopsy sample), Indonesia (30 biopsy samples), Pakistan (8 biopsy samples), and Philippines (28 biopsy samples). The Thai 53 strain of M. leprae, which was obtained from a patient with lepromatous leprosy in Thailand, was established at the Leprosy Research Center, National Institute of Infectious Diseases, Japan, by passages in nude mice. Informed consent was obtained from all patients, and human experimentation guidelines of the Ministry of Health, Labour and Welfare, Japan, were followed.
TABLE 1.
Treatment histories of leprosy patients
| Isolate(s) | MDT | Antimicrobial treatmenta | Status |
|---|---|---|---|
| Japan | |||
| Airaku-2 | Yes | RIF, DDS, CLF, OFX | Relapse |
| Airaku-3 | Yes | RIF, DDS, INH, CLF, OFX | Relapse |
| Hoshizuka-4 | No | OFX | Relapse |
| Shinsei-1 | Yes | RIF, DDS, CLF, OFX, SPFX | Relapse |
| Zensho-2 | Yes | RIF, DDS, PZ, PM | Relapse |
| Zensho-4 | Yes | RIF, DDS, OFX | Relapse |
| Zensho-5 | Yes | RIF, DDS, CLF | Relapse |
| Other isolates | —b | —b | Relapse |
| Philippines, all isolates | Yes | WHO regimen (MB) | New case |
| Indonesia, all isolates | Yes | WHO regimen (MB) | New case |
| Haiti, Haiti-1 | Yes | WHO regimen (MB) | Intractable |
| Pakistan | |||
| Peshawar-5 | No | DDS | Intractable |
| Other isolates | Yes | WHO regimen (MB) | New case |
CLF, clofazimine; DDS, dapsone; INH, isoniazid; OFX, ofloxacin; RIF, rifampcin; SPFX, sparfloxacin; MB, multibacillary leprosy.
No data available.
Sample preparation for PCR.
Thai 53 was used as an antimicrobial-sensitive standard. Leprosy bacilli were purified from infected tissues, and extraction of chromosomal DNA was carried out as previously described (2, 3).
PCR amplification and DNA sequencing.
PCR was carried out using Ex Taq DNA polymerase (Takara Shuzo Co., Shiga, Japan) in a 50-μl volume containing 150 ng of genomic DNA and 1 μM concentrations of the primers, which were designed according to the sequence of the folP1 (accession no. AL023093), rpoB (Z14314), and gyrA (Z70722) genes of M. leprae. The primers used for amplification of the folP1 gene were folP1-1 (5′-GCTTCTCGTGCCGAAGCGCTCG-3′) and folP1-2 (5′-GCCATCGCGGGATCTGCTCGCCCA-3′). The primers for the rpoB gene were rpoB-1 (5′-CAGACGCTGATCAATATCCGT-3′) and rpoB-2 (5′-TACGGTGTTTCGATGAACCCG-3′). For the gyrA gene, gyrA-1 (5′-ATGACTGATATCACGCTGCCA-3′) and gyrA-2 (5′-ATAACGCATCGCTGCCGGTGG-3′) were used. The target regions of the rpoB and gyrA genes were amplified by a PC 800 thermal cycler (Astech Co., Fukuoka, Japan) with a program of 30 s at 95°C, 2 min at 50°C, and 3 min at 72°C for 40 cycles. For amplification of the target region of the folP1 gene, the cycling conditions were 95°C for 30 s, 60°C for 2 min, and 72°C for 3 min for 35 cycles. DNA samples for sequencing were recovered from agarose gels using an Easy Trap DNA purification kit (Takara Shuzo) after electrophoresis and ligated to pGEM-T vector (Promega Co., Madison, Wis.). At least two independent clones were subjected to sequencing. Both strands of recombinant plasmids were sequenced with a BigDye terminator cycle sequencing FS Ready Reaction kit (Perkin-Elmer Applied Bio systems, Norwalk, Conn.) and an ABI Prism 310 genetic analyzer (Perkin-Elmer). The nucleotide sequences obtained were analyzed by the DNASIS computer program (Hitachi Software Engineering, Yokohama, Japan).
Mouse footpad assay.
The in vivo drug susceptibility of M. leprae was determined by the method of Shepard (14). The conditions of the assay were as previously described (10).
