Abstract
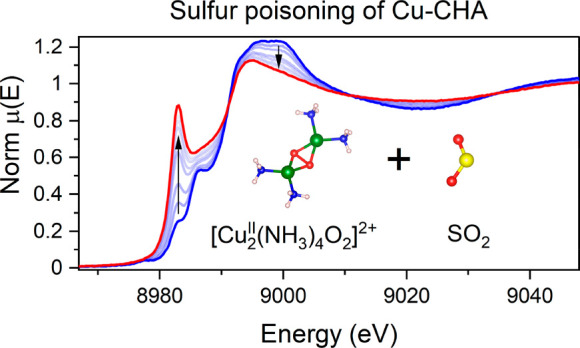
Cu-exchanged chabazite zeolites (Cu-CHA) are effective catalysts for the NH3-assisted selective catalytic reduction of NO (NH3-SCR) for the abatement of NOx emission from diesel vehicles. However, the presence of a small amount of SO2 in diesel exhaust gases leads to a severe reduction in the low-temperature activity of these catalysts. To shed light on the nature of such deactivation, we characterized a Cu-CHA catalyst under well-defined exposures to SO2 using in situ X-ray absorption spectroscopy. By varying the pretreatment procedure prior to the SO2 exposure, we have selectively prepared CuI and CuII species with different ligations, which are relevant for the NH3-SCR reaction. The highest reactivity toward SO2 was observed for CuII species coordinated to both NH3 and extraframework oxygen, in particular for [CuII2(NH3)4O2]2+ complexes. Cu species without either ammonia or extraframework oxygen ligands were much less reactive, and the associated SO2 uptake was significantly lower. These results explain why SO2 mostly affects the low-temperature activity of Cu-CHA catalysts, since the dimeric complex [CuII2(NH3)4O2]2+ is a crucial intermediate in the low-temperature NH3-SCR catalytic cycle.
Keywords: selective catalytic reduction, Cu-CHA, deNOx catalysis, sulfur poisoning, X-ray absorption spectroscopy, X-ray adsorbate quantification, XAS, XAQ
The emission of nitrogen oxides (NOx) from diesel vehicles is a global environmental challenge.1,2 State of the art exhaust gas aftertreatment systems contain catalysts for selective catalytic reduction of NOx by ammonia (NH3-SCR), capable of reducing well over 90% of the NOx emitted by the engine. In the NH3-SCR reaction, NO reacts with NH3 in the presence of O2 to form N2 and H2O. At present, Cu-exchanged chabazites (Cu-CHA) are the preferred catalysts for NH3-SCR, due to their superior low-temperature activity (150–350 °C)3,4 and hydrothermal stability.5,6 The temperature dependence of the NH3-SCR activity of Cu-CHA catalysts shows a minimum at around 350 °C, which indicates that the reaction mechanism at low temperatures is different from that at higher temperatures.7
The NH3-SCR reaction cycle for the low-temperature activity is a redox cycle, consisting of a series of oxidation and reduction steps, in which the oxidation state of Cu changes between CuI and CuII. The NO and NH3 coordinate to Cu in the zeolite, giving rise to a variety of Cu species along the NH3-SCR cycle.8−11 The low-temperature activity of Cu-CHA catalysts originates from the ability to form mobile CuI(NH3)2 complexes under SCR conditions. Pairs of these species constitute the active Cu sites capable of O2 activation via the formation of [CuII2(NH3)4O2]2+ dimers around 200 °C, which is a crucial step in the NH3-SCR reaction cycle.12,13
In practice, the application of Cu-CHA catalysts for the NH3-SCR is restricted to ultralow-sulfur diesel fuels, due to the fact that a few ppm of SO2 present in the exhaust gas drastically reduces the activity at low temperatures.3,4,14 Multiple studies show that SO2 affects the Cu mobility, the amount of Cu active sites,14 and the redox behavior of the Cu in the NH3-SCR cycle.9,15 Most studies have focused on the overall effect of SO2 on the performance of the catalysts,14−21 while the chemistry behind SO2 poisoning at the molecular level remains poorly understood. To determine a mechanism for SO2 poisoning, one must identify the species in the Cu-CHA catalysts that interact with SO2. To this end, we have selectively prepared well-defined CuI and CuII species with different ligands inside the pores of the Cu-CHA catalyst and exposed them to SO2 under well-defined conditions. We monitored the changes in the Cu K-edge X-ray absorption spectra (XAS) during the absorption of SO2. This allowed us to determine the chemical state of the Cu that interacts with SO2. The results were corroborated by X-ray emission spectroscopy (XES) and measurements of the SO2 uptake using temperature-programmed desorption (TPD) of SO2.
