Abstract
Background
There are limited and nonconcordant data on the rapidity and safety of blood pressure response to clonidine in the setting of asymptomatic severe hypertension. We evaluated the blood pressure response to clonidine in hospitalized patients with asymptomatic severe hypertension.
Methods
We performed a review of hospitalized, noncritically ill patients receiving clonidine within 6 hours of developing asymptomatic severe hypertension (systolic blood pressure [SBP] >180 or diastolic blood pressure [DBP] >110 mm Hg in the absence of acute hypertension-mediated target organ damage). The incidence of mean arterial pressure (MAP) reduction by ≥30% at 4 hours after clonidine was the primary endpoint.
Results
We identified 200 relevant patient encounters (median age 63 years, 48.5% women). Median time to clonidine following asymptomatic severe hypertension was 2.8 hours. A total of 20 (10%) patients had ≥30% MAP reduction within 4 hours after clonidine, and 32 (16%) patients had ≥30% reduction in either SBP, DBP, or MAP. Older age, female sex, and preexisting vascular disease were associated with ≥30% MAP reductions (P < 0.05). Only patient sex and clonidine dose of 0.3 mg were significant in multivariable models. There were 14 adverse events observed within 24 hours of administration of clonidine; most (9) were acute kidney injury. There were no ischemic (myocardial, cerebrovascular) events.
Conclusions
A substantial minority of hospitalized patients with asymptomatic severe hypertension experience precipitous blood pressure decline with clonidine, and though blood pressure declines more precipitously in women and those receiving higher doses (0.3 mg specifically), the response to clonidine is generally not predictable on clinical grounds.
Keywords: asymptomatic severe hypertension, blood pressure, clonidine, hypertension, inpatient hypertension
Graphical Abstract
Graphical Abstract.
Chronic hypertension is a well-established risk factor of cardiovascular disease.1,2 Though not as well-studied, blood pressure elevations to severe levels are common in the acute setting.3 Despite lack of high-quality evidence of morbidity and mortality benefit, guidelines consistently recommend prompt blood pressure control in patients with acute severe hypertension associated with acute hypertension-mediated target organ damage (hypertensive emergency).4–6 However, severe but asymptomatic elevations in blood pressure are subject of greater controversy. There is lack of guidance regarding approach to therapy and therapeutic goals. Current recommendations do not encourage rapid blood pressure lowering in the absence of end organ damage, which has no proven benefit and may have complications, including cerebral hypoperfusion.7–12
Acute severe hypertension without target organ damage often poses a dilemma to the clinician. Severely elevated pressures may be alarming in acutely ill inpatients and may be associated with nonspecific symptoms such as headache and atypical chest pain.4 Moreover, clinicians may fear progression to true hypertensive emergency in certain scenarios. Previous clinical trials suggested that clonidine was a safe option which may avoid precipitous declines in blood pressure in hypertensive urgencies.4,13–15 However, recently published individual patient data from patients treated in the emergency department indicate that clonidine may be associated with excessive reductions in blood pressure to an extent similar to those observed with intravenous hydralazine.9
In this observational study of acute severe hypertension in hospitalized patients without target organ damage, we examined the blood pressure response to clonidine using time-varying blood pressure data. We evaluated the proportion of patients treated with clonidine that had precipitous blood pressure decline and adverse events, and explored clinical characteristics associated with blood pressure changes.
METHODS
Data source
This cohort was extracted from extensive electronic medical record data for 304,695 adult inpatient admissions at 1 of 5 network hospitals from 1/6/2016 to 3/31/2020. Following exclusions, we performed manual chart review for each subject in the final cohort.
Study population
We studied adult inpatients treated with clonidine within 6 hours of developing asymptomatic severe hypertension without end organ damage. We defined asymptomatic severe hypertension as blood pressure elevation to systolic blood pressure (SBP) >180 mm of Hg or diastolic blood pressure (DBP) >110 mm of Hg after admission. Patients with end organ damage were excluded based on the International Classification of Diseases—10 codes for hypertensive emergency, hypertensive urgency, or hypertensive crisis.
