Abstract
Objective:
The aim of this study was to describe the association of individual-level characteristics (sex, race/ethnicity, birth weight, maternal education) with child BMI within each US Census region and variation in child BMI by region.
Methods:
This study used pooled data from 25 prospective cohort studies. Region of residence (Northeast, Midwest, South, West) was based on residential zip codes. Age- and sex-specific BMI z scores were the outcome.
Results:
The final sample included 14,313 children with 85,428 BMI measurements, 49% female and 51% non-Hispanic White. Males had a lower average BMI z score compared with females in the Midwest (β = −0.12, 95% CI: −0.19 to −0.05) and West (β = −0.12, 95% CI: −0.20 to −0.04). Compared with non-Hispanic White children, BMI z score was generally higher among children who were Hispanic and Black but not across all regions. Compared with the Northeast, average BMI z score was significantly higher in the Midwest (β = 0.09, 95% CI: 0.05 to 0.14) and lower in the South (β = −0.12, 95% CI: −0.16 to −0.08) and West (β = −0.14, 95% CI: −0.19 to −0.09) after adjustment for age, sex, race/ethnicity, and birth weight.
Conclusions:
Region of residence was associated with child BMI z scores, even after adjustment for sociodemographic characteristics. Understanding regional influences can inform targeted efforts to mitigate BMI-related disparities among children.
INTRODUCTION
In the United States (US), 35% of children have overweight or obesity (1). Increased childhood BMI is associated with multiple adverse medical and psychological outcomes (2). There has been a growing recognition that child weight gain, and hence obesity risk, is highly responsive to social, cultural, and physical environments (3,4). This recognition has spurred the development of clinical and public health interventions, with a focus on mitigating the obesity-promoting features of children’s home, school, and neighborhood environments (5,6). Despite substantial investment in these areas, the prevalence of childhood obesity has not declined for 15 years (1,7). Reducing obesity prevalence will require continued investment in the most proximal exposures, as well as consideration of the more distal environments that contribute to increases in BMI.
Disparities in child BMI or overweight/obesity prevalence by individual sociodemographic characteristics are well documented. Children who are Hispanic or Black have significantly higher rates of obesity than non-Hispanic White children (1). Lower family socioeconomic status and low maternal education also have been shown to increase the risk for childhood overweight/obesity (8,9). In addition, children with a higher birth weight were shown to be more likely to have obesity in elementary school compared with their normal-birth weight counterparts (10). Known risk factors explained only a small proportion of racial/ethnic disparities in obesity in the US (11). Studies are needed to identify unknown factors that contribute to disparities in BMI, including regional influences (11).
A second stream of research points to regional differences in BMI. In health-related research, US Census regions are a commonly used unit of analysis because of their historical, economic, agricultural, topographical, and cultural significance (12,13). Regional-level environmental features, such as climate or cuisine, may influence BMI. If those features vary by region, then we would expect children’s region of residence to explain some of the variation in child BMI in the US. Using data collected in 2003, the National Survey of Children’s Health (NSCH), a nationally representative sample of children by race/ethnicity, showed higher risk of overweight among children in the southeastern region of the US compared with the Rocky Mountain West (14). Surprisingly, previous research has not considered whether regional predictors are independently related to child BMI. For example, the NSCH did not examine whether regional differences persisted after adjustment for differences across regions in individual sociodemographic characteristics (e.g., sex, race/ethnicity, other markers of socioeconomic status) (14). Therefore, it is not known whether regional differences are explained by sociodemographic differences across regions. In addition, it is not known whether the magnitude, or even the direction, of associations between socioeconomic factors, race, and child BMI are similar across different regions. Among adults, for example, the difference in obesity prevalence between non-Hispanic Black and non-Hispanic White individuals was shown to vary by US state (11). The answers to these questions hold important implications for intervention and prevention efforts. Specifically, should such efforts be tailored to US region? Or should such efforts focus on individual-level sociodemographic factors without considering region? Evidence of regional variation in child BMI that occurs independently of the sociodemographic composition of each region could help prioritize the distribution of limited resources for obesity prevention to regions with the greatest need and ultimately inform the development of customized clinical and public health interventions. Evidence of regional differences in BMI could also spur the development of novel research questions related to region-specific cultural, physical, or economic attributes that can influence child BMI (15).
The objectives of this study were to (1) describe the variation in average BMI levels among children aged <15 years by US Census region (Northeast, Midwest, West, and South), (2) determine to what extent the regional differences may be accounted for by differences in individual sociodemographic characteristics (sex, race/ethnicity, birth weight, maternal education), and (3) describe the association between individual sociodemographic characteristics and child BMI within each US Census region. Understanding variation in childhood BMI by region is the first step in uncovering region-specific environmental factors or policies that may support or adversely affect child growth.
These objectives are examined within the Environmental Influences on Child Health Outcomes (ECHO) Program (http://echochildren.org) (16). ECHO includes a large sample of children from 72 community- and clinic-based cohorts with longitudinal data across the US. As such, it is uniquely equipped to explore regional and sociodemographic differences in BMI from infancy through late childhood.
METHODS
Study population
The study population was drawn from the ECHO Program (16). The goal of ECHO is to investigate the effects of environmental exposures on child health. Human participant activities are overseen by both single and site-specific institutional review boards. Participants provided informed consent.