The Cu-CHA catalyst used in this study had a Si/Al ratio of 6.7 and a Cu loading of 3.2 wt % (Cu/Al = 0.24). The Cu K-edge XAS and Cu Kβ valence-to-core XES measurements were carried out at the BM2322 and ID2623 beamlines of the European Synchrotron Radiation Facility (ESRF), respectively. Sample treatment protocols consisted of three distinct steps. First, all samples were heated to 550 °C in a 10% O2/He flow, removing water and forming CuII species bound to the framework of the zeolite (fw-CuII). Then, the specific state of Cu was prepared, using one of the six different pretreatment procedures summarized in Table 1. Finally, the catalyst was exposed to 400 ppm SO2/He flow at 200 °C for 3 h until no visible changes in the spectra occurred. Further experimental details are given in the Supporting Information.
Table 1. Pretreatment Procedures and Resulting Cu Species.
| procedure | conditions | dominant Cu state | designation in the text and figures | ref |
|---|---|---|---|---|
| 1 | 1% H2 at 400 °C; cooling to 200 °C in He | fw-CuI | fw-CuI | (24) |
| 2 | 500 ppm of NO + 600 ppm of NH3 at 200 °C | mobile [CuI(NH3)2]+ | [CuI(NH3)2]+ | (24) |
| 3 | 500 ppm of NO + 600 ppm of NH3 at 200 °C; heating to 550 °C in He; cooling back to 200 °C in He | fw-CuI (after thermal treatment of [CuI(NH3)2]+) | [CuI(NH3)2]+ + T | (24) |
| 4 | 10% O2 at 200 °C | fw-CuII | fw-CuII | (25) |
| 5 | 500 ppm of NO + 600 ppm of NH3 at 200 °C; He purge; 10% O2 at 200 °C | mobile [CuII2(NH3)4O2]2+ dimer | [CuII2(NH3)4O2]2+ | (13) |
| 6 | 600 ppm of NH3 at 200 °C | mixeda | CuII + NH3 | this work |
Procedure 6 results in a mixture of two NH3-coordinated Cu species, as discussed further in the text.
The Cu species formed with the pretreatments differ in three aspects: (1) the oxidation state of Cu (CuI or CuII), (2) the coordination of the Cu (NH3 or/and O), and (3) the interaction of the Cu with the framework (fw-coordinated or mobile species).
Figure 1 shows the evolution of Cu K-edge XANES and EXAFS spectra during the exposure of the pretreated Cu-CHA catalyst to 400 ppm SO2/He flow at 200 °C. For all CuI species and fw-CuII species (procedures 1–4 in Table 1), only minor changes are observed upon SO2 exposure, indicating that these species are not very reactive toward SO2. In contrast, for CuII species in the presence of NH3 (procedures 5 and 6) significant changes are observed in the spectra. In these cases, the exposure to SO2 results in a pronounced increase of the XANES peak at 8983 eV, characteristic for linear CuI complexes,9,26,27 and a decrease in the intensity of the first shell in the EXAFS FT. This means that some of the CuII species are reduced to CuI upon interaction with SO2. The decrease in the first-shell intensity indicates a reduction of the coordination number for the Cu ions, which is also in line with the formation of a linear CuI species.
Figure 1.
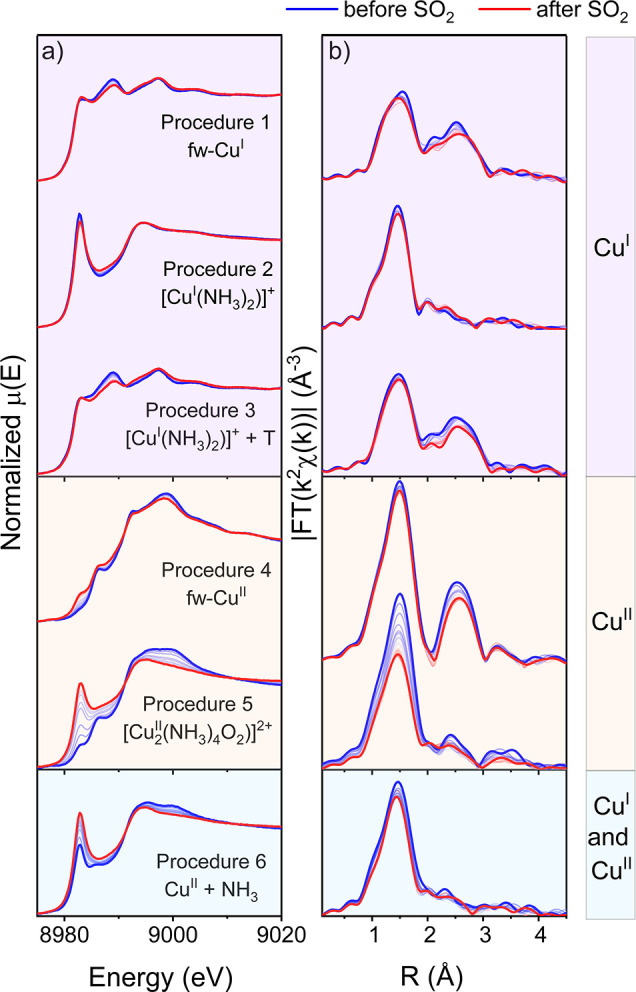
Cu K-edge XANES (a) and FT-EXAFS spectra (b) collected in situ during the exposure of Cu species obtained in procedures 1–6 to SO2 at 200 °C.