Patients were excluded if: (i) length of stay <2 or >30 days, (ii) admitted to the intensive care, maternity, or research units, (iii) no available blood pressure measurements following clonidine administration (i.e., no blood pressure measurements at all for remainder of the hospitalization; patients with blood pressure measurements >4 hours after clonidine administration were still included, as detailed below), (iv) vasopressors were administered within 6 hours prior to developing severe hypertension, (v) falsely elevated measurements, defined as drop in pressure to SBP <180 or DBP <110 mm Hg within 1 hour of the index severe pressure elevation in the absence of antihypertensive therapy, and (vi) incident episode of acute severe hypertension developed within 1 hour of admission (Figure 1, flow chart).
Figure 1.
Study flow diagram.
Study covariates
We identified the following covariates: (i) patient demographics (age at admission, sex), (ii) medical comorbidities as mentioned in physician notes (past history of hypertension, diabetes, smoking, cardiovascular disease [history of coronary disease corroborated by past cardiac catheterizations or stress tests], heart failure [both with reduced and preserved ejection fraction, corroborated by past echocardiograms], atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, opioid use disorder, alcohol use disorder), (iii) home antihypertensive medications (outpatient clonidine prescription prior to admission), (iv) inpatient antihypertensive therapies (inpatient antihypertensive medications in addition to clonidine, including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta blockers, diuretics, and alpha blockers within 4 hours before and after receiving clonidine), (v) measures of patient discomfort (administration of analgesics [narcotics or nonsteroidal anti-inflammatory drugs] and sedatives), (vi) admission blood pressure measurements, and (vii) dose of clonidine received after developing asymptomatic severe hypertension.
Study outcomes
The primary study outcome was the proportion of patients with ≥30% reduction in mean arterial pressure (MAP) within 4 hours of clonidine administration. Guidelines recommend avoiding more rapid reductions in MAP.4,5 MAP was defined as 2/3 of the diastolic pressure plus 1/3 of the systolic pressure. We used 4 hours as a cutoff as clonidine reaches peak effect within 2–4 hours.16 A proportion (25%) of patients did not have blood pressure measurements within 4 hours of receiving clonidine. For those patients, we used the first available blood pressure after receiving clonidine to determine response to clonidine.
There were 2 secondary outcomes. We created a composite outcome comprised of ≥30% reduction in either SBP, DBP, or MAP. Second, we obtained data directly from inpatient notes and lab results regarding adverse events occurring within 24 hours following clonidine administration (Figure 2). The following adverse events were considered possible sequalae of rapid blood pressure reduction: myocardial infarction (defined as evidence of myocardial infarction and ischemia), myocardial ischemia, stroke, acute kidney injury (defined as increase in serum creatinine ≥0.3 mg/dl), acute heart failure (defined as acute development of heart failure symptoms), and altered mental status, syncope, or dizziness.7
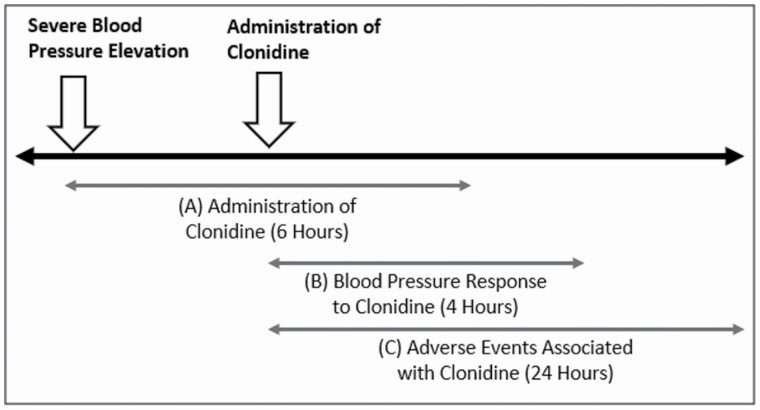
Figure 2.
Timeline schematic. This figure displays 3 time cutoffs used in the study: (a) administration of clonidine, which must have occurred within 6 hours after severe blood pressure elevation, (b) blood pressure response to clonidine, which was examined within 4 hours after clonidine administration, and (c) adverse events, which were examined within 24 hours after clonidine administration.