Of the 72 cohorts participating in the observational arm of ECHO, 34 cohorts were excluded from the analyses because they did not assess height and weight. Of the remaining 38 cohorts (Supporting Information Figure S1), 13 were excluded because they assessed height and weight at one time point only (n = 2 cohorts), enrolled only children born before 2000 (n = 2), enrolled preterm births only (n = 1), could not submit individual-level data for analysis (n = 4), or did not assess key sociodemographic characteristics (n = 1). Cohorts that oversampled children with asthma and autism were also excluded (n = 3), as studies have shown differences in body weight for children with asthma and autism compared with children without (17,18). Within cohorts, children were included if they were a singleton birth between 2000 and 2018, had weight at birth, and had weight and length or height from at least one other time point before age 15. Children were excluded if they had a BMI > 70 kg/m2 (n = 140) or were missing sex, birth year, birth weight or gestational age data (n = 325).
There were fewer observations after age 10, and data were sparse after age 16. Thus, this report only included participants age ≤15 years. More participants in the Northeast were born before 2005, and more participants in the South, Midwest, and West were born after 2005 (Supporting Information Figure S2 and Table 1).
TABLE 1.
Number and characteristics of participating children and contributing cohorts by US region
| Overall | Northeast | South | Midwest | West | |
|---|---|---|---|---|---|
| Number of children | 14,313 | 3,407 | 5,912 | 2,562 | 2,432 |
| Number of cohorts | 25 | 12 | 17 | 13 | 13 |
| Birth year | |||||
| <2005 | 3,841 (27%) 8 cohorts | 2,370 (70%) 5 cohorts | 840 (14%) 4 cohorts | 544 (21%) 4 cohorts | 87 (4%) 3 cohorts |
| ≥2005 | 10,472 (73%) 21 cohorts | 1,037 (30%) 9 cohorts | 5,072 (86%) 14 cohorts | 2,018 (79%) 11 cohorts | 2,345 (96%) 12 cohorts |
| Sex at birth | |||||
| Male | 7,333 (51%) 25 cohorts | 1,770 (52%) 11 cohorts | 2,999 (51%) 16 cohorts | 1,310 (51%) 11 cohorts | 1,254 (52%) 13 cohorts |
| Female | 6,980 (49%) 25 cohorts | 1,637 (48%) 12 cohorts | 2,913 (49%) 15 cohorts | 1,252 (49%) 12 cohorts | 1,178 (48%) 11 cohorts |
| Race and ethnicity | |||||
| Non-Hispanic White | 7,342 (51%) 21 cohorts | 1,676 (49%) 10 cohorts | 2,482 (42%) 10 cohorts | 1,914 (75%) 11 cohorts | 1,270 (52%) 11 cohorts |
| Non-Hispanic Black | 2,360 (16%) 20 cohorts | 531 (16%) 9 cohorts | 1,509 (26%) 10 cohorts | 132 (5%) 8 cohorts | 188 (8%) 7 cohorts |
| Hispanic White | 871 (6%) 22 cohorts | 207 (6%) 10 cohorts | 298 (5%) 11 cohorts | 40 (2%) 6 cohorts | 326 (13%) 10 cohorts |
| Hispanic Black | 153 (1%) 14 cohorts | 94 (3%) 7 cohorts | 37 (<1%) 6 cohorts | <5 (<1%) 1 cohort | 21 (<1%) 4 cohorts |
| Other | 3,587 (25%) 24 cohorts | 899 (26%) 11 cohorts | 1,586 (27%) 16 cohorts | 475 (19%) 9 cohorts | 627 (26%) 12 cohorts |
| Hispanic ethnicity | |||||
| Not Hispanic | 11,029 (77%) 23 cohorts | 2,425 (71%) 11 cohorts | 4,462 (75%) 14 cohorts | 2,405 (94%) 11 cohorts | 1,737 (71%) 11 cohorts |
| Hispanic | 2,016 (14%) 23 cohorts | 772 (23%) 10 cohorts | 689 (12%) 13 cohorts | 105 (4%) 8 cohorts | 450 (19%) 11 cohorts |
| Unknown | 1,268 (9%) 14 cohorts | 210 (6%) 7 cohorts | 761 (13%) 5 cohorts | 52 (2%) 2 cohorts | 245 (10%) 5 cohorts |
| Birth weight in grams, mean (SD) | |||||
| Overall | 3,417 (476) 25 cohorts | 3,501 (488) 12 cohorts | 3,397 (472) 17 cohorts | 3,500 (472) 13 cohorts | 3,294 (437) 13 cohorts |
| Male | 3,478 (485) 25 cohorts | 3,569 (500) 11 cohorts | 3,460 (479) 16 cohorts | 3,558 (481) 11 cohorts | 3,349 (443) 13 cohorts |
| Female | 3,351 (457) 25 cohorts | 3,428 (464) 12 cohorts | 3,333 (456) 15 cohorts | 3,437 (453) 12 cohorts | 3,229 (421) 11 cohorts |
| Maternal education | |||||
| Less than high school | 1,096 (8%) 17 cohorts | 215 (6%) 8 cohorts | 469 (8%) 10 cohorts | 136 (5%) 7 cohorts | 276 (11%) 8 cohorts |
| High school | 1,625 (11%) 8 cohorts | 148 (4%) 4 cohorts | 1,061 (18%) 4 cohorts | 185 (7%) 3 cohorts | 231 (9%) 3 cohorts |
| Some college | 2,096 (15%) 10 cohorts | 759 (22%) 5 cohorts | 595 (10%) 3 cohorts | 433 (17%) 2 cohorts | 309 (13%) 5 cohorts |
| College degree and above | 3,749 (26%) 11 cohorts | 815 (24%) 5 cohorts | 1,171 (20%) 7 cohorts | 943 (37%) 3 cohorts | 820 (34%) 5 cohorts |
| Missing | 5,747 (40%) 23 cohorts | 1,470 (43%) 12 cohorts | 2,616 (44%) 13 cohorts | 865 (34%) 11 cohorts | 796 (33%) 10 cohorts |
| Child BMI z score, mean (SD) | 0.23 (1.45) | 0.37 (1.34) | 0.25 (1.59) | 0.36 (1.51) | −0.03 (1.37) |
Number of cohorts in each region (12 in the Northeast, 17 in the South, 13 in the Midwest, 13 in the West) exceeded the total number of cohorts (n = 25) because 14 cohorts enrolled children from multiple US Census regions.