The species obtained in procedure 5 are the oxygen-bridged diamine dicopper complexes [CuII2(NH3)4O2]2+, which are formed by the reaction of O2 with a pair of [CuI(NH3)2]+ complexes.13 In the reaction cycle for the low-temperature NH3-SCR reaction,11,13,28 the [CuII2(NH3)4O2]2+ complexes react with NO, which eventually leads to the production of N2 and H2O. The observation that the [CuII2(NH3)4O2]2+ complexes are reactive toward SO2 is therefore a good explanation for the SO2-induced deactivation of Cu-CHA catalysts for NH3-SCR: the reaction with SO2 interrupts the NH3-SCR cycle, thereby decreasing the activity of the catalyst.
The other case where Cu reacts with SO2 is obtained in procedure 6 by exposure of the fw-CuII species to NH3. Previously, a similar pretreatment resulted in a mixture of linear [CuI(NH3)2]+ and either square-planar [CuII(NH3)4]2+ complexes or mixed-ligand [CuIIOx(NH3)y]2+ moieties.9,24 For the sample reported in this work, the mixed-ligand configuration is more likely. Indeed, linear combination fits of the XANES data on the basis of references for the linear [CuI(NH3)2]+ complex and pure CuII(NH3)4 groups (aqueous [CuII(NH3)4]2+ or solid-state [CuII(NH3)4]SO4·H2O) resulted in visible discrepancies with the data (Figure S7 in the Supporting Information). A better agreement is obtained when the spectrum of oxygen-bridged diamine dicopper complex [CuII2(NH3)4O2]2+ is used as a CuII reference in combination with [CuI(NH3)2]+, with approximately equal weights for each component (Figure 2). The necessary stock of available oxygen needed for the formation of the mixed-ligand species is expected to be present in the sample, as a wavelet analysis of the EXAFS collected after heating to 550 °C and cooling to 200 °C in 10% O2/He flow reveals the presence of Cu–Cu scattering usually attributed to the oxygen-containing dimers29,30 (Figure S8 in the Supporting Information), which may be susceptible to form mixed-ligand species upon exposure to NH3.
Figure 2.
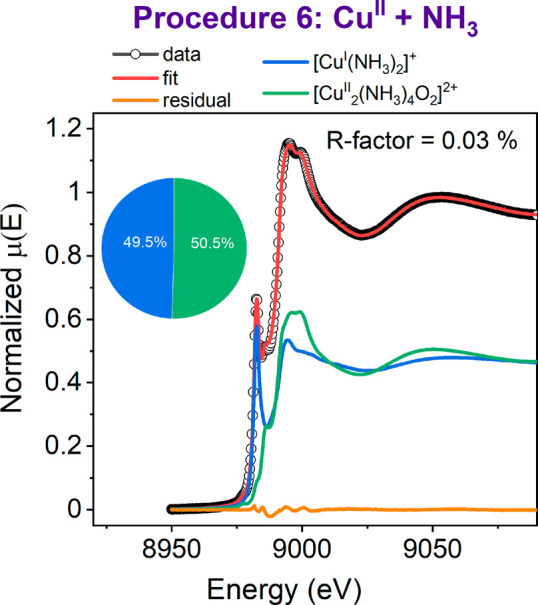
Linear combination fit of Cu K-edge XANES spectra obtained in Cu-CHA after exposing fw-CuII species to NH3 at 200 °C (CuII + NH3 pretreatment).
The evolution of XANES spectra upon interaction with SO2 shows that the most susceptible species are CuII with mixed (NH3)xOy ligation, whereas CuI species or CuII in the absence of NH3 are much less affected. These findings are supported by X-ray adsorbate quantification (XAQ) data,31 collected simultaneously with the XAS measurements during the exposure to SO2, and a TPD analysis of a parallel set of catalyst samples, exposed to the same pretreatments used in XANES experiments (Figure 3a). We find the highest sulfur content (S/Cu ratio) for the [CuII2(NH3)4O2]2+ and CuII + NH3 procedures. The sulfur uptake of the [CuI(NH3)2]+ and fw-CuII moieties was ca. 3 times lower, and for the bare fw-CuI species, it was ca. 6 times lower. These results show that the reaction between the [CuII2(NH3)4O2]2+ species and SO2 contributes the most to the accumulation of SO2 in the Cu-CHA catalyst.
