Abstract
BACKGROUND
The purpose of this document is to provide clinicians with guidance, using expert consensus, to help summarize evidence and offer practical recommendations.
METHODS
Expert Consensus Documents are intended to provide guidance for clinicians in areas in which there are no clinical practice guidelines, especially for new and evolving tests such as arterial stiffness measurements, until any formal guidelines are released.
RESULTS
This expert consensus document is intended as a source of information for decision-making and to guide clinician–patient discussions in various clinical scenarios.
CONCLUSIONS
The goal is to help clinicians and patients make a more informed decision together.
Keywords: arterial stiffness, blood pressure, cardio-ankle vascular index, clinical, endothelial function, expert consensus, hypertension, vascular disease
Graphical Abstract
Graphical Abstract.
Prior to understanding the potential clinical utility of vascular measures, it is imperative to understand the basis and definition of arterial stiffness. The concept of stiffness in a flexible conduit is derived from the physical relationships between stress and strain. There are extensive reviews of these components.1–3 Briefly, stress is the result of an application of force to a unit of area, and strain is a measurement of the deformation due to the stress. In the arterial circulation, stiffness measures are usually presented in 1 of 2 ways. One method uses the change in diameter of an arterial vessel at a certain pressure. This is represented mathematically as a fractional change in diameter (ΔD/D) at a certain pressure (P), for example as reflected in Peterson’s elastic modulus: .4 The other, and more commonly used method, of defining arterial stiffness utilizes the velocity at which the arterial pressure wave travels in large vessels (pulse wave velocity, PWV).5 Stiffer arteries conduct the pulse wave at a higher velocity. Much of the research establishing the role of arterial stiffness in circulatory pathophysiology has been generated through measurement of the velocity of pulse wave travel, particularly in the aorta.6,7
BRIEF HISTORY
The idea of “hardening” of the pulse is a concept shrouded in antiquity. The palpation of the wrist, a time honored tradition in medicine, and symbolized by the picture/logo of the Royal College of Physicians, was used for centuries to estimate the health of the circulation. In the 19th century, a series of ingenious devices were invented and used to describe more fully the contour of the pulse.8 This gave rise to the science of sphygmomanometry (sphygmo = beat or throb; manometry = measurement). Along with the study of pulse contours, the science of fluid dynamics also advanced rapidly in the 19th century, allowing for the pursuit of mechanisms to understand the nature of fluid travel in tubes. Despite this beginning, the field of sphygmomanometry seemed to pause for nearly a century with the introduction of the measurement of brachial blood pressure (BP). During this time, some investigators continued to study basic and clinical aspects of arterial function, but the clinical integration of such findings needed a clarion call. With the reemergence of the importance of systolic BP in the late 1990s,9 the field of arterial stiffness measurement was reinvigorated. In the past several decades, there has been a proliferation of the number of devices used clinically to estimate arterial stiffness in humans, which incorporate tonometric, oscillometric, combined phonocardiographic and oscillometric, ultrasonographic and nuclear magnetic resonance imaging methods. These have resulted in a robust literature on this topic, as well as societies devoted to arterial function measurement in Europe (Artery Society), the United States/Canada (North American Artery), and Asia (Pulse of Asia).7,10,11
TECHNIQUES TO MEASURE ARTERIAL STIFFNESS
Arterial stiffness data can be obtained through a multitude of different noninvasive procedures that could potentially be performed in the clinic. The most common procedure providing a measure of aortic stiffness is the determination of PWV.4 Although PWV can be accurately determined using magnetic resonance imaging, this is an expensive method that is not available in-house in most clinics. Instead, PWV is commonly measured through tonometry of the carotid and femoral arteries (namely carotid–femoral PWV [cfPWV]), often with ECG as a timing marker, though other validated pulse transit routes are also used.
The distance (L) between the measuring sites is assessed with a measuring tape, a caliper, or a segmometer, and velocity is calculated (PWV = L/Δt).4 Sometimes subject height may provide an estimate of “L.” The instruments required are portable and can easily be used in most standard clinic exam rooms. Tonometry, however, requires substantial training for accurate measurements, and the equipment can be expensive. This has led to the development of cuff-based (oscillometric) methods that require much less operator skill. Some manufacturers also provide a combination of cuff- and tonometry-based measures.4
Most cuff-based methods measure brachial–ankle PWV (baPWV), which incorporates several arterial segments, including elastic large arteries such as the descending aorta, and stiffer peripheral arteries in a combined value. There is a substantial amount of evidence that baPWV is of clinical value5,6 but to date, most of research data are from Asian countries.4,5 A relatively new method, Cardio-Ankle Vascular Index (CAVI7,8), is measured using cuffs on all 4 limbs, coupled with a microphone on the chest. Measuring CAVI using peripheral cuffs and a chest microphone provides several logistical advantages that are beneficial for clinical use. It is primarily operator independent, does not require exposure of the groin area (which some tonometric methods use for femoral pulse reference points), is a very reliable measure, and automated for greater ease-of-use and reproducibility. In addition to including the more peripheral arteries of the legs, the entire aorta is represented in the CAVI measurement, whereas other measurements omit significant portions of the aorta, particularly the ascending aorta, where the earliest aging-related changes are seen. The pulse transit of CAVI is from the heart (aortic valve) to the ankle. CAVI is also less dependent on BP at the time of measurement, compared with other PWV-based methods, because CAVI incorporates the stiffness parameter “β.” 9 In addition, CAVI does not require a cumbersome distance measurement, as its algorithm can make use of patient’s height as a substitute. This simplifies measurements in the clinic. CAVI is a measure that can be easily tracked between clinic visits as a measure of arterial health. The main limitation of CAVI is that it incorporates long muscular arterial segments, therefore not being a pure index of large artery stiffness.
SIGNIFICANCE OF ARTERIAL STIFFNESS
Arterial stiffness measurements have been incorporated into a number of longitudinal cohort studies in the United States, Europe, and Asia. Generally speaking, measures of arterial stiffness complement routine office brachial BPs, showing an independent prediction of future cardiovascular and renal events.12,13 The following are selected examples highlighting the clinical value of arterial stiffness measurements:
Predicting cardiovascular morbidity and mortality in otherwise healthy older adults16
Predicting mortality in patients with hypertension17
Predicting death and kidney disease progression in patients with chronic kidney disease18
Predicting incident cognitive decline and dementia19
Predicting cardiovascular and total mortality in patients with diabetes and glucose intolerance20
Predicting coronary artery disease (CAD) in patients with and without diabetes
Although much of the epidemiology of arterial stiffness has been studied using cfPWV,13 data supporting the predictive value of arterial stiffness have also been obtained through baPWV,20 CAVI,21 and arterial distensibility using either ultrasound22 or magnetic resonance imaging.23
ASSOCIATION BETWEEN CAVI AND OTHER ARTERIAL STIFFNESS MEASURES
A variety of arterial stiffness measures have been proposed and used in the research settings. How well is CAVI related to other measures of arterial stiffness? CAVI is significantly associated with cfPWV (r = 0.74) and baPWV (r = 0.82).24 Similar to other segmental measures of arterial stiffness (cfPWV), associations of CAVI with local measures of arterial stiffness derived from ultrasound-based assessment (e.g., arterial compliance) are weak.24 CAVI has been introduced as a BP independent measure of arterial stiffness as CAVI has been derived from the concept of β-stiffness index.25 Several studies have verified the BP independence of CAVI25,26 and have reported a strong association between CAVI and the stiffness parameter β obtained with transesophageal echocardiography27 although a conflicting finding has been reported.24,28
TESTING IN THE CLINIC
Currently, very few clinics in the United States perform and/or offer arterial stiffness measurements. At the time of this article, a major limitation is still the lack of insurance reimbursement as the Centers for Medicare & Medicaid Services has not yet recognized arterial stiffness testing as a reimbursable procedure. However, there are several efforts underway to create a suitable and reimbursable procedure code for arterial stiffness testing. There is a CPT code (93050) for arterial waveform and central BP determination. However, the reimbursement rate is low, and reimbursability of this procedure varies across states. Thus, even this approved procedure (which is related to and often performed in conjunction with arterial stiffness measures in research settings) is rarely performed in the clinic setting. However, mounting evidence for the clinical importance of arterial stiffness may lead to future reimbursement for this particular procedure, thus increasing the probability that arterial stiffness measurement will become more widely available.