Statistical analysis
We examined the univariate association between clonidine-related blood pressure reductions and patient characteristics including demographics, comorbidities, and home clonidine therapy. We performed chi-squared tests to compare categorical variables and Mann–Whitney U to compare continuous variables with the primary outcome.
To examine independent association of clinical variables with precipitous blood pressure drops, we created multivariable logistic regression models with MAP reduction ≥30% as the outcome and patient characteristics as the independent variables. We repeated all analyses for the composite secondary outcome of ≥30% reduction in SBP, DBP, or MAP.
We examined the association between clonidine dose and blood pressure drop in a secondary analysis. We used the chi-squared test to examine the univariate association between dose and precipitous drop in blood pressure and constructed 2 additional logistic regression models adjusting for dose, one with the primary outcome and the other with the composite secondary outcome of SBP, DBP, and MAP as dependent variables.
All analyses were 2-sided and performed using R 4.0.3 (CRAN) with statistical significance set at 0.05. This study was approved by the Yale University Human Investigation Committee.
RESULTS
Data were available for 304,695 adult inpatient admissions over the study period. A total of 23,147 unique encounters documented asymptomatic severe hypertension. After exclusions, patients received clonidine within 6 hours of developing asymptomatic severe hypertension during 200 unique encounters (median time to clonidine 2.8 hours, interquartile range [IQR] 1.7–4.4 hours). Median patient age was 63.0 (IQR 52.3–76.8) years and 48.5% were female. Of the 200 clonidine-receiving patients, 125 (62.5%) received additional antihypertensive medications within 4 hours (before and after) of clonidine administration. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Characteristic | Total | MAP drop ≤30% | MAP drop ≥30% | P |
|---|---|---|---|---|
| N (% or median) | N (% or median) | N (% or median) | ||
| Total | 200 (100) | 180 (90) | 20 (10) | |
| Demographics | ||||
| Age (years) | 200 (63.0) | 180 (78.0) | 20 (62.1) | 0.01 |
| Sex (female) | 97 (48.5) | 81 (45.0) | 16 (80.0) | 0.006 |
| Medical history | ||||
| Outpatient clonidine prescription | 128 (64.0) | 114 (63.3) | 14 (70.0) | 0.73 |
| Hypertension | 167 (83.5) | 149 (82.8) | 18 (90.0) | 0.61 |
| Diabetes | 91 (45.5) | 82 (45.5) | 9 (45.0) | 1 |
| Ever smoker | 120 (60.0) | 107 (59.4) | 13 (65.0) | 0.81 |
| Vascular disease | 73 (36.5) | 61 (33.9) | 12 (60.0) | 0.04 |
| Heart failure | 35 (17.5) | 30 (16.8) | 5 (25.0) | 0.54 |
| Atrial fibrillation | 29 (14.5) | 23 (79.3) | 6 (30.0) | 0.08 |
| Chronic obstructive pulmonary disease | 24 (12.0) | 12 (12.8) | 3 (15.0) | 0.94 |
| Pulmonary hypertension | 10 (5.0) | 10 (5.6) | 0 (0.0) | 0.59 |
| Obstructive sleep apnea | 32 (16) | 28 (15.6) | 4 (20.0) | 0.85 |
| Chronic kidney disease | 37 (18.5) | 65 (36.1) | 6 (30.0) | 0.78 |
| Malignancy | 40 (20.0) | 31 (17.2) | 6 (30.0) | 0.27 |
| Alcohol use disorder | 40 (20.0) | 38 (21.1) | 2 (10.0) | 0.38 |
| Opioid use disorder | 32 (16.0) | 31 (17.2) | 1 (5.0) | 0.27 |
| Inpatient therapy | ||||
| Analgesic or sedative | 105 (52.5) | 96 (53.3) | 9 (45.0) | 0.64 |
| Nonclonidine antihypertensives | 125 (62.5) | 110 (61.1) | 15 (75.0) | 0.33 |
| Admission blood pressure | ||||
| Mean arterial pressure (mm Hg) | 200 (105.0) | 180 (105.0) | 20 (103.7) | 0.8 |
| Systolic blood pressure (mm Hg) | 200 (151.5) | 180 (151.0) | 20 (156.0) | 0.75 |
| Diastolic blood pressure (mm Hg) | 200 (80) | 180 (80.0) | 20 (82.5) | 0.8 |
Patient characteristics by drop in mean arterial pressure (MAP) ≥30% within 4 hours following clonidine administration.