Figure 3.
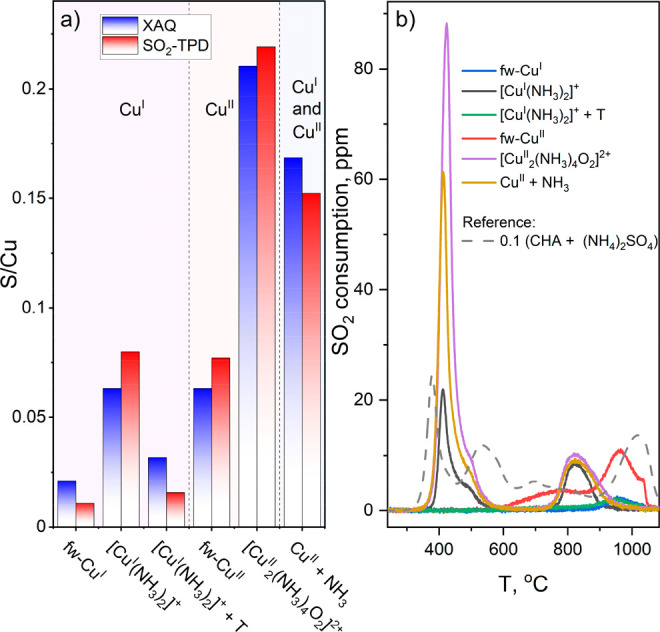
(a) S/Cu ratios in the samples after exposure to SO2 obtained from SO2-TPD and XAQ. (b) SO2-TPD profiles collected after exposure of the species obtained in procedures 1–6 to SO2 in comparison to a reference SO2-TPD curve of a CHA zeolite without Cu impregnated with 20 wt % (NH4)2SO4, downscaled ×10.
Interestingly, the sulfur content in the CuII + NH3 sample lies between those for samples with pure [CuI(NH3)2]+ and [CuII2(NH3)4O2]2+ species, which in combination with the linear combination fit shown in Figure 2 suggests that the reactivity of the CuII(NH3)xOy species obtained after CuII + NH3 treatment toward SO2 is similar to that of [CuII2(NH3)4O2]2+.
By comparing the SO2-TPD curves of Cu-CHA samples with that of (NH4)2SO4, adsorbed on Cu-free CHA (Figure 3b), we can also deduce that the elevated sulfur content in the samples with the CuII(NH3)xOy species is due to the reactivity toward SO2 and not to the formation of (NH4)2SO4 in a reaction of SO2 with NH3 and NH4+ groups stored in the zeolite framework. For the adsorbed (NH4)2SO4, we observe SO2 desorption at around 380, 530, and 1000 °C (gray curve in Figure 3b). The desorption at 380 °C matches the known thermal decomposition of (NH4)2SO4;32 the other two peaks are probably due to the interaction of either (NH4)2SO4 or products of its decomposition with the zeolite, their precise interpretation being beyond the scope of the present argument. For all three Cu-CHA samples containing NH3 before exposure to SO2 ([CuI(NH3)2]+, [CuII2(NH3)4O2]2+, and (CuII + NH3) procedures), we observe SO2 desorption at around 420 °C (Figure 3b). As this does not match any of the observed desorption characteristics of (NH4)2SO4 in Cu-free Cu-CHA, the SO2-TPD feature at 420 °C reflects an interaction of Cu with SO2. Interestingly, the SO2-TPD curve for the sample with the dominant fw-CuII species shows a significant SO2 desorption peak close to 1000 °C, which, together with the lack of changes in Cu K-edge XANES upon exposure to SO2, indicates the formation of some sulfur deposits not directly coordinated to Cu.
The presence of Cu–N and Cu–O bonds in the [CuII2(NH3)4O2]2+ complex has been independently confirmed by valence-to-core XES.27,33,34 XES spectra at different stages of pretreatment leading to the formation of [CuII2(NH3)4O2]2+ dimers are reported in Figure 4. The origin of the Kβ′′ satellite peak is the transition from the ligand s orbitals to Cu 1s, which makes its position sensitive to the species directly coordinated to Cu and allows it to discriminate among Cu–O, Cu–N, and Cu–S bonds.35−37Figure 4 shows that after heating in O2 Cu is predominantly coordinated by oxygens (as expected for the fw-CuII species), whereas after exposure to NO + NH3 N ligands are dominating, as expected for a [CuI(NH3)2]+ linear complex. After subsequent exposure to O2 and formation of [CuII2(NH3)4O2]2+ dimers, the peak broadens, confirming the presence of both Cu–N and Cu–O bonds. These bonds remain after exposure to SO2, while no significant contribution from Cu–S bonds38 is observed, suggesting that the possible SO2 binding to the Cu is carried out through an oxygen atom.