With CAVI, information on the ankle–brachial index (ABI) can be acquired simultaneously. ABI is a reimbursable test procedure (CPT codes 93922 and 93924). Some expert organizations recommend screening peripheral artery disease with ABI in high-risk populations.29 Also, the American Heart Association and the American College of Cardiology 2018 Cholesterol Management Guideline recognizes ABI ≤0.9 as a risk enhancing factor.30 Thus, in future (assuming arterial stiffness will eventually be reimbursable), simultaneously measuring CAVI and ABI could be an attractive, useful, and time-saving clinic option in appropriate patient populations.
There are several practical and environmental factors that should be controlled to obtain an accurate arterial stiffness measurement.25,31–37 These include availability of a dimly lit and quiet room, with stable temperature, regardless of climate and season. Patients also need to be adequately prepared prior to attending the clinic. Avoidance of alcohol, vasoactive medications, and vigorous exercise for 12 hours or more is necessary, as is avoiding food, smoking, and caffeine-containing beverages for at least 4 hours before testing. Ideally, patients should be rested in a supine position for 10 minutes before measures are obtained. All measurements should also be obtained in the supine position, as body position affects CAVI and PWV substantially.25,31–37 Although it is not difficult to meet these requirements for accurate measurements in controlled research settings, keeping these conditions may be considerably more challenging in a clinic setting.
As arterial stiffness predicts cardiovascular risk independently of traditional risk factors, it has a significant impact on decision-making in various clinical scenarios. Several studies have reported the association between CAVI and future cardiovascular events.38–42 The participants in these studies were at moderate- to high risk for cardiovascular disease, such as having hypertension, diabetes, and obesity. In a study including 626 patients with type 2 diabetes, a CAVI >9.0 was independently associated with increased cardiovascular events, as compared with a CAVI <9.0 (heart rate [HR] 1.23).38 In another study involving patients with cardiovascular risk factors, a CAVI ≥10 was associated with a higher incidence of cardiovascular events (HR 2.25) than a CAVI <9.0.39 A CAVI ≥10.1 was also associated with more cardiovascular events in metabolic syndrome.41 Recently, the Japanese Society for Vascular Failure proposed the cutoff values for CAVI: <8 for normal, ≥8 to <9 for borderline, and ≥9 for abnormal.43 Since these criteria were based on data from studies mainly from Asian countries, it is uncertain if these cutoff values may be applicable to other racial/ethnic groups. Nevertheless, a number of cross-sectional and longitudinal studies have indicated that CAVI can be used to evaluate CAD and that it is a predictive marker of future cardiovascular events.
In clinical practice, CAVI can be suitable as an early screening tool. Patients with CAVI scores ≥9 can be considered to be at high cardiovascular risk, and clinicians and patients should take this information into account for further risk stratification. Some clinical scenarios in which arterial stiffness measurements can aid in risk stratification have been discussed previously (Table 1).44
Table 1.
Suggested clinical applications of measurements of CAVI in primordial and primary prevention of cardiovascular disease
| Clinical scenario | Rationale | Impact |
|---|---|---|
| Hypertension | ||
| ACC/AHA Stage 1 Hypertension (130–139/80–89 mm Hg) with PCE-calculated 10-yr ASCVD risk ~10% without diabetes or CKD. | LAS can be useful to refine risk stratification when PCE-calculated 10-yr ASCVD risk is close to the threshold for treatment, after an informed clinician patient discussion. | • Initiation of pharmacologic antihypertensive therapy |
| Stage 2 isolated systolic hypertension (>140 mm Hg) in very young adults with paucity of other cardiovascular risk factors. | The combination of high pulse pressure amplification (with normal central systolic pressure) and low or normal LAS for age support a low CV risk. | • Withholding of pharmacologic antihypertensive therapy |
| Nonhypertensive adults <40 yr of age with family histories of ISH. | LAS is partially heritable. LAS precedes and predicts the development of ISH, a potentially avoidable threshold in the life course of cardiovascular disease. A high PWV for age is consistent with early vascular aging. | • Guide clinician–patient risk discussions • Intensification of lifestyle interventions • More frequent assessments of cardiovascular risk before age 40 (<4 to 6 yr) |
| Other CV risk-assessment scenarios | ||
| Refinement of cardiovascular risk assessment in nondiabetic adults 40–75 yr of age at intermediate PCE-calculated 10-yr ASCVD risk. | In this group of patients, risk-based decisions for preventative intervention may be uncertain, and LAS measurements can be used to refine risk assessment (particularly if various “risk-enhancing” clinical parameters do not clearly favor a specific course of action). | • Guide clinician–patient risk discussion • Guide decision-making regarding initiation of pharmacologic therapy (i.e., statins) |
| Refinement of cardiovascular risk assessment in middle-aged nondiabetic adults at borderline PCE-calculated 10-yr ASCVD (5% to <7.5%) who also have other factors that increase their ASCVD risk (“risk enhancers”). | In this group of patients, LAS measurements may be useful to improve risk-based decisions as an alternative or as a “gate-keeper” for coronary calcium score testing, particularly when concerns about radiation exposure (younger age overweight/obese) or about cost are present. | • Guide clinician–patient risk discussion • Guide decision-making regarding further testing (coronary calcium score) or initiation of therapy (i.e., statins) |
| Assessment of CV risk in special populations. | PCE-calculated 10-yr risk estimations can provide notoriously miscalibrated estimates in non-US populations, particularly those at earlier stages of the epidemiologic transition. This may also apply to immigrants from those populations in the United States. | • Guide clinician–patient risk discussion and various interventions • An abnormal PWV for age indicates subclinical arterial damage and suggests a higher risk. Expert clinical judgment must guide result interpretation and decision-making depending on the specific clinical scenario |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; CAVI, Cardio-Ankle Vascular Index; CKD, chronic kidney disease; CV, cardiovascular; ISH, isolated systolic hypertension; LAS, left atrial size; PCE, pulled cohort equations; PWV, pulse wave velocity. Modified from ref. 44.
FACTORS ASSOCIATED WITH CAVI
Association with usual clinical factors
Age is the variable most strongly associated with CAVI, and CAVI is higher in men than in women.45,46 In 1 study, age, sex, body mass index (BMI), and systolic BP accounted for 54% of the interindividual variation in CAVI; while hypertension, diabetes, and chronic kidney disease accounted for an additional 2%.47 The modest effect of cardiovascular risk factors on interindividual variation in CAVI highlights the distinct pathophysiology of arterial stiffness and demonstrates that arterial stiffness may be an early signal of vascular dysfunction.
Similar to other measures of arterial stiffness, CAVI is inversely, rather than directly, associated with BMI. This has been attributed to the vascular adaptation to obesity48 that includes decreased peripheral vascular resistance and vasodilation to accommodate greater hemodynamic arterial load for a larger body size. Additionally, increased intraabdominal pressure may partially offset the effect of intraluminal pressure on the arterial wall (i.e., distending pressure). However, the association between arterial stiffness and obesity remains controversial.49–51 Insulin is a potent vasodilator, and the association of obesity-related insulin resistance with lesser insulin-induced reduction of arterial stiffness has been reported.52 Arterial stiffness is greater in patients with chronic kidney disease due to endothelial dysfunction, increased oxidative stress, and/or the activation of the renin–angiotensin–aldosterone system.53 CAVI is more strongly associated with chronic kidney disease than factors such as hypertension, hyperlipidemia, smoking, or diabetes. The kidneys are a low-resistance and high-flow organ that receives ~20% of cardiac output. Arterial stiffening can damage glomeruli by increasing renal pulsatile stress.
Association with peripheral artery disease. ABI
Since CAVI can be obtained simultaneously with ABI, it is important to note the utility of this additional measure. ABI is considered a useful indicator for lower-extremity peripheral artery disease.54 An ABI ≤0.9 is considered low and ABI >0.9 to 1.0 borderline low. High ABI (>1.4) indicates the presence of medial arterial calcification and is also associated with poor prognosis.55 ABI is associated with CAD risk; most commonly among men, the effect is to down-classify high-risk men; among women, the effect is to up-classify low-risk women.
Furthermore, ABI tends to be lower in women and in African Americans, and sex- and race-specific cutoffs for defining an abnormal ABI may be needed. Finally, in people with diabetes, ABI might be falsely elevated due to medial arterial calcification.