Dapsone resistance.
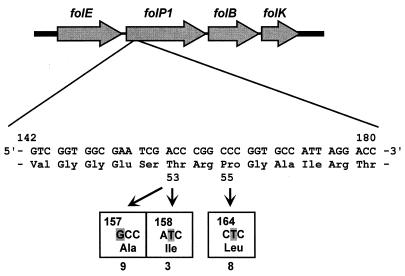
The involvement of two mutations in the folP1 gene of dapsone-resistant M. leprae has been reported (9, 17). In this study, 19 isolates showed the reported mutations in the folP1 gene. One isolate (Zensho-3) showed mutations at positions 157 and 164. They corresponded to Thr→Ala and Pro→Leu at amino acid positions 53 and 55 in dihydropteroate synthase (DHPS), respectively (Table 2). Nine isolates showed an A→G mutation in codon 157 (M. leprae numbering [9]), and three isolates had a C→T mutation in codon 158 (Fig. 1). These mutations caused Thr→Ala and Thr→Ile changes at position 53 in DHPS, respectively. Eight isolates (one isolate overlaps because of a double mutation) showed a C-to-T mutation (Pro→Leu) at codon 164 (position 55 of DHPS).
TABLE 2.
Mutations in folP1, gyrA, and rpoB genes related to drug resistance in clinical isolates of M. leprae
| Isolate | Mutation (position) ina:
|
Origin | ||
|---|---|---|---|---|
| folp1 (dapsone resistance) | rpoB (rifampcin resistance) | gyrA (ofloxacin resistance) | ||
| Airaku-2 | Pro→Leu (55); R | Ser→Leu (531) | None | Japan |
| Airaku-3 | Thr→Ile (53); R | None; R | None; S | Japan |
| Airaku-4 | None | None | Ala→Val (91) | Japan |
| Hoshizuka-1 | None | ND | Gly→Cys (89) | Japan |
| Hoshizuka-3 | Thr→Ile (53) | Ser→Leu (531) | ND | Japan |
| Hoshizuka-4 | ND | Ser→Leu (531) | ND | Japan |
| Kusatsu-1 | Pro→Leu (55) | Ser→Trp (531) | None | Japan |
| Kusatsu-2 | Pro→Leu (55) | Ser→Leu (531) | None | Japan |
| Kusatsu-3 | Pro→Leu (55) | None | ND | Japan |
| Kusatsu-4 | Thr→Ala (53) | Ser→Leu (531) | ND | Japan |
| Kusatsu-5 | Thr→Ala (53) | None | ND | Japan |
| Shinsei-1 | Thr→Ala (53) | Asp→Asn (516); Leu→Pro (533) | Ala→Val (91) | Japan |
| Zensho-2 | Pro→Leu (55) | None | None | Japan |
| Zensho-3 | Thr→Ala (53); Pro→Leu (55) | None | Ala→Val (91) | Japan |
| Zensho-4 | Thr→Ile (53); R | Ser→Leu (531); R | Ala→Val (91); R | Japan |
| Zensho-5 | Pro→Leu (55); R | Ser→Leu (531); R | None; S | Japan |
| Zensho-6 | None | Ser→Leu (531) | None | Japan |
| Zensho-8 | ND | Ser→Leu (531) | ND | Japan |
| Zensho-9 | None | His→Tyr (526) | ND | Japan |
| Phil-5 | None | His→Tyr (526) | ND | Philippines |
| Phil-6 | None | His→Tyr (526) | ND | Philippines |
| Phil-7 | None | His→Tyr (526) | ND | Philippines |
| Phil-10 | Thr→Ala (53) | Ser→Leu (531) | ND | Philippines |
| Phil-12 | Thr→Ala (53) | Ser→Leu (531) | ND | Philippines |
| Phil-17 | None | His→Tyr (526) | ND | Philippines |
| Phil-22 | Thr→Ala (53) | His→Tyr (526) | ND | Philippines |
| Sur2-1 | None | His→Tyr (526) | ND | Indonesia |
| Sur2-6 | None | His→Tyr (526) | ND | Indonesia |
| Sur2-10 | None | Asp→Asn (516) | ND | Indonesia |
| Sur2-14 | ND | His→Tyr (526) | ND | Indonesia |
| Sur2-19 | Thr→Ala (53) | None | ND | Indonesia |
| Sur2-22 | None | His→Tyr (526) | ND | Indonesia |
| Sur2-30 | None | His→Tyr (526) | ND | Indonesia |
| Haiti-1 | None | Ser→Leu (531) | Ala→Val (91) | Haiti |
| Karachi-5 | Thr→Ala (53) | None | ND | Pakistan |
| Peshawar-5 | Pro→Leu (55) | None | ND | Pakistan |
The drug susceptibilities of Airaku-2, Airaku-3, Zensho-4, and Zensho-5 were confirmed in a mouse footpad assay. R, resistant; S, susceptible; None, no mutation in the gene positions which were previously reported for other bacteria, such as E. coli and M. tuberculosis; ND, not determined.