Figure 4.
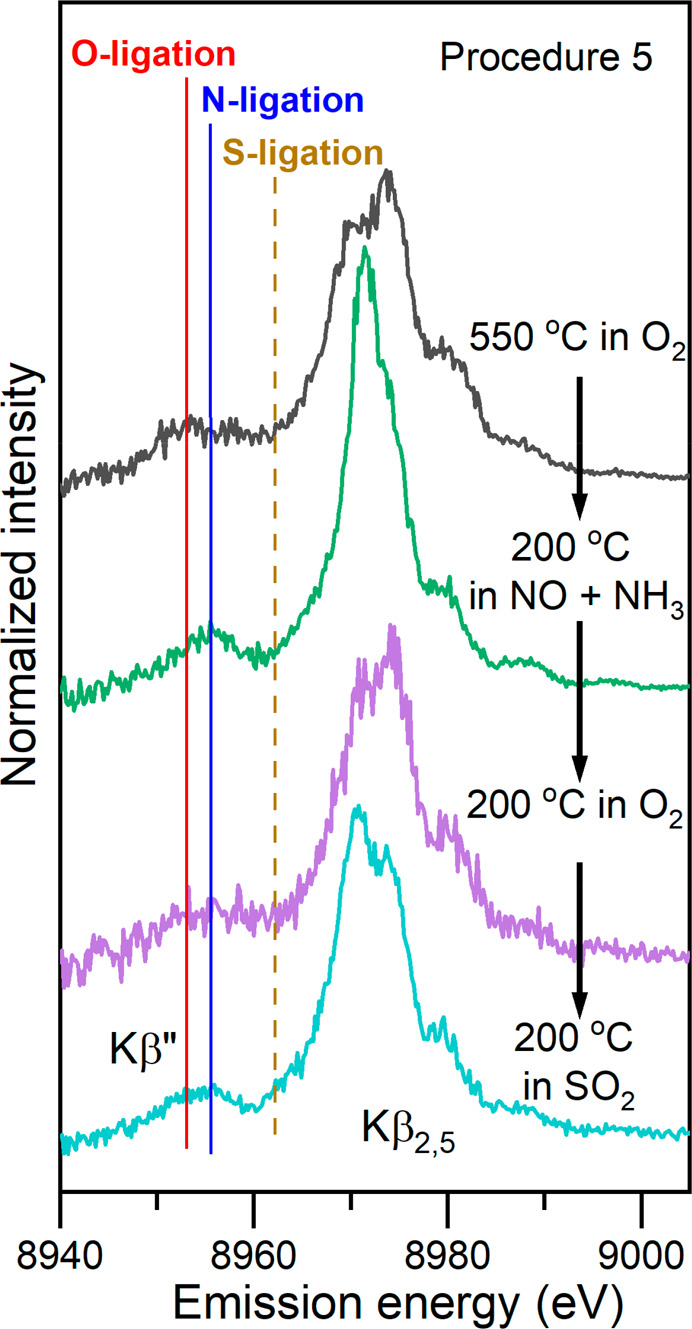
Background-subtracted Cu Kβ valence-to-core XES spectra for different stages of procedure 5 leading to the formation of the [CuII2(NH3)4O2]2+ complex and its exposure to SO2.
In conclusion, the in situ XAS and XES measurements of different Cu intermediates formed in a Cu-CHA catalyst exposed to SO2 demonstrate that CuII species with mixed NH3 and O ligation of Cu are particularly reactive toward SO2, whereas CuI species and CuII without NH3 are much less affected by it. In particular, the [CuII2(NH3)4O2]2+ complex, which is formed upon activation of O2 in the NH3-SCR cycle, shows a clear reaction with SO2, resulting in a partial reduction of the CuII and accumulation of sulfur in the zeolite. Therefore, we conclude that this reaction is responsible for the poisoning of Cu-CHA catalysts in NH3-SCR by SO2.
Acknowledgments
ESRF is kindly acknowledged for the provision of beamtime at the BM23 and ID26 beamlines. We thank N. Daffé and B. Detlefs for help during the XES experiment at ID26.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00053.