Arterial stiffness as a tool to assess individuals at risk or patients with hypertension
Individuals at risk of hypertension may benefit from the evaluation of arterial stiffness since arterial stiffness appears to be positioned upstream of hypertension. Indeed, current literature indicates that elevated arterial stiffness (e.g., cfPWV) predates the development of hypertension.15 Also, the evaluation of arterial stiffness may be informative in patients with hypertension since elevated arterial stiffness will result in the transmission of pulsatile pressure and flow into the end organs. Indeed, a meta-analysis has demonstrated the independent associations of arterial stiffness with incident cardiovascular outcomes such as CAD and stroke.56 Results from the Framingham Heart Study, higher arterial stiffness assessed by cfPWV was associated with increased cardiovascular risk, and improved risk prediction when added to standard risk factors in a large population.57 Of note, a recent study has demonstrated that heart failure (HF) is the cardiovascular outcome most potently related to elevated arterial stiffness.58 Several studies have also reported the link between arterial stiffness and other adverse outcomes such as kidney disease, carotid stiffness/stroke, and dementia.
Most of the aforementioned studies examined cfPWV, but several studies have confirmed similar results for CAVI. For example, a recent meta-analysis has identified several prospective studies for cardiovascular outcomes, and a few studies have reported the associations of higher CAVI with reduced kidney function and impaired cognitive function.59,60 However, it should be noted that measures of arterial stiffness including CAVI have been less strongly associated with cardiovascular outcomes in older adults compared with younger populations.35,56 This suggests that the evaluation of CAVI may be especially useful in younger adults not only because arterial stiffness appears as more of an anomaly in the young but also because arterial stiffness identified at a relatively early stage can be associated with a longer course of interplay throughout individual lifespan, resulting in more profound downstream changes in health. This concept aligns with the fact that arterial stiffness tends to occur in the upstream of the continuum of pathophysiology for a subset of the hypertensive population.
Associations with cardiac structure and function changes
Associations with left ventricular function and geometry
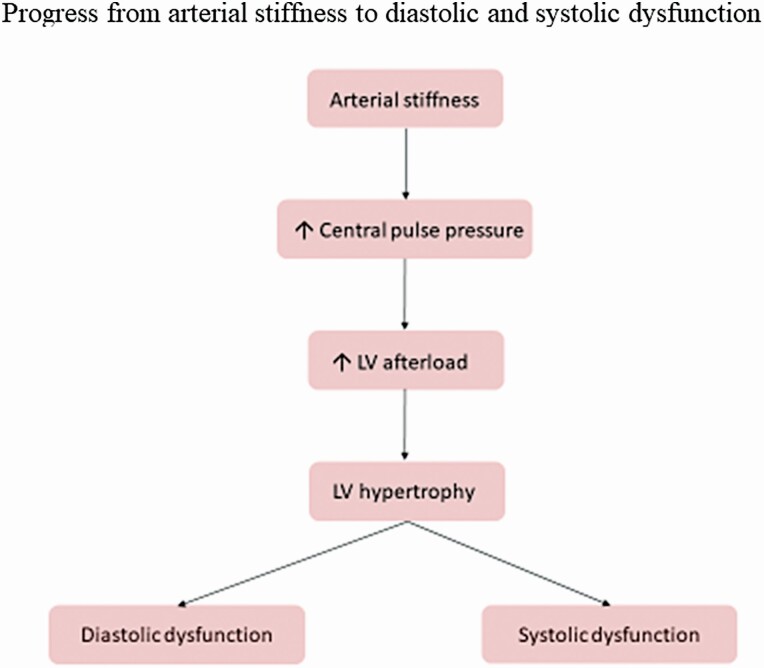
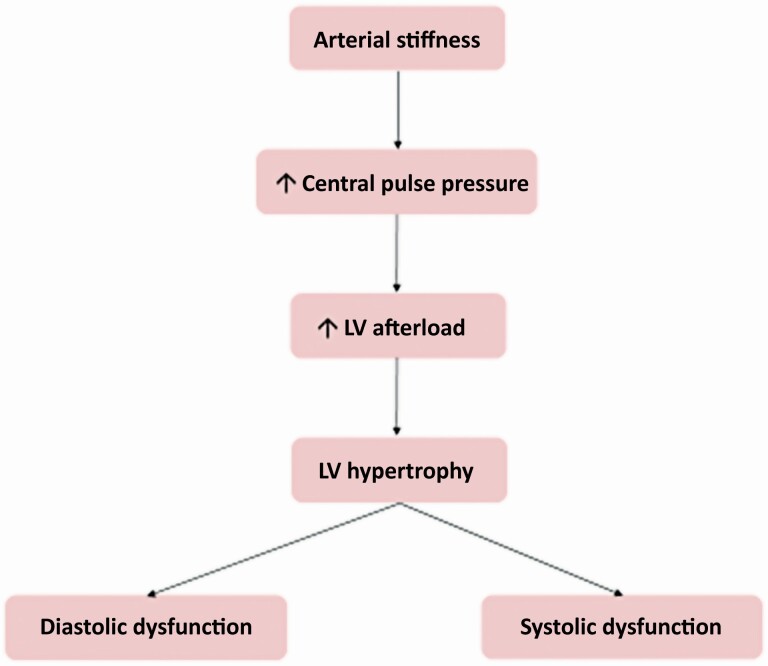
Arterial stiffness increases central pulse pressure and left ventricular (LV) afterload as suggested by the findings of an association between PWV and LV hypertrophy in patients with obstructive sleep apnea and/or hypertension and of a correlation between plasma B natriuretic peptide levels and pulse pressure; this stimulates the development of LV hypertrophy, fibrosis, diastolic and systolic dysfunction (Figure 1).61,62
Figure 1.
A demonstration of the typical progression clinically from arterial stiffness to heart failure, both preserved (diastolic dysfunction) and reduced (systolic dysfunction).
In a prospective study of individuals without known cardiovascular disease, higher CAVI was associated with greater relative wall thickness, worse systolic function and worse diastolic relaxation assessed by tissue Doppler, independent of bloods pressure and conventional risk factors. In addition, sex differences for associations of CAVI with cardiac function were present. CAVI was associated with LV mass index in women, whereas a stronger association of higher CAVI with greater early diastolic filling was present in men than in women.
Higher CAVI was associated with impaired LV longitudinal movement independent of LV ejection fraction.63 Interestingly, despite the association of higher CAVI with worse e′ (mitral annular early diastolic velocity) in both sexes, the association of CAVI with diastolic filling was stronger in men than in women. Increased diastolic filling is an adaptive response of LV to compensate for impaired diastolic relaxation. This study also demonstrated a stronger association of higher CAVI with greater LV mass index in women than men, suggesting greater susceptibility of diastolic relaxation and concentric geometry to altered arterial stiffness. CAVI can be used to assess the impact of LV afterload on cardiac function and structure, and may predict adverse cardiovascular outcomes.