Blood pressure response to clonidine
Of the total cohort, 20 (10%) patients had ≥30% MAP reduction within 4 hours after receiving clonidine (or at the first documented blood pressure), while 6.7% of patients who received clonidine without additional antihypertensive therapy had ≥30% MAP reductions. Of the subgroup that received clonidine plus an additional antihypertensive drug, 12.0% (15 of 125) had ≥30% MAP reduction. We observed 11 (14.7%) clonidine-only receiving patients that had a ≥30% reduction in either SBP, DBP, or MAP within 4 hours of receiving clonidine. Similarly, 32 (16%) patients in the total cohort had a ≥30% reduction in SBP, DBP, or MAP (Figure 3).
Figure 3.
Change in blood pressure over time. These figures display change in (a) mean arterial pressure (MAP), (b) systolic blood pressure (SBP), and (c) diastolic blood pressure (DBP) at each blood pressure check within 4 hours after receiving clonidine (time = 0). Cases with ≥30% drop in pressure are colored in gray.
Patient characteristics and blood pressure response
Patients with ≥30% reduction in MAP were older, with a median age of 78.0 (IQR 61.2–84.4) years, while those with <30% drop had a median age of 62.1 (IQR 51.8–75.1) years (P = 0.01). Women were also more likely to have precipitous declines in MAP (8.0% vs. 2.0% for men, P = 0.01). Finally, patients with vascular disease had a greater proportion of ≥30% MAP drops (6.0% vs. 4.0% without vascular disease, P < 0.05). There were no significant differences in the proportion of ≥30% reductions in MAP across other medical comorbidities (Table 1).
There were no significant differences in the proportion of patients with ≥30% MAP reductions between groups who received analgesics or sedatives and groups that did not receive these drug classes. Likewise, there was no statistically significant difference in the proportion with ≥30% MAP drops between patients who received nonclonidine antihypertensives within 4 hours of clonidine and those who received only clonidine (12.0% vs. 6.7%; P = 0.33 with chi-square testing).
The only statistically significant difference in the proportion of the composite blood pressure outcome between groups was in patient age, with older patients more likely to drop either SBP, DBP, or MAP ≥30% (P < 0.05).
Adverse events
There were several documented adverse events within 24 hours of receiving clonidine with 14 (7.0%) cases from the total cohort having at least 1 event, of which 7 were clonidine-only receiving patients. Most (9 of 14) events were acute kidney injury. One patient developed acute heart failure within 24 hours of receiving clonidine though this was likely high output heart failure owing primarily to severe anemia. One patient developed hypotension necessitating intravenous fluids. There were no documented ischemic events. Overall, adverse events were more common in patients with MAP decline ≥30% (8.0% vs. 2.0% in patients without ≥30% MAP reductions; P < 0.05).
Independent association of precipitous blood pressure reduction
In multivariable logistic regression models with ≥30% MAP reduction as the dependent variable and patient characteristics, additional drug therapy, home clonidine therapy, and admission pressure measurements as the independent variables, the solo administration of clonidine (i.e., no other antihypertensives administered within 4 hours of the index event) was not associated with fewer episodes of MAP reductions ≥30%. Only patient sex was independently associated with large reductions in MAP. Men were less likely to have precipitous MAP reductions as compared with women (odds ratio 0.19, 95% confidence interval 0.05–0.61; P = 0.01).
In models that controlled for the same covariates and used the composite blood pressure outcome as the dependent variable, none of the covariates we assessed were significantly associated with a ≥30% reduction in SBP, DBP, or MAP.