Measures
Weight, length for children <2 years, and height for those age ≥2 years were used to calculate BMI as weight (kilograms)/height or length (meters squared). We used the Centers for Disease Control and Prevention (CDC) SAS macro to calculate the sex-specific BMI z scores for children age ≥2 years (19). As recommended by the CDC, we used the SAS macro provided by the World Health Organization to calculate BMI z scores for children age <2 (20). We defined region of residence using the US Census Bureau regions: Northeast, Midwest, South, and West (21) (Figure 1). Children were assigned to a region based on their residential zip code at cohort enrollment. A subset of cohorts collected maternal education at offspring’s birth. Race and ethnicity data were collapsed into the categories non-Hispanic White, non-Hispanic Black, Hispanic White, Hispanic Black, and other (including children with two or more races, or unknown race/ethnicity). Maternal education was categorized as less than high school, high school, some college, or college degree and above.
FIGURE 1.
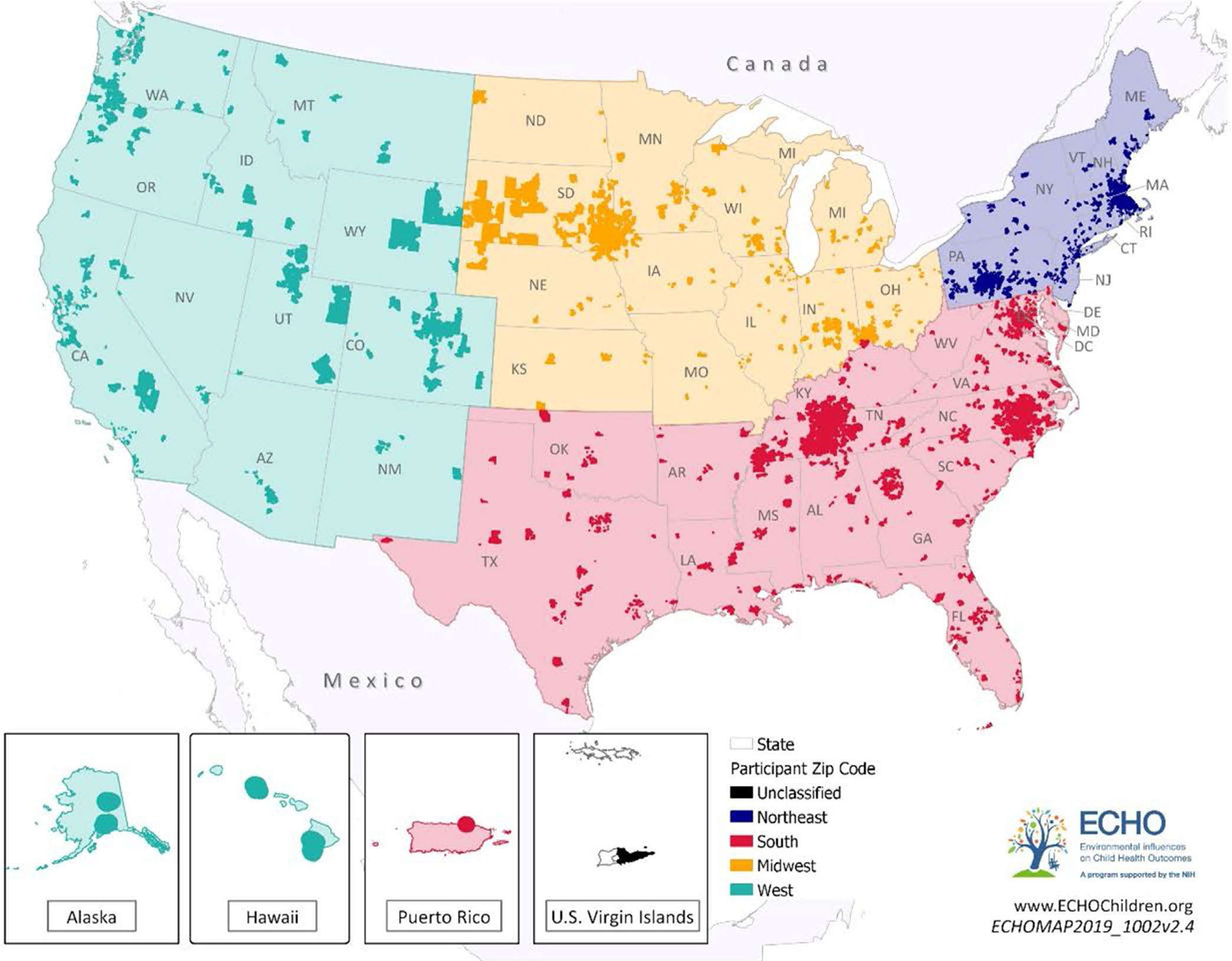
Residential codes of participants, 2000 to 2018. Among the 14,313 children included in our study population, 12,943 had 5-digit zip codes of residence (and are included in this map), hailing from 3,014 unique zip codes across the United States. ECHO, Environmental influences on Child Health Outcomes
Statistical analyses
Analyses were conducted by the ECHO Data Analysis Center. To estimate differences in average BMI z scores by region, we used linear regression models with generalized estimating equations specifying an exchangeable working covariance matrix with a robust variance estimator to account for the possible correlation from repeated measurements (22). We explored the effect of adjustment for sociodemographic variables. Model 1 included region and BMI z score only. Model 2 was fully adjusted and included in addition to region, child sex, birth weight, race/ethnicity and three parameterizations of age (age, age-squared, and age-cubed) to account for nonlinear effects across maturation. The QIC fit statistic was used to determine the best fitting model (23). Similar analyses, additionally adjusting for maternal education (Model 3), were conducted for the subsample of participants with non-missing data on this variable. Given the regional variation in birth year and age (Supporting Information Figure S2), two sensitivity analyses were conducted: (1) restricting the sample to children born after 2005, and (2) restricting the sample to children age ≤10 years.