Diastolic dysfunction
LV diastolic dysfunction is common in patients with hypertension.64 Diastolic dysfunction is also an important characteristic of heart failure with preserved ejection fraction (HFpEF).65 Arterial stiffness has an independent relationship with diastolic dysfunction in a number of studies. In patients with hypertensive heart disease, arterial stiffness (measured using the pulse pressure method) was directly and significantly related to diastolic dysfunction. This relationship persisted after adjustment for age, sex, body size, BP, and LV hypertrophy.66 Arterial stiffness and BP were the only independent predictors of diastolic dysfunction in this study.66
Data from a cross-sectional study of patients undergoing echocardiography showed that arterial stiffness determined by baPWV was an independent predictor of early diastolic mitral velocity and LV diastolic dysfunction, on multivariate analysis.67 Higher baPWV values were associated with lower early diastolic mitral velocity and worse LV diastolic function.67 Using baPWV, a Chinese study of older adults with a normal LV ejection fraction found that arterial stiffness was a better predictor of LV diastolic dysfunction than both pulse pressure and mean arterial pressure.68 Another study conducted in healthy individuals found an independent association between baPWV and measures of LV diastolic function in elderly women.69 Furthermore, the risk of moderate LV diastolic dysfunction was higher in the presence of increased PWV in a population study.70 In patients with HF or HF risk factors who had a normal ejection fraction, arterial stiffness measured using the cfPWV and age were the strongest independent predictors of LV filling pressure (a marker of diastolic dysfunction).71
CAVI has been shown to have better accuracy for detecting diastolic dysfunction in patients with hypertension than assessment of the ambulatory arterial stiffness index (determined using ambulatory BP monitoring).72 In a cross-sectional study of patients with clinical cardiovascular disease that used CAVI, arterial stiffness was independently associated with LV diastolic dysfunction.73 Similar findings were reported in another study of patients with cardiovascular risk factors.74 In treated hypertensive patients, CAVI was independently associated with the Tei index, a measure of LV systolic and diastolic function.44 Significant relationships between CAVI and echocardiographic parameters of LV diastolic dysfunction have also been documented in subjects referred for echocardiography.75 The odds ratio for the prevalence of LV diastolic dysfunction increased in parallel with increasing CAVI, even after adjustment for confounding variables, including age, BMI, hypertension, use of renin–angiotensin system inhibitors, and left atrial diameter. The adjusted odds ratio (95% confidence interval) for having diastolic dysfunction, when CAVI was in the highest (9.6–11.4) vs. lowest (6.7–7.7) quartile, was 4.94 (1.02–23.4).75 In patients with HFpEF, CAVI was significantly associated with echocardiographic measures of LV diastolic dysfunction.76
Taken together, available data suggest that increased arterial stiffness provides a potential mechanistic link between vascular changes and the development of diastolic dysfunction. The effect of arterial stiffness on LV diastolic function is likely mediated by premature wave reflection, which increases mid-to-late systolic load. Late systolic load has been shown to cause profound diastolic dysfunction and remodeling in experimental studies, and these observations are supported by multiple human studies as reviewed in.
Left atrial remodeling
Left atrial volume index (LAVI) is a useful predictor of cardiovascular risk and outcomes.77 In a study of young adults with suspected CAD, those with CAVI above age- and sex-specific cutoff values had a significantly higher LAVI than patients with a normal CAVI.78 In addition, a significant correlation between arterial stiffness and left atrial diameter has been documented in patients with hypertension, independent of traditional risk factors including age, sex, BMI, ventricular remodeling, and filling pressure.79 Also in patients with hypertension, data from a cross-sectional study showed that arterial stiffness, determined using PWV, was an independent predictor of LAVI—increased arterial stiffness was correlated with left atrial enlargement.80 Arterial stiffness also appears to be related to left atrial structure and function in the absence of clinically evident LV dysfunction. A study in healthy women showed that cfPWV was significantly correlated with relative wall thickness, global circumferential strain, apical rotation, and LV twist (all after adjustment for age, BMI, BP, and heart rate).81 Arterial stiffness (determined using baPWV or cfPWV) has been shown to predict readmission in patients with HF.82–86 Associations have also been reported between PWV and HF-related events in both HFpEF and heart failure with reduce ejection fraction (HFrEF).87,88 Therefore, measures of arterial stiffness could be useful for a better risk stratification in HF patients. Further research will help to clarify the potential of arterial stiffness as a biomarker and therapeutic target in this setting.
Association with cardiovascular autonomic neuropathy
Cardiac autonomic neuropathy (CAN) plays a significant role in cardiovascular outcomes, and more so where arterial stiffness is present. Additionally, autonomic tone can affect arterial stiffness acutely. In diabetes, CAN is defined as an impairment of autonomic control of the cardiovascular system, which typically manifests initially as a vagal defect and sympathetic overactivity, and at later stages, as a progressing attenuation of sympathetic activity.89 Some experimental data in rats support the protective role of vagal activity and an aggravating role of sympathetic predominance in arterial stiffness.90,91 CAN may contribute to cardiovascular changes and impair the prognosis. A meta-analysis of 15 longitudinal studies showed that the presence of CAN was associated with a relative risk of mortality of 3.45.92 Several disorders associated with subclinical CAN may contribute to a negative cardiovascular prognosis in the people with diabetes.
In a study including patients with type 1 diabetes and normoalbuminuria, the presence of CAN was associated with higher pulse pressure, an indirect marker of arterial stiffness, and more prevalent nondipping, together with alterations of systolic and diastolic function of the left ventricle.93 In obese and diabetic hypertensive patients, power spectral analysis of recorded continuous BP measurements showed that the low-frequency peak of systolic BP variations in the standing position, which reflects sympathetic activity, correlated significantly with pulse pressure measured in the supine position. This represents further evidence for the role of a higher sympathetic activity in arterial stiffness.94 In healthy individuals, resting muscle sympathetic nerve activity was found to correlate with PWV, and an acute elevation of muscle sympathetic nerve activity elicited by lower body negative pressure produced significant increase in PWV.95,96 In the Pittsburgh Epidemiology of Diabetes Complications study, CAN was assessed in a childhood-onset population of patients with type 1 diabetes, and was associated with the increased arterial stiffness measured 18 years later.97 In patients with type 2 diabetes arterial stiffness was also found to be associated with peripheral neuropathy and retinopathy, and might be a marker of microangiopathic complications.98 An association between endothelial dysfunction and arterial stiffness evaluated by mean 24-hour pulse pressure has been reported in hypertensive and diabetic patients.99,100 Type 2 diabetes on top of hypertension might worsen arterial stiffness by endothelium-related mechanisms.101
Association with CAD. Clinical studies using CAVI and computed tomography angiography
CAVI has been applied clinically to assess arterial stiffness in patients who were diagnosed with CAD, stroke, and those at risk. Structural and vascular changes of the vessel wall have been implicated in the pathogenesis of atherosclerosis, which results in reduced vascular distensibility and elevated arterial stiffness.102,103 Arterial stiffness is an independent predictor of CAD, and is correlated with the severity of coronary stenosis.104
Several studies have demonstrated the relationship of CAVI with atherosclerosis and obstructive artery disease. CAVI is independently associated with the progression and severity of coronary atherosclerosis.105 Furthermore, the significance of CAVI >8 with obstructive CAD (degree of stenosis >50%—CAVI cutoff value: 7.6 and coronary artery calcium [CAC] score >300—CAVI cutoff value: 8.1) was reported in Korean population.106 In a recent study that enrolled 285 individuals clinically referred for CAC scoring and Coronary Computed Tomography Angiography (CCTA), the association of CAVI with severe stenosis (>50%) and CAC >100 was evaluated. The degree of CAC and severe coronary stenosis demonstrated significant correlation with CAVI. Receiver operating characteristic curve analysis indicated that a CAVI measure of 7.8 was an optimal cut-point for sensitivity and specificity in detecting obstructive CAD. Logistic regression demonstrated that CAVI >7.8 is significantly associated with obstructive CAD and CAC score >100. Arterial stiffness, as represented by CAVI is significantly and strongly correlated with both CAC and coronary stenosis.107 Thus, CAVI may be used as screening tool for early prediction of obstructive CAD and plays a critical role in preventive cardiology to optimize management. This approach may be especially relevant in younger adults since most of them have zero CAC.
AVAILABLE TREATMENTS
The field of large artery stiffness has witnessed a large number of epidemiological investigations and a large body of basic science using a variety of animal models and ex vivo studies. The entanglement of arterial stiffness, especially when expressed by PWV, with mean arterial pressure has challenged investigators in differentiating the effect of an intervention on large artery stiffness independent of the effect of an intervention on BP.31 In addition, true improvements in arterial stiffness, not just declines in PWV that occur with BP lowering, likely require interventions employed over months or even years to allow sufficient remodeling of large arteries.
Physical exercise
Regular exercise provides a number of health benefits including improvements in functional capacity, traditional risk factors, and mental health. Various exercise protocols have been employed to determine the effect on arterial stiffness. These fall into 2 broad categories: (i) aerobic exercise that is characterized by dynamic, rhythmic, whole-body physical activities that increase oxygen consumption and (ii) resistance exercise that is characterized by muscle contracting activities against an external resistance such as barbell weights or stretch bands.