Clonidine dose and precipitous blood pressure decline
The median dose of clonidine received after developing asymptomatic severe hypertension was 0.1 (IQR 0.1–0.2) mg. There was no significant difference by dose in the proportion of patients with ≥30% drop in MAP (P = 0.07) or the composite outcome of ≥30% drop in MAP, SBP, or DBP (P = 0.80). In secondary models which included clonidine dose as a covariate, only a dose of 0.3 mg was significantly associated with ≥30% MAP reduction as compared with a dose of 0.1 mg (odds ratio 5.96, 95% confidence interval 1.44–29.0; P = 0.02). Dose was not significantly associated with ≥30% reduction in SBP, DBP, or MAP as a composite outcome in logistic regression models. The median maximum drop in blood pressure within 4 hours following each dose of clonidine is given in Table 2.
Table 2.
Maximum drop in blood pressure by dose
| Total cohort | 0.05 mg | 0.1 mg | 0.2 mg | 0.3 mg | |
|---|---|---|---|---|---|
| N = 200 | N = 7 | N = 101 | N = 61 | N = 31 | |
| Median (interquartile range) in mm Hg | |||||
| Mean arterial pressure | −10.0 (−23.4, 0) | −3.3 (−23.8, 0) | −9.7 (−23.7, 0) | −7.3 (−14.7, 0) | −18.0 (−27.8, −5.2) |
| Systolic blood pressure | −15.0 (−35.3, 0) | 0.0 (−31.5, 0) | −13.0 (−36.0, 0) | −14.0 (−25.0, 0) | −29.0 (−48.5, −12.5) |
| Diastolic blood pressure | −8.0 (−18.0, 0) | −8.0 (16.0, 0) | −8.0 (−19.0, 0) | −3.0 (−13.0, 0) | −14.0 (−25.0, −0.5) |
Maximum drop in blood pressure within 4 hours following clonidine administration, according to dose of clonidine.
Discussion
In this study of clonidine utilization for asymptomatic severe hypertension, we observed that 10% of patients had a precipitous decline in blood pressure after receiving clonidine. Women were especially susceptible to rapid blood pressure decline. Moreover, there were a number of adverse events, especially acute kidney injury, within 24 hours after receiving clonidine, which were associated with precipitous pressure reductions. These data contribute to past reports urging restraint when managing asymptomatic severe hypertension and contradict previous assertions that blood pressure responds more smoothly to clonidine than other antihypertensive drugs.4,14,15
Rapid correction of blood pressure raises concern because of the potential for organ hypoperfusion, including of the brain. Classic studies demonstrated that autoregulation of blood flow in hypertensive patients, which prevents cerebral sequalae of chronically elevated pressures, makes such patients susceptible to hypoperfusion at higher pressures than normal controls.4,17,18 Therefore, even if overt hypotension is avoided, complications may occur at technically (numerically) hypertensive levels. These observations underpin recommendations for careful blood pressure correction in severely hypertensive patients without compelling conditions (aortic dissection, preeclampsia/eclampsia, and pheochromocytoma crisis) for more aggressive reduction.5 Several studies have demonstrated no benefit of immediate correction in asymptomatic patients and therefore, guidelines do not recommend immediate therapy in such patients.5
Nonetheless, many clinical situations are neither hypertensive emergency nor completely asymptomatic. Patients may have nonspecific symptoms such as atypical chest pain and epistaxis, and there may be scenarios where clinicians suspect the possibility of evolution into hypertensive emergency.4 Clinicians would ideally have access to safe and effective means for blood pressure control when clinical judgment warrants treatment. Data from 2 older trials suggest that clonidine elicits smooth blood pressure responses in the setting of acute severe hypertension. In a trial of 36 patients randomized to labetalol or clonidine, the mean reduction in blood pressure with clonidine was <30% (average reduction in 6 hours was 57/32 mm Hg). Another trial of 51 patients comparing clonidine with nifedipine also observed average pressure reductions of <30% and showed reductions to be similar between nifedipine and clonidine after 6 hours (clonidine produced blood pressure reduction more slowly than nifedipine).14,15 However, though the mean reduction across patients may be acceptable, the proportion of patients who experience precipitous pressure drops may not be. The benefit of treating asymptomatic severe hypertension may not outweigh the risk. A recent observational study documented precipitous pressure reductions in a high proportion of clonidine-receiving patients presenting to the emergency department with hypertensive urgency.9 Over 30% of patients had SBP drops to below 140 mm Hg (from starting pressures >220/120). Our study examined percent pressure change rather than absolute cutoffs and 15%–20% of patients had ≥30% reductions in either the mean arterial, systolic, or diastolic pressures. Fortunately, no patients experienced ischemic events in either study, though we noted a number of other associated adverse events which may have been related to pressure fluctuations, particularly acute kidney injury. Prior studies have observed large reductions in cerebral blood flow with antihypertensive therapy, including 30% reduction with clonidine, though data are needed regarding the risk of clinically significant ischemic events from large pressure reductions.10
Blood pressure response to clonidine may not be predictable on clinical grounds.