Finally, data were stratified by region to estimate the differences in average BMI z score by sex, race/ethnicity, birth weight, and maternal education within each region. All models also adjusted for the other sociodemographic variables, including age, age-squared, and age-cubed. A likelihood ratio test was used to determine if the model with interaction terms for region and race/ethnicity had a better fit than the model without interaction terms. All statistical analyses were performed in R 3.5.2 using the geepack 1.2 package. A 2-sided p < 0.05 was considered statistically significant.
RESULTS
The analytic sample included 25 cohorts and 14,313 children with 85,428 anthropometric measurements (Supporting Information Figure S1). Eighty-two percent of weight and height measurements were extracted from the medical record or measured at the research visit. Participants were under observation between 2000 and 2018 for a median of 4 years (interquartile range: 1.25–6.92) and contributed a median of 4 measurements (interquartile range: 3–7). Participants were residents of 3,014 unique zip codes (Figure 1); 89% of children had no difference in their classification of region at the time of birth compared with the time of cohort enrollment.
There were fewer female participants than male participants in each region (Table 1). The mean birth weight was highest in the Northeast (3,501 g) and the Midwest (3,500 g), followed by the South (3,397 g) and the West (3,294 g), although the mean gestational age was not different by region. The proportion of non-Hispanic White children varied by region, from 42% in the South to 75% in the Midwest. The ECHO Program leverages existing clinical and community-based cohorts and thus, was not designed to provide a nationally representative sample. Nevertheless, the analytic sample for the present study was similar to the racial/ethnic composition of the general US pediatric population for each region. There were a few exceptions. The ECHO study population had fewer Hispanic White children in all regions, fewer non-Hispanic Black children in the Midwest, and more children with “other/unknown” race/ethnicities across all regions (Supporting Information Table S1) (24).
Observed BMI levels
The BMI levels (birth to age 15) for each participant, stratified by region and sex, are shown in Supporting Information Figure S3. The distribution of BMI levels was concentrated between the CDC’s BMI 5th and 95th percentiles, although more children had levels above the 95th than below the 5th percentile.
Estimated differences in BMI z score by region
Children in the West had a substantially and significantly lower average BMI z score compared with children in the Northeast (Table 2). This difference was statistically significant in both models, although adjustment for sociodemographic differences attenuated it substantially: −0.29 (95% CI: −0.34 to −0.24) (Model 1, unadjusted) to −0.14 (95% CI: −0.19 to −0.09) (Model 2, fully adjusted). Children in the South also had a lower average BMI z score compared with children in the Northeast, and adjustment for sociodemographic differences had minimal impact: −0.14 (95% CI: −0.18 to −0.10) (Model 1, unadjusted) to −0.12 (95% CI: −0.16 to −0.08) (Model 2, fully adjusted). In contrast, children in the Midwest had a higher average BMI z score compared with children in the Northeast but only in the fully adjusted model: 0.02 (−0.03 to 0.07) (Model 1, unadjusted) and 0.09 (95% CI: 0.05 to 0.14) (Model 2, fully adjusted). Sensitivity analysis restricted to children born after 2005 and children age ≤10 years showed similar findings to those observed in the full sample (Supporting Information Table S2).
TABLE 2.
Estimated regional differences (and 95% CIs) in average BMI z scores for ECHO children from birth to age 15, using two models
| Models | Children (observations) | South | Midwest | West | QIC |
|---|---|---|---|---|---|
| Model 1: Region | 14,313 (83,464) | −0.14 (−0.18 to −0.10) | 0.02 (−0.03 to 0.07) | −0.29 (−0.34 to −0.24) | 174,507 |
| Model 2: Region, child sex, birth weight, race and ethnicity, age, age-squared, age-cubed | 14,313 (83,464) | −0.12 (−0.16 to −0.08) | 0.09 (0.05–0.14) | −0.14 (−0.19 to −0.09) | 162,556 |
Bolded numbers indicate a statistically significant difference (p < 0.05). QIC is a model fit statistic; lower value suggests a better fit. Northeast is the reference.
Abbreviation: ECHO, Environmental influences on Child Health Outcomes.
Similar models were conducted among the subgroup of participants (N = 8,566) with data on maternal education at birth (Table 3). In this subset, in the fully adjusted model (Model 3, additionally adjusting for maternal education), the difference in average BMI z score remained null among children in the South versus the Northeast (β = −0.01, 95% CI: −0.08 to 0.06). The associations of region and average BMI z score strengthened among children in the Midwest (β = 0.17, 95% CI: 0.11 to 0.23) and the West (β = −0.22, 95% CI: −0.29 to −0.16) compared with the children in the Northeast. Sensitivity analysis restricted to children born after 2005 and children age ≤10 years showed robust findings.
TABLE 3.