In the Baltimore Longitudinal Study of Aging, older adults with a history of intense habitual aerobic exercise (running) for years demonstrated 25% lower artery stiffness as assessed by aortic PWV compared with age and BP matched sedentary counterparts.108 Similarly, in participants aged 66–90 years in the Atherosclerosis Risk in Communities study, habitual physical activity was associated with a reduced change in cfPWV with age.109 In a European study of older people without hypertension at the time of study enrollment, habitual aerobic exercise blunted the age-related increases in large artery stiffness.110
The findings in the exercise intervention studies are generally consistent with the community-based observational studies.111 Reductions in arterial stiffness have been observed in absence of BP decrease. Older age (the eighth decade of life)112 and comorbidities like hypertension may require longer intervention periods to give rise to clinically significant changes in arterial stiffness. One notion that should be kept in mind when prescribing exercise is that “In the pharmacological treatment of hypertension, it is a standard practice to change the class of antihypertensive medications if one drug is found to be ineffective. In the exercise prescription, however, it is not a common practice to change the mode of exercise when one mode of exercise fails to improve.” 111
Resistance exercise studies initially suggested that arterial stiffness increases after a few months of these interventions.112 However, a meta-analysis of literature on resistance training and arterial stiffness observed that this was more likely to occur in younger adults with lower initial arterial stiffness, and less likely to occur when the intensity of the resistance training is moderate compared with intense.113 Two recent meta-analyses found that resistance exercise did not alter arterial stiffness in healthy subjects or in persons at risk for cardiovascular disease.114,115
Physiological mechanisms by which aerobic exercise interventions could improve arterial stiffness include reductions in sympathetic vasoconstrictor tone on large arteries, and improvements in the biomarker profiles of agents such as endothelins and inflammatory cytokines.111
Weight loss
In a population of relatively young (20–45 years) adults enrolled in the Slow Adverse Vascular Effects of excess weight study (SAVE), a 7% decrease in body weight was associated with a reduction of ~0.5 m/s in cfPWV.116 Reductions in body weight are typically accompanied by the corresponding decrease in BP in obese patients with hypertension.11 Disentangling the benefits of weight loss on BP from the potential benefits of weight loss on arterial stiffness can be challenging. One way of possibly evaluating improvements in arterial stiffness independently of BP changes is using the CAVI measurement, which incorporates a factor to dampen the role of BP in assessing vascular stiffness.117 A 12-week diet intervention produced a decrease in BMI from 33.3 to 30.7 kg/m2, and this was associated with a significant reduction in CAVI from 8.4 to 7.9 in a study of obese Japanese subjects.118 Mechanisms linking weight loss to improvements in arterial stiffness include reductions in inflammatory cytokines like C-reactive protein and interleukin-6. However, a recent study demonstrated that CAVI and heart-ankle PWV increased after bariatric surgery, probably reflecting the complex association between adiposity and arterial stiffness measures, as noted above.
Salt restriction
A reduction in sodium intake has been a consistent recommendation in BP guidelines to improve BP control in hypertensive patients.11 A meta-analysis of studies designed to examine the effect of a reduction in sodium intake on PWV noted a small but significant fall in PWV with a 90 mEq/day sodium intake on average.119 In these studies, the effect of sodium reduction on arterial stiffness was independent of BP changes.119
Pharmacological agents
The literature describing the effect of drugs on arterial stiffness is large and challenging to summarize. Table 2 summarizes pharmacologic agent classes and their effects on arterial stiffness. We made an attempt to evaluate the effect of the agent class independent of the effects on BP or heart rate. Generally, when mean arterial pressure is reduced by a drug, arterial stiffness will be lowered as well.31
Table 2.
| Agent class | Effect(s) on arterial stiffness | Comments |
|---|---|---|
| ACE inhibitors | ↓ | Likely among the strongest reducers of arterial stiffness |
| Aldosterone antagonist | ↓ | |
| α-Blockers | ↓ | |
| ARB | ↓ | Likely among the strongest reducers of arterial stiffness |
| β-Blockers | ↔/↓ | Heterogenous group of drugs |
| CCB | ↔/↓ | ? arterial stiffness improvement offset by sympathetic activation |
| Diuretics | ↔/↓ | Long-term trials show arterial stiffness reduction |
| Antihyperglycemics | ↔/↓ | Thioglitazones, metformin, and SGLT2 inhibitors seem to improve stiffness |
| Nitrates | ↓ | Short acting |
| Statins | ↔/↓ | Probably have greater destiffening effect on peripheral vs central arteries; seem more effective when inflammation also present |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blockers; SGLT2, sodium glucose cotransporter 2.
CURRENT GUIDELINES
The accurate assessment of cardiovascular risk is a cornerstone of contemporary preventive medicine. The current cardiovascular risk prediction includes scoring systems built on a variety of cardiovascular risk factors. The classical approach has been less than optimal, since current population-based risk algorithms tend to overestimate risk, and none of the predictors included in the scoring systems addresses cardiovascular structure or function. Could a risk stratification using arterial stiffness lead to a better classification of cardiovascular risk? Arterial stiffness, including CAVI, has an independent predictive value for cardiovascular events as an intermediate endpoint, above and beyond traditional risk factors.56 CAVI is elevated in a variety of populations that are at high risk of developing cardiovascular disease.121,122 Additionally, as described below in more detail, CAVI has been demonstrated to respond to a number of interventions and treatments that act to reduce cardiovascular risks, including smoking cessation, exercise training, weight reduction, and various pharmacological agents.121,123
In 2013, the European Society of Hypertension/European Society of Cardiology published guidelines for the management of arterial hypertension.124 In these guidelines, the measurement of arterial stiffness based on cfPWV was recommended for evaluation of “subclinical organ damage in patients with hypertension.” 124 In the updated guidelines in 2018, however, cfPWV was not recommended for routine clinical settings as it was deemed not clinically practical.10 Although cfPWV is considered a reference standard measure of arterial stiffness, this methodology is hindered by the technical precision required for carotid pulse acquisition and the intimate nature of femoral pulse acquisition on the groin area. This is one of the reasons why many devices designed to measure aortic PWV have evolved to incorporate the use of thigh cuffs to replace the tonometric transducers for detecting pulses at the 2 femoral pulsation points. CAVI, as measured using the VaSera device (model VS-1500 or VS-2000, Fukuda Denshi), has been proposed as an arterial stiffness index with a lesser dependence on BP at the time of measurement. Because of the procedural advantage of its ease-of-use, it is one of few methodologies that has a higher potential for being incorporated into routine clinical settings. Large studies using CAVI in the United States and Europe are ongoing.
CLINICAL PRACTICE GUIDANCE USING CAVI
Detection of arterial stiffness now has strong implications for enriching the assessment of individuals with hypertension, obesity, and diabetes. Published research spanning more than 30 years and published medical guidelines specific to arterial stiffness have clearly defined its clinical value. However, arterial stiffness is still a new and underused metric for clinics of the United States.125
This proof of concept was just published in the SPARTE (Strategy for Preventing cardiovascular and renal events based on ARTErial stiffness) study.126 Patients with primary hypertension were assigned to a strategy targeting the normalization of PWV measured every 6 months (PWV group, n = 264) vs. a strategy implementing the European Guidelines for Hypertension Treatment (conventional group, n = 272). In the PWV group, renin–angiotensin–system blockers and calcium channel blockers were prescribed at higher dosage (P = 0.028), PWV increased less (P = 0.0003) than in the conventional group, and office and ambulatory systolic BP and diastolic BP decreased more (P < 0.001 and P < 0.01, respectively). The SPARTE study demonstrated that a PWV-driven treatment for hypertension allows for further reduction in office and ambulatory systolic and diastolic BPs and prevent vascular aging in patients with hypertension.
Data collected from 663 hospitals in the American College of Cardiology National Cardiovascular Data Registry on 400,000 patients revealed that the yield of positive elective cardiac catheterizations for patients without known CAD is only a little more than one third.127 Having the means to improving medical evaluations at outpatient clinics and doctors’ offices can improve this yield by as much as 27% as was demonstrated in a large population study in Thailand where CAVI was coupled to a Framingham-based risk score,21 and thereby lower a portion of healthcare costs, and improve referral decisions, from general practice to cardiology.
For CAVI, a cutoff value of 8.0 is suggested, based on prior published research, and best suited for general practice, and frontline clinical assessments of patients with unknown cardiac status, and who are asymptomatic for cardiovascular disease. This guidance for the management echoes the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension,10 in identifying and risk-stratifying patients whose hypertension is more likely linked to greater cardiovascular disease risk, having hypertension-mediated organ damage, and where underlying arteriosclerosis may be assessed with a PWV-based measurement. Though US medical guidelines for management of hypertension already categorize risk according to hypertensive class, our algorithm suggests that inclusions of arterial stiffness testing with CAVI can further subclassify “heightened risk,” where CAD and HF may be more likely comorbidities.