Experimental details, XANES linear combination fits before SO2 exposure, wavelet transform analysis of the sample heated in O2, and EXAFS fitting results (PDF)
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 847439.
The authors declare no competing financial interest.
Supplementary Material
References
- Lambert C. K. Perspective on SCR NOx control for diesel vehicles. React. Chem. Eng. 2019, 4 (6), 969–974. 10.1039/C8RE00284C. [DOI] [Google Scholar]
- Gounder R.; Moini A. Automotive NOx abatement using zeolite-based technologies. React. Chem. Eng. 2019, 4 (6), 966–968. 10.1039/C9RE90030F. [DOI] [Google Scholar]
- Hammershoi P. S.; Jensen A. D.; Janssens T. V. W. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl. Catal., B 2018, 238, 104–110. 10.1016/j.apcatb.2018.06.039. [DOI] [Google Scholar]
- Hammershoi P. S.; Jangjou Y.; Epling W. S.; Jensen A. D.; Janssens T. V. W. Reversible and irreversible deactivation of Cu-CHA NH3-SCRcatalysts by SO2 and SO3. Appl. Catal., B 2018, 226, 38–45. 10.1016/j.apcatb.2017.12.018. [DOI] [Google Scholar]
- Peden C. H. F. Cu/Chabazite catalysts for ’Lean-Burn’ vehicle emission control. J. Catal. 2019, 373, 384–389. 10.1016/j.jcat.2019.04.046. [DOI] [Google Scholar]
- Borfecchia E.; Lomachenko K. A.; Giordanino F.; Falsig H.; Beato P.; Soldatov A. V.; Bordiga S.; Lamberti C. Revisiting the nature of Cu-sites in activated Cu-SSZ-13 catalyst for SCR reaction. Chem. Sci. 2015, 6, 548–563. 10.1039/C4SC02907K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.; Walter E. D.; Kollar M.; Wang Y.; Szanyi J.; Peden C. H. F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. 10.1016/j.jcat.2014.08.010. [DOI] [Google Scholar]
- Feng Y.; Wang X.; Janssens T. V. W.; Vennestrøm P. N. R.; Jansson J.; Skoglundh M.; Grönbeck H. First-Principles Microkinetic Model for Low-Temperature NH3-Assisted Selective Catalytic Reduction of NO over Cu-CHA. ACS Catal. 2021, 11, 14395–14407. 10.1021/acscatal.1c03973. [DOI] [Google Scholar]
- Janssens T. V. W.; Falsig H.; Lundegaard L. F.; Vennestrom P. N. R.; Rasmussen S. B.; Moses P. G.; Giordanino F.; Borfecchia E.; Lomachenko K. A.; Lamberti C.; Bordiga S.; Godiksen A.; Mossin S.; Beato P. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5 (5), 2832–2845. 10.1021/cs501673g. [DOI] [Google Scholar]
- Gao F.; Mei D.; Wang Y.; Szanyi J.; Peden C. H. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139 (13), 4935–4942. 10.1021/jacs.7b01128. [DOI] [PubMed] [Google Scholar]
- Paolucci C.; Khurana I.; Parekh A. A.; Li S. C.; Shih A. J.; Li H.; Di Iorio J. R.; Albarracin-Caballero J. D.; Yezerets A.; Miller J. T.; Delgass W. N.; Ribeiro F. H.; Schneider W. F.; Gounder R. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357 (6354), 898–903. 10.1126/science.aan5630. [DOI] [PubMed] [Google Scholar]
- Jones C. B.; Khurana I.; Krishna S. H.; Shih A. J.; Delgass W. N.; Miller J. T.; Ribeiro F. H.; Schneider W. F.; Gounder R. Effects of dioxygen pressure on rates of NOx selective catalytic reduction with NH3 on Cu-CHA zeolites. J. Catal. 2020, 389, 140–149. 10.1016/j.jcat.2020.05.022. [DOI] [Google Scholar]
- Negri C.; Selleri T.; Borfecchia E.; Martini A.; Lomachenko K. A.; Janssens T. V. W.; Cutini M.; Bordiga S.; Berlier G. Structure and Reactivity of Oxygen-Bridged Diamino Dicopper(II) Complexes in Cu-Ion-Exchanged Chabazite Catalyst for NH3-Mediated Selective Catalytic Reduction. J. Am. Chem. Soc. 2020, 142 (37), 15884–15896. 10.1021/jacs.0c06270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. D.; Wang D.; Wang X.; Zha Y. H.; An H. M.; Kamasamudram K.; Yezerets A. Impact of low temperature sulfur exposure on the aging of small pore Cu-zeolite SCR catalyst. Catal. 2021, 360, 234–240. 10.1016/j.cattod.2020.04.033. [DOI] [Google Scholar]