Estimated regional differences (and 95% CIs) in average BMI z scores for the subsample of children with available data on maternal education by region, using three models
| Models | Children (observations) | South | Midwest | West | QIC |
|---|---|---|---|---|---|
| Model 1: Region | 8,566 (52,003) | −0.017 (−0.076 to 0.042) | 0.049 (−0.014 to 0.11) | −0.37 (−0.43 to −0.3) | 95,529 |
| Model 2: Region, child sex, birth weight, race and ethnicity, age, age squared, age cubed | 8,566 (52,003) | 0.02 (−0.041 to 0.082) | 0.18 (0.12 to 0.23) | −0.21 (−0.27 to −0.14) | 87,335 |
| Model 3: Region, child sex, birth weight, race and ethnicity, maternal education, age, age-squared, age-cubed | 8,566 (52,003) | −0.01 (−0.077 to 0.057) | 0.17 (0.11 to 0.23) | −0.22 (−0.29 to −0.16) | 86,811 |
Bolded numbers indicate a statistically significant difference (p < 0.05). QIC is a model fit statistic; lower value suggests a better fit. Northeast is the reference.
Estimated differences in BMI z score by sex, race/ethnicity, birth weight, and maternal education within each region
Males had a lower BMI z score compared with females in the Midwest and West, after accounting for age, birth weight, and race/ethnicity (Table 4). BMI z score did not differ by sex in South or Northeast. Non-Hispanic Black children had a higher average BMI z score compared with non-Hispanic White children in the Northeast, South, and Midwest, after accounting for age, sex, and birth weight (Table 4 and Supporting Information Figure S4). Hispanic Black children had a higher average BMI z score compared with non-Hispanic White children in the Northeast and Midwest after adjustment. Hispanic White children had a higher average BMI z score compared with non-Hispanic White children in the South and West after adjustment. There was a positive association between birth weight and BMI z score in the Northeast, South, Midwest, and West. In all four regions, among the subsample of participants with maternal education data, children with mothers with less than a high school degree had a higher average BMI z score compared with children with mothers with a college degree (Table 4).
TABLE 4.
Estimated differences (and 95% CIs) in average BMI z score by sex, race and ethnicity, birth weight, and maternal education (subsample) within each US Census Region
| Northeast | South | Midwest | West | |
|---|---|---|---|---|
| Number of children | 3,407 | 5,912 | 2,562 | 2,432 |
| Number of observations | 27,872 | 25,385 | 8,714 | 21,493 |
| Female | reference | reference | reference | reference |
| Male | −0.03 (−0.09 to 0.03) | −0.04 (−0.09 to 0.01) | −0.12 (−0.19 to −0.05) | −0.12 (−0.20 to −0.04) |
| Race and ethnicity | ||||
| Non-Hispanic White | reference | reference | reference | reference |
| Non-Hispanic Black | 0.16 (0.05 to 0.27) | 0.19 (0.14 to 0.25) | 0.49 (0.25 to 0.72) | −0.032 (−0.16 to 0.091) |
| Hispanic Black | 0.32 (0.07 to 0.57) | 0.40 (−0.04 to 0.84) | −0.54 (−0.61 to −0.47) | 0.15 (−0.15 to 0.44) |
| Hispanic White | 0.07 (−0.06 to 0.20) | 0.16 (0.04 to 0.28) | −0.03 (−0.22 to 0.17) | 0.16 (0.06 to 0.26) |
| Other | 0.16 (0.08 to 0.24) | 0.03 (−0.03 to 0.09) | 0.28 (0.18 to 0.38) | 0.01 (−0.09 to 0.11) |
| Birth weight (g)/100 | 0.07 (0.06 to 0.08) | 0.08 (0.08 to 0.09) | 0.08 (0.08 to 0.09) | 0.08 (0.07 to 0.09) |
| Maternal education | ||||
| College degree and above | reference | reference | reference | reference |
| Some college | 0.12 (0.033 to 0.2) | 0.071 (−0.014 to 0.16) | 0.032 (−0.041 to 0.1) | 0.12 (0.018 to 0.21) |
| High school | 0.06 (−0.41 to 0.53) | 0.12 (0.039 to 0.2) | 0.069 (−0.041 to 0.18) | 0.28 (0.17 to 0.38) |
| Less than high school | 0.26 (0.089 to 0.43) | 0.16 (0.056 to 0.26) | 0.4 (0.18 to 0.62) | 0.56 (0.42 to 0.7) |
All four models are adjusted for the variables in the table and age, age-squared, and age-cubed. Bolded numbers indicate a statistically significant difference (p < 0.05).
DISCUSSION
Among 14,313 youth, average BMI from birth to age 15 was lower in the West and South and higher in the Midwest, compared with the Northeast, independent of regional differences in sociodemographic characteristics. Adjustment for age, sex, race/ethnicity, birth weight, and maternal education did not fully explain the regional differences in child BMI. Within each region, BMI was higher among children who were larger at birth and had less educated mothers. There was also a trend toward higher BMI among Hispanic and Black youth compared with non-Hispanic White youth and females compared with males, but these trends were not uniform across regions. Regional differences in average BMI z scores, although modest, are likely clinically meaningful (25,26), especially the lower average BMI z score in the West.
In the 2003 NSCH, obesity was highest in the South and lowest in the Rocky Mountain West (14). This is consistent with our finding that average child BMI levels were lowest in the West. However, we found that BMI levels were lower in the South compared with the Northeast and Midwest. There are several potential reasons for these discrepancies. First, the regional variation observed in the NSCH may have resulted from variation in the sociodemographic makeup of the population within each region, which was not accounted for. Another explanation is that the demographic characteristics of NSCH children may have differed from the ECHO sample. NSCH randomly selected children by race/ethnicity within regions, whereas ECHO did not. Our outcome was average child BMI, not overweight. Finally, it is also possible that the burden of childhood overweight and obesity may be slowly shifting across US regions.