Detecting arterial stiffness and other early indicators of cardiovascular disease is a reasonable first-level approach to medical management of people at risk of cardiovascular events. To the degree that measurements of CAVI relate to cardiovascular risk independently of traditional risk factors, they can be used to enhance cardiovascular risk assessments in specific clinical scenarios for primary and primordial prevention. Suggested clinical applications are summarized in Table 2. These include clinical scenarios in which a refinement of cardiovascular risk can decision-making for intensification of lifestyle interventions, pharmacologic therapy (with statins or antihypertensive agents), guide clinician–patient discussions and/or impact the frequency of cardiovascular risk assessments (particularly in younger individuals). CAVI may also aid in the evaluation of younger individuals with a family history of isolated systolic hypertension, given that a substantial proportion of the variability in large artery stiffness is inheritable.9
CONCLUSIONS AND SUMMARY
Having the means to improving medical evaluations at outpatient clinics and doctors’ offices, can improve risk stratification and utilization of additional testing. Detecting arterial stiffness and other early indicators of cardiovascular disease is a reasonable first-level approach to medical management of people at risk of cardiovascular events. We suggest that CAVI is a useful frontline test, appropriate for outpatient clinics, for identifying patients who might otherwise go unnoticed for coronary vessel risk. As such, CAVI could be used effectively to improve referral decisions to more centralized cardiac evaluation. This proposed scenario is even more pertinent to patients with diabetes, hypertension, and kidney dysfunction, where cardiovascular risk is higher.
FUNDING
None.
DISCLOSURE
K. Kario received research grant from Fukuda Denshi. I. Kullo is a Consultant for Fukuda Denshi. No other author has conflicts of interest.
REFERENCES
- 1. Segers P, Rietzschel ER, Chirinos JA. How to measure arterial stiffness in humans. Arterioscler Thromb Vasc Biol 2020; 40:1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avolio A. Arterial stiffness. Pulse (Basel) 2013; 1:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nichols WW, O'Rourke M, Vlachopoulos C.. McDonald’s Blood Flow in Arteries. Hodder Arnold: London, 2011. [Google Scholar]
- 4. Peterson LH, Jensen RE, Parnell J. Mechanical properties of arteries in vivo. Circ Res 1960; 8:622–639. [Google Scholar]
- 5. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 7. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 8. Karamanou M, Papaioannou TG, Tsoucalas G, Tousoulis D, Stefanadis C, Androutsos G. Blood pressure measurement: lessons learned from our ancestors. Curr Pharm Des 2015; 21:700–704. [DOI] [PubMed] [Google Scholar]
- 9. He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219. [DOI] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members: . 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 11. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 12. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 13. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Hansen TW, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2008; 51:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study . Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005; 111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 17. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 18. Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY; CRIC Study Investigators . Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 2018; 71:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pase MP, Herbert A, Grima NA, Pipingas A, O’Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern Med J 2012; 42:808–815. [DOI] [PubMed] [Google Scholar]
- 20. Munakata M, Konno S, Miura Y, Yoshinaga K; J-TOPP Study Group . Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res 2012; 35:839–842. [DOI] [PubMed] [Google Scholar]
- 21. Yingchoncharoen T, Limpijankit T, Jongjirasiri S, Laothamatas J, Yamwong S, Sritara P. Arterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score). Heart Asia 2012; 4:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002; 106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 23. Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson 2014; 16:33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships among various measures of central artery stiffness. Am J Hypertens 2016; 29:1024–1028. [DOI] [PubMed] [Google Scholar]
- 25. Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb 2011; 18:924–938. [DOI] [PubMed] [Google Scholar]
- 26. Ibata J, Sasaki H, Kakimoto T, Matsuno S, Nakatani M, Kobayashi M, Tatsumi K, Nakano Y, Wakasaki H, Furuta H, Nishi M, Nanjo K. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res Clin Pract 2008; 80:265–270. [DOI] [PubMed] [Google Scholar]
- 27. Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J 2007; 71:1710–1714. [DOI] [PubMed] [Google Scholar]
- 28. Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol 2015; 309:R1540–R1545. [DOI] [PubMed] [Google Scholar]
- 29. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017; 69:e71–e126. [DOI] [PubMed] [Google Scholar]
- 30. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 31. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroeder EC, Rosenberg AJ, Hilgenkamp TIM, White DW, Baynard T, Fernhall B. Effect of upper body position on arterial stiffness: influence of hydrostatic pressure and autonomic function. J Hypertens 2017; 35:2454–2461. [DOI] [PubMed] [Google Scholar]
- 33. Otsuka K, Nakanishi K, Shimada K, Nakamura H, Inanami H, Nishioka H, Fujimoto K, Kasayuki N, Yoshiyama M. Ankle-brachial index, arterial stiffness, and biomarkers in the prediction of mortality and outcomes in patients with end-stage kidney disease. Clin Cardiol 2019; 42:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taniguchi Y, Kitamura A, Shinozaki T, Seino S, Yokoyama Y, Narita M, Amano H, Matsuyama Y, Fujiwara Y, Shinkai S. Trajectories of arterial stiffness and all-cause mortality among community-dwelling older Japanese. Geriatr Gerontol Int 2018; 18:1108–1113. [DOI] [PubMed] [Google Scholar]
- 35. Matsushita K, Ding N, Kim ED, Budoff M, Chirinos JA, Fernhall B, Hamburg NM, Kario K, Miyoshi T, Tanaka H, Townsend R. Cardio-ankle vascular index and cardiovascular disease: systematic review and meta-analysis of prospective and cross-sectional studies. J Clin Hypertens (Greenwich) 2019; 21:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz JE, Feig PU, Izzo JL Jr. Pulse wave velocities derived from cuff ambulatory pulse wave analysis. Hypertension 2019; 74:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kario K, Kanegae H, Oikawa T, Suzuki K. Hypertension is predicted by both large and small artery disease. Hypertension 2019; 73:75–83. [DOI] [PubMed] [Google Scholar]
- 38. Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary artery calcium score compared with cardio-ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb 2015; 22:1255–1265. [DOI] [PubMed] [Google Scholar]
- 39. Kubota Y, Maebuchi D, Takei M. Cardio-Ankle Vascular Index is a predictor of cardiovascular events. Artery Res 2011; 5:91–96. [Google Scholar]
- 40. Laucevičius A, Ryliškytė L, Balsytė J, Badarienė J, Puronaitė R, Navickas R, Solovjova S. Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina (Kaunas) 2015; 51:152–158. [DOI] [PubMed] [Google Scholar]
- 41. Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I. Cardio-Ankle Vascular Index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb 2016; 23:596–605. [DOI] [PubMed] [Google Scholar]
- 42. Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A; Japan Obesity and Metabolic Syndrome Study (JOMS) Group . Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis 2015; 242:461–468. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, Ueda S, Vlachopoulos C, Higashi Y, Inoue T, Node K; Physiological Diagnosis Criteria for Vascular Failure Committee . Physiological diagnostic criteria for vascular failure. Hypertension 2018; 72:1060–1071. [DOI] [PubMed] [Google Scholar]
- 44. Masugata H, Senda S, Okuyama H, Murao K, Inukai M, Hosomi N, Yukiiri K, Nishiyama A, Kohno M, Goda F. Comparison of central blood pressure and cardio-ankle vascular index for association with cardiac function in treated hypertensive patients. Hypertens Res 2009; 32:1136–1142. [DOI] [PubMed] [Google Scholar]