FIG. 1.
Nucleotide sequence of the folP1 gene from Thai 53 and clinical isolates of M. leprae. A DNA fragment of folP1 (388 bp) was amplified by PCR and sequenced. In 19 isolates of M. leprae, mutations at positions 53 and 55, which were found to be associated with dapsone resistance, were detected (the numbering system of reference 9 was used). The numbers of cases which had a mutation are shown. One isolate was overlapping in this figure because it had mutations at positions 157 and 164.
Rifampin resistance.
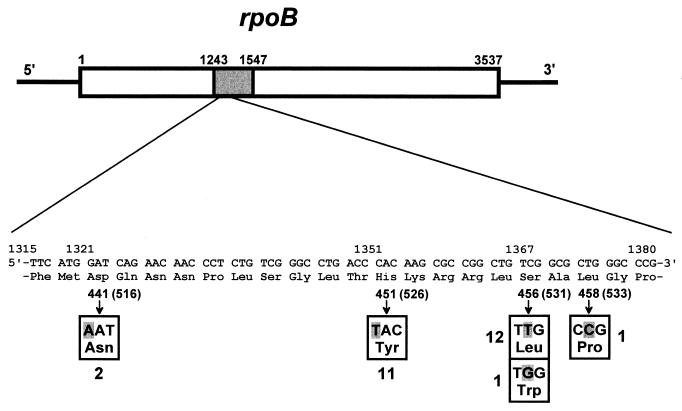
Mutations in the rpoB gene, encoding the β subunit of RNA polymerase, were reported to result in resistance to rifampin in several mycobacterial species, including M. leprae (4, 18). In the present study, 26 isolates showed the mutations which had already been observed in rifampin-resistant bacteria (Fig. 2). A mutation at histidine 526 (numbering system used in reference 16) was reported frequently in Mycobacterium tuberculosis (16, 18) but not in M. leprae. The change from aspartic acid to asparagine at position 516 has not been reported for mycobacteria but has been observed in Escherichia coli (8). This is the first report that these mutations (His to Tyr at 526 and Asp to Asn at 516) occur in rifampin-resistant M. leprae. Among 26 isolates harboring mutations in rpoB, two exhibited a mutation at 516 and 11 showed the mutation at histidine 526, showing a tendency similar to that seen in M. tuberculosis. Thirteen isolates had mutations (Ser to Leu or Trp) at position 531. One isolate (Shinsei-1) showed a double mutation, Asp→Asn at 516 and Leu→Pro at position 533 (Table 2).
FIG. 2.
DNA sequence of the rpoB gene from clinical isolates of M. leprae. The rpoB sequence from positions 1243 to 1547 was amplified and analyzed. Twenty-six isolates had a mutation at position 441 (516), 451 (526), 456 (531), or 458 (533) (E. coli numbering in parentheses [16]). One isolate had mutations at positions 441 (516) and 458 (533).
Ofloxacin resistance.
Mutations in the quinolone resistance-determining region of gyrA were also reported for quinolone-resistant mycobacteria (15). Only one case of quinolone resistance in leprosy was reported, from Mali (1). One mutation (Ala→Val at position 91) was detected in five isolates of M. leprae, and another mutation (Gly→Cys at position 89) was found as in quinolone-resistant M. tuberculosis (15) (Fig. 3).
FIG. 3.