- Mesilov V. V.; Bergman S. L.; Dahlin S.; Yang X.; Xi S. B.; Ma Z. R.; Lian X.; Wei C.; Pettersson L. J.; Bernasek S. L. Differences in oxidation-reduction kinetics and mobility of Cu species in fresh and SO2-poisoned Cu-SSZ-13 catalysts. Appl. Catal., B 2021, 284, 119756. 10.1016/j.apcatb.2020.119756. [DOI] [Google Scholar]
- Mesilov V.; Xiao Y.; Dahlin S.; Bergman S. L.; Pettersson L. J.; Bernasek S. L. First-Principles Calculations of Condition-Dependent Cu/Fe Speciation in Sulfur-Poisoned Cu- and Fe-SSZ-13 Catalysts. J. Phys. Chem. C 2021, 125 (8), 4632–4645. 10.1021/acs.jpcc.1c01016. [DOI] [Google Scholar]
- Feng Y.; Janssens T. V. W.; Vennestrøm P. N. R.; Jansson J.; Skoglundh M.; Grönbeck H. The Role of H+- and Cu+-Sites for N2O Formation during NH3-SCR over Cu-CHA. J. Phys. Chem. C 2021, 125 (8), 4595–4601. 10.1021/acs.jpcc.0c11008. [DOI] [Google Scholar]
- Millan R.; Cnudde P.; van Speybroeck V.; Boronat M. Mobility and Reactivity of Cu+ Species in Cu-CHA Catalysts under NH3-SCR-NOx Reaction Conditions: Insights from AIMD Simulations. JACS Au 2021, 1 (10), 1778–1787. 10.1021/jacsau.1c00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S. H.; Jones C. B.; Gounder R. Temperature dependence of Cu(I) oxidation and Cu(II) reduction kinetics in the selective catalytic reduction of NOx with NH3 on Cu-chabazite zeolites. J. Catal. 2021, 404, 873–882. 10.1016/j.jcat.2021.08.042. [DOI] [Google Scholar]
- Mesilov V. V.; Dahlin S.; Bergman S. L.; Xi S.; Han J.; Olsson L.; Pettersson L. J.; Bernasek S. L. Regeneration of sulfur-poisoned Cu-SSZ-13 catalysts: Copper speciation and catalytic performance evaluation. Appl. Catal., B 2021, 299, 120626. 10.1016/j.apcatb.2021.120626. [DOI] [Google Scholar]
- Negri C.; Borfecchia E.; Martini A.; Deplano G.; Lomachenko K. A.; Janssens T. V. W.; Berlier G.; Bordiga S. In situ X-ray absorption study of Cu species in Cu-CHA catalysts for NH3-SCR during temperature-programmed reduction in NO/NH3. Rev. Chem. Intermed. 2021, 47 (1), 357–375. 10.1007/s11164-020-04350-1. [DOI] [Google Scholar]
- Mathon O.; Beteva A.; Borrel J.; Bugnazet D.; Gatla S.; Hino R.; Kantor I.; Mairs T.; Munoz M.; Pasternak S.; Perrin F.; Pascarelli S. The time-resolved and extreme conditions XAS (TEXAS) facility at the European Synchrotron Radiation Facility: the general-purpose EXAFS bending-magnet beamline BM23. J. Synchrotron Radiat. 2015, 22 (6), 1548–1554. 10.1107/S1600577515017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzel P.; Harris A.; Marion P.; Sikora M.; Weng T. C.; Guilloud C.; Lafuerza S.; Rovezzi M.; Detlefs B.; Ducotte L. The five-analyzer point-to-point scanning crystal spectrometer at ESRF ID26. J. Synchrotron Radiat. 2021, 28, 362–371. 10.1107/S1600577520015416. [DOI] [PubMed] [Google Scholar]
- Borfecchia E.; Negri C.; Lomachenko K. A.; Lamberti C.; Janssens T. V. W.; Berlier G. Temperature-dependent dynamics of NH3-derived Cu species in the Cu-CHA SCR catalyst. React. Chem. Eng. 2019, 4 (6), 1067–1080. 10.1039/C8RE00322J. [DOI] [Google Scholar]
- Martini A.; Borfecchia E.; Lomachenko K. A.; Pankin I. A.; Negri C.; Berlier G.; Beato P.; Falsig H.; Bordiga S.; Lamberti C. Composition-driven Cu-speciation and reducibility in Cu-CHA zeolite catalysts: a multivariate XAS/FTIR approach to complexity. Chem. Sci. 2017, 8 (10), 6836–6851. 10.1039/C7SC02266B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau L. S.; Spira-Solomon D. J.; Penner-Hahn J. E.; Hodgson K. O.; Solomon E. I. X-ray absorption edge determination of the oxidation state and coordination number of copper. Application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 1987, 109 (21), 6433–6442. 10.1021/ja00255a032. [DOI] [Google Scholar]