There were few associated clinical characteristics with precipitous pressure decline. Older patients, women, and patients with known vascular disease had higher proportions of patients with unfavorable pressure responses. However, only sex and a dose of 0.3 mg were significant in multivariable regression models. These observations emphasize the lack of predictability of blood pressure response and encourage additional caution when treating women with asymptomatic severe hypertension.
It is notable that no ischemic events, especially stroke, a particularly feared complication of such pressure fluctuations, were noted despite the 10% of patients that experienced precipitous decline in blood pressure following clonidine administration. This is in contrast to a number of acute kidney injuries that were documented. We speculate that the differences in rate were observed because acute kidney injury is more readily detected, and more frequently measured, with daily creatinine assessments. Neurologic injury, in contrast, is not routinely measured in the absence of symptoms. Another possible contributor is the brain’s unique capability of substantial increases in oxygen extraction, which maintains tissue oxygenation despite reductions in cerebral blood flow.19
Despite the observations detailed above, we emphasize that the study design cannot sufficiently identify clonidine administration as a causal factor of adverse events and blood pressure drops to prohibit its use. We do not recommend against clonidine use in this population in lieu of a randomized trial to sufficiently control for possible confounders, though these data highlight the need for caution and further investigation.
Our study has several strengths. This was the largest study of clonidine utilization for asymptomatic severe hypertension. We conducted manual chart review to avoid the potential for error associated with automatic data extraction from electronic medical records. The inpatient ward is a common backdrop for severe asymptotic hypertension, and our study is one of the few which examines clonidine use in hospitalized patients. The study also has several limitations, most importantly its retrospective design, which cannot establish causation. Adverse events were observed following clonidine administrations though there are many confounders which may contribute to such events. Furthermore, though larger than other similar studies, this study contains a small number of patients that received only clonidine, though we did control for multiple antihypertensive therapy in multivariable analysis and it was not significantly associated with precipitous blood pressure decline. Finally, our study does not contain a control group and therefore, we cannot compare to rates of blood pressure decline in the absence of therapy. Comparable rates of precipitous decline in an untreated group would suggest that clonidine therapy is superfluous rather than directly harmful, though either case argues against clonidine use. Prospective data are needed to guide effective guidelines for the treatment of asymptomatic severe hypertension.
A substantial minority of hospitalized patients with asymptomatic severe hypertension experience precipitous blood pressure decline following clonidine therapy. The risk of rapid blood pressure decline with clonidine therapy in these patients may not outweigh the benefits. Women and those receiving higher doses (0.3 mg specifically) of clonidine may be particularly susceptible to dramatic blood pressure responses though significant drops in blood pressure are generally unpredictable on clinical grounds. A number of adverse events may be associated with clonidine therapy, especially acute kidney injury. A randomized controlled trial is needed to sufficiently control for confounders and therefore, we do not recommend against clonidine use in this population based on these data alone, but rather urge caution and the need for further investigation.
Contributor Information
Jonathan Hanna, Department of Internal Medicine, Yale School of Medicine, Yale University, Haven, Connecticut, USA.
Lama Ghazi, Department of Internal Medicine, Clinical and Translational Research Accelerator, Yale University, New Haven, Connecticut, USA.