Structure and nucleotide sequence of the gyrA gene from clinical isolates of M. leprae. To analyze the sequence, the gyrA sequence from positions 1 to 390 was amplified by PCR. Mutations at positions 89 and 91 were found in ofloxacin-resistant M. leprae. The numbering system of reference 1 was used.
The drug susceptibilities of Airaku-2, Airaku-3, Zensho-4, and Zensho-5 were confirmed by mouse footpad assay. In the assay, both Airaku-2 and Airaku-3 were dapsone resistant (Table 2). They showed a mutation in the folP1 gene that has been demonstrated to be responsible for dapsone resistance. In Airaku-3, rpoB and gyrA had a wild-type sequence. However, the results of the footpad assay showed that Airaku-3 was rifampin resistant and ofloxacin susceptible. The mutations in rpoB did not account for rifampin resistance in Airaku-3. Therefore, it is likely that Airaku-3 acquired rifampin resistance by other mechanisms, such as changes in membrane permeability and function of the efflux pump. In Zensho-4, mutations were detected in the folP1, rpoB, and gyrA genes. The mouse footpad assay revealed that the isolate had acquired dapsone, rifampin, and ofloxacin resistance (10) as a result of these mutations. Also, it was found that the mutations in the folP1 and rpoB genes brought on resistance to dapsone and rifampin in Zensho-5, as confirmed in the mouse footpad assay.
The most striking finding was that M. leprae frequently harbored more than two mutations, suggesting multidrug resistance in this cohort of patients who did not respond to treatment. As shown in Table 2, 11 isolates had mutations in two genes (resistance to two drugs), and 2 strains (Shinsei-1 and Zensho-4) showed mutations in three genes (resistance to three drugs). MDR M. leprae (resistant to dapsone, rifampin, and ofloxacin) was first reported in 1997 (1). However, the DNA sequence of folP1 was not analyzed for confirmation of dapsone resistance in that study. We clearly showed both genetic and mouse footpad confirmation for MDR leprosy (Zensho-4).
Generally, discontinuation of treatments and monotherapy play a major role in production of MDR bacilli. The drug-resistant M. leprae generated in the past may recur in Japanese patients. Furthermore, new cases in developing countries had drug-resistant M. leprae. MDR M. tuberculosis has become a major problem globally. We should take prompt measures against MDR M. leprae, because leprosy is caused by mycobacteria like M. tuberculosis. However, WHO has not yet considered drug-resistant M. leprae. Our results strongly suggest the importance of a survey of drug resistance in leprosy as well as the establishment of rapid methods for detection of drug-resistant leprosy bacilli. Genetic analysis may provide a powerful tool for rapid detection of drug-resistant M. leprae and important information on chemotherapy.
In this study, other mutations were also detected in folP1, rpoB, and gyrA (data not shown). However, it is not clear that such mutations are linked to drug resistance in M. leprae, because there have been no reports on the positions of mutations which are responsible for drug resistance. We are investigating the relationship between genotypic mutations and phenotypic resistance using M. leprae isolates in the mouse footpad assay.
Acknowledgments
We thank Eiji Nagao (National Sanatorium Oshimaseishoen, Kagawa, Japan), Kunihiro Kinjoh (National Sanatorium Okinawa Airakuen, Okinawa, Japan), Masako Namisato (National Sanatorium Tamazenshoen, Tokyo, Japan), Masamichi Goto (Faculty of Medicine, Kagoshima University, Kagoshima, Japan), Atsushi Hosokawa (Faculty of Medicine, University of the Ryukyu, Okinawa, Japan), Akiko Obara (Faculty of Medicine, Kyoto University, Kyoto, Japan), Motoaki Ozaki (Hyogo Prefectural Amagasaki Hospital, Hyogo, Japan), Tsugio Yanagihashi (National Sanatorium Tohoku-shinseien, Miyagi, Japan), Abraham T. Agdamag (Dr. Jose Rodrigues Memorial Hospital, Manila, Philippines), Indropo Agusni (Dr. Soetomo General Hospital, Faculty of Medicine, Airlangga University, Surabesi, Indonesia), Mohamed Ali Abassi (KMC Leprosy Hospital, Karachi, Pakistan), Mohammad Zubair Khan (Lady Reading Hospital, Peshawar, Pakistan), and Akira Kobayashi (Peshawar-Kai Hospital, Peshawar, Pakistan) for supplying clinical samples.