The pathways responsible for the association between geographic region and BMI are not well understood. Adult data provide some insight into potential mechanisms, but findings are mixed. Diet quality and time spent in moderate-to-vigorous activity are higher among adults in the West and Northeast compared with the South and Midwest, which suggests lifestyle behaviors may play a role (27,28). Yet other studies indicate no regional variation in physical activity (29). Environmental features such as the density of fast food restaurants, supermarkets, and recreation facilities have been linked to childhood BMI (30), but it is unclear how they vary by region (31). State and regional regulations for the types of foods and beverages offered in childcare and schools have been linked to BMI, but there is little evidence of regional variation (32,33). Regional variation in BMI has been observed in Canada, and these studies have generated hypotheses about the role of regional differences in food prices, childcare policies, attitudes toward physical activity, and the built environment as potential mediators (15). More work is needed to test these hypotheses in the US and other countries and ultimately inform policy and public health interventions at the regional level. Future studies should explore whether lifestyle behaviors, degree of rurality, and modifiable environmental factors may be acting at the regional level (34–37).
BMI was inversely associated with maternal education and positively associated with birth weight, in all regions. Similar trends were documented in the US and Europe, although mostly among children under age 12 (10,38,39). We build on that literature by showing similar findings among a sample that also includes adolescents and demonstrating uniformity across regions. Males had lower BMI z scores compared with females but in the Midwest and West only. Higher BMI z scores among females is likely driven by cultural factors (e.g., less socialization toward sports participation) (40), differences in lifestyle behaviors, and biological differences in body composition and fat distribution (41,42). We do not expect biologically based sex differences to vary by region. However, regional differences in sex-specific lifestyle behaviors (e.g., females may be more active in the Northeast compared with the Midwest) may explain why males have a lower BMI in some regions but not others.
Higher BMI or obesity among Hispanic and Black children has been documented in nationally representative samples (43,44). The present study is the first to suggest that this racial and ethnic patterning of child BMI may not be uniform across regions. For example, compared with non-Hispanic White children, Hispanic Black children had a higher BMI in the Northeast but a lower BMI in the Midwest. Yet in the West and South, BMI did not differ between non-Hispanic White and Hispanic Black children. This suggests that regional factors may protect against or exacerbate BMI-related racial/ethnic disparities. Risk factors related to racial/ethnic differences in child BMI include duration and/or exclusivity of breastfeeding, age at introduction of solid foods, parent feeding practices, intake of sugar-sweetened beverages and fast food, sleep duration, and having a television in the bedroom (45). Future studies that examine regional variation in these factors, including consideration of regional differences in policies that mitigate systemic racism, may provide insight into regional differences in the association between race/ethnicity and BMI.
This study has several strengths. First, the large, diverse sample provides adequate statistical power to detect small regional differences in BMI after adjustment for individual characteristics. This is important because regional differences that persist after adjustment for age, sex, race/ethnicity, birth weight, and maternal education provide evidence for the effects of place itself versus population composition. Second, participants were drawn from all four US Census regions, and the racial/ethnic composition of the sample resembles (although it is not identical to) that of the general US pediatric population.
This study also has limitations. This was not a population-based study, and the sample represents a mix of community- and clinic-based cohorts. However, the racial/ethnic composition of the sample resembles that of the general US pediatric population. Although our sample has a larger proportion of children with race/ethnicity labeled as “Other” compared with the underlying population; this does not vary by region. Thus, it is unlikely a source of selection bias. The observational design prohibits cause- and-effect conclusions about the association between region and BMI. The broad age range of children included may have masked age-specific nuances in the region-BMI association. Future analyses should focus on exploring how the environment shapes BMI trajectories across specific life-stages. Data from children aged 10 to 15 years were sparse, so the findings may be less precise in this age group. Most children in the Northeast sample were born before 2005, whereas most children in all other regions were born after 2005. However, sensitivity analyses restricted to children born after 2005 or age ≤10 showed similar findings. Finally, missing sociodemographic data were common, and the degree of missingness differed by region. Nevertheless, the analysis among children with maternal education data demonstrated similar results, although the lower BMI in the South compared with Northeast was no longer significant.
The outcome measure also has limitations. BMI z score is a measure of age- and sex-specific weight-for-height but does not distinguish between lean and fat tissue. It is therefore only a proxy for excess adiposity (46). Nevertheless, some studies have demonstrated a strong correlation between BMI and other adiposity indicators, such as overall fat mass and/or visceral fat mass (47). It has recently been suggested that BMI z score may be inaccurate above the 97th percentile (48). This is because child BMI in the US is skewed, so transforming BMI to a z score shrinks the scale at the upper end. This compression can result in similar BMI z scores among different children of the same age with different BMI levels. Although this is a potential limitation of BMI z score, we believe this limitation is mostly relevant for studies in which a high proportion of the study population has obesity or even severe obesity. In contrast, our sample had a mean BMI z score of 0.23.
Ongoing prospective data collection in ECHO using a standardized protocol that includes maternal education, degree of rurality, anthropometrics, and other key characteristics will likely overcome these limitations in the future. Future studies could also advance the science by considering the association between child BMI and finer-tuned geographic units of analysis, such as census tract or zip code, which reflect diverse built, economic, social, and cultural microenvironments (30) that may be masked at the regional level. Of particular interest are innovative indices of social deprivation that can quantify levels of disadvantage across small geographic areas (49) and indices of neighborhood resources that promote healthy lifestyle behaviors and growth (50). ECHO and other population-based studies that assess weight and height among children across the US (e.g., the National Health and Nutrition Examination Survey) are well poised to examine these associations using geocoded participant data.