- Giordanino F.; Borfecchia E.; Lomachenko K. A.; Lazzarini A.; Agostini G.; Gallo E.; Soldatov A. V.; Beato P.; Bordiga S.; Lamberti C. Interaction of NH3 with Cu-SSZ-13 Catalyst: A Complementary FTIR, XANES, and XES Study. J. Phys. Chem. Lett. 2014, 5 (9), 1552–1559. 10.1021/jz500241m. [DOI] [PubMed] [Google Scholar]
- Chen L.; Janssens T. V. W.; Vennestrom P. N. R.; Jansson J.; Skoglundh M.; Gronbeck H. A Complete Multisite Reaction Mechanism for Low-Temperature NH3-SCR over Cu-CHA. ACS Catal. 2020, 10 (10), 5646–5656. 10.1021/acscatal.0c00440. [DOI] [Google Scholar]
- Martini A.; Signorile M.; Negri C.; Kvande K.; Lomachenko K. A.; Svelle S.; Beato P.; Berlier G.; Borfecchia E.; Bordiga S. EXAFS wavelet transform analysis of Cu-MOR zeolites for the direct methane to methanol conversion. Phys. Chem. Chem. Phys. 2020, 22 (34), 18950–18963. 10.1039/D0CP01257B. [DOI] [PubMed] [Google Scholar]
- Sushkevich V. L.; Safonova O. V.; Palagin D.; Newton M. A.; van Bokhoven J. A. Structure of copper sites in zeolites examined by Fourier and wavelet transform analysis of EXAFS. Chem. Sci. 2020, 11 (20), 5299–5312. 10.1039/D0SC01472A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomachenko K. A.; Molokova A. Y.; Atzori C.; Mathon O. Quantification of Adsorbates by X-ray Absorption Spectroscopy: Getting TGA-like Information for Free. J. Phys. Chem. C 2022, 126 (11), 5175–5179. 10.1021/acs.jpcc.2c00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyoura R.; Urano K. Mechanism, Kinetics, and Equilibrium of Thermal Decomposition of Ammonium Sulfate. Ind. Eng. Chem. Process 1970, 9 (4), 489–494. 10.1021/i260036a001. [DOI] [Google Scholar]
- Gunter T.; Carvalho H. W. P.; Doronkin D. E.; Sheppard T.; Glatzel P.; Atkins A. J.; Rudolph J.; Jacob C. R.; Casapu M.; Grunwaldt J. D. Structural snapshots of the SCR reaction mechanism on Cu-SSZ-13. Chem. Commun. 2015, 51 (44), 9227–9230. 10.1039/C5CC01758K. [DOI] [PubMed] [Google Scholar]
- Gunter T.; Doronkin D. E.; Boubnov A.; Carvalho H. W. P.; Casapu M.; Grunwaldt J. D. The SCR of NOx with NH3 Examined by Novel X-ray Emission and X-ray Absorption Methods. Top. Catal. 2016, 59 (10–12), 866–874. 10.1007/s11244-016-0561-7. [DOI] [Google Scholar]
- Lomachenko K. A.; Borfecchia E.; Negri C.; Berlier G.; Lamberti C.; Beato P.; Falsig H.; Bordiga S. The Cu-CHA deNOx catalyst in action: temperature-dependent NH3-assisted selective catalytic reduction monitored by operando XAS and XES. J. Am. Chem. Soc. 2016, 138 (37), 12025–12028. 10.1021/jacs.6b06809. [DOI] [PubMed] [Google Scholar]
- Glatzel P.; Bergmann U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes - electronic and structural information. Coord. Chem. Rev. 2005, 249 (1–2), 65–95. 10.1016/j.ccr.2004.04.011. [DOI] [Google Scholar]
- Vegelius J. R.; Kvashnina K. O.; Klintenberg M.; Soroka I. L.; Butorin S. M. Cu Kβ2,5 X-ray emission spectroscopy as a tool for characterization of monovalent copper compounds. J. Anal. At. Spectrom. 2012, 27 (11), 1882–1888. 10.1039/c2ja30095h. [DOI] [Google Scholar]
- Muller P.; Neuba A.; Florke U.; Henkel G.; Kuhne T. D.; Bauer M. Experimental and Theoretical High Energy Resolution Hard X-ray Absorption and Emission Spectroscopy on Biomimetic Cu2S2 Complexes. J. Phys. Chem. A 2019, 123 (16), 3575–3581. 10.1021/acs.jpca.9b00463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