- 45. Choi SY, Oh BH, Bae Park J, Choi DJ, Rhee MY, Park S. Age-associated increase in arterial stiffness measured according to the cardio-ankle vascular index without blood pressure changes in healthy adults. J Atheroscler Thromb 2013; 20:911–923. [DOI] [PubMed] [Google Scholar]
- 46. Kadota K, Takamura N, Aoyagi K, Yamasaki H, Usa T, Nakazato M, Maeda T, Wada M, Nakashima K, Abe K, Takeshima F, Ozono Y. Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circ J 2008; 72:304–308. [DOI] [PubMed] [Google Scholar]
- 47. Ye Z, Pellikka PA, Kullo IJ. Sex differences in associations of cardio-ankle vascular index with left ventricular function and geometry. Vasc Med 2017; 22:465–472. [DOI] [PubMed] [Google Scholar]
- 48. Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging 2008; 28:287–293. [DOI] [PubMed] [Google Scholar]
- 49. Dahlén EM, Bjarnegård N, Länne T, Nystrom FH, Ostgren CJ. Sagittal abdominal diameter is a more independent measure compared with waist circumference to predict arterial stiffness in subjects with type 2 diabetes—a prospective observational cohort study. Cardiovasc Diabetol 2013; 12:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scuteri A, Orru’ M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis 2012; 221:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nordstrand N, Gjevestad E, Dinh KN, Hofso D, Roislien J, Saltvedt E, Os I, Hjelmesaeth J. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord 2011; 11:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Westerbacka J, Seppälä-Lindroos A, Yki-Järvinen H. Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab 2001; 86:5262–5268. [DOI] [PubMed] [Google Scholar]
- 53. Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 2004; 43:163–168. [DOI] [PubMed] [Google Scholar]
- 54. Kullo IJ, Rooke TW. Clinical Practice. Peripheral artery disease. N Engl J Med 2016; 374:861–871. [DOI] [PubMed] [Google Scholar]
- 55. Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, Kullo IJ. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol 2012; 59:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim ED, Ballew SH, Tanaka H, Heiss G, Coresh J, Matsushita K. Short-Term prognostic impact of arterial stiffness in older adults without prevalent cardiovascular disease. Hypertension 2019; 74:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yukutake T, Yamada M, Fukutani N, Nishiguchi S, Kayama H, Tanigawa T, Adachi D, Hotta T, Morino S, Tashiro Y, Arai H, Aoyama T. Arterial stiffness determined according to the cardio-ankle vascular index(CAVI) is associated with mild cognitive decline in community-dwelling elderly subjects. J Atheroscler Thromb 2014; 21:49–55. [DOI] [PubMed] [Google Scholar]
- 60. Satirapoj B, Triwatana W, Supasyndh O. Arterial Stiffness predicts rapid decline in glomerular filtration rate among patients with high cardiovascular risks. J Atheroscler Thromb 2020; 27:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schillaci G, Battista F, Settimi L, Anastasio F, Pucci G. Cardio-ankle vascular index and subclinical heart disease. Hypertens Res 2015; 38:68–73. [DOI] [PubMed] [Google Scholar]
- 62. Namba T, Masaki N, Takase B, Adachi T. Arterial stiffness assessed by cardio-ankle vascular index. Int J Mol Sci 2019; 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc 2016; 5:e002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Galderisi M. Diagnosis and management of left ventricular diastolic dysfunction in the hypertensive patient. Am J Hypertens 2011; 24:507–517. [DOI] [PubMed] [Google Scholar]
- 65. Redfield MM. Heart Failure with preserved ejection fraction. N Engl J Med 2016; 375:1868–1877. [DOI] [PubMed] [Google Scholar]
- 66. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart 2005; 91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hsu PC, Tsai WC, Lin TH, Su HM, Voon WC, Lai WT, Sheu SH. Association of arterial stiffness and electrocardiography-determined left ventricular hypertrophy with left ventricular diastolic dysfunction. PLoS One 2012; 7:e49100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu L, Jiang CQ, Lam TH, Yue XJ, Lin JM, Cheng KK, Liu B, Li Jin Y, Zhang WS, Thomas GN. Arterial stiffness and left-ventricular diastolic dysfunction: Guangzhou Biobank Cohort Study-CVD. J Hum Hypertens 2011; 25:152–158. [DOI] [PubMed] [Google Scholar]
- 69. Kim HL, Lim WH, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between arterial stiffness and left ventricular diastolic function in relation to gender and age. Medicine (Baltimore) 2017; 96:e5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cauwenberghs N, Knez J, Tikhonoff V, D’hooge J, Kloch-Badelek M, Thijs L, Stolarz-Skrzypek K, Haddad F, Wojciechowska W, Swierblewska E, Casiglia E, Kawecka-Jaszcz K, Narkiewicz K, Staessen JA, Kuznetsova T; European Project On Genes in Hypertension (EPOGH) Investigators . Doppler indexes of left ventricular systolic and diastolic function in relation to the arterial stiffness in a general population. J Hypertens 2016; 34:762–771. [DOI] [PubMed] [Google Scholar]
- 71. Lüers C, Trippel TD, Seeländer S, Wachter R, Hasenfuss G, Lindhorst R, Bobenko A, Nolte K, Pieske B, Edelmann F. Arterial stiffness and elevated left ventricular filling pressure in patients at risk for the development or a previous diagnosis of HF—a subgroup analysis from the DIAST-CHF study. J Am Soc Hypertens 2017; 11:303–313. [DOI] [PubMed] [Google Scholar]
- 72. Kim H, Kim HS, Yoon HJ, Park HS, Cho YK, Nam CW, Hur SH, Kim YN, Kim KB. Association of cardio-ankle vascular index with diastolic heart function in hypertensive patients. Clin Exp Hypertens 2014; 36:200–205. [DOI] [PubMed] [Google Scholar]
- 73. Namba T, Masaki N, Matsuo Y, Sato A, Kimura T, Horii S, Yasuda R, Yada H, Kawamura A, Takase B, Adachi T. Arterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular disease. Int Heart J 2016; 57:729–735. [DOI] [PubMed] [Google Scholar]
- 74. Mizuguchi Y, Oishi Y, Tanaka H, Miyoshi H, Ishimoto T, Nagase N, Oki T. Arterial stiffness is associated with left ventricular diastolic function in patients with cardiovascular risk factors: early detection with the use of cardio-ankle vascular index and ultrasonic strain imaging. J Card Fail 2007; 13:744–751. [DOI] [PubMed] [Google Scholar]
- 75. Sakane K, Miyoshi T, Doi M, Hirohata S, Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S, Yamamoto K. Association of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic function. J Atheroscler Thromb 2008; 15:261–268. [DOI] [PubMed] [Google Scholar]
- 76. Noguchi S, Masugata H, Senda S, Ishikawa K, Nakaishi H, Tada A, Inage T, Kajikawa T, Inukai M, Himoto T, Hosomi N, Murakami K, Noma T, Kohno M, Okada H, Goda F, Murao K. Correlation of arterial stiffness to left ventricular function in patients with reduced ejection fraction. Tohoku J Exp Med 2011; 225:145–151. [DOI] [PubMed] [Google Scholar]
- 77. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006; 47:1018–1023. [DOI] [PubMed] [Google Scholar]
- 78. Osawa K, Nakanishi R, Miyoshi T, Rahmani S, Ceponiene I, Nezarat N, Kanisawa M, Qi H, Jayawardena E, Kim N, Ito H, Budoff MJ. Correlation of arterial stiffness with left atrial volume index and left ventricular mass index in young adults: evaluation by coronary computed tomography angiography. Heart Lung Circ 2019; 28:932–938. [DOI] [PubMed] [Google Scholar]
- 79. Lantelme P, Laurent S, Besnard C, Bricca G, Vincent M, Legedz L, Milon H. Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis 2008; 101:35–40. [DOI] [PubMed] [Google Scholar]
- 80. Janwanishstaporn S, Boonyasirinant T. Correlation between aortic stiffness and left atrial volume index in hypertensive patients. Clin Exp Hypertens 2016; 38:160–165. [DOI] [PubMed] [Google Scholar]
- 81. Zhang J, Chowienczyk PJ, Spector TD, Jiang B. Relation of arterial stiffness to left ventricular structure and function in healthy women. Cardiovasc Ultrasound 2018; 16:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meguro T, Nagatomo Y, Nagae A, Seki C, Kondou N, Shibata M, Oda Y. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J 2009; 73:673–680. [DOI] [PubMed] [Google Scholar]