In summary, we observed significant and clinically meaningful regional variation in child BMI levels that persisted after adjustment for individual characteristics. This provides evidence that features of the regional environment, and not just the sociodemographic composition of the population in each region, may influence child BMI. The next step is to identify factors responsible for the association between region of residence and child BMI. Importantly, the association between race/ethnicity and child BMI varied by region. Thus, understanding regional influences may inform efforts to mitigate BMI-related health disparities.
Supplementary Material
Study Importance.
What is already known?
In the United States, pediatric obesity remains unacceptably high. Among adults, obesity and related diseases vary by US Census region. There is regional variation in environmental factors linked to obesity, including cuisine, food price, nutrition-related regulations for childcare centers, and exercise opportunities.
What does this study add?
Among children and adolescents (N = 14,313), average BMI z score was higher in the Midwest and lower in the South and West compared with the Northeast. These findings persisted after sociodemographic factors were considered, suggesting that other environmental factors explain regional differences. Compared with children who were non-Hispanic White, BMI z score was generally higher among children who were Hispanic and Black but not across all regions.
How might these results change the direction of research or the focus of clinical practice?
Understanding regional influences can inform targeted efforts to mitigate BMI-related disparities among children.
Understanding regional influences may inform efforts to mitigate BMI-related health disparities.
ACKNOWLEDGMENTS
The authors wish to thank our ECHO colleagues and the medical, nursing, and program staff, as well as the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators:
ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, Benjamin DK; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Parker CB.
ECHO Awardees and Cohorts: Columbia University, New York, New York: Perera FP, Herbstman JB; Kaiser Permanente, Oakland, California: Croen LA; New York University, New York, New York: Blair CB; New York University School of Medicine, New York, New York: Trasande L; Northeastern University, Boston, Massachusetts: Alshawabkeh AN; University of Utah, Salt Lake City: Stanford JB, Clark EB, Porucznik C; University of Wisconsin-Madison: Gern J; Washington University, St. Louis, Missouri: Bacharier L
Per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public. Requests to access the data sets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org
Funding information
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, NIH, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and UG3/UH3 OD023279 (AJE), UG3/UH3 OD-023253 (CAC), UG3/UH3OD023328 (CSD), UG3/UH3 OD023318 (ALD), UG3/UH3 OD023289 (AF), UH3 OD023282 (DRG), UG3/UH3 OD023282 (TH), UG3/UH3 OD023244 (AEH), UG3 OD023365 04 (IH-P), UG3/UH3OD023337 (KH), UG3/UH3OD023285, UG3/UH3OD023282 (CCJ and EZ), UG3/UH3 OD023275 (MRK), 5UH3 OD023282-04 (GKKH), UG3/UH3 OD023389 (LL and JN), UG3/UH3 OD023288 (CTE), UG3/UH3OD023337 (RW), UG3/UH3 023286 (EO), UH3OD023290 (AR), UG3OD023271 4UH3OD023271-03 (CJK and SS), 1UG3/1UH3 OD023271-01 (FAT), 8UG1OD024956 (CT), and 1UG1HD090904-01 (SEW). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
CONFLICT OF INTEREST
Dr. Althoff reports personal fees from The All of Us study and from Trio Health. The other authors declared no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JD, Fu E, Kobayashi MA. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol. 2020;16:351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman T, Cushing RA, Jackson RJ. Contributions of built environment to childhood obesity. Mt Sinai J Med. 2011;78:49–57. [DOI] [PubMed] [Google Scholar]
- 4.Caprio S, Daniels SR, Drewnowski A, et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment. Obesity (Silver Spring). 2008;16(12):2566–2577. [DOI] [PubMed] [Google Scholar]
- 5.Ash T, Agaronov A, Young T, Aftosmes-Tobio A, Davison KK. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis. Int J Behav Nutr Phys Act. 2017;14(1):113. doi: 10.1186/s12966-017-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16(7):547–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver RG, Brazendale K, Hunt E, Sarzynski MA, Beets MW, White K. Disparities in childhood overweight and obesity by income in the United States: an epidemiological examination using three nationally representative datasets. Int J Obes (Lond). 2019;43(6):1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthuri SK, Onywera VO, Tremblay MS, et al. Relationships between parental education and overweight with childhood overweight and physical activity in 9–11 year old children: results from a 12-country study. PLoS One. 2016;11(8):e0147746. doi: 10.1371/journal.pone.0147746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapral N, Miller SE, Scharf RJ, Gurka MJ, DeBoer MD. Associations between birthweight and overweight and obesity in school-age children. Pediatr Obes. 2018;13(6):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh K, Elder TE, Grady SC, Darden JT, Vojnovic I. Explained and unexplained racial and regional inequality in obesity prevalence in the United States. Ethn Health. 2020;25(5):665–678. [DOI] [PubMed] [Google Scholar]
- 12.Parker MG, Greenberg LT, Edwards EM, Ehret D, Belfort MB, Horbar JD. National trends in the provision of human milk at hospital discharge among very low-birth-weight infants. JAMA Pediatr. 2019;173(10):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Census Bureau. The Geographic Areas Reference Manual, Chapter 2: Geographic Overview. Washington, DC: US Department of Commerce; 1994. [Google Scholar]
- 14.Tudor-Locke C, Kronenfeld JJ, Kim SS, Benin M, Kuby M. A geographical comparison of prevalence of overweight school-aged children: the National Survey of Children’s Health 2003. Pediatrics. 2007;120(4):e1043–e1050. [DOI] [PubMed] [Google Scholar]
- 15.Dutton DJ, McLaren L. Explained and unexplained regional variation in Canadian obesity prevalence. Obesity (Silver Spring). 2011;19(7):1460–1468. [DOI] [PubMed] [Google Scholar]
- 16.Tylavsky FA, Ferrara A, Catellier DJ et al. Understanding childhood obesity in the US: the NIH Environmental influences on Child Health Outcomes (ECHO) program. Int J Obes (Lond). 2020;44(3):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalid F, Holguin F. A review of obesity and asthma across the life span. J Asthma. 2018;55(12):1286–1300. [DOI] [PubMed] [Google Scholar]
- 18.Kahathuduwa CN, West BD, Blume J, Dharavath N, Moustaid-Moussa N, Mastergeorge A. The risk of overweight and obesity in children with autism spectrum disorders: a systematic review and meta-analysis. Obes Rev. 2019;20(12):1667–1679. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. A SAS program for the 2000 CDC Growth Charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Published 2019. Accessed June 2020.