This work was supported by a Health Research Grant of Research on Emerging and Re-emerging Infectious Diseases, Ministry of Health, Labour and Welfare, Government of Japan, and partly by a grant for Research on International Collaboration in Medicine of International Medical Center, Ministry of Health, Labour and Welfare. Also, this work was partly supported by the U.S.-Japan Cooperative Medical Science Program.
REFERENCES
- 1.Cambau E, Perani E, Guillemin I, Jamet P, Ji B. Multidrug-resistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae. Lancet. 1997;349:103–104. doi: 10.1016/S0140-6736(05)60888-4. [DOI] [PubMed] [Google Scholar]
- 2.de Wit M Y, Faber W R, Krieg S R, Douglas J T, Lucas S B, Montreewasuwat N, Pattyn S R, Hussain R, Ponnighaus J M, Hartskeerl R A, Klatser P R. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J Clin Microbiol. 1991;29:906–910. doi: 10.1128/jcm.29.5.906-910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhandayuthapani S, Banu M J, Kashiwabara Y. Cloning and sequence determination of the gene coding for the elongation factor Tu of Mycobacterium leprae. J Biochem (Tokyo) 1994;115:664–669. doi: 10.1093/oxfordjournals.jbchem.a124393. [DOI] [PubMed] [Google Scholar]
- 4.Honore N, Cole S T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993;37:414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson R R, Hastings R C. Rifampin-resistant leprosy. Lancet. 1976;ii:1304–1305. doi: 10.1016/s0140-6736(76)92071-7. [DOI] [PubMed] [Google Scholar]
- 6.Jamal M A, Maeda S, Nakata N, Kai M, Fukuchi K, Kashiwabara Y. Molecular basis of clarithromycin-resistance in Mycobacterium avium-intracellulare complex. Tuber Lung Dis. 2000;80:1–4. doi: 10.1054/tuld.1999.0227. [DOI] [PubMed] [Google Scholar]
- 7.Ji B, Perani E G, Petinom C, Grosset J H. Bactericidal activities of combinations of new drugs against Mycobacterium leprae in nude mice. Antimicrob Agents Chemother. 1996;40:393–399. doi: 10.1128/aac.40.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin D J, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 9.Kai M, Matsuoka M, Nakata N, Maeda S, Gidoh M, Maeda Y, Hashimoto K, Kobayashi K, Kashiwabara Y. Diaminodiphenylsulfone resistance of Mycobacterium leprae due to mutations in the dihydropteroate synthase gene. FEMS Microbiol Lett. 1999;177:231–235. doi: 10.1111/j.1574-6968.1999.tb13737.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka M, Kashiwabara Y, Namisato M. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int J Lepr Other Mycobact Dis. 2001;68:452–455. [PubMed] [Google Scholar]
- 11.Musser J M. Antimicrobial agent resistance in mycobacteria: molecular insights. Clin Mol Microbiol Rev. 1995;8:496–514. doi: 10.1128/cmr.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettit J H S, Rees R J W. Sulphone resistance in leprosy. An experimental and clinical study. Lancet. 1964;ii:673–674. doi: 10.1016/s0140-6736(64)92482-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Musser J M. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis. Tuber Lung Dis. 1998;79:3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 14.Shepard C C. A kinetic method for the study of drugs against Mycobacterium leprae in mice. Int J Lepr Other Mycobact Dis. 1967;35:429–435. [PubMed] [Google Scholar]
- 15.Takiff H E, Salazar L, Guerrero C, Philipp W, Huang W M, Kreiswirth B, Cole S T, Jacobs W R, Jr, Telenti A. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob Agents Chemother. 1994;38:773–780. doi: 10.1128/aac.38.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopher K, Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 17.Williams D L, Spring L, Harris E, Roche P, Gillis T P. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob Agents Chemother. 2000;44:1530–1537. doi: 10.1128/aac.44.6.1530-1537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams D L, Waguespack C, Eisenach K, Crawford J T, Postaels F, Salfinger M, Nolan C, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Leprosy—global situation. Wkly Epidemiol Rec. 2000;75:226–231. [PubMed] [Google Scholar]