Yu Yamamoto, Department of Internal Medicine, Clinical and Translational Research Accelerator, Yale University, New Haven, Connecticut, USA.
Michael Simonov, Department of Internal Medicine, Clinical and Translational Research Accelerator, Yale University, New Haven, Connecticut, USA.
Tayyab Shah, Department of Internal Medicine, Yale School of Medicine, Yale University, Haven, Connecticut, USA.
Francis P Wilson, Department of Internal Medicine, Clinical and Translational Research Accelerator, Yale University, New Haven, Connecticut, USA.
Aldo J Peixoto, Department of Internal Medicine, Section of Nephrology, Yale School of Medicine, and the Hypertension Program, Yale New Haven Hospital Heart and Vascular Center, New Haven, Connecticut, USA.
FUNDING
This work was supported by National Institutes of Health (P30DK079310, R01DK113191), American Heart Association (829804), and Robert E. Leet and Clara Guthrie Patterson Trust.
DISCLOSURE
The authors declared no conflict of interest.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author, AJP, upon reasonable request.
REFERENCES
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med 2001; 161:1501–1508. [DOI] [PubMed] [Google Scholar]
- 3. Astarita A, Covella M, Vallelonga F, Cesareo M, Totaro S, Ventre L, Aprà F, Veglio F, Milan A. Hypertensive emergencies and urgencies in emergency departments: a systematic review and meta-analysis. J Hypertens 2020; 38:1203–1210. [DOI] [PubMed] [Google Scholar]
- 4. Peixoto AJ. Acute severe hypertension. N Engl J Med 2019; 381:1843–1852. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 6. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members . 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 7. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA 1996; 276:1328–1331. [PubMed] [Google Scholar]
- 8. Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: a systematic review of the literature. J Gen Intern Med 2002; 17:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Preston RA, Arciniegas R, DeGraff S, Materson BJ, Bernstein J, Afshartous D. Outcomes of minority patients with very severe hypertension (>220/>120 mmHg). J Hypertens 2019; 37:415–425. [DOI] [PubMed] [Google Scholar]
- 10. Bertel O, Marx BE, Conen D. Effects of antihypertensive treatment on cerebral perfusion. Am J Med 1987; 82:29–36. [DOI] [PubMed] [Google Scholar]
- 11. Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, Ferrari AJ, Hyman S, Laxminarayan R, Levin C, Lund C, Medina Mora ME, Petersen I, Scott J, Shidhaye R, Vijayakumar L, Thornicroft G, Whiteford H; DCP MNS Author Group . Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 2016; 387:1672–1685. [DOI] [PubMed] [Google Scholar]
- 12. Levy PD, Mahn JJ, Miller J, Shelby A, Brody A, Davidson R, Burla MJ, Marinica A, Carroll J, Purakal J, Flack JM, Welch RD. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med 2015; 33:1219–1224. [DOI] [PubMed] [Google Scholar]
- 13. Greene CS, Gretler DD, Cervenka K, McCoy CE, Brown FD, Murphy MB. Cerebral blood flow during the acute therapy of severe hypertension with oral clonidine. Am J Emerg Med 1990; 8:293–296. [DOI] [PubMed] [Google Scholar]
- 14. Atkin SH, Jaker MA, Beaty P, Quadrel MA, Cuffie C, Soto-Greene ML. Oral labetalol versus oral clonidine in the emergency treatment of severe hypertension. Am J Med Sci 1992; 303:9–15. [DOI] [PubMed] [Google Scholar]
- 15. Jaker M, Atkin S, Soto M, Schmid G, Brosch F. Oral nifedipine vs oral clonidine in the treatment of urgent hypertension. Arch Intern Med 1989; 149:260–265. [PubMed] [Google Scholar]
- 16. Administration FD. Catapres (Clonidine Hydrochloride) Label. Silver Springs, MD: Federal Drug Administration, 2021. [Google Scholar]
- 17. Reed G, Devous M. Cerebral blood flow autoregulation and hypertension. Am J Med Sci 1985; 289:37–44. [DOI] [PubMed] [Google Scholar]
- 18. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1973; 1:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 2002; 125:595–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, AJP, upon reasonable request.