- 20.Grummer-Strawn LM, Reinold C, Krebs NF; Centers for Disease Control and Prevention. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5909a1.htm [PubMed] [Google Scholar]
- 21.US Census Bureau. Census regions and divisions of the United States. https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html. Published 2010. Updated 2018. Accessed June 2020. [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 23.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics (NCHS). Bridged-race population estimates - data files and documentation, 2010–2018. https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm. Published 2018. Accessed June 2020.
- 25.Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. 2016;101(8):3171–3179. [DOI] [PubMed] [Google Scholar]
- 26.Kolsgaard ML, Joner G, Brunborg C, Anderssen SA, Tonstad S, Andersen LF. Reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo Adiposity Intervention Study - a hospital/public health nurse combined treatment. BMC Pediatr. 2011;11(1):47. doi: 10.1186/1471-2431-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, McGuire LC, Galuska DA. Regional differences in sugar-sweetened beverage intake among US adults. J Acad Nutr Diet. 2015;115(12):1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troost JP, Rafferty AP, Luo Z, Reeves MJ. Temporal and regional trends in the prevalence of healthy lifestyle characteristics: United States, 1994–2007. Am J Public Health. 2012;102(7):1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohn EK, Porch T, Hill S, Thorpe RJ Jr. Geography, race/ethnicity, and physical activity among men in the United States. Am J Mens Health. 2017;11(4):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll-Scott A, Gilstad-Hayden K, Rosenthal L, et al. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: the role of built, socioeconomic, and social environments. Soc Sci Med. 2013;95:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers CA, Slack T, Martin CK, Broyles ST, Heymsfield SB. Regional disparities in obesity prevalence in the United States: a spatial regime analysis. Obesity (Silver Spring). 2015;23(2):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datar A, Nicosia N. The effect of state competitive food and beverage regulations on childhood overweight and obesity. J Adolesc Health. 2017;60(5):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin SE, Taveras EM, Cradock AL, Walker EM, Slining MM, Gillman MW. State and regional variation in regulations related to feeding infants in child care. Pediatrics. 2009;124(1):e104–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaparro MP, Anderson CE, Crespi CM, Wang MC, Whaley SE. The new child food package is associated with reduced obesity risk among formula fed infants participating in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC) in Los Angeles County, California, 2003–2016. Int J Behav Nutr Phys Act. 2020;17(1):18. doi: 10.1186/s12966-020-0921-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega Hinojosa AM, MacLeod KE, Balmes J, Jerrett M. Influence of school environments on childhood obesity in California. Environ Res. 2018;166:100–107. [DOI] [PubMed] [Google Scholar]
- 36.Findholt NE, Michael YL, Jerofke LJ, Brogoitti VW. Environmental influences on children’s physical activity and eating habits in a rural Oregon County. Am J Health Promot. 2011;26(2):e74–e85. [DOI] [PubMed] [Google Scholar]
- 37.Warner ML, Harley K, Bradman A, Vargas G, Eskenazi B. Soda consumption and overweight status of 2-year-old Mexican-American children in california. Obesity (Silver Spring). 2006;14(11):1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz M, Goldblatt P, Morrison J, et al. Impact of low maternal education on early childhood overweight and obesity in Europe. Paediatr Perinat Epidemiol. 2016;30(3):274–284. [DOI] [PubMed] [Google Scholar]
- 39.Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of obesity among youths by household income and education level of head of household - United States 2011–2014. MMWR Morb Mortal Wkly Rep. 2018;67(6):186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeting HN. Gendered dimensions of obesity in childhood and adolescence. Nutr J. 2008;7(1):1. doi: 10.1186/1475-2891-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring). 2010;18(7):1410–1416. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RW, Gold E, Manning P, Goulding A. Gender differences in body fat content are present well before puberty. Int J Obes Relat Metab Disord. 1997;21(11):1082–1084. [DOI] [PubMed] [Google Scholar]
- 43.Datar A, Chung PJ. Changes in socioeconomic, racial/ethnic, and sex disparities in childhood obesity at school entry in the United States. JAMA Pediatr. 2015;169(7):696–697. [DOI] [PubMed] [Google Scholar]
- 44.Weaver RG, Beets MW, Brazendale K, Hunt E. Disparities by household income and race/ethnicity: the utility of BMI for surveilling excess adiposity in children. Ethn Health. 2021;26(8):1180–1195. [DOI] [PubMed] [Google Scholar]
- 45.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167(8):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–147. [DOI] [PubMed] [Google Scholar]
- 47.Bouchard C BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond). 2007;31(10):1552–1553. [DOI] [PubMed] [Google Scholar]
- 48.Freedman DS, Woo JG, Ogden CL, Xu JH, Cole TJ. Distance and percentage distance from median BMI as alternatives to BMI z score. Br J Nutr. 2020;124(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2pt1):539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acevedo-Garcia D, Noelke C, McArdle N, et al. Racial and ethnic inequities in children’s neighborhoods: evidence from the new child opportunity index 2.0. Health Aff (Millwood). 2020;39(10):1693–1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