- 83. Cong T, Jiang S, Wang K, Zhong L, Wu J, Su D. Predictive value of brachial-ankle artery pulse wave velocity to heart failure with preserved ejection fraction in hospitalised patients with acute dyspnoea. Pak J Med Sci 2015; 31:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, Berry JD. Arterial Stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC Study (Health, Aging, and Body Composition). Hypertension 2017; 69:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 2015; 4:e002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators; CRIC Study Investigators . Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail 2014; 7:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takae M, Yamamoto E, Tokitsu T, Oike F, Nishihara T, Fujisue K, Sueta D, Usuku H, Motozato K, Ito M, Kanazawa H, Araki S, Nakamura T, Arima Y, Takashio S, Suzuki S, Sakamoto K, Soejima H, Yamabe H, Kaikita K, Tsujita K. Clinical significance of brachial-ankle pulse wave velocity in patients with heart failure with reduced left ventricular ejection fraction. Am J Hypertens 2019; 32:657–667. [DOI] [PubMed] [Google Scholar]
- 88. Tokitsu T, Yamamoto E, Oike F, Hirata Y, Tsujita K, Yamamuro M, Kaikita K, Hokimoto S. Clinical significance of brachial-ankle pulse-wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens 2018; 36:560–568. [DOI] [PubMed] [Google Scholar]
- 89. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P; Toronto Consensus Panel on Diabetic Neuropathy . Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27:639–653. [DOI] [PubMed] [Google Scholar]
- 90. Cosson E, Herisse M, Laude D, Thomas F, Valensi P, Attali JR, Safar ME, Dabire H. Aortic stiffness and pulse pressure amplification in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2007; 292:H2506–H2512. [DOI] [PubMed] [Google Scholar]
- 91. Cosson E, Valensi P, Laude D, Mesangeau D, Dabire H. Arterial stiffness and the autonomic nervous system during the development of Zucker diabetic fatty rats. Diabetes Metab 2009; 35:364–370. [DOI] [PubMed] [Google Scholar]
- 92. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003; 26:1895–1901. [DOI] [PubMed] [Google Scholar]
- 93. Mogensen UM, Jensen T, Køber L, Kelbæk H, Mathiesen AS, Dixen U, Rossing P, Hilsted J, Kofoed KF. Cardiovascular autonomic neuropathy and subclinical cardiovascular disease in normoalbuminuric type 1 diabetic patients. Diabetes 2012; 61:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brahimi M, Dabire H, Platon P, Hadj-Brahim F, Attali JR, Valensi P. [Arterial rigidity and cardiovascular vagosympathetic activity in normotensive and hypertensive obese patients and type 2 diabetics]. Arch Mal Coeur Vaiss 2001; 94:944–946. [PubMed] [Google Scholar]
- 95. Nardone M, Incognito AV, Millar PJ. Evidence for pressure-independent sympathetic modulation of central pulse wave velocity. J Am Heart Assoc 2018; 7:e007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, Fadel PJ, Abboud FM, Pierce GL. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. Hypertension 2019; 73:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010; 33:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen Q, Chiheb S, Fysekidis M, Jaber Y, Brahimi M, Nguyen MT, Millasseau S, Cosson E, Valensi P. Arterial stiffness is elevated in normotensive type 2 diabetic patients with peripheral neuropathy. Nutr Metab Cardiovasc Dis 2015; 25:1041–1049. [DOI] [PubMed] [Google Scholar]
- 99. Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, Sesti G, Perticone F. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol 2003; 41:1753–1758. [DOI] [PubMed] [Google Scholar]
- 100. Brahimi M, Le Clésiau H, Ouazen Z, Soufi K, Michault A, Pariès J, Cosson E, Valensi P. [Microalbuminuria, a marker of artery rigidity and cardiac dysfunction]. Arch Mal Coeur Vaiss 2007; 100:673–676. [PubMed] [Google Scholar]
- 101. Bruno RM, Penno G, Daniele G, Pucci L, Lucchesi D, Stea F, Landini L, Cartoni G, Taddei S, Ghiadoni L, Del Prato S. Type 2 diabetes mellitus worsens arterial stiffness in hypertensive patients through endothelial dysfunction. Diabetologia 2012; 55:1847–1855. [DOI] [PubMed] [Google Scholar]
- 102. Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006; 113:657–663. [DOI] [PubMed] [Google Scholar]
- 103. Matsumoto S, Nakanishi R, Luo Y, Kim M, Alani A, Nezarat N, Dailing C, Budoff MJ. The relationship between cardio-ankle vascular index and subclinical atherosclerosis evaluated by cardiac computed tomographic angiography. Clin Cardiol 2017; 40:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J 2008; 72:598–604. [DOI] [PubMed] [Google Scholar]
- 105. Izuhara M, Shioji K, Kadota S, Baba O, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Relationship of cardio-ankle vascular index (CAVI) to carotid and coronary arteriosclerosis. Circ J 2008; 72:1762–1767. [DOI] [PubMed] [Google Scholar]
- 106. Park JB, Park HE, Choi SY, Kim MK, Oh BH. Relation between cardio-ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J Atheroscler Thromb 2013; 20:557–567. [DOI] [PubMed] [Google Scholar]
- 107. Birudaraju D, Cherukuri L, Kinninger A, Chaganti BT, Haroun P, Pidikiti S, Lakshmanan S, Hamal S, Flores F, Dailing C, Shaikh K, Roy SK, Budoff MJ. Relationship between cardio-ankle vascular index and obstructive coronary artery disease. Coron Artery Dis 2020; 31:550–555. [DOI] [PubMed] [Google Scholar]
- 108. Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993; 88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 109. Tanaka H, Palta P, Folsom AR, Meyer ML, Matsushita K, Evenson KR, Aguilar D, Heiss G. Habitual physical activity and central artery stiffening in older adults: the Atherosclerosis Risk in Communities study. J Hypertens 2018; 36:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ahmadi-Abhari S, Sabia S, Shipley MJ, Kivimaki M, Singh-Manoux A, Tabak A, McEniery C, Wilkinson IB, Brunner EJ. Physical activity, sedentary behavior, and long-term changes in aortic stiffness: the Whitehall II Study. J Am Heart Assoc 2017; 6:e005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tanaka H. Antiaging effects of aerobic exercise on systemic arteries. Hypertension 2019; 74:237–243. [DOI] [PubMed] [Google Scholar]
- 112. Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 2003; 41:130–135. [DOI] [PubMed] [Google Scholar]
- 113. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 2013; 47:393–396. [DOI] [PubMed] [Google Scholar]
- 114. Evans W, Willey Q, Hanson ED, Stoner L. Effects of resistance training on arterial stiffness in persons at risk for cardiovascular disease: a meta-analysis. Sports Med 2018; 48:2785–2795. [DOI] [PubMed] [Google Scholar]
- 115. Ceciliato J, Costa EC, Azevêdo L, Sousa JC, Fecchio RY, Brito LC. Effect of resistance training on arterial stiffness in healthy subjects: a systematic review and meta-analysis. Curr Hypertens Rep 2020; 22:51–55. [DOI] [PubMed] [Google Scholar]
- 116. Cooper JN, Buchanich JM, Youk A, Brooks MM, Barinas-Mitchell E, Conroy MB, Sutton-Tyrrell K. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis 2012; 223:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 118. Nagayama D, Endo K, Ohira M, Yamaguchi T, Ban N, Kawana H, Nagumo A, Saiki A, Oyama T, Miyashita Y, Shirai K. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes Res Clin Pract 2013; 7:e139–e145. [DOI] [PubMed] [Google Scholar]
- 119. D’Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J Hypertens 2018; 36:734–743. [DOI] [PubMed] [Google Scholar]
- 120. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens 2015; 33:1742–1757; discussion 1757. [DOI] [PubMed] [Google Scholar]
- 122. Asmar R. Principles and usefulness of the cardio-ankle vascular index (CAVI): a new global arterial stiffness index. Eur Heart J 2017; 19:B4–B10. [Google Scholar]
- 123. Dobšák P, Tomandl J, Spinarova L, Vitovec J, Dusek L, Novakova M, Jarkovsky J, Krejci J, Hude P, Honek T, Siegelova J, Homolka P. Effects of neuromuscular electrical stimulation and aerobic exercise training on arterial stiffness and autonomic functions in patients with chronic heart failure. Artif Organs 2012; 36:920–930. [DOI] [PubMed] [Google Scholar]
- 124. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 125. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and control among adults: United States, 2015–2016. NCHS Data Brief 2017; 289:1–8. [PubMed] [Google Scholar]
- 126. Laurent S, Chatellier G, Azizi M, Calvet D, Choukroun G, Danchin N, Delsart P, Girerd X, Gosse P, Khettab H, London G, Mourad JJ, Pannier B, Pereira H, Stephan D, Valensi P, Cunha P, Narkiewicz K, Bruno RM, Boutouyrie P; SPARTE Investigators . SPARTE Study: normalization of arterial stiffness and cardiovascular events in patients with hypertension at medium to very high risk. Hypertension 2021; 78:983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010; 362